Mucormycosis of the Central Nervous System
Abstract
:1. Introduction
2. Epidemiology
2.1. Diabetes Mellitus
2.2. Malignancy
2.3. Trauma
2.4. Injection Drug Use
3. Pathogenesis
4. Microbiology
5. Clinical Features
5.1. Rhino-Orbito-Cerebral Mucormycosis (ROCM)
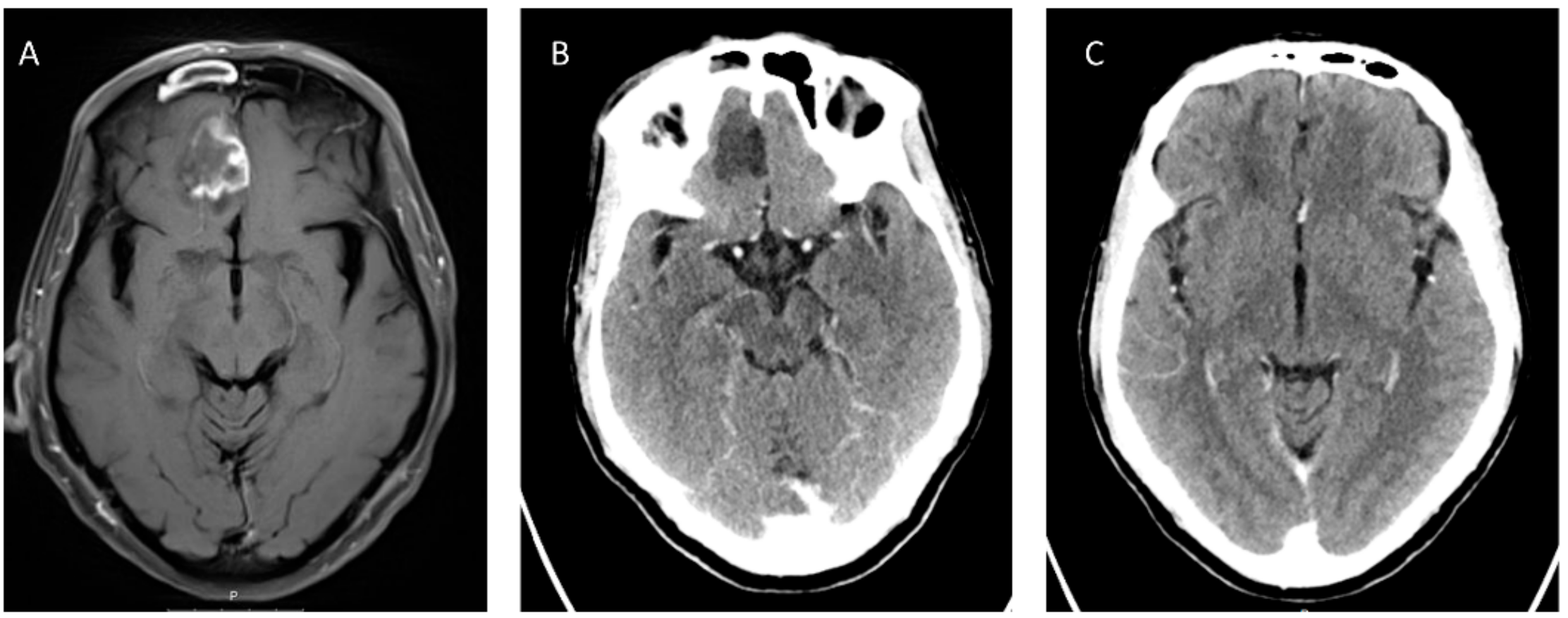
5.2. Radiological Findings
5.3. Pulmonary Mucormycosis
5.4. Isolated Cerebral Mucormycosis
5.5. Intracranial Granulomatous Mucormycosis
6. Diagnosis
7. Treatment
7.1. Surgery
7.2. Antifungal Drugs
8. Adjunctive Therapeutic Modalities
8.1. Iron Chelation
8.2. Hyperbaric Oxygen (HBO)
8.3. Echinocandins
9. Future Directions
Funding
Acknowledgments
Conflicts of Interest
References
- Neofytos, D.; Horn, D.; Anaissie, E.; Steinbach, W.; Olyaei, A.; Fishman, J.; Pfaller, M.; Chang, C.; Webster, K.; Marr, K. Epidemiology and outcome of invasive fungal infection in adult hematopoietic stem cell transplant recipients: Analysis of Multicenter Prospective Antifungal Therapy (PATH) Alliance registry. Clin. Infect. Dis. 2009, 48, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Kume, H.; Yamazaki, T.; Abe, M.; Tanuma, H.; Okudaira, M.; Okayasu, I. Increase in aspergillosis and severe mycotic infection in patients with leukemia and MDS: Comparison of the data from the Annual of the Pathological Autopsy Cases in Japan in 1989, 1993 and 1997. Pathol. Int. 2003, 53, 744–750. [Google Scholar] [CrossRef] [PubMed]
- Kontoyiannis, D.P.; Marr, K.A.; Park, B.J.; Alexander, B.D.; Anaissie, E.J.; Walsh, T.J.; Ito, J.; Andes, D.R.; Baddley, J.W.; Brown, J.M.; et al. Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001–2006: Overview of the Transplant-Associated Infection Surveillance Network (TRANSNET) Database. Clin. Infect. Dis. 2010, 50, 1091–1100. [Google Scholar] [CrossRef] [PubMed]
- Bitar, D.; Van Cauteren, D.; Lanternier, F.; Dannaoui, E.; Che, D.; Dromer, F.; Desenclos, J.C.; Lortholary, O. Increasing incidence of zygomycosis (mucormycosis), France, 1997–2006. Emerg. Infect. Dis. 2009, 15, 1395–1401. [Google Scholar] [CrossRef] [PubMed]
- Chamilos, G.; Luna, M.; Lewis, R.E.; Bodey, G.P.; Chemaly, R.; Tarrand, J.J.; Safdar, A.; Raad, II.; Kontoyiannis, D.P. Invasive fungal infections in patients with hematologic malignancies in a tertiary care cancer center: An autopsy study over a 15-year period (1989–2003). Haematologica 2006, 91, 986–989. [Google Scholar]
- Lewis, R.E.; Cahyame-Zuniga, L.; Leventakos, K.; Chamilos, G.; Ben-Ami, R.; Tamboli, P.; Tarrand, J.; Bodey, G.P.; Luna, M.; Kontoyiannis, D.P. Epidemiology and sites of involvement of invasive fungal infections in patients with haematological malignancies: A 20-year autopsy study. Mycoses 2013, 56, 638–645. [Google Scholar] [CrossRef] [PubMed]
- Guinea, J.; Escribano, P.; Vena, A.; Munoz, P.; Martinez-Jimenez, M.D.C.; Padilla, B.; Bouza, E. Increasing incidence of mucormycosis in a large Spanish hospital from 2007 to 2015: Epidemiology and microbiological characterization of the isolates. PLoS ONE 2017, 12, e0179136. [Google Scholar] [CrossRef]
- Roden, M.M.; Zaoutis, T.E.; Buchanan, W.L.; Knudsen, T.A.; Sarkisova, T.A.; Schaufele, R.L.; Sein, M.; Sein, T.; Chiou, C.C.; Chu, J.H.; et al. Epidemiology and outcome of zygomycosis: A review of 929 reported cases. Clin. Infect. Dis. 2005, 41, 634–653. [Google Scholar] [CrossRef]
- Lanternier, F.; Dannaoui, E.; Morizot, G.; Elie, C.; Garcia-Hermoso, D.; Huerre, M.; Bitar, D.; Dromer, F.; Lortholary, O.; French Mycosis Study Group. A global analysis of mucormycosis in France: The RetroZygo Study (2005–2007). Clin. Infect. Dis. 2012, 54, S35–S43. [Google Scholar] [CrossRef]
- Ben-Ami, R.; Luna, M.; Lewis, R.E.; Walsh, T.J.; Kontoyiannis, D.P. A clinicopathological study of pulmonary mucormycosis in cancer patients: Extensive angioinvasion but limited inflammatory response. J. Infect. 2009, 59, 134–138. [Google Scholar] [CrossRef]
- Bannykh, S.I.; Hunt, B.; Moser, F. Intra-arterial spread of Mucormycetes mediates early ischemic necrosis of brain and suggests new venues for prophylactic therapy. Neuropathology 2018, 38, 539–541. [Google Scholar] [CrossRef] [PubMed]
- Economides, M.P.; Ballester, L.Y.; Kumar, V.A.; Jiang, Y.; Tarrand, J.; Prieto, V.; Torres, H.A.; Kontoyiannis, D.P. Invasive mold infections of the central nervous system in patients with hematologic cancer or stem cell transplantation (2000–2016): Uncommon, with improved survival but still deadly often. J. Infect. 2017, 75, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Higo, T.; Kobayashi, T.; Yamazaki, S.; Ando, S.; Gonoi, W.; Ishida, M.; Okuma, H.; Nakamura, F.; Ushiku, T.; Ohtomo, K.; et al. Cerebral embolism through hematogenous dissemination of pulmonary mucormycosis complicating relapsed leukemia. Int. J. Clin. Exp. Pathol. 2015, 8, 13639–13642. [Google Scholar] [PubMed]
- Manesh, A.; Rupali, P.; Sullivan, M.O.; Mohanraj, P.; Rupa, V.; George, B.; Michael, J.S. Mucormycosis—A clinicoepidemiological review of cases over 10 years. Mycoses 2019, 62, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Torres-Narbona, M.; Guinea, J.; Martinez-Alarcon, J.; Munoz, P.; Gadea, I.; Bouza, E.; Group, M.Z.S. Impact of zygomycosis on microbiology workload: A survey study in Spain. J. Clin. Microbiol. 2007, 45, 2051–2053. [Google Scholar] [CrossRef] [PubMed]
- Rees, J.R.; Pinner, R.W.; Hajjeh, R.A.; Brandt, M.E.; Reingold, A.L. The epidemiological features of invasive mycotic infections in the San Francisco Bay area, 1992–1993: Results of population-based laboratory active surveillance. Clin. Infect. Dis. 1998, 27, 1138–1147. [Google Scholar] [CrossRef] [PubMed]
- Shimodaira, K.; Okubo, Y.; Nakayama, H.; Wakayama, M.; Shinozaki, M.; Ishiwatari, T.; Sasai, D.; Nemoto, T.; Takahashi, K.; Ishii, T.; et al. Trends in the prevalence of invasive fungal infections from an analysis of annual records of autopsy cases of Toho University. Mycoses 2012, 55, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Skiada, A.; Pagano, L.; Groll, A.; Zimmerli, S.; Dupont, B.; Lagrou, K.; Lass-Florl, C.; Bouza, E.; Klimko, N.; Gaustad, P.; et al. Zygomycosis in Europe: Analysis of 230 cases accrued by the registry of the European Confederation of Medical Mycology (ECMM) Working Group on Zygomycosis between 2005 and 2007. Clin. Microbiol. Infect. 2011, 17, 1859–1867. [Google Scholar] [CrossRef]
- Pagano, L.; Offidani, M.; Fianchi, L.; Nosari, A.; Candoni, A.; Picardi, M.; Corvatta, L.; D’Antonio, D.; Girmenia, C.; Martino, P.; et al. Mucormycosis in hematologic patients. Haematologica 2004, 89, 207–214. [Google Scholar]
- Lelievre, L.; Garcia-Hermoso, D.; Abdoul, H.; Hivelin, M.; Chouaki, T.; Toubas, D.; Mamez, A.C.; Lantieri, L.; Lortholary, O.; Lanternier, F.; et al. Posttraumatic mucormycosis: A nationwide study in France and review of the literature. Medicine (Baltimore) 2014, 93, 395–404. [Google Scholar] [CrossRef]
- Hagensee, M.E.; Bauwens, J.E.; Kjos, B.; Bowden, R.A. Brain abscess following marrow transplantation: Experience at the Fred Hutchinson Cancer Research Center, 1984–1992. Clin. Infect. Dis. 1994, 19, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Heimann, S.M.; Vehreschild, M.; Cornely, O.A.; Heinz, W.J.; Gruner, B.; Silling, G.; Kessel, J.; Seidel, D.; Vehreschild, J.J. Healthcare burden of probable and proven invasive mucormycosis: A multi-centre cost-of-illness analysis of patients treated in tertiary care hospitals between 2003 and 2016. J. Hosp. Infect. 2019, 101, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Ruping, M.J.; Heinz, W.J.; Kindo, A.J.; Rickerts, V.; Lass-Florl, C.; Beisel, C.; Herbrecht, R.; Roth, Y.; Silling, G.; Ullmann, A.J.; et al. Forty-one recent cases of invasive zygomycosis from a global clinical registry. J. Antimicrob. Chemother. 2010, 65, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Prakash, H.; Ghosh, A.K.; Rudramurthy, S.M.; Singh, P.; Xess, I.; Savio, J.; Pamidimukkala, U.; Jillwin, J.; Varma, S.; Das, A.; et al. A prospective multicenter study on mucormycosis in India: Epidemiology, diagnosis, and treatment. Med. Mycol. 2018, 57, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, A.; Chatterjee, S.S.; Das, A.; Panda, N.; Shivaprakash, M.R.; Kaur, A.; Varma, S.C.; Singhi, S.; Bhansali, A.; Sakhuja, V. Invasive zygomycosis in India: Experience in a tertiary care hospital. Postgrad. Med. J. 2009, 85, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Bhatkar, S.; Goyal, M.K.; Takkar, A.; Mukherjee, K.K.; Singh, P.; Singh, R.; Lal, V. Cavernous sinus syndrome: A prospective study of 73 cases at a tertiary care centre in Northern India. Clin. Neurol. Neurosurg. 2017, 155, 63–69. [Google Scholar] [CrossRef]
- Kontoyiannis, D.P.; Lionakis, M.S.; Lewis, R.E.; Chamilos, G.; Healy, M.; Perego, C.; Safdar, A.; Kantarjian, H.; Champlin, R.; Walsh, T.J.; et al. Zygomycosis in a tertiary-care cancer center in the era of Aspergillus-active antifungal therapy: A case-control observational study of 27 recent cases. J. Infect. Dis. 2005, 191, 1350–1360. [Google Scholar] [CrossRef]
- Chayakulkeeree, M.; Ghannoum, M.A.; Perfect, J.R. Zygomycosis: The re-emerging fungal infection. Eur. J. Clin. Microbiol. Infect. Dis. 2006, 25, 215–229. [Google Scholar] [CrossRef]
- Kontoyiannis, D.P.; Lewis, R.E. How I treat mucormycosis. Blood 2011, 118, 1216–1224. [Google Scholar] [CrossRef]
- Marr, K.A.; Carter, R.A.; Crippa, F.; Wald, A.; Corey, L. Epidemiology and outcome of mould infections in hematopoietic stem cell transplant recipients. Clin. Infect. Dis. 2002, 34, 909–917. [Google Scholar] [CrossRef]
- Kontoyiannis, D.P. Decrease in the number of reported cases of zygomycosis among patients with diabetes mellitus: A hypothesis. Clin. Infect. Dis. 2007, 44, 1089–1090. [Google Scholar] [CrossRef] [PubMed]
- Candoni, A.; Klimko, N.; Busca, A.; Di Blasi, R.; Shadrivova, O.; Cesaro, S.; Zannier, M.E.; Verga, L.; Forghieri, F.; Calore, E.; et al. Fungal infections of the central nervous system and paranasal sinuses in onco-haematologic patients. Epidemiological study reporting the diagnostic-therapeutic approach and outcome in 89 cases. Mycoses 2019, 62, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Muggeo, P.; Calore, E.; Decembrino, N.; Frenos, S.; De Leonardis, F.; Colombini, A.; Petruzziello, F.; Perruccio, K.; Berger, M.; Burnelli, R.; et al. Invasive mucormycosis in children with cancer: A retrospective study from the Infection Working Group of Italian Pediatric Hematology Oncology Association. Mycoses 2019, 62, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Lionakis, M.S.; Dunleavy, K.; Roschewski, M.; Widemann, B.C.; Butman, J.A.; Schmitz, R.; Yang, Y.; Cole, D.E.; Melani, C.; Higham, C.S.; et al. Inhibition of B Cell Receptor Signaling by Ibrutinib in Primary CNS Lymphoma. Cancer Cell 2017, 31, 833–843. [Google Scholar] [CrossRef] [PubMed]
- Teh, B.W.; Chui, W.; Handunnetti, S.; Tam, C.; Worth, L.J.; Thursky, K.A.; Slavin, M.A. High rates of proven invasive fungal disease with the use of ibrutinib monotherapy for relapsed or refractory chronic lymphocytic leukemia. Leuk. Lymphoma 2019, 60, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Chamilos, G.; Lionakis, M.S.; Kontoyiannis, D.P. Call for Action: Invasive Fungal Infections Associated With Ibrutinib and Other Small Molecule Kinase Inhibitors Targeting Immune Signaling Pathways. Clin. Infect. Dis. 2018, 66, 140–148. [Google Scholar] [CrossRef]
- Ghez, D.; Calleja, A.; Protin, C.; Baron, M.; Ledoux, M.P.; Damaj, G.; Dupont, M.; Dreyfus, B.; Ferrant, E.; Herbaux, C.; et al. Early-onset invasive aspergillosis and other fungal infections in patients treated with ibrutinib. Blood 2018, 131, 1955–1959. [Google Scholar] [CrossRef]
- Arthurs, B.; Wunderle, K.; Hsu, M.; Kim, S. Invasive aspergillosis related to ibrutinib therapy for chronic lymphocytic leukemia. Respir. Med. Case Rep. 2017, 21, 27–29. [Google Scholar] [CrossRef]
- Ruchlemer, R.; Ben Ami, R.; Lachish, T. Ibrutinib for Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2016, 374, 1593–1594. [Google Scholar] [CrossRef]
- Grossi, O.; Pineau, S.; Sadot-Lebouvier, S.; Hay, B.; Delaunay, J.; Miailhe, A.F.; Bretonniere, C.; Jeddi, F.; Lavergne, R.A.; Le Pape, P. Disseminated mucormycosis due to Lichtheimia corymbifera during ibrutinib treatment for relapsed chronic lymphocytic leukaemia: A case report. Clin. Microbiol. Infect. 2019, 25, 261–263. [Google Scholar] [CrossRef]
- Kreiniz, N.; Bejar, J.; Polliack, A.; Tadmor, T. Severe pneumonia associated with ibrutinib monotherapy for CLL and lymphoma. Hematol. Oncol. 2018, 36, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Pouvaret, A.; Guery, R.; Montillet, M.; Molina, T.J.; Dureault, A.; Bougnoux, M.E.; Galliot, R.; Lanternier, F.; Delarue, R.; Lortholary, O. Concurrent cerebral aspergillosis and abdominal mucormycosis during ibrutinib therapy for chronic lymphocytic leukaemia. Clin. Microbiol. Infect. 2019, 25, 771–773. [Google Scholar] [CrossRef] [PubMed]
- Varughese, T.; Taur, Y.; Cohen, N.; Palomba, M.L.; Seo, S.K.; Hohl, T.M.; Redelman-Sidi, G. Serious Infections in Patients Receiving Ibrutinib for Treatment of Lymphoid Malignancies. Clin. Infect. Dis. 2018, 67, 687–692. [Google Scholar] [CrossRef] [PubMed]
- Chamdine, O.; Gaur, A.H.; Broniscer, A. Effective treatment of cerebral mucormycosis associated with brain surgery. Pediatr. Infect. Dis. J. 2015, 34, 542–543. [Google Scholar] [CrossRef] [PubMed]
- Hussain, F.S.; Hussain, N.S. A Unique Case of Intracranial Mucormycosis Following an Assault. Cureus 2016, 8, e696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melsom, S.M.; Khangure, M.S. Craniofacial mucormycosis following assault: An unusual presentation of an unusual disease. Australas. Radiol. 2000, 44, 104–106. [Google Scholar] [CrossRef] [PubMed]
- Kerezoudis, P.; Watts, C.R.; Bydon, M.; Dababneh, A.S.; Deyo, C.N.; Frye, J.M.; Kelley, P.C.; Kemp, A.M.; Palraj, B.V.; Pupillo, G.T. Diagnosis and Treatment of Isolated Cerebral Mucormycosis: Patient-Level Data Meta-Analysis and Mayo Clinic Experience. World Neurosurg. 2019, 123, 425–434. [Google Scholar] [CrossRef]
- Gebremariam, T.; Liu, M.; Luo, G.; Bruno, V.; Phan, Q.T.; Waring, A.J.; Edwards, J.E.; Filler, S.G.; Yeaman, M.R.; Ibrahim, A.S. CotH3 mediates fungal invasion of host cells during mucormycosis. J. Clin. Investig. 2014, 124, 237–250. [Google Scholar] [CrossRef]
- Liu, M.; Spellberg, B.; Phan, Q.T.; Fu, Y.; Fu, Y.; Lee, A.S.; Edwards, J.E., Jr.; Filler, S.G.; Ibrahim, A.S. The endothelial cell receptor GRP78 is required for mucormycosis pathogenesis in diabetic mice. J. Clin. Investig. 2010, 120, 1914–1924. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, A.S.; Spellberg, B.; Avanessian, V.; Fu, Y.; Edwards, J.E., Jr. Rhizopus oryzae adheres to, is phagocytosed by, and damages endothelial cells in vitro. Infect. Immun. 2005, 73, 778–783. [Google Scholar] [CrossRef]
- Ibrahim, A.S.; Gebremariam, T.; Lin, L.; Luo, G.; Husseiny, M.I.; Skory, C.D.; Fu, Y.; French, S.W.; Edwards, J.E., Jr.; Spellberg, B. The high affinity iron permease is a key virulence factor required for Rhizopus oryzae pathogenesis. Mol. Microbiol. 2010, 77, 587–604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levitz, S.M.; Selsted, M.E.; Ganz, T.; Lehrer, R.I.; Diamond, R.D. In vitro killing of spores and hyphae of Aspergillus fumigatus and Rhizopus oryzae by rabbit neutrophil cationic peptides and bronchoalveolar macrophages. J. Infect. Dis. 1986, 154, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Waldorf, A.R.; Ruderman, N.; Diamond, R.D. Specific susceptibility to mucormycosis in murine diabetes and bronchoalveolar macrophage defense against Rhizopus. J. Clin. Investig. 1984, 74, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Chinn, R.Y.; Diamond, R.D. Generation of chemotactic factors by Rhizopus oryzae in the presence and absence of serum: Relationship to hyphal damage mediated by human neutrophils and effects of hyperglycemia and ketoacidosis. Infect. Immun. 1982, 38, 1123–1129. [Google Scholar] [PubMed]
- Sheldon, W.H. The Development of the Acute Inflammatory Response to Experimental Cutaneous Mucormycosis in Normal and Diabetic Rabbits. J. Exp. Med. 1959, 110, 845–852. [Google Scholar] [CrossRef]
- McCarthy, M.; Rosengart, A.; Schuetz, A.N.; Kontoyiannis, D.P.; Walsh, T.J. Mold infections of the central nervous system. N. Engl. J. Med. 2014, 371, 150–160. [Google Scholar] [CrossRef]
- Ochiai, H.; Iseda, T.; Miyahara, S.; Goya, T.; Wakisaka, S. Rhinocerebral mucormycosis—Case report. Neurol. Med. Chir. 1993, 33, 373–376. [Google Scholar] [CrossRef]
- Mantadakis, E.; Samonis, G. Clinical presentation of zygomycosis. Clin. Microbiol. Infect. 2009, 15 (Suppl. 5), 15–20. [Google Scholar] [CrossRef] [Green Version]
- Terry, A.R.; Kahle, K.T.; Larvie, M.; Vyas, J.M.; Stemmer-Rachamimov, A. Case Records of the Massachusetts General Hospital. Case 5-2016. A 43-Year-Old Man with Altered Mental Status and a History of Alcohol Use. N. Engl. J. Med. 2016, 374, 671–680. [Google Scholar] [CrossRef]
- Dusart, A.; Duprez, T.; Van Snick, S.; Godfraind, C.; Sindic, C. Fatal rhinocerebral mucormycosis with intracavernous carotid aneurysm and thrombosis: A late complication of transsphenoidal surgery? Acta Neurol. Belg. 2013, 113, 179–184. [Google Scholar] [CrossRef]
- Malik, A.N.; Bi, W.L.; McCray, B.; Abedalthagafi, M.; Vaitkevicius, H.; Dunn, I.F. Isolated cerebral mucormycosis of the basal ganglia. Clin. Neurol. Neurosurg. 2014, 124, 102–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tseng, H.K.; Huang, T.Y.; Wu, A.Y.; Chen, H.H.; Liu, C.P.; Jong, A. How Cryptococcus interacts with the blood-brain barrier. Future Microbiol. 2015, 10, 1669–1682. [Google Scholar] [CrossRef] [PubMed]
- Artis, W.M.; Fountain, J.A.; Delcher, H.K.; Jones, H.E. A mechanism of susceptibility to mucormycosis in diabetic ketoacidosis: Transferrin and iron availability. Diabetes 1982, 31, 1109–1114. [Google Scholar] [CrossRef] [PubMed]
- de Locht, M.; Boelaert, J.R.; Schneider, Y.J. Iron uptake from ferrioxamine and from ferrirhizoferrin by germinating spores of Rhizopus microsporus. Biochem. Pharmacol. 1994, 47, 1843–1850. [Google Scholar] [CrossRef]
- Alvarez, E.; Sutton, D.A.; Cano, J.; Fothergill, A.W.; Stchigel, A.; Rinaldi, M.G.; Guarro, J. Spectrum of zygomycete species identified in clinically significant specimens in the United States. J. Clin. Microbiol. 2009, 47, 1650–1656. [Google Scholar] [CrossRef]
- Gomes, M.Z.; Lewis, R.E.; Kontoyiannis, D.P. Mucormycosis caused by unusual mucormycetes, non-Rhizopus, -Mucor, and -Lichtheimia species. Clin. Microbiol. Rev. 2011, 24, 411–445. [Google Scholar] [CrossRef] [PubMed]
- Warkentien, T.; Rodriguez, C.; Lloyd, B.; Wells, J.; Weintrob, A.; Dunne, J.R.; Ganesan, A.; Li, P.; Bradley, W.; Gaskins, L.J.; et al. Invasive mold infections following combat-related injuries. Clin. Infect. Dis. 2012, 55, 1441–1449. [Google Scholar] [CrossRef]
- Neblett Fanfair, R.; Benedict, K.; Bos, J.; Bennett, S.D.; Lo, Y.C.; Adebanjo, T.; Etienne, K.; Deak, E.; Derado, G.; Shieh, W.J.; et al. Necrotizing cutaneous mucormycosis after a tornado in Joplin, Missouri, in 2011. N. Engl. J. Med. 2012, 367, 2214–2225. [Google Scholar] [CrossRef]
- Chakrabarti, A.; Shivaprakash, M.R.; Curfs-Breuker, I.; Baghela, A.; Klaassen, C.H.; Meis, J.F. Apophysomyces elegans: Epidemiology, amplified fragment length polymorphism typing, and in vitro antifungal susceptibility pattern. J. Clin. Microbiol. 2010, 48, 4580–4585. [Google Scholar] [CrossRef]
- Yohai, R.A.; Bullock, J.D.; Aziz, A.A.; Markert, R.J. Survival factors in rhino-orbital-cerebral mucormycosis. Surv. Ophthalmol. 1994, 39, 3–22. [Google Scholar] [CrossRef]
- Walsh, T.J.; Skiada, A.; Cornely, O.A.; Roilides, E.; Ibrahim, A.; Zaoutis, T.; Groll, A.; Lortholary, O.; Kontoyiannis, D.P.; Petrikkos, G. Development of new strategies for early diagnosis of mucormycosis from bench to bedside. Mycoses 2014, 57, 2–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raab, P.; Sedlacek, L.; Buchholz, S.; Stolle, S.; Lanfermann, H. Imaging Patterns of Rhino-Orbital-Cerebral Mucormycosis in Immunocompromised Patients: When to Suspect Complicated Mucormycosis. Clin. Neuroradiol. 2017, 27, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Safder, S.; Carpenter, J.S.; Roberts, T.D.; Bailey, N. The “Black Turbinate” sign: An early MR imaging finding of nasal mucormycosis. AJNR Am. J. Neuroradiol. 2010, 31, 771–774. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kang, B.C.; Lee, J.H.; Jang, Y.J.; Lee, B.J.; Chung, Y.S. The prognostic value of gadolinium-enhanced magnetic resonance imaging in acute invasive fungal rhinosinusitis. J. Infect. 2015, 70, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Son, J.H.; Lim, H.B.; Lee, S.H.; Yang, J.W.; Lee, S.B. Early Differential Diagnosis of Rhino-Orbito-Cerebral Mucormycosis and Bacterial Orbital Cellulitis: Based on Computed Tomography Findings. PLoS ONE 2016, 11, e0160897. [Google Scholar] [CrossRef] [PubMed]
- Cunnane, M.B.; Curtin, H.D. Imaging of orbital disorders. Handb. Clin. Neurol. 2016, 135, 659–672. [Google Scholar] [CrossRef] [PubMed]
- Kursun, E.; Turunc, T.; Demiroglu, Y.Z.; Aliskan, H.E.; Arslan, A.H. Evaluation of 28 cases of mucormycosis. Mycoses 2015, 58, 82–87. [Google Scholar] [CrossRef]
- Horger, M.; Hebart, H.; Schimmel, H.; Vogel, M.; Brodoefel, H.; Oechsle, K.; Hahn, U.; Mittelbronn, M.; Bethge, W.; Claussen, C.D. Disseminated mucormycosis in haematological patients: CT and MRI findings with pathological correlation. Br. J. Radiol. 2006, 79, e88–e95. [Google Scholar] [CrossRef]
- Siegal, J.A.; Cacayorinb, E.D.; Nassif, A.S.; Rizk, D.; Galambos, C.; Levy, B.; Kennedy, D.; Visconti, J.; Perman, W. Cerebral mucormycosis: Proton MR spectroscopy and MR imaging. Magn. Reson. Imaging 2000, 18, 915–920. [Google Scholar] [CrossRef]
- Lin, E.; Moua, T.; Limper, A.H. Pulmonary mucormycosis: Clinical features and outcomes. Infection 2017, 45, 443–448. [Google Scholar] [CrossRef]
- Skiada, A.; Vrana, L.; Polychronopoulou, H.; Prodromou, P.; Chantzis, A.; Tofas, P.; Daikos, G.L. Disseminated zygomycosis with involvement of the central nervous system. Clin. Microbiol. Infect. 2009, 15, 46–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albrecht, P.; Stettner, M.; Husseini, L.; Macht, S.; Jander, S.; Mackenzie, C.; Oesterlee, U.; Slotty, P.; Methner, A.; Hartung, H.P.; et al. An emboligenic pulmonary abscess leading to ischemic stroke and secondary brain abscess. BMC Neurol. 2012, 12, 133. [Google Scholar] [CrossRef] [PubMed]
- Chamilos, G.; Marom, E.M.; Lewis, R.E.; Lionakis, M.S.; Kontoyiannis, D.P. Predictors of pulmonary zygomycosis versus invasive pulmonary aspergillosis in patients with cancer. Clin. Infect. Dis. 2005, 41, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Legouge, C.; Caillot, D.; Chretien, M.L.; Lafon, I.; Ferrant, E.; Audia, S.; Pages, P.B.; Roques, M.; Estivalet, L.; Martin, L.; et al. The reversed halo sign: Pathognomonic pattern of pulmonary mucormycosis in leukemic patients with neutropenia? Clin. Infect. Dis. 2014, 58, 672–678. [Google Scholar] [CrossRef] [PubMed]
- Bourcier, J.; Heudes, P.M.; Morio, F.; Gastinne, T.; Chevallier, P.; Rialland-Battisti, F.; Garandeau, C.; Danner-Boucher, I.; Le Pape, P.; Frampas, E.; et al. Prevalence of the reversed halo sign in neutropenic patients compared with non-neutropenic patients: Data from a single-centre study involving 27 patients with pulmonary mucormycosis (2003–2016). Mycoses 2017, 60, 526–533. [Google Scholar] [CrossRef] [PubMed]
- Wahba, H.; Truong, M.T.; Lei, X.; Kontoyiannis, D.P.; Marom, E.M. Reversed halo sign in invasive pulmonary fungal infections. Clin. Infect. Dis. 2008, 46, 1733–1737. [Google Scholar] [CrossRef] [PubMed]
- Lass-Florl, C.; Resch, G.; Nachbaur, D.; Mayr, A.; Gastl, G.; Auberger, J.; Bialek, R.; Freund, M.C. The value of computed tomography-guided percutaneous lung biopsy for diagnosis of invasive fungal infection in immunocompromised patients. Clin. Infect. Dis. 2007, 45, e101–e104. [Google Scholar] [CrossRef]
- Hazama, A.; Galgano, M.; Fullmer, J.; Hall, W.; Chin, L. Affinity of Mucormycosis for Basal Ganglia in Intravenous Drug Users: Case Illustration and Review of Literature. World Neurosurg. 2017, 98, 872.e1–872.e3. [Google Scholar] [CrossRef]
- Dubey, A.; Patwardhan, R.V.; Sampth, S.; Santosh, V.; Kolluri, S.; Nanda, A. Intracranial fungal granuloma: Analysis of 40 patients and review of the literature. Surg. Neurol. 2005, 63, 254–260, discussion 260. [Google Scholar] [CrossRef]
- Sharma, B.S.; Khosla, V.K.; Kak, V.K.; Banerjee, A.K.; Vasishtha, R.K.; Prasad, K.S.; Sharma, S.C.; Mathuriya, S.N.; Tewari, M.K.; Pathak, A. Intracranial fungal granuloma. Surg. Neurol. 1997, 47, 489–497. [Google Scholar] [CrossRef]
- Dadwal, S.S.; Kontoyiannis, D.P. Recent advances in the molecular diagnosis of mucormycosis. Expert Rev. Mol. Diagn. 2018, 18, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Schelenz, S.; Barnes, R.A.; Barton, R.C.; Cleverley, J.R.; Lucas, S.B.; Kibbler, C.C.; Denning, D.W. British Society for Medical Mycology best practice recommendations for the diagnosis of serious fungal diseases. Lancet Infect. Dis. 2015, 15, 461–474. [Google Scholar] [CrossRef]
- Burnham-Marusich, A.R.; Hubbard, B.; Kvam, A.J.; Gates-Hollingsworth, M.; Green, H.R.; Soukup, E.; Limper, A.H.; Kozel, T.R. Conservation of Mannan Synthesis in Fungi of the Zygomycota and Ascomycota Reveals a Broad Diagnostic Target. mSphere 2018, 3, e00094-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernal-Martinez, L.; Buitrago, M.J.; Castelli, M.V.; Rodriguez-Tudela, J.L.; Cuenca-Estrella, M. Development of a single tube multiplex real-time PCR to detect the most clinically relevant Mucormycetes species. Clin. Microbiol. Infect. 2013, 19, E1–E7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baldin, C.; Soliman, S.S.M.; Jeon, H.H.; Alkhazraji, S.; Gebremariam, T.; Gu, Y.; Bruno, V.M.; Cornely, O.A.; Leather, H.L.; Sugrue, M.W.; et al. PCR-Based Approach Targeting Mucorales-Specific Gene Family for Diagnosis of Mucormycosis. J. Clin. Microbiol. 2018, 56, e00746-18. [Google Scholar] [CrossRef]
- Jung, J.; Park, Y.S.; Sung, H.; Song, J.S.; Lee, S.O.; Choi, S.H.; Kim, Y.S.; Woo, J.H.; Kim, S.H. Using immunohistochemistry to assess the accuracy of histomorphologic diagnosis of aspergillosis and mucormycosis. Clin. Infect. Dis. 2015, 61, 1664–1670. [Google Scholar] [CrossRef] [PubMed]
- Kasapoglu, F.; Coskun, H.; Ozmen, O.A.; Akalin, H.; Ener, B. Acute invasive fungal rhinosinusitis: Evaluation of 26 patients treated with endonasal or open surgical procedures. Otolaryngol. Head Neck Surg. 2010, 143, 614–620. [Google Scholar] [CrossRef]
- Gillespie, M.B.; O’Malley, B.W., Jr.; Francis, H.W. An approach to fulminant invasive fungal rhinosinusitis in the immunocompromised host. Arch. Otolaryngol. Head Neck Surg. 1998, 124, 520–526. [Google Scholar] [CrossRef]
- Davoudi, S.; Kumar, V.A.; Jiang, Y.; Kupferman, M.; Kontoyiannis, D.P. Invasive mould sinusitis in patients with haematological malignancies: A 10 year single-centre study. J. Antimicrob. Chemother. 2015, 70, 2899–2905. [Google Scholar] [CrossRef]
- Turner, J.H.; Soudry, E.; Nayak, J.V.; Hwang, P.H. Survival outcomes in acute invasive fungal sinusitis: A systematic review and quantitative synthesis of published evidence. Laryngoscope 2013, 123, 1112–1118. [Google Scholar] [CrossRef]
- Zuniga, M.G.; Turner, J.H. Treatment outcomes in acute invasive fungal rhinosinusitis. Curr. Opin. Otolaryngol. Head Neck Surg. 2014, 22, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Middelhof, C.A.; Loudon, W.G.; Muhonen, M.D.; Xavier, C.; Greene, C.S., Jr. Improved survival in central nervous system aspergillosis: A series of immunocompromised children with leukemia undergoing stereotactic resection of aspergillomas. Report of four cases. J. Neurosurg. 2005, 103, 374–378. [Google Scholar] [CrossRef] [PubMed]
- Ben-Ami, R.; Halaburda, K.; Klyasova, G.; Metan, G.; Torosian, T.; Akova, M. A multidisciplinary team approach to the management of patients with suspected or diagnosed invasive fungal disease. J. Antimicrob. Chemother. 2013, 68, iii25–iii33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kyvernitakis, A.; Torres, H.A.; Jiang, Y.; Chamilos, G.; Lewis, R.E.; Kontoyiannis, D.P. Initial use of combination treatment does not impact survival of 106 patients with haematologic malignancies and mucormycosis: A propensity score analysis. Clin. Microbiol. Infect. 2016, 22, 811.e1–811.e8. [Google Scholar] [CrossRef] [PubMed]
- Pagano, L.; Cornely, O.A.; Busca, A.; Caira, M.; Cesaro, S.; Gasbarrino, C.; Girmenia, C.; Heinz, W.J.; Herbrecht, R.; Lass-Florl, C.; et al. Combined antifungal approach for the treatment of invasive mucormycosis in patients with hematologic diseases: A report from the SEIFEM and FUNGISCOPE registries. Haematologica 2013, 98, e127–e130. [Google Scholar] [CrossRef] [PubMed]
- Cornely, O.A.; Arikan-Akdagli, S.; Dannaoui, E.; Groll, A.H.; Lagrou, K.; Chakrabarti, A.; Lanternier, F.; Pagano, L.; Skiada, A.; Akova, M.; et al. ESCMID and ECMM joint clinical guidelines for the diagnosis and management of mucormycosis 2013. Clin. Microbiol. Infect. 2014, 20, 5–26. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.S.; Avanessian, V.; Spellberg, B.; Edwards, J.E., Jr. Liposomal amphotericin B, and not amphotericin B deoxycholate, improves survival of diabetic mice infected with Rhizopus oryzae. Antimicrob. Agents Chemother. 2003, 47, 3343–3344. [Google Scholar] [CrossRef]
- Lewis, R.E.; Albert, N.D.; Liao, G.; Hou, J.; Prince, R.A.; Kontoyiannis, D.P. Comparative Pharmacodynamics of Amphotericin B Lipid Complex and Liposomal Amphotericin B in a Murine Model of Pulmonary Mucormycosis. Antimicrob. Agents Chemother. 2009, 54, 1298–1304. [Google Scholar] [CrossRef]
- Reed, C.; Bryant, R.; Ibrahim, A.S.; Edwards, J., Jr.; Filler, S.G.; Goldberg, R.; Spellberg, B. Combination polyene-caspofungin treatment of rhino-orbital-cerebral mucormycosis. Clin. Infect. Dis. 2008, 47, 364–371. [Google Scholar] [CrossRef]
- Chamilos, G.; Lewis, R.E.; Kontoyiannis, D.P. Delaying amphotericin B-based frontline therapy significantly increases mortality among patients with hematologic malignancy who have zygomycosis. Clin. Infect. Dis. 2008, 47, 503–509. [Google Scholar] [CrossRef]
- Lanternier, F.; Poiree, S.; Elie, C.; Garcia-Hermoso, D.; Bakouboula, P.; Sitbon, K.; Herbrecht, R.; Wolff, M.; Ribaud, P.; Lortholary, O.; et al. Prospective pilot study of high-dose (10 mg/kg/day) liposomal amphotericin B (L-AMB) for the initial treatment of mucormycosis. J. Antimicrob. Chemother. 2015, 70, 3116–3123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spellberg, B.; Ibrahim, A.S.; Chin-Hong, P.V.; Kontoyiannis, D.P.; Morris, M.I.; Perfect, J.R.; Fredricks, D.; Brass, E.P. The Deferasirox-AmBisome Therapy for Mucormycosis (DEFEAT Mucor) study: A randomized, double-blinded, placebo-controlled trial. J. Antimicrob. Chemother. 2012, 67, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Collette, N.; van der Auwera, P.; Lopez, A.P.; Heymans, C.; Meunier, F. Tissue concentrations and bioactivity of amphotericin B in cancer patients treated with amphotericin B-deoxycholate. Antimicrob. Agents Chemother. 1989, 33, 362–368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collette, N.; Van der Auwera, P.; Meunier, F.; Lambert, C.; Sculier, J.P.; Coune, A. Tissue distribution and bioactivity of amphotericin B administered in liposomes to cancer patients. J. Antimicrob. Chemother. 1991, 27, 535–548. [Google Scholar] [CrossRef] [PubMed]
- Groll, A.H.; Giri, N.; Petraitis, V.; Petraitiene, R.; Candelario, M.; Bacher, J.S.; Piscitelli, S.C.; Walsh, T.J. Comparative efficacy and distribution of lipid formulations of amphotericin B in experimental Candida albicans infection of the central nervous system. J. Infect. Dis. 2000, 182, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Day, J.N.; Chau, T.T.H.; Wolbers, M.; Mai, P.P.; Dung, N.T.; Mai, N.H.; Phu, N.H.; Nghia, H.D.; Phong, N.D.; Thai, C.Q.; et al. Combination Antifungal Therapy for Cryptococcal Meningitis. N. Engl. J. Med. 2013, 368, 1291–1302. [Google Scholar] [CrossRef] [PubMed]
- Einstein, H.E.; Holeman, C.W., Jr.; Sandidge, L.L.; Holden, D.H. Coccidioidal meningitis. The use of amphotericin B in treatment. Calif. Med. 1961, 94, 339–343. [Google Scholar]
- Grannan, B.L.; Yanamadala, V.; Venteicher, A.S.; Walcott, B.P.; Barr, J.C. Use of external ventriculostomy and intrathecal anti-fungal treatment in cerebral mucormycotic abscess. J. Clin. Neurosci. 2014, 21, 1819–1821. [Google Scholar] [CrossRef]
- Ho, J.; Fowler, P.; Heidari, A.; Johnson, R.H. Intrathecal Amphotericin B: A 60-Year Experience in Treating Coccidioidal Meningitis. Clin. Infect. Dis. 2017, 64, 519–524. [Google Scholar] [CrossRef]
- Lamoth, F.; Mercier, T.; Andre, P.; Pagani, J.L.; Pantet, O.; Maduri, R.; Guery, B.; Decosterd, L.A. Isavuconazole brain penetration in cerebral aspergillosis. J. Antimicrob. Chemother. 2019, 74, 1751–1753. [Google Scholar] [CrossRef]
- Schmitt-Hoffmann, A.H.; Kato, K.; Townsend, R.; Potchoiba, M.J.; Hope, W.W.; Andes, D.; Spickermann, J.; Schneidkraut, M.J. Tissue Distribution and Elimination of Isavuconazole following Single and Repeat Oral-Dose Administration of Isavuconazonium Sulfate to Rats. Antimicrob. Agents Chemother. 2017, 61, e01292-17. [Google Scholar] [CrossRef] [PubMed]
- Marty, F.M.; Ostrosky-Zeichner, L.; Cornely, O.A.; Mullane, K.M.; Perfect, J.R.; Thompson, G.R.; Alangaden, G.J.; Brown, J.M.; Fredricks, D.N.; Heinz, W.J.; et al. Isavuconazole treatment for mucormycosis: A single-arm open-label trial and case-control analysis. Lancet Infect. Dis. 2016, 16, 828–837. [Google Scholar] [CrossRef]
- Reinwald, M.; Uharek, L.; Lampe, D.; Grobosch, T.; Thiel, E.; Schwartz, S. Limited penetration of posaconazole into cerebrospinal fluid in an allogeneic stem cell recipient with invasive pulmonary aspergillosis. Bone Marrow Transplant. 2009, 44, 269–270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruping, M.J.; Albermann, N.; Ebinger, F.; Burckhardt, I.; Beisel, C.; Muller, C.; Vehreschild, J.J.; Kochanek, M.; Fatkenheuer, G.; Bangard, C.; et al. Posaconazole concentrations in the central nervous system. J. Antimicrob. Chemother. 2008, 62, 1468–1470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calcagno, A.; Baietto, L.; De Rosa, F.G.; Tettoni, M.C.; Libanore, V.; Bertucci, R.; D’Avolio, A.; Di Perri, G. Posaconazole cerebrospinal concentrations in an HIV-infected patient with brain mucormycosis. J. Antimicrob. Chemother. 2011, 66, 224–225. [Google Scholar] [CrossRef] [PubMed]
- Pitisuttithum, P.; Negroni, R.; Graybill, J.R.; Bustamante, B.; Pappas, P.; Chapman, S.; Hare, R.S.; Hardalo, C.J. Activity of posaconazole in the treatment of central nervous system fungal infections. J. Antimicrob. Chemother. 2005, 56, 745–755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tarani, L.; Costantino, F.; Notheis, G.; Wintergerst, U.; Venditti, M.; Di Biasi, C.; Friederici, D.; Pasquino, A.M. Long-term posaconazole treatment and follow-up of rhino-orbital-cerebral mucormycosis in a diabetic girl. Pediatric Diabetes 2009, 10, 289–293. [Google Scholar] [CrossRef]
- Davoudi, S.; Anderlini, P.; Fuller, G.N.; Kontoyiannis, D.P. A long-term survivor of disseminated Aspergillus and mucorales infection: An instructive case. Mycopathologia 2014, 178, 465–470. [Google Scholar] [CrossRef]
- Ibrahim, A.S.; Gebremariam, T.; Luo, G.; Fu, Y.; French, S.W.; Edwards, J.E., Jr.; Spellberg, B. Combination therapy of murine mucormycosis or aspergillosis with iron chelation, polyenes, and echinocandins. Antimicrob. Agents Chemother. 2011, 55, 1768–1770. [Google Scholar] [CrossRef]
- Ibrahim, A.S.; Gebermariam, T.; Fu, Y.; Lin, L.; Husseiny, M.I.; French, S.W.; Schwartz, J.; Skory, C.D.; Edwards, J.E., Jr.; Spellberg, B.J. The iron chelator deferasirox protects mice from mucormycosis through iron starvation. J. Clin. Investig. 2007, 117, 2649–2657. [Google Scholar] [CrossRef] [Green Version]
- Reed, C.; Ibrahim, A.; Edwards, J.E., Jr.; Walot, I.; Spellberg, B. Deferasirox, an iron-chelating agent, as salvage therapy for rhinocerebral mucormycosis. Antimicrob. Agents Chemother. 2006, 50, 3968–3969. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, J.P.; Lahav, M. Deferasirox as adjunctive therapy for mucormycosis. J. Antimicrob. Chemother. 2012, 67, 519–520. [Google Scholar] [CrossRef] [PubMed]
- Gudewicz, T.M.; Mader, J.T.; Davis, C.P. Combined effects of hyperbaric oxygen and antifungal agents on the growth of Candida albicans. Aviat. Space Environ. Med. 1987, 58, 673–678. [Google Scholar] [PubMed]
- Dhingra, S.; Buckey, J.C.; Cramer, R.A. Hyperbaric Oxygen Reduces Aspergillus fumigatus Proliferation In Vitro and Influences In Vivo Disease Outcomes. Antimicrob. Agents Chemother. 2018, 62, e01953-17. [Google Scholar] [CrossRef] [PubMed]
- Couch, L.; Theilen, F.; Mader, J.T. Rhinocerebral mucormycosis with cerebral extension successfully treated with adjunctive hyperbaric oxygen therapy. Arch. Otolaryngol. Head Neck Surg. 1988, 114, 791–794. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, B.J.; Mitchell, T.G.; Moon, R.; Camporesi, E.M.; Farmer, J. Adjunctive hyperbaric oxygen for treatment of rhinocerebral mucormycosis. Rev. Infect. Dis. 1988, 10, 551–559. [Google Scholar] [CrossRef] [PubMed]
- John, B.V.; Chamilos, G.; Kontoyiannis, D.P. Hyperbaric oxygen as an adjunctive treatment for zygomycosis. Clin. Microbiol. Infect. 2005, 11, 515–517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibrahim, A.S.; Gebremariam, T.; Fu, Y.; Edwards, J.E., Jr.; Spellberg, B. Combination echinocandin-polyene treatment of murine mucormycosis. Antimicrob. Agents Chemother. 2008, 52, 1556–1558. [Google Scholar] [CrossRef]
- Felton, T.; Troke, P.F.; Hope, W.W. Tissue penetration of antifungal agents. Clin. Microbiol. Rev. 2014, 27, 68–88. [Google Scholar] [CrossRef]
- Gebremariam, T.; Alkhazraji, S.; Lin, L.; Wiederhold, N.P.; Garvey, E.P.; Hoekstra, W.J.; Schotzinger, R.J.; Patterson, T.F.; Filler, S.G.; Ibrahim, A.S. Prophylactic Treatment with VT-1161 Protects Immunosuppressed Mice from Rhizopus arrhizus var. arrhizus Infection. Antimicrob. Agents Chemother. 2017, 61, e00390-17. [Google Scholar] [CrossRef] [Green Version]
| Underlying Condition | Proportion of CNS Involvement | Form of CNS Involvement | Reference | ||
|---|---|---|---|---|---|
| Total | Rhinocerebral | Hematogenous | Isolated CNS | ||
| Diabetes mellitus | 43% | 43–52% | 0% | 0% | [8,18] |
| Malignancy | 4–19% | 4–15% | 12% | 0% | [8,18,19] |
| Stem cell transplantation | 11% | 0% | [8] | ||
| Trauma | 1%> | 1%> | [20] | ||
| Injection drug use | 67% | 5% | 62% | [8] | |
| Overall | 12.8–44.1% | 11.3% | 7.8% | 2% | [8,9,14,18,22] |
| Recommendation | |
|---|---|
| Surgical treatment | Debridement of extracranial site of infection: Sinus debridement using endoscopic approach for early disease and open surgery for extensive disease. |
| Consider indications for neurosurgery: Increased intracranial pressure (e.g. hemispheric stroke) Obstructive hydrocephalus Lesions compressing the spinal cord | |
| Antifungal treatment | Initial treatment: Liposomal amphotericin B 5-10 mg/kg/day IV for initial 28 days. Alternative: Isavuconazole 300mg TID for 2 days followed by 300mg QD, IV or PO. Step-down: Isavuconazole 300mg TID for 2 days followed by 300mg QD PO. |
| Duration of treatment: at least 6 months. Factors affecting treatment duration are the extent of surgery done and immune status of the patient. | |
| Ancillary treatment | Correction of hyperglycemia and ketoacidosis. |
| Discontinue or reduce dose of immunosuppressive drugs, when possible. | |
| Consider hyperbaric oxygen for rhino-orbito-cerebral mucormycosis. |
| Development of a reproducible and relevant animal model of CNS mucormycosis with validated endpoints of measurement of outcome (e.g., PCR, antigen, and/or histopathology) to offer insights on the pathogenesis and appropriate management of this infection. |
| Investigate if there is Mucorales species-specific or isolate- specific CNS tropism. |
| Study the role of brain immune effector cell activity against Mucorales. |
| Study strategies employed by Mucorales to access the CNS (e.g., through GRP78 attachment, hijacking of host phagocytes and endocytosis). |
| Development of validated non-culture-based biomarkers in blood and CSF (e.g., based on antigen detection, PCR, volatile organic compounds derived from Mucorales metabolism). |
| Development of validated neuroimaging readouts that differentiate CNS mucormycosis from other fungal and non-fungal CNS diseases. |
| Development of rapidly cidal agents that penetrate the blood–brain barrier. |
| Effect of metabolic derangements (glycemia, ketoacidosis and iron overload) on the degree and rate of CNS involvement. |
| Risk stratification models to examine the benefit of surgery (type, timing). |
| Immuno-adjunctive strategies that enhance Mucorales killing in the brain microenvironment without resulting in excess inflammation. |
| Robust registries of CNS mucormycosis cases. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chikley, A.; Ben-Ami, R.; Kontoyiannis, D.P. Mucormycosis of the Central Nervous System. J. Fungi 2019, 5, 59. https://doi.org/10.3390/jof5030059
Chikley A, Ben-Ami R, Kontoyiannis DP. Mucormycosis of the Central Nervous System. Journal of Fungi. 2019; 5(3):59. https://doi.org/10.3390/jof5030059
Chicago/Turabian StyleChikley, Amanda, Ronen Ben-Ami, and Dimitrios P Kontoyiannis. 2019. "Mucormycosis of the Central Nervous System" Journal of Fungi 5, no. 3: 59. https://doi.org/10.3390/jof5030059
APA StyleChikley, A., Ben-Ami, R., & Kontoyiannis, D. P. (2019). Mucormycosis of the Central Nervous System. Journal of Fungi, 5(3), 59. https://doi.org/10.3390/jof5030059





