Activation of TGF-? Canonical and Noncanonical Signaling in Bovine Lactoferrin-Induced Osteogenic Activity of C3H10T1/2 Mesenchymal Stem Cells
Abstract
:1. Introduction
2. Results
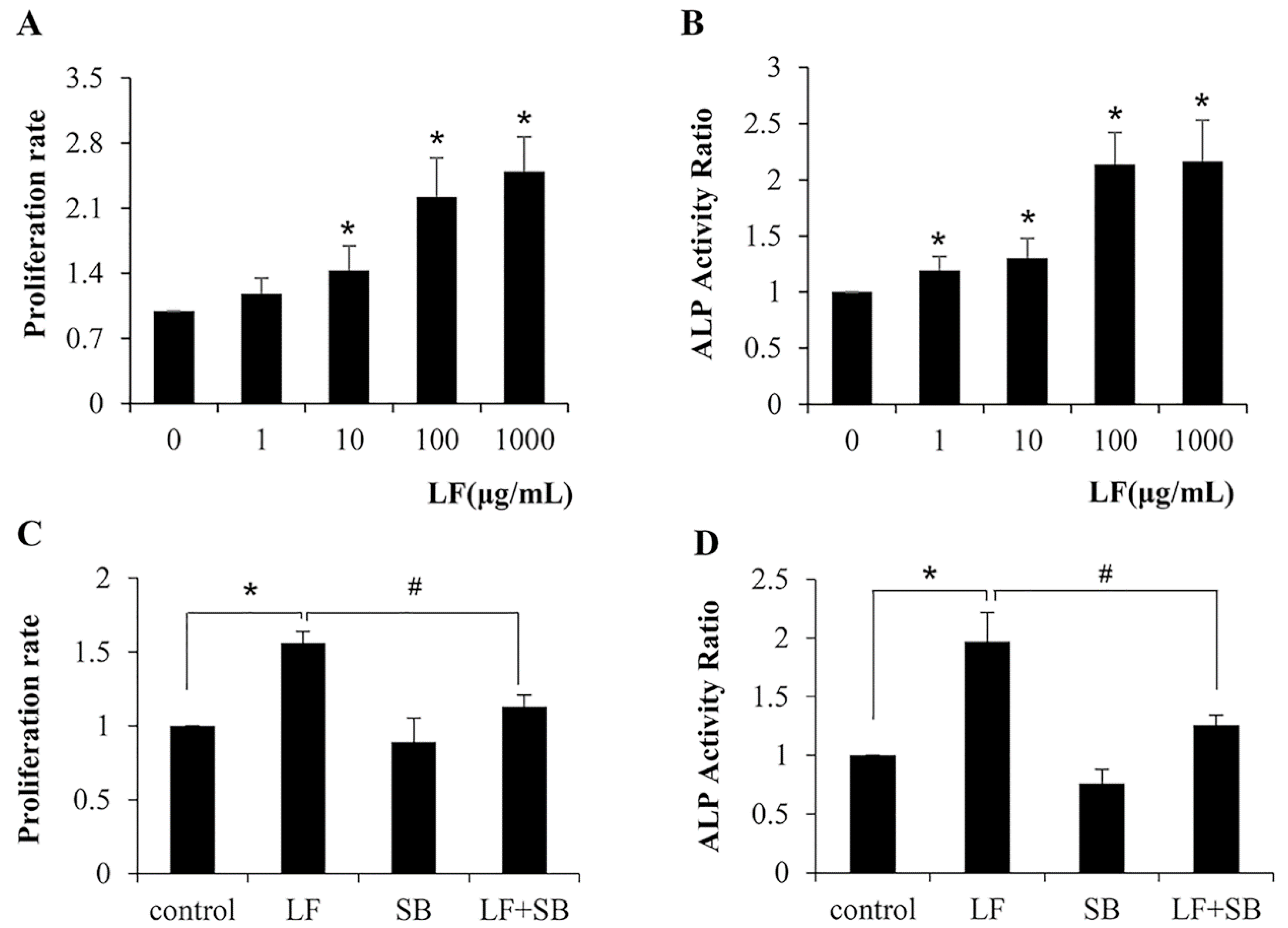
2.1. TGF-β Pathway Is Required for LF-Induced Proliferation and Differentiation in Mesenchymal Stem Cells C3H10T1/2
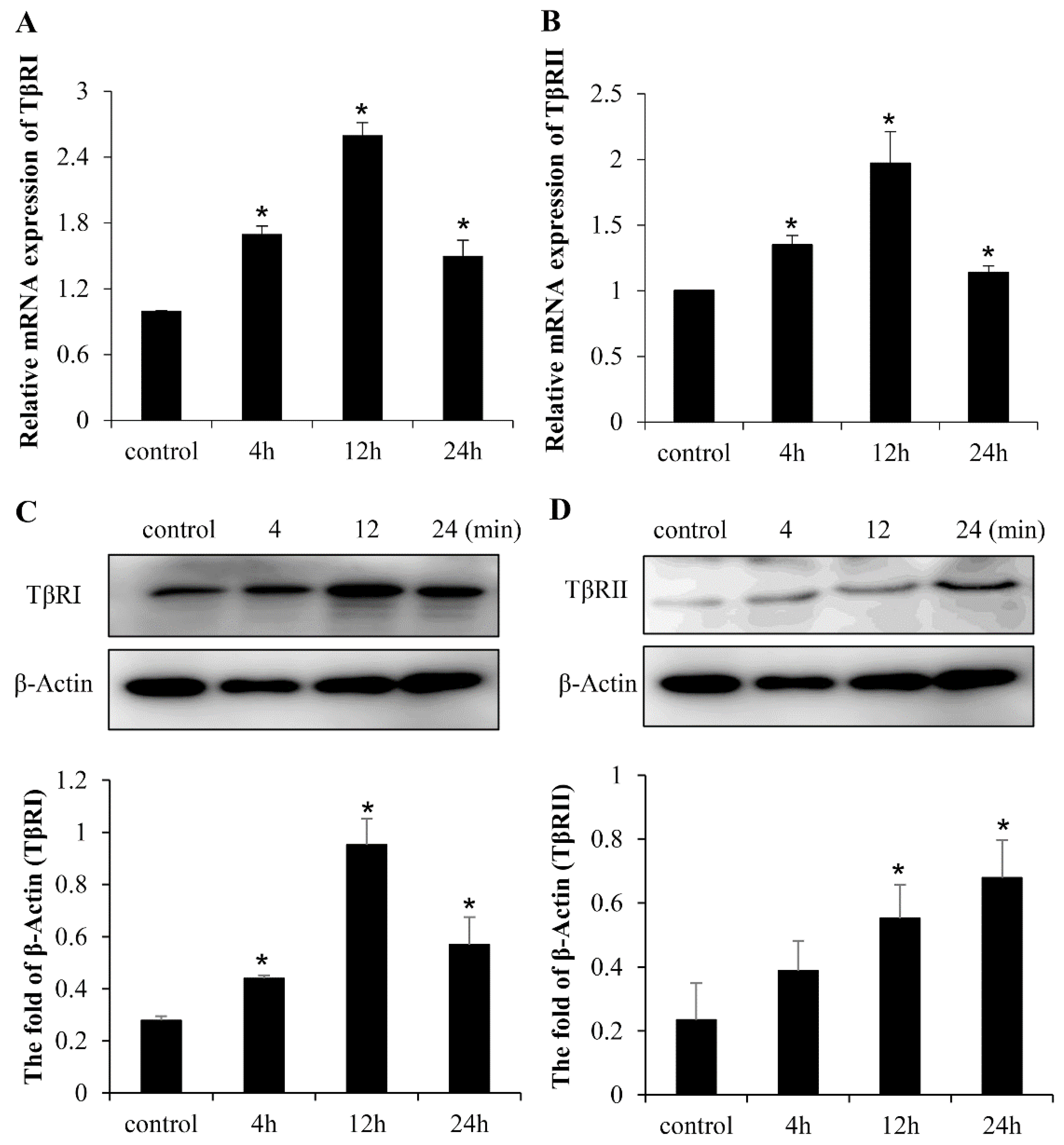
2.2. TGF-β Pathway Is Involved in the LF-Induced Expression of Gene Markers for the Osteogenic Activity in Mesenchymal Stem Cells
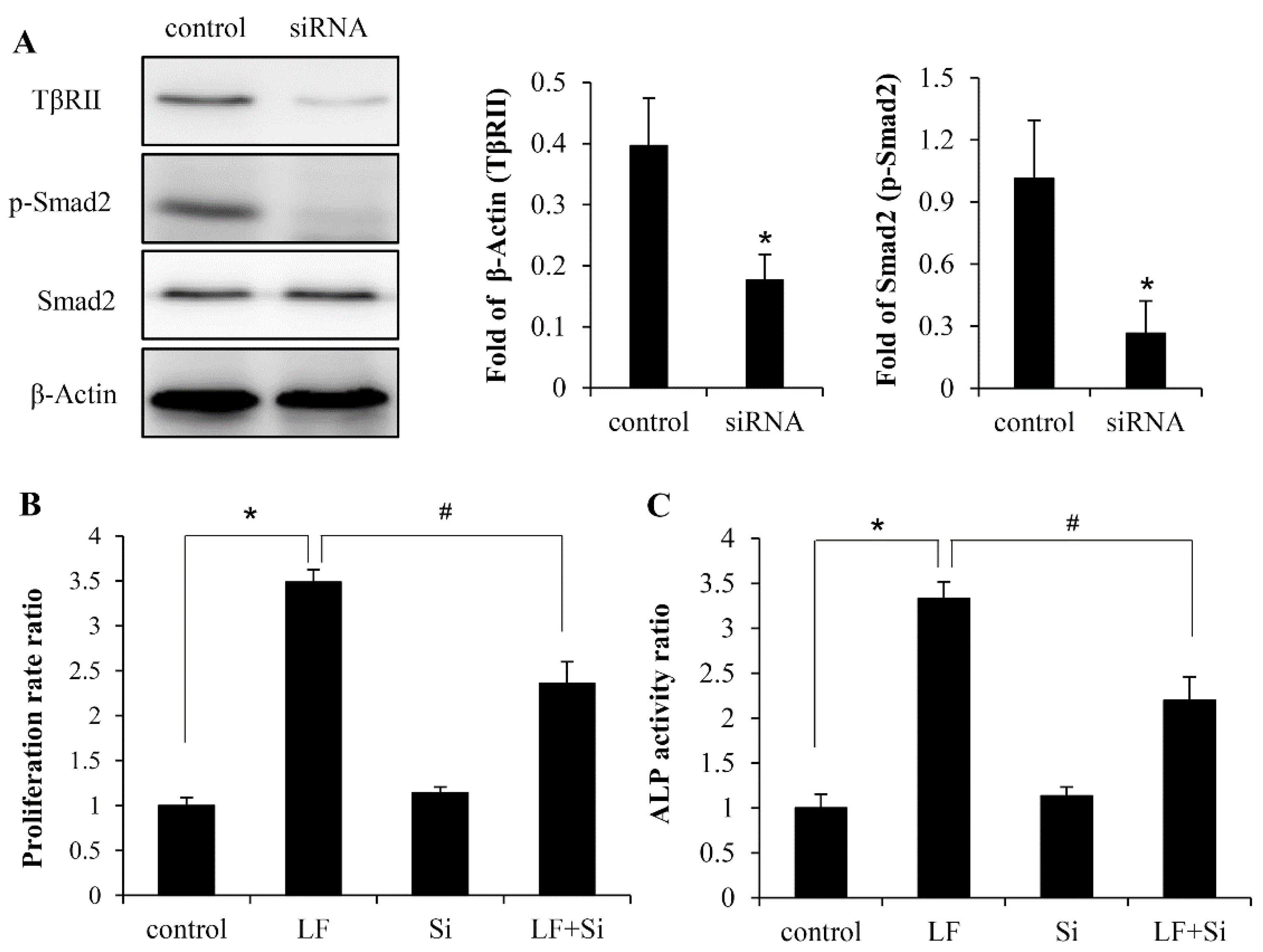
2.3. LF Induces Osteogenic Activity in Mesenchymal Stem Cells Is Mediated by TGF-β Receptors
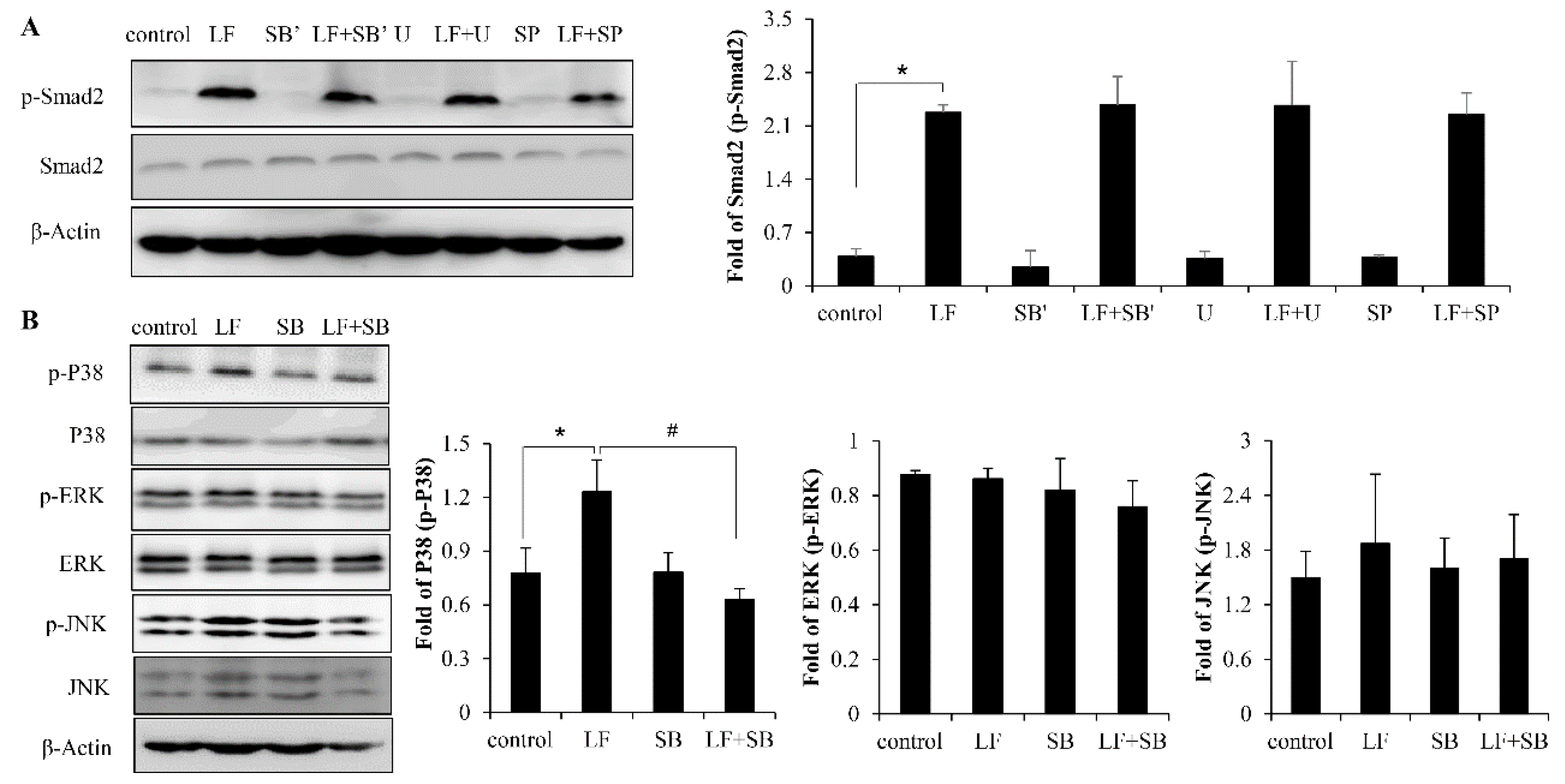
2.4. LF Activates TGF-β/smad2 Signaling Pathway in Mesenchymal Stem Cells
2.5. LF Activates TGF-β/smad2 Pathway Dependent on p38 MAPK Signaling Pathway
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cells Culture and Treatment
4.3. Cell Proliferation Assay
4.4. ALP Activity Assay
4.5. Western Blot
4.6. Quantitative Real-Time PCR Analysis
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Alexander, D.B.; Iigo, M.; Yamauchi, K.; Suzui, M.; Tsuda, H. Lactoferrin: An alternative view of its role in human biological fluids. Biochem. Cell Biol. 2012, 90, 279–306. [Google Scholar] [CrossRef] [PubMed]
- Legrand, D. Lactoferrin, a key molecule in immune and inflammatory processes. Biochem. Cell Biol. 2012, 90, 252–268. [Google Scholar] [CrossRef] [PubMed]
- Ward, P.P.; Paz, E.; Conneely, O.M. Multifunctional roles of lactoferrin: A critical overview. Cell Mol. Life Sci. 2005, 62, 2540–2548. [Google Scholar] [CrossRef] [PubMed]
- Tomita, M.; Wakabayashi, H.; Shin, K.; Yamauchi, K.; Yaeshima, T.; Iwatsuki, K. Twenty-five years of research on bovine lactoferrin applications. Biochimie 2009, 91, 52–57. [Google Scholar] [CrossRef]
- Cornish, J.; Callon, K.E.; Naot, D.; Palmano, K.P.; Banovic, T.; Bava, U.; Watson, M.; Lin, J.M.; Tong, P.C.; Chen, Q.; et al. Lactoferrin is a potent regulator of bone cell activity and increases bone. formation in vivo. Endocrinology 2004, 145, 4366–4374. [Google Scholar] [CrossRef] [PubMed]
- Amini, A.A.; Nair, L.S. Lactoferrin: A Biologically Active Molecule for Bone Regeneration. Curr. Med. Chem. 2011, 18, 1220–1229. [Google Scholar] [CrossRef] [PubMed]
- Grey, A.; Zhu, Q.; Watson, M.; Callon, K.; Cornish, J. Lactoferrin potently inhibits osteoblast apoptosis, via an LRP1-independent pathway. Mol. Cell. Endocrinol. 2006, 251, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Takayama, Y.; Mizumachi, K. Effect of lactoferrin-embedded collagen membrane on osteogenic differentiation of human osteoblast-like cells. J. Biosci. Bioeng. 2009, 107, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.M.; Wu, M.; Lin, Q.M.; Lin, F.; Xue, Y.; Lan, X.H.; Chen, E.Y.; Wang, M.L.; Yang, H.Y.; Wang, F.X. Lactoferrin promote primary rat osteoblast proliferation and differentiation via up-regulation of insulin-like growth factor-1 expression (Retraction of vol 41, pg 5019, 2014). Mol. Biol. Rep. 2015, 42, 1467. [Google Scholar] [CrossRef]
- Guo, H.Y.; Jiang, L.; Ibrahim, S.A.; Zhang, L.; Zhang, H.; Zhang, M.; Ren, F.Z. Orally Administered Lactoferrin Preserves Bone Mass and Microarchitecture in Ovariectomized Rats. J. Nutr. 2009, 139, 958–964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bharadwaj, S.; Naidu, A.G.T.; Betageri, G.V.; Prasadarao, N.V.; Naidu, A.S. Milk ribonuclease-enriched lactoferrin induces positive effects on bone turnover markers in postmenopausal women. Osteoporos. Int. 2009, 20, 1603–1611. [Google Scholar] [CrossRef] [PubMed]
- Aly, E.; Lopez-Nicolas, R.; Darwish, A.A.; Frontela-Saseta, C.; Ros-Berruezo, G. Supplementation of infant formulas with recombinant human lactoferrin and/or galactooligosaccharides increases iron bioaccessibility as measured by ferritin formed in Caco-2 cell model. Food Res. Int. 2016, 89, 1048–1055. [Google Scholar] [CrossRef]
- Chen, K.; Chai, L.Y.; Li, H.; Zhang, Y.; Xie, H.M.; Shang, J.; Tian, W.Z.; Yang, P.; Jiang, A.C. Effect of bovine lactoferrin from iron-fortified formulas on diarrhea and respiratory tract infections of weaned infants in a randomized controlled trial. Nutrition 2016, 32, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Jovani, A.; Barbera, R.; Farre, R. Lactoferrin and its possible role in iron enrichment of infant formulas. Food Sci. Technol. Int. 2001, 7, 97–103. [Google Scholar] [CrossRef]
- Aly, E.; Frontela, C.; Martinez, C.; Ros, G. Mineral Availability of Infant Formulas Supplemented with Lactoferrin and / or Gos after in Vitro Gastrointestinal Digestion. Ann. Nutr. Metab. 2013, 63, 1669. [Google Scholar]
- Katagiri, T.; Takahashi, N. Regulatory mechanisms of osteoblast and osteoclast differentiation. Oral. Dis. 2002, 8, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Ripamonti, U.; Duarte, R.; Parak, R.; Dickens, C.; Dix-Peek, T.; Klar, R.M. Redundancy and Molecular Evolution: The Rapid Induction of Bone Formation by the Mammalian Transforming Growth Factor-beta(3) Isoform. Front. Physiol. 2016, 7, 396. [Google Scholar] [CrossRef] [PubMed]
- Derynck, R.; Akhurst, R.J. Differentiation plasticity regulated by TGF-beta family proteins in development and disease. Nat. Cell Biol. 2007, 9, 1000–1004. [Google Scholar] [CrossRef] [PubMed]
- Querques, F.; Cantilena, B.; Cozzolino, C.; Esposito, M.T.; Passaro, F.; Parisi, S.; Lombardo, B.; Russo, T.; Pastore, L. Angiotensin Receptor I Stimulates Osteoprogenitor Proliferation Through TGF beta-Mediated Signaling. J. Cell Physiol. 2015, 230, 1466–1474. [Google Scholar] [CrossRef]
- Zeng, H.C.; Bae, Y.; Dawson, B.C.; Chen, Y.Q.; Bertin, T.; Munivez, E.; Campeau, P.M.; Tao, J.N.; Chen, R.; Lee, B.H. MicroRNA miR-23a cluster promotes osteocyte differentiation by regulating TGF-beta signalling in osteoblasts. Nat. Commun. 2017, 8, 15000. [Google Scholar] [CrossRef]
- Chakravorty, N.; Hamlet, S.; Jaiprakash, A.; Crawford, R.; Oloyede, A.; Alfarsi, M.; Xiao, Y.; Ivanovski, S. Pro-osteogenic topographical cues promote early activation of osteoprogenitor differentiation via enhanced TGF beta, Wnt, and Notch signaling. Clin. Oral. Implan. Res. 2014, 25, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.S.; Hong, S.H.; Bae, S.C. Both the Smad and p38 MAPK pathways play a crucial role in Runx2 expression following induction by transforming growth factor-beta and bone morphogenetic protein. Oncogene 2002, 21, 7156–7163. [Google Scholar] [CrossRef] [PubMed]
- Fong, D.; Bisson, M.; Laberge, G.; McManus, S.; Grenier, G.; Faucheux, N.; Roux, S. Bone morphogenetic protein-9 activates Smad and ERK pathways and supports human osteoclast function and survival in vitro. Cell Signal. 2013, 25, 717–728. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.X.; Wang, J.X.; Ren, F.Z.; Zhang, W.; Zhang, H.; Zhao, L.; Zhang, M.; Cui, W.; Wang, X.B.; Guo, H.Y. Lactoferrin Promotes Osteogenesis through TGF-ss Receptor II Binding in Osteoblasts and Activation of Canonical TGF-ss Signaling in MC3T3-E1 Cells and C57BL/6J Mice. J. Nutr. 2018, 148, 1285–1292. [Google Scholar] [CrossRef] [PubMed]
- Grey, A.; Banovic, T.; Zhu, Q.; Watson, M.; Callon, K.; Palmano, K.; Ross, J.; Naot, D.; Reid, I.R.; Cornish, J. The low-density lipoprotein receptor-related protein 1 is a mitogenic receptor for lactoferrin in osteoblastic cells. Mol. Endocrinol. 2004, 18, 2268–2278. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Guo, H.Y.; Jing, H.; Li, Y.X.; Wang, X.Y.; Zhang, H.; Jiang, L.; Ren, F.Z. Lactoferrin Stimulates Osteoblast Differentiation Through PKA and p38 Pathways Independent of Lactoferrin’s Receptor LRP1. J. Bone Miner. Res. 2014, 29, 1232–1243. [Google Scholar] [CrossRef] [PubMed]
- Gazit, D.; Ebner, R.; Kahn, A.J.; Derynck, R. Modulation of Expression and Cell-Surface Binding of Members of the Transforming Growth-Factor-Beta Superfamily during Retinoic Acid-Induced Osteoblastic Differentiation of Multipotential Mesenchymal Cells. Mol. Endocrinol. 1993, 7, 189–198. [Google Scholar] [PubMed]
- Chen, D.; Ye, W.S.; Cai, F. Bone morphogenetic protein receptor and signal transduction. Prog. Biochem. Biophys. 1999, 26, 141–143. [Google Scholar]
- Lee, J.S.; Park, J.H.; Kwon, I.K.; Lim, J.Y. Retinoic acid inhibits BMP4-induced C3H10T1/2 stem cell commitment to adipocyte via downregulating Smad/p38MAPK signaling. Biochem. Biophys. Res. Commun. 2011, 409, 550–555. [Google Scholar] [CrossRef]
- Makhijani, N.S.; Bischoff, D.S.; Yamaguchi, D.T. Regulation of proliferation and migration in retinoic acid treated C3H10T1/2 cells by TGF-beta Isoforms. J. Cell Physiol. 2005, 202, 304–313. [Google Scholar] [CrossRef]
- Higuchi, C.; Myoui, A.; Hashimoto, N.; Kuriyama, K.; Yoshioka, K.; Yoshikawa, H.; Itoh, K. Continuous inhibition of MAPK signaling promotes the early osteoblastic differentiation and mineralization of the extracellular matrix. J. Bone Miner. Res. 2002, 17, 1785–1794. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, M. Matrix proteins versus cytokines in the regulation of osteoblast function and bone formation. Calcif. Tissue Int. 2003, 72, 5–7. [Google Scholar] [CrossRef] [PubMed]
- Lian, J.B.; Javed, A.; Zaidi, S.K.; Lengner, C.; Montecino, M.; van Wijnen, A.J.; Stein, J.L.; Stein, G.S. Regulatory controls for osteoblast growth and differentiation: Role of Runx/Cbfa/AML factors. Crit. Rev. Eukar. Gene 2004, 14, 1–41. [Google Scholar] [CrossRef]
- Karsenty, G. Transcriptional Control of Osteoblast Differentiation. Cold Spring Harb. Mon. 2009, 53, 205–217. [Google Scholar] [CrossRef]
- Marie, P.J. Transcription factors controlling osteoblastogenesis. Arch. Biochem. Biophys. 2008, 473, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Marie, P.J. Growth factors and osteogenesis: Applications to bone reconstruction. Innov. Tech. Biol. Med. 1995, 16, 67–76. [Google Scholar]
- Andrades, J.A.; Nimni, M.E.; Becerra, J. The use of modified recombinant growth factors in osteogenesis. Bone 1999, 24, 428. [Google Scholar]
- Shao, P.L.; Wu, S.C.; Lin, Z.Y.; Ho, M.L.; Chen, C.H.; Wang, C.Z. Alpha-5 Integrin Mediates Simvastatin-Induced Osteogenesis of Bone Marrow Mesenchymal Stem Cells. Int. J. Mol. Sci. 2019, 20, 506. [Google Scholar] [CrossRef]
- ten Dijke, P.; Korchynskyi, O.; Valdimarsdottir, G.; Goumans, M.J. Controlling cell fate by bone morphogenetic protein receptors. Mol. Cell. Endocrinol. 2003, 211, 105–113. [Google Scholar] [CrossRef]
- Huang, Y.F.; Lin, J.J.; Lin, C.H.; Su, Y.; Hung, S.C. c-Jun N-terminal kinase 1 negatively regulates osteoblastic differentiation induced by BMP2 via phosphorylation of Runx2 at Ser104. J. Bone Miner. Res. 2012, 27, 1093–1105. [Google Scholar] [CrossRef]
- Liu, H.; Liu, Y.S.; Viggeswarapu, M.; Zheng, Z.M.; Titus, L.; Boden, S.D. Activation of c-Jun NH2-Terminal Kinase 1 Increases Cellular Responsiveness to BMP-2 and Decreases Binding of Inhibitory Smad6 to the Type 1 BMP Receptor. J. Bone Miner. Res. 2011, 26, 1122–1132. [Google Scholar] [CrossRef]
- Nakayama, K.; Tamura, Y.; Suzawa, M.; Harada, S.; Fukumoto, S.; Kato, M.; Miyazono, K.; Rodan, G.A.; Takeuchi, Y.; Fujita, T. Receptor tyrosine kinases inhibit bone morphogenetic protein-smad responsive promoter activity and differentiation of murine MC3T3-E1 osteoblast-like cells. J. Bone Miner. Res. 2003, 18, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Greenblatt, M.B.; Shim, J.H.; Zou, W.G.; Sitara, D.; Schweitzer, M.; Hu, D.; Lotinun, S.; Sano, Y.; Baron, R.; Park, J.M.; et al. The p38 MAPK pathway is essential for skeletogenesis and bone homeostasis in mice. J. Clin. Investig. 2010, 120, 2457–2473. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.Y.; Chan, E.; Wang, S.X.; Li, B.J. Activation of p38 mitogen-activated protein kinase is required for osteoblast differentiation. Endocrinology 2003, 144, 2068–2074. [Google Scholar] [CrossRef]
- Ortuno, M.J.; Ruiz-Gaspa, S.; Rodriguez-Carballo, E.; Susperregui, A.R.G.; Bartrons, R.; Rosa, J.L.; Ventura, F. p38 Regulates Expression of Osteoblast-specific Genes by Phosphorylation of Osterix. J. Biol. Chem. 2010, 285, 31985–31994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuguchi, T.; Chiba, N.; Bandow, K.; Kakimoto, K.; Masuda, A.; Ohnishi, T. JNK Activity Is Essential for Atf4 Expression and Late-Stage Osteoblast Differentiation. J. Bone Miner. Res. 2009, 24, 398–410. [Google Scholar] [CrossRef]
- Xiao, G.Z.; Jiang, D.; Thomas, P.; Benson, M.D.; Guan, K.L.; Karsenty, G.; Franceschi, R.T. MAPK pathways activate and phosphorylate the osteoblast-specific transcription factor, Cbfa1. J. Biol. Chem. 2000, 275, 4453–4459. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.Y.; Song, T.J.; Li, X.; Hu, L.L.; He, Q.; Liu, M.; Lane, M.D.; Tang, Q.Q. BMP signaling pathway is required for commitment of C3H10T1/2 pluripotent stem cells to the adipocyte lineage. Proc. Natl. Acad. Sci. USA 2009, 106, 12670–12675. [Google Scholar] [CrossRef] [Green Version]
- Qin, W.; Lin, Z.M.; Deng, R.; Li, D.D.; Song, Z.; Tian, Y.G.; Wang, R.F.; Ling, J.Q.; Zhu, X.F. p38a MAPK is involved in BMP-2-induced odontoblastic differentiation of human dental pulp cells. Int. Endod. J. 2012, 45, 224–233. [Google Scholar] [CrossRef]
- Greenblatt, M.B.; Shim, J.H.; Glimcher, L.H. TAK1 mediates BMP signaling in cartilage. Skelet. Biol. Med. 2010, 1192, 385–390. [Google Scholar] [CrossRef] [Green Version]
- Haynes, T.; Gutierrez, C.; Aycinena, J.C.; Tsonis, P.A.; Del Rio-Tsonis, K. BMP signaling mediates stem/progenitor cell-induced retina regeneration. Proc. Natl. Acad. Sci. USA 2007, 104, 20380–20385. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Guo, H.Y.; Zhang, W.; Wen, P.C.; Zhang, H.; Guo, Z.R.; Ren, F.Z. Effect of iron saturation level of lactoferrin on osteogenic activity in vitro and in vivo. J. Dairy Sci. 2013, 96, 33–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Gene Name | Primers Sequences |
|---|---|
| OCN | forward 5′-TGCTTGTGACGAGCTATCAG-3′ reverse 5′-GAGGACAGGGAGGATCAAGT-3′ |
| OPN | forward 5′-ATCTCACCATTCGGATGAGTCT-3′ reverse 5′-TGTAGGGACGATTGGAGTGAAA-3′ |
| Col2a1 | forward 5′-GGGTCACAGAGGTTACCCAG-3′ reverse 5′- ACCAGGGGAACCACTCTCAC-3′ |
| FGF2 | forward 5′-GCGACCCACACGTCAAACTA-3′ reverse 5′-CCGTCCATCTTCCTTCATAGC-3′ |
| GAPDH | forward 5′-TGGCAAAGTGGAGATTGTTGC-3′ reverse 5′-AAGATGGTGATGGGCTTCCCG-3′ |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Zhang, W.; Ren, F.; Guo, H. Activation of TGF-? Canonical and Noncanonical Signaling in Bovine Lactoferrin-Induced Osteogenic Activity of C3H10T1/2 Mesenchymal Stem Cells. Int. J. Mol. Sci. 2019, 20, 2880. https://doi.org/10.3390/ijms20122880
Li Y, Zhang W, Ren F, Guo H. Activation of TGF-? Canonical and Noncanonical Signaling in Bovine Lactoferrin-Induced Osteogenic Activity of C3H10T1/2 Mesenchymal Stem Cells. International Journal of Molecular Sciences. 2019; 20(12):2880. https://doi.org/10.3390/ijms20122880
Chicago/Turabian StyleLi, Yixuan, Wei Zhang, Fazheng Ren, and Huiyuan Guo. 2019. "Activation of TGF-? Canonical and Noncanonical Signaling in Bovine Lactoferrin-Induced Osteogenic Activity of C3H10T1/2 Mesenchymal Stem Cells" International Journal of Molecular Sciences 20, no. 12: 2880. https://doi.org/10.3390/ijms20122880
APA StyleLi, Y., Zhang, W., Ren, F., & Guo, H. (2019). Activation of TGF-? Canonical and Noncanonical Signaling in Bovine Lactoferrin-Induced Osteogenic Activity of C3H10T1/2 Mesenchymal Stem Cells. International Journal of Molecular Sciences, 20(12), 2880. https://doi.org/10.3390/ijms20122880




