The Effects of Oral l-Arginine and l-Citrulline Supplementation on Blood Pressure
Abstract
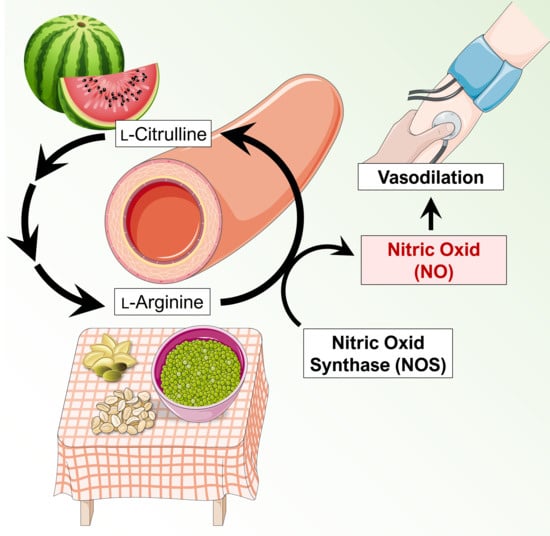
:1. Introduction
2. Nitric Oxide in Blood Pressure Regulation
2.1. Nitric Oxide-Mediated Vasodilation
2.2. Mechanisms Mediated by Oral Administration of l-Arginine and l-Citrulline on the Vasculature
3. l-Citrulline and l-Arginine as Antihypertensive Compounds
3.1. Pharmacokinetics of l-Citrulline and l-Arginine
3.2. Results of Clinical Trials
4. Discussion
5. Conclusions and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey, D.E., Jr.; Collins, K.J.; Dennison Himmelfarb, C.; DePalma, S.M.; Gidding, S.; Jamerson, K.A.; Jones, D.W.; et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: Executive summary: A report of the american college of cardiology/american heart association task force on clinical practice guidelines. Hypertension 2018, 71, 1269–1324. [Google Scholar]
- Carretero, O.A.; Oparil, S. Essential hypertension. Part I: Definition and etiology. Circulation 2000, 101, 329–335. [Google Scholar] [CrossRef]
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in blood pressure from 1975 to 2015: A pooled analysis of 1479 population-based measurement studies with 19.1 million participants. Lancet 2017, 389, 37–55. [Google Scholar] [CrossRef]
- Lim, S.S.; Vos, T.; Flaxman, A.D.; Danaei, G.; Shibuya, K.; Adair-Rohani, H.; Amann, M.; Anderson, H.R.; Andrews, K.G.; Aryee, M.; et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2224–2260. [Google Scholar] [CrossRef]
- Saxena, T.; Ali, A.O.; Saxena, M. Pathophysiology of essential hypertension: An update. Expert Rev. Cardiovasc. 2018, 16, 879–887. [Google Scholar] [CrossRef]
- Schwedhelm, E.; Maas, R.; Freese, R.; Jung, D.; Lukacs, Z.; Jambrecina, A.; Spickler, W.; Schulze, F.; Boger, R.H. Pharmacokinetic and pharmacodynamic properties of oral L-citrulline and L-arginine: Impact on nitric oxide metabolism. Br. J. Clin. Pharm. 2008, 65, 51–59. [Google Scholar] [CrossRef]
- Zhao, Y.; Vanhoutte, P.M.; Leung, S.W. Vascular nitric oxide: Beyond eNOS. J. Pharm. Sci 2015, 129, 83–94. [Google Scholar] [CrossRef] [Green Version]
- Van Faassen, E.E.; Bahrami, S.; Feelisch, M.; Hogg, N.; Kelm, M.; Kim-Shapiro, D.B.; Kozlov, A.V.; Li, H.; Lundberg, J.O.; Mason, R.; et al. Nitrite as regulator of hypoxic signaling in mammalian physiology. Med. Res. Rev. 2009, 29, 683–741. [Google Scholar] [CrossRef] [Green Version]
- Ignarro, L.J.; Buga, G.M.; Wood, K.S.; Byrns, R.E.; Chaudhuri, G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc. Natl. Acad. Sci. USA 1987, 84, 9265–9269. [Google Scholar] [CrossRef]
- Palmer, R.M.; Rees, D.D.; Ashton, D.S.; Moncada, S. L-arginine is the physiological precursor for the formation of nitric oxide in endothelium-dependent relaxation. Biochem. Biophys. Res. Commun. 1988, 153, 1251–1256. [Google Scholar] [CrossRef]
- Rees, D.D.; Palmer, R.M.; Moncada, S. Role of endothelium-derived nitric oxide in the regulation of blood pressure. Proc. Natl. Acad. Sci. USA 1989, 86, 3375–3378. [Google Scholar] [CrossRef] [PubMed]
- Dessy, C.; Feron, O. Pathophysiological roles of nitric oxide: In the heart and the coronary vasculature. Curr. Med. Chem. Anti-Inflamm. Anti Allergy Agents 2004, 3, 207–216. [Google Scholar] [CrossRef]
- Pepke-Zaba, J.; Higenbottam, T.W.; Dinh-Xuan, A.T.; Stone, D.; Wallwork, J. Inhaled nitric oxide as a cause of selective pulmonary vasodilatation in pulmonary hypertension. Lancet 1991, 338, 1173–1174. [Google Scholar] [CrossRef]
- Forstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012, 33, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Denninger, J.W.; Marletta, M.A. Guanylate cyclase and the NO/cGMP signaling pathway. Biochim. Biophys. Acta 1999, 1411, 334–350. [Google Scholar] [CrossRef]
- Carvajal, J.A.; Germain, A.M.; Huidobro-Toro, J.P.; Weiner, C.P. Molecular mechanism of cGMP-mediated smooth muscle relaxation. J. Cell Physiol. 2000, 184, 409–420. [Google Scholar] [CrossRef]
- Huang, P.L.; Huang, Z.; Mashimo, H.; Bloch, K.D.; Moskowitz, M.A.; Bevan, J.A.; Fishman, M.C. Hypertension in mice lacking the gene for endothelial nitric oxide synthase. Nature 1995, 377, 239–242. [Google Scholar] [CrossRef]
- Forte, P.; Copland, M.; Smith, L.M.; Milne, E.; Sutherland, J.; Benjamin, N. Basal nitric oxide synthesis in essential hypertension. Lancet 1997, 349, 837–842. [Google Scholar] [CrossRef]
- Tain, Y.L.; Huang, L.T.; Lee, C.T.; Chan, J.Y.; Hsu, C.N. Maternal citrulline supplementation prevents prenatal N(G)-nitro-L-arginine-methyl ester (L-NAME)-induced programmed hypertension in rats. Biol. Reprod. 2015, 92, 7. [Google Scholar] [CrossRef]
- Schlaich, M.P.; Parnell, M.M.; Ahlers, B.A.; Finch, S.; Marshall, T.; Zhang, W.Z.; Kaye, D.M. Impaired L-arginine transport and endothelial function in hypertensive and genetically predisposed normotensive subjects. Circulation 2004, 110, 3680–3686. [Google Scholar] [CrossRef]
- Van de Poll, M.C.; Ligthart-Melis, G.C.; Boelens, P.G.; Deutz, N.E.; van Leeuwen, P.A.; Dejong, C.H. Intestinal and hepatic metabolism of glutamine and citrulline in humans. J. Physiol. 2007, 581, 819–827. [Google Scholar] [CrossRef]
- Romero, M.J.; Platt, D.H.; Caldwell, R.B.; Caldwell, R.W. Therapeutic use of citrulline in cardiovascular disease. Cardiovasc. Drug Rev. 2006, 24, 275–290. [Google Scholar] [CrossRef]
- El-Kirsh, A.A.; Abd El-Wahab, H.M.; Abd-Ellah Sayed, H.F. The effect of L-arginine or L-citrulline supplementation on biochemical parameters and the vascular aortic wall in high-fat and high-cholesterol-fed rats. Cell Biochem. Funct. 2011, 29, 414–428. [Google Scholar] [CrossRef]
- Morita, M.; Hayashi, T.; Ochiai, M.; Maeda, M.; Yamaguchi, T.; Ina, K.; Kuzuya, M. Oral supplementation with a combination of L-citrulline and L-arginine rapidly increases plasma L-arginine concentration and enhances NO bioavailability. Biochem. Biophys. Res. Commun. 2014, 454, 53–57. [Google Scholar] [CrossRef]
- Jourdan, M.; Nair, K.S.; Carter, R.E.; Schimke, J.; Ford, G.C.; Marc, J.; Aussel, C.; Cynober, L. Citrulline stimulates muscle protein synthesis in the post-absorptive state in healthy people fed a low-protein diet—A pilot study. Clin. Nutr. 2015, 34, 449–456. [Google Scholar] [CrossRef]
- Li, H.; Poulos, T.L. Structure-function studies on nitric oxide synthases. J. Inorg. Biochem. 2005, 99, 293–305. [Google Scholar] [CrossRef]
- Wascher, T.C.; Bachernegg, M.; Kickenweiz, A.; Stark, G.; Stark, U.; Toplak, H.; Graier, W.F. Involvement of the L-arginine-nitric oxide pathway in hyperglycaemia-induced coronary artery dysfunction of isolated guinea pig hearts. Eur. J. Clin. Investig. 1996, 26, 707–712. [Google Scholar] [CrossRef]
- Bode-Boger, S.M.; Boger, R.H.; Galland, A.; Tsikas, D.; Frolich, J.C. L-arginine-induced vasodilation in healthy humans: Pharmacokinetic-pharmacodynamic relationship. Br. J. Clin. Pharm. 1998, 46, 489–497. [Google Scholar] [CrossRef]
- Boger, R.H. Asymmetric dimethylarginine, an endogenous inhibitor of nitric oxide synthase, explains the “L-arginine paradox” and acts as a novel cardiovascular risk factor. J. Nutr. 2004, 134, 2842S–2847S, discussion 2853S. [Google Scholar] [CrossRef]
- Ito, A.; Tsao, P.S.; Adimoolam, S.; Kimoto, M.; Ogawa, T.; Cooke, J.P. Novel mechanism for endothelial dysfunction: Dysregulation of dimethylarginine dimethylaminohydrolase. Circulation 1999, 99, 3092–3095. [Google Scholar] [CrossRef]
- Antoniades, C.; Shirodaria, C.; Leeson, P.; Antonopoulos, A.; Warrick, N.; Van-Assche, T.; Cunnington, C.; Tousoulis, D.; Pillai, R.; Ratnatunga, C.; et al. Association of plasma asymmetrical dimethylarginine (ADMA) with elevated vascular superoxide production and endothelial nitric oxide synthase uncoupling: Implications for endothelial function in human atherosclerosis. Eur. Heart J. 2009, 30, 1142–1150. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Youn, J.Y.; Cai, H. Mechanisms and consequences of endothelial nitric oxide synthase dysfunction in hypertension. J. Hypertens 2015, 33, 1128–1136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xuan, C.; Lun, L.M.; Zhao, J.X.; Wang, H.W.; Wang, J.; Ning, C.P.; Liu, Z.; Zhang, B.B.; He, G.W. L-citrulline for protection of endothelial function from ADMA-induced injury in porcine coronary artery. Sci. Rep. 2015, 5, 10987. [Google Scholar] [CrossRef] [PubMed]
- Ananthakrishnan, M.; Barr, F.E.; Summar, M.L.; Smith, H.A.; Kaplowitz, M.; Cunningham, G.; Magarik, J.; Zhang, Y.; Fike, C.D. L-Citrulline ameliorates chronic hypoxia-induced pulmonary hypertension in newborn piglets. Am. J. Physiol. Lung Cell Mol. Physiol. 2009, 297, L506–L511. [Google Scholar] [CrossRef] [PubMed]
- Chien, S.J.; Lin, K.M.; Kuo, H.C.; Huang, C.F.; Lin, Y.J.; Huang, L.T.; Tain, Y.L. Two different approaches to restore renal nitric oxide and prevent hypertension in young spontaneously hypertensive rats: L-citrulline and nitrate. Transl. Res. 2014, 163, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Allerton, T.D.; Proctor, D.N.; Stephens, J.M.; Dugas, T.R.; Spielmann, G.; Irving, B.A. l-Citrulline Supplementation: Impact on Cardiometabolic Health. Nutrients 2018, 10, 921. [Google Scholar] [CrossRef] [PubMed]
- McRae, M.P. Therapeutic Benefits of l-Arginine: An Umbrella Review of Meta-analyses. J. Chiropr. Med. 2016, 15, 184–189. [Google Scholar] [CrossRef] [Green Version]
- King, D.E.; Mainous, A.G., 3rd; Geesey, M.E. Variation in L-arginine intake follow demographics and lifestyle factors that may impact cardiovascular disease risk. Nutr. Res. 2008, 28, 21–24. [Google Scholar] [CrossRef] [Green Version]
- Cynober, L.A. Plasma amino acid levels with a note on membrane transport: Characteristics, regulation, and metabolic significance. Nutrition 2002, 18, 761–766. [Google Scholar] [CrossRef]
- Tangphao, O.; Grossmann, M.; Chalon, S.; Hoffman, B.B.; Blaschke, T.F. Pharmacokinetics of intravenous and oral L-arginine in normal volunteers. Br. J. Clin. Pharm. 1999, 47, 261–266. [Google Scholar] [CrossRef]
- Castillo, L.; Chapman, T.E.; Yu, Y.M.; Ajami, A.; Burke, J.F.; Young, V.R. Dietary arginine uptake by the splanchnic region in adult humans. Am. J. Physiol. 1993, 265, E532–E539. [Google Scholar] [CrossRef] [PubMed]
- Papadia, C.; Osowska, S.; Cynober, L.; Forbes, A. Citrulline in health and disease. Review on human studies. Clin. Nutr. 2018, 37, 1823–1828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, T.; Morita, M.; Hayashi, T.; Kamimura, A. The effects on plasma L-arginine levels of combined oral L-citrulline and L-arginine supplementation in healthy males. Biosci. Biotechnol. Biochem. 2017, 81, 372–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shearer, J.D.; Richards, J.R.; Mills, C.D.; Caldwell, M.D. Differential regulation of macrophage arginine metabolism: A proposed role in wound healing. Am. J. Physiol. 1997, 272, E181–E190. [Google Scholar] [CrossRef] [PubMed]
- Moinard, C.; Nicolis, I.; Neveux, N.; Darquy, S.; Benazeth, S.; Cynober, L. Dose-ranging effects of citrulline administration on plasma amino acids and hormonal patterns in healthy subjects: The Citrudose pharmacokinetic study. Br. J. Nutr. 2008, 99, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.Y.; Qin, L.Q.; Zhang, Z.; Zhao, Y.; Wang, J.; Arigoni, F.; Zhang, W. Effect of oral L-arginine supplementation on blood pressure: A meta-analysis of randomized, double-blind, placebo-controlled trials. Am. Heart J. 2011, 162, 959–965. [Google Scholar] [CrossRef] [PubMed]
- Mirenayat, M.S.; Moradi, S.; Mohammadi, H.; Rouhani, M.H. Effect of L-Citrulline Supplementation on Blood Pressure: A Systematic Review and Meta-Analysis of Clinical Trials. Curr. Hypertens Rep. 2018, 20, 98. [Google Scholar] [CrossRef]
- Barkhidarian, B.; Khorshidi, M.; Shab-Bidar, S.; Hashemi, B. Effects of L-citrulline supplementation on blood pressure: A systematic review and meta-analysis. Avicenna J. Phytomed. 2019, 9, 10–20. [Google Scholar]
- Mahboobi, S.; Tsang, C.; Rezaei, S.; Jafarnejad, S. Effect of L-citrulline supplementation on blood pressure: A systematic review and meta-analysis of randomized controlled trials. J. Hum. Hypertens 2019, 33, 10–21. [Google Scholar] [CrossRef]
- Reule, C.A.; Goyvaerts, B.; Schoen, C. Effects of an L-arginine-based multi ingredient product on endothelial function in subjects with mild to moderate hypertension and hyperhomocysteinemia—A randomized, double-blind, placebo-controlled, cross-over trial. BMC Complement. Altern. Med. 2017, 17, 92. [Google Scholar] [CrossRef]
- Sanchez-Gonzalez, M.A.; Koutnik, A.P.; Ramirez, K.; Wong, A.; Figueroa, A. The effects of short term L-citrulline supplementation on wave reflection responses to cold exposure with concurrent isometric exercise. Am. J. Hypertens 2013, 26, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Balderas-Munoz, K.; Castillo-Martinez, L.; Orea-Tejeda, A.; Infante-Vazquez, O.; Utrera-Lagunas, M.; Martinez-Memije, R.; Keirns-Davis, C.; Becerra-Luna, B.; Sanchez-Vidal, G. Improvement of ventricular function in systolic heart failure patients with oral L-citrulline supplementation. Cardiol. J. 2012, 19, 612–617. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.; Chernykh, O.; Figueroa, A. Chronic l-citrulline supplementation improves cardiac sympathovagal balance in obese postmenopausal women: A preliminary report. Auton. Neurosci. 2016, 198, 50–53. [Google Scholar] [CrossRef] [PubMed]
- Saneei, P.; Salehi-Abargouei, A.; Esmaillzadeh, A.; Azadbakht, L. Influence of Dietary Approaches to Stop Hypertension (DASH) diet on blood pressure: A systematic review and meta-analysis on randomized controlled trials. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 1253–1261. [Google Scholar] [CrossRef] [PubMed]
- Whelton, S.P.; Chin, A.; Xin, X.; He, J. Effect of aerobic exercise on blood pressure: A meta-analysis of randomized, controlled trials. Ann. Intern. Med. 2002, 136, 493–503. [Google Scholar] [CrossRef]


| Compound | Dose (mg) × Number of Daily Administrations | Plasma [arginine] AUC (μmol·h·L−1) ± SEM |
|---|---|---|
| l-citrulline | 750 × 2 | 271 ± 38 |
| lL-citrulline | 1500 × 2 | 421 ± 65 |
| lL-citrulline | 3000 × 2 | 898 ± 67 |
| lL-arginine slow release | 1600 × 2 | 289 ± 50 |
| lL-arginine immediate release | 1000 × 3 | 283 ± 51 |
| Title and trial identifier | Objective | Design | Status and results |
|---|---|---|---|
| l-arginine treatment in mild hypertension (LAHN) NCT02894723 | To evaluate the efficacy of Arg treatment on blood pressure control patients with stage 1 hypertension | Dietary Supplement: Arg Dietary Supplement: syrup Patients will receive Arg 30 mL twice a day for eight weeks. Phase 4 | Not yet recruiting |
| l-arginine effects on chronic hypertension in pregnancy NCT00974714 | To evaluate the effects of oral Arg administration on pregnant women at second trimester of gestation with chronic hypertension, with respect with placebo. | Oral Arg 2 g twice a day, for 14 weeks Placebo-controlled Phase 3 | Completed (2010) |
| l-Arginine metabolism in essential hypertension NCT00137124 | This study determines whether metabolism and transport of Arg are altered in patients with essential hyper-tension and whether these potential alterations can be targeted therapeutically. | Interventional, randomized trial with 120 participants, oral administration of Arg for 4 weeks Phase 2 | Completed (2009) |
| Effects of oral l-arginine on chronic hypertension in pregnancy NCT00571766 | To evaluate the effects of oral Arg in pregnant women with chronic hypertension. | Interventional (Clinical Trial), randomized with 80 participants, oral Arg 2 g twice a day for 14 weeks Phase 3 | Completed (2008) |
| Effect of l-arginine and pycnogenol on light to moderate hypertension and endothelial function NCT02392767 | To evaluate the effect of a combination product (Verum) with Arg, Pycnogenol, vitamin K2, R-(+)-alpha-lipoic acid, and vitamins B6, B12, and folic acid | A randomized, double-blind, placebo-controlled cross-over study Two tablets twice a day for four weeks. Verum/Placebo | Completed (2015) Systolic blood pressure decreased significantly under the Arg-based multi-ingredient product (AbMIP) [50] |
| Impact of citrulline and arginine supplementation on the post-exercise hypotension (PEH) NCT03378596 | To increase the knowledge regarding non-pharmacological models aimed at the prevention and treatment of hypertension in normotensive and hypertensive patients. Cit (6 g) Arg (8 g) | Interventional (clinical trial), randomized, 20 participants, ambulatorial blood pressure monitoring | Recruiting |
| Effects of inhibition of NO synthesis on renal hemodynamics and sodium excretion in patients with essential hypertension and healthy controls NCT00345150 | To test the hypothesis that systemic and renal nitric oxide synthesis is changed in essential hypertension by investigating the effects of a non-selective nitric oxide inhibitor on renal hemodynamics and sodium excretion in patients with essential hypertension. NG-monomethyl- l-arginine | Interventional (clinical trial), randomized, 30 participants Phase 1 | Completed (2006) |
| l-arginine, vascular response and mechanisms NCT01482247 | To employ the supplement Arg to test the hypothesis that the activation of blood flow to the brain during cognitive tasks is regulated by nitric oxide in older subjects with diabetes mellitus and/or hypertension. Arg as supplement | Interventional (Clinical Trial), randomized, 25 participants. Phase 2 | Completed (2014) |
| Author and Publication Year | Supplement | Total Number of Trials and Participants | Reduction in SBP | Reduction in DBP | Dose and Duration |
|---|---|---|---|---|---|
| Dong et al. 2011 [46] | l-arginine | 11 trials 387 participants | 5.39 mmHg (95% CI: 2.25–8.54, p = 0.001) | 2.66 mmHg (95% CI:1.54–3.77, p < 0.001) | 2–24 weeks 4–24 g/day |
| Mahboobi et al. 2019 [49] | l-citrulline or watermelon extract | 15 trials 424 participants | 7.54 mmHg (95% CI: 5.63-9.44, p = 0.0001) | 3.77 mmHg for DBP (95% CI: 1.86–5.67, p = 0.0001) | 1–16 weeks 2.7–8.4 g/day |
| Barkhidarian et al. 2019 [48] | l-citrulline | 8 trials 190 participants | 4.10 mmHg (95% CI: 0.26–7.94. p = 0.037) | 2.08 mmHg (95% CI: −0.16–4.32. p = 0.069) | 1–17 weeks 3–9 g/day |
| Mirenayat et al. 2018 [47] | l-citrulline | 5 trials 114 participants | Brachial: 0.28 mmHg (95% CI: −2.87 to 2.31) Aortic: 0.22 mmHg (95% CI: −4.81 to 4.38) | Brachial: −1.56 mmHg (95% CI: −4.30 to 1.20) Aortic: 0.26 mmHg (95% CI: −2.27 to 2.80) | 1–8 weeks 3–6 g/day |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalaf, D.; Krüger, M.; Wehland, M.; Infanger, M.; Grimm, D. The Effects of Oral l-Arginine and l-Citrulline Supplementation on Blood Pressure. Nutrients 2019, 11, 1679. https://doi.org/10.3390/nu11071679
Khalaf D, Krüger M, Wehland M, Infanger M, Grimm D. The Effects of Oral l-Arginine and l-Citrulline Supplementation on Blood Pressure. Nutrients. 2019; 11(7):1679. https://doi.org/10.3390/nu11071679
Chicago/Turabian StyleKhalaf, David, Marcus Krüger, Markus Wehland, Manfred Infanger, and Daniela Grimm. 2019. "The Effects of Oral l-Arginine and l-Citrulline Supplementation on Blood Pressure" Nutrients 11, no. 7: 1679. https://doi.org/10.3390/nu11071679
APA StyleKhalaf, D., Krüger, M., Wehland, M., Infanger, M., & Grimm, D. (2019). The Effects of Oral l-Arginine and l-Citrulline Supplementation on Blood Pressure. Nutrients, 11(7), 1679. https://doi.org/10.3390/nu11071679