Association of Lifelong Intake of Barley Diet with Healthy Aging: Changes in Physical and Cognitive Functions and Intestinal Microbiome in Senescence-Accelerated Mouse-Prone 8 (SAMP8)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Diet
2.3. Animals
2.4. Food Consumption, Liquid Consumption, Body Weight, and Survival Analysis
2.5. Plasma Cholesterol in SAMP8 Mice Consuming Rice or Barley Diets
2.6. Blood Glucose Analysis
2.7. Changes in Aging-Related Scores in SAMP8 Mice Consuming Rice or Barley Diets
2.8. CHANGES in Age-Related Physical Activities in SAMP8 Mice Consuming Rice or Barley Diets
2.8.1. Locomotor Activities
2.8.2. Wire Hanging Test and Balancing Abilities on an Acryl Rod
2.8.3. Foot Print Test
2.9. Changes in Object Recognition (Long-Term Object Memory) and Spatial Recognition (Long-Term Location Memory) in SAMP8 Mice Consuming Rice or Barley Diets
2.10. Changes in Intestinal Microbiome in SAMP8 Consuming Rice or Barley Diets
2.11. Statistical Analyses
3. Results
3.1. Food Consumption, Liquid Consumption, Body Weight, and Number of Surviving in SAMP8 Mice Consuming Rice or Barley Diets
3.2. Plasma Cholesterol Levels at 16 Weeks Old in SAMP8 Consuming Rice or Barley Diets
3.3. Changes in Blood Glucose Levels in SAMP8 Mice Consuming Rice or Barley Diets
3.4. Changes in Aging-Related Scores in SAMP8 Mice Consuming Rice or Barley Diets
3.5. Changes in Age-Related Physical Activities in SAMP8 Mice Consuming Rice or Barley Diets
3.5.1. Locomotor Activities
3.5.2. Wire Hanging Test and Balancing Ability on an Acryl Rod
3.5.3. Foot Print Test
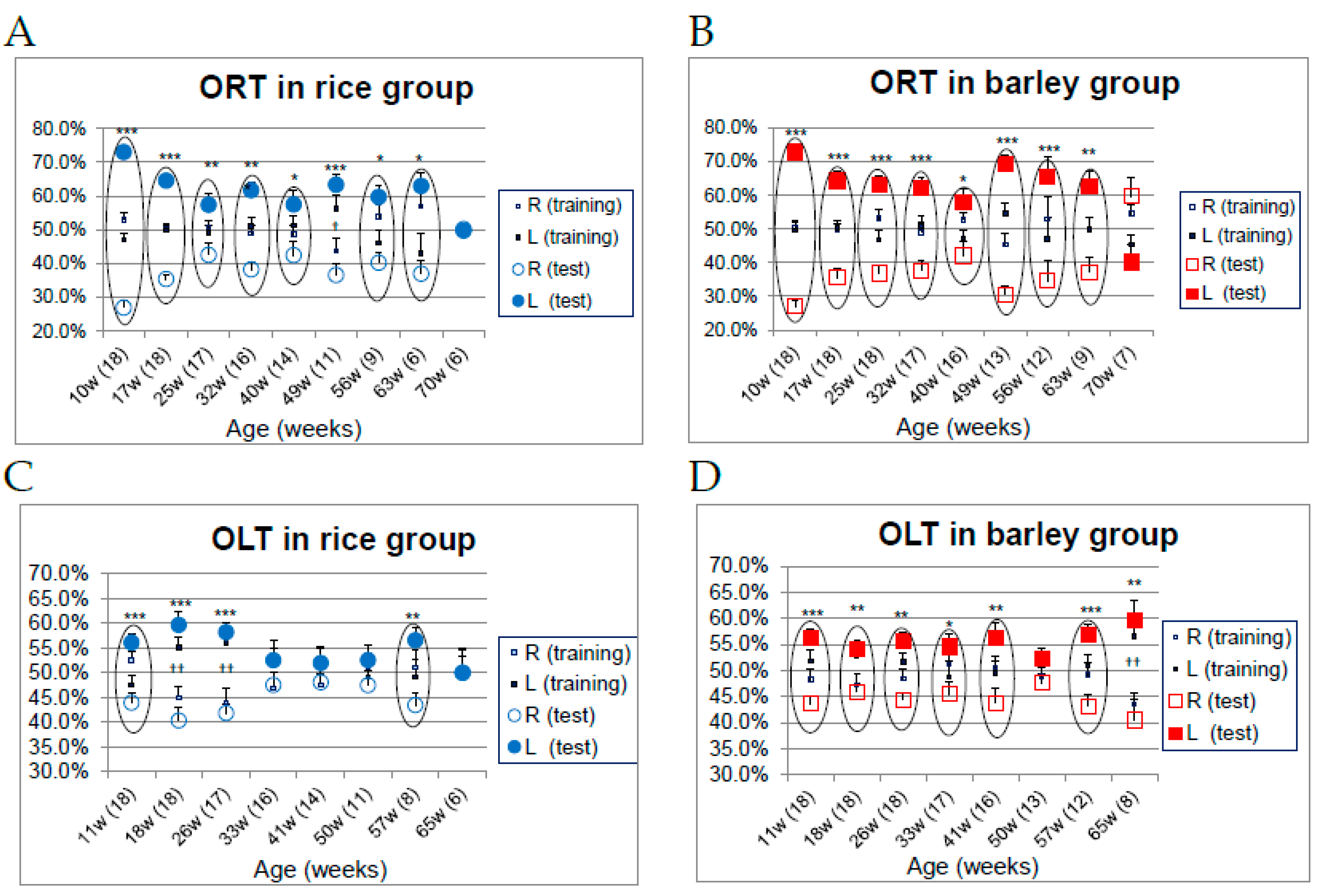
3.6. Changes in Object Recognition (Long-Term Object Memory) and Spatial Recognition (Long-Term Location Memory) in SAMP8 Mice Consuming Rice or Barley Diets
3.7. Changes in Intestinal Microbiome in SAMP8 Consuming Rice or Barley Diets
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ullrich, S.E.; Clancy, J.A.; Eslick, R.F.; Lance, R.C.M. β-Glucan content and viscosity of extracts from waxy barley. J. Cereal Sci. 1986, 4, 279–285. [Google Scholar] [CrossRef]
- Burton, R.A.; Fincher, G.B. Evolution and development of cell walls in cereal grains. Front. Plant Sci. 2014, 5, 456. [Google Scholar] [CrossRef] [PubMed]
- Behall, K.M.; Scholfield, D.J.; Hallfrisch, J. Diets containing barley significantly reduce lipids in mildly hypercholesterolemic men and women. Am. J. Clin. Nutr. 2004, 80, 1185–1193. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, C.; Kihara, M.; Aoe, S.; Araki, S.; Ito, K.; Hayashi, K.; Watari, J.; Sakata, Y.; Ikegami, S. Effect of high beta-glucan barley on serum cholesterol concentrations and visceral fat area in Japanese men—a randomized, double-blinded, placebo-controlled trial. Plant Foods Hum. Nut. 2008, 63, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Granfeldt, Y. Glucose and insulin responses to barley products: Influence of food structure and amylose-amylopectin ratio. Amer. J. Clin. Nutr. 1994, 59, 1075–1082. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.H.; Macfarlane, G.T.; Englyst, H.N. Prebiotic digestion and fermentation. Am. J. Clin. Nutr. 2001, 73, 415S–420S. [Google Scholar] [CrossRef]
- Food and Drug Administration, HHS. Food labeling: Health claims; soluble fiber from certain foods and risk of coronary heart disease. Interim final rule. Fed. Regist. 2008, 73, 9938–9947. [Google Scholar]
- Ames, N.P.; Rhymer, C.R. Issues surrounding health claims for barley. J. Nutr. 2008, 138, 1237S–1243S. [Google Scholar] [CrossRef]
- Food Standards Australia New Zealand (FSANZ). Available online: http://www.foodstandards.gov.au/consumer/labelling/nutrition/Pages/Consultation-about-beta-glucan-and-blood-cholesterol-health-claims.aspx (accessed on 26 June 2019).
- Ministry of Agriculture, Forestry and Fisheries of Japan. e-STAT. Available online: http://www.maff.go.jp/j/tokei/kouhyou/sakumotu/sakkyou_kome/index.html (accessed on 26 June 2019). (In Japanese)
- Aune, D.; Keum, N.; Giovannucci, E.; Fadnes, L.T.; Boffetta, P.; Greenwood, D.C.; Tonstad, S.; Vatten, L.J.; Riboli, E.; Norat, T. Whole grain consumption and risk of cardiovascular disease, cancer, and all cause and cause specific mortality: Systematic review and dose-response meta-analysis of prospective studies. BMJ 2016, 353, i2716. [Google Scholar] [CrossRef]
- Aune, D.; Norat, T.; Romundstad, P.; Vatten, L.J. Whole grain and refined grain consumption and the risk of type 2 diabetes: A systematic review and dose-response meta-analysis of cohort studies. Eur. J. Epidemiol. 2013, 28, 845–858. [Google Scholar] [CrossRef]
- Ma, X.; Tang, W.G.; Yang, Y.; Zhang, Q.L.; Zheng, J.L.; Xiang, Y.B. Association between whole grain intake and all-cause mortality: A meta-analysis of cohort studies. Oncotarget. 2016, 7, 61996–62005. [Google Scholar] [CrossRef]
- World Report on Ageing and Health - World Health Organization. Chapter 2, 2015. Available online: https://apps.who.int/iris/bitstream/handle/10665/186463/9789240694811_eng.pdf (accessed on 26 June 2019).
- Shimizu, C.; Oki, Y.; Mitani, Y.; Tsuchiya, Y.; Nabeshima, T. Moderate-dose regular lifelong alcohol intake changes the intestinal flora, protects against aging, and keeps spatial memory in the senescence-accelerated mouse prone 8 (SAMP8) model. J. Pharm. Pharm. Sci. 2016, 19, 430–447. [Google Scholar] [CrossRef]
- Shimizu, C.; Wakita, Y.; Inoue, T.; Hiramitsu, M.; Okada, M.; Mitani, Y.; Segawa, S.; Tsuchiya, Y.; Nabeshima, T. Effects of lifelong intake of lemon polyphenols on aging and intestinal microbiome in the senescence-accelerated mouse prone 1 (SAMP1). Sci. Rep. 2019, 9, 3671. [Google Scholar] [CrossRef] [Green Version]
- Takeda, T.; Hosokawa, M.; Takeshita, S.; Irino, M.; Higuchi, K.; Matushita, T.; Tomita, Y.; Yasuhira, K.; Hamamoto, H.; Shimizu, K.; et al. A new murine model of accelerated senescence. Mech. Ageing Dev. 1981, 17, 183–194. [Google Scholar] [CrossRef]
- Akiguchi, I.; Pallàs, M.; Budka, H.; Akiyama, H.; Ueno, M.; Han, J.; Yagi, H.; Nishikawa, T.; Chiba, Y.; Sugiyama, H.; et al. SAMP8 mice as a neuropathological model of accelerated brain aging and dementia: Toshio Takeda’s legacy and future directions. Neuropathology 2017, 37, 293–305. [Google Scholar] [CrossRef]
- Onishi, S.; Ishino, M.; Kitazawa, H.; Yoto, A.; Shimba, Y.; Mochizuki, Y.; Unno, K.; Meguro, S.; Tokimitsu, I.; Miura, S. Green tea extracts ameliorate high-fat diet-induced muscle atrophy in senescence-accelerated mouse prone-8 mice. PLoS ONE 2018, 13, e0195753. [Google Scholar] [CrossRef]
- Matsubara, K.; Okuda, M.; Shibata, S.; Miyaki, S.; Ohkubo, T.; Izu, H.; Fujii, T. The delaying effect of alpha-glycerophosphocholine on senescence, transthyretin deposition, and osteoarthritis in senescence-accelerated mouse prone 8 mice. Biosci. Biotechnol. Biochem. 2018, 82, 647–653. [Google Scholar] [CrossRef] [Green Version]
- Toshima, G.; Iwama, Y.; Kimura, F.; Matsumoto, Y.; Miura, M.; Takahashi, J.; Yasuda, H.; Arai, N.; Mizutani, H.; Hata, K. LipoSEARCH®; Analytical GP-HPLC method for lipoprotein profiling and its applications. J. Biol. Macromol. 2013, 13, 21–32. [Google Scholar]
- Heinecke, J.W. The not-so-simple HDL story: A new era for quantifying HDL and cardiovascular risk? Nat. Med. 2012, 18, 1346–1347. [Google Scholar] [CrossRef]
- Modified Prosky Method, AOAC Official Method 991.43 Total, Soluble, and Insoluble Dietary Fibre in Foods. Available online: https://acnfp.food.gov.uk/sites/default/files/mnt/drupal_data/sources/files/multimedia/pdfs/annexg.pdf (accessed on 26 June 2019).
- Okazaki, M.; Usui, S.; Fukui, A.; Kubota, I.; Tomoike, H. Component analysis of HPLC profiles of unique lipoprotein subclass cholesterols for detection of coronary artery disease. Clin. Chem. 2006, 52, 2049–2053. [Google Scholar] [CrossRef]
- Chei, C.L.; Yamagishi, K.; Kitamura, A.; Kiyama, M.; Imano, H.; Ohira, T.; Cui, R.; Tanigawa, T.; Sankai, T.; Ishikawa, Y.; et al. CIRCS Investigators. High-density lipoprotein subclasses and risk of stroke and its subtypes in Japanese population: The Circulatory Risk in Communities Study. Stroke. 2013, 44, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Hosokawa, M.; Kasai, R.; Higuchi, K.; Takeshita, S.; Shimizu, K.; Hamamoto, H.; Honma, A.; Irino, M.; Toda, K.; Matsumura, A.; et al. Grading score system: A method for evaluation of the degree of senescence in senescence accelerated mouse (SAM). Mech. Ageing Dev. 1984, 26, 91–102. [Google Scholar] [CrossRef]
- Garneau, A.P.; Marcoux, A.A.; Noël, M.; Frenette-Cotton, R.; Drolet, M.C.; Couet, J.; Larivière, R.; Isenring, P. Ablation of potassium-chloride cotransporter type 3 (Kcc3) in mouse causes multiple cardiovascular defects and isosmotic polyuria. PLoS One. 2016, 11, e0154398. [Google Scholar] [CrossRef]
- Tai, C.Y.; Nabeshima, T.; Sivam, S.; Ho, I.K. Effects of acute and continuous administration of pentobarbital on phencyclidine response. Res. Comm. Sub. Abuse. 1981, 2, 239–252. [Google Scholar]
- Ko, S.u.; Ling, S.M.; Winters, J.; Ferrucci, L. Age-related mechanical work expenditure during normal walking: The Baltimore Longitudinal Study of Aging. J. Biomech. 2009, 42, 1834–1839. [Google Scholar] [CrossRef] [Green Version]
- Blizzard, C.A.; Southam, K.A.; Dawkins, E.; Lewis, K.E.; King, A.E.; Clark, J.A.; Dickson, T.C. Identifying the primary site of pathogenesis in amyotrophic lateral sclerosis - vulnerability of lower motor neurons to proximal excitotoxicity. Dis. Model Mech. 2015, 8, 215–224. [Google Scholar] [CrossRef]
- Murai, T.; Okuda, S.; Tanaka, T.; Ohta, H. Characteristics of object location memory in mice: Behavioral and pharmacological studies. Physiol. Behav. 2007, 90, 116–124. [Google Scholar] [CrossRef]
- Matsuki, T.; Watanabe, K.; Fujimoto, J.; Kado, Y.; Takada, T.; Matsumoto, K.; Tanaka, R. Quantitative PCR with 16S rRNA-gene-targeted species-specific primers for analysis of human intestinal bifidobacteria. Appl. Environ. Microbiol. 2004, 70, 167–173. [Google Scholar] [CrossRef]
- Lozupone, C.; Lladser, M.E.; Knights, D.; Stombaugh, J.; Knight, R. UniFrac: An effective distance metric for microbial community comparison. ISME J. 2011, 5, 169–172. [Google Scholar] [CrossRef]
- Yamamoto, K.; Hatakeyama, Y.; Sakamoto, Y.; Tsuduki, T. High-fat diet intake from senescence inhibits the attenuation of cell functions and the degeneration of villi with aging in the small intestine, and inhibits the attenuation of lipid absorption ability in SAMP8 mice. J. Clin. Biochem. Nutr. 2015, 57, 204–211. [Google Scholar] [CrossRef] [Green Version]
- Miyamoto, J.; Watanabe, K.; Taira, S.; Kasubuchi, M.; Li, X.; Irie, J.; Itoh, H.; Kimura, I. Barley β-glucan improves metabolic condition via short-chain fatty acids produced by gut microbial fermentation in high fat diet fed mice. PLoS One. 2018, 13, e0196579. [Google Scholar] [CrossRef] [PubMed]
- Mehla, J.; Chauhan, B.C.; Chauhan, N.B. Experimental induction of type 2 diabetes in aging-accelerated mice triggered Alzheimer-like pathology and memory deficits. J. Alzheimers Dis. 2014, 39, 145–162. [Google Scholar] [CrossRef] [PubMed]
- Panahi, S.; Ezatagha, A.; Jovanovski, E.; Jenkins, A.; Temelli, F.; Vasanthan, T.; Vuksan, V. Glycemic effect of oat and barley beta-glucan when incorporated into a snack bar: A dose escalation study. J. Am. Coll. Nutr. 2014, 33, 442–449. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Obesity and Overweight. Available online: https://www.who.int/en/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 26 June 2019).
- Matsuo, T.; Sairenchi, T.; Iso, H.; Irie, F.; Tanaka, K.; Fukasawa, N.; Ota, H.; Muto, T. Age- and gender-specific BMI in terms of the lowest mortality in Japanese general population. Obesity 2008, 16, 2348–2355. [Google Scholar] [CrossRef] [PubMed]
- Tamakoshi, A.; Yatsuya, H.; Lin, Y.; Tamakoshi, K.; Kondo, T.; Suzuki, S.; Yagyu, K.; Kikuchi, S.; JACC Study Group. BMI and all-cause mortality among Japanese older adults: Findings from the Japan collaborative cohort study. Obesity 2010, 18, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Sun, X.; Wang, M.; Zhang, C.; Cao, Y.; Mo, G.; Liang, J.; Zhu, S. Quantitative assessment of the effects of beta-glucan consumption on serum lipid profile and glucose level in hypercholesterolemic subjects. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 714–723. [Google Scholar] [CrossRef] [PubMed]
- Ho, H.V.; Sievenpiper, J.L.; Zurbau, A.; Blanco Mejia, S.; Jovanovski, E.; Au-Yeung, F.; Jenkins, A.L.; Vuksan, V. A systematic review and meta-analysis of randomized controlled trials of the effect of barley β-glucan on LDL-C., non-HDL-C and apoB for cardiovascular disease risk reductioni-iv. Eur. J. Clin. Nutr. 2016, 70, 1239–1245. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.W.; Chan, Y.C.; Wei, C.C.; Chen, Y.A.; Wang, M.F.; Chang, S.J. An alternative model for studying age-associated metabolic complications: Senescence-accelerated mouse prone 8. Exp. Gerontol. 2017, 99, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Tosh, S.M. Review of human studies investigating the post-prandial blood-glucose lowering ability of oat and barley food products. Eur. J. Clin. Nutr. 2013, 67, 310–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schulze, M.B.; Schulz, M.; Heidemann, C.; Schienkiewitz, A.; Hoffmann, K.; Boeing, H. Fiber and magnesium intake and incidence of type 2 diabetes: A prospective study and meta-analysis. Arch. Intern. Med. 2007, 167, 956–965. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, S.; Hanafusa, M.; Tsubone, H.; Takimoto, H.; Yamanaka, D.; Kuwahara, M.; Ito, K. Age-dependency of the serum oxidative level in the senescence-accelerated mouse prone 8. J. Vet. Med. Sci. 2016, 78, 1369–1371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chou, S.T.; Tseng, S.T. Oxidative stress markers in type 2 diabetes patients with diabetic neppathy. Clin. Exp. Nephrol. 2017, 21, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Bavkar, L.N.; Patil, R.S.; Rooge, S.B.; Nalawade, M.L.; Arvindekar, A.U. Acceleration of protein glycation by oxidative stress and comparative role of antioxidant and protein glycation inhibitor. Mol. Cell Biochem 2019. [Google Scholar] [CrossRef] [PubMed]
- Matilainen, V.; Koskela, P.; Keinänen-Kiukaanniemi, S. Early androgenetic alopecia as a marker of insulin resistance. Lancet 2000, 356, 1165–1166. [Google Scholar] [CrossRef]
- Meng, S.J.; Yu, L.J. Oxidative stress, molecular inflammation and sarcopenia. Int. J. Mol. Sci. 2010, 11, 1509–1526. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Fischer, K.E.; Soto, V.; Liu, Y.; Sosnowska, D.; Richardson, A.; Salmon, A.B. Obesity-induced oxidative stress, accelerated functional decline with age and increased mortality in mice. Arch. Biochem. Biophys. 2015, 576, 39–48. [Google Scholar] [CrossRef] [Green Version]
- Kalyani, R.R.; Corriere, M.; Ferrucci, L. Age-related and disease-related muscle loss: The effect of diabetes, obesity, and other diseases. Lancet Diabetes Endocrinol. 2014, 2, 819–829. [Google Scholar] [CrossRef]
- Guo, A.Y.; Leung, K.S.; Siu, P.M.; Qin, J.H.; Chow, S.K.; Qin, L.; Li, C.Y.; Cheung, W.H. Muscle mass, structural and functional investigations of senescence-accelerated mouse P8 (SAMP8). Exp. Anim. 2015, 64, 425–433. [Google Scholar] [Green Version]
- Wu, I.C.; Chang, H.Y.; Hsu, C.C.; Chiu, Y.F.; Yu, S.H.; Tsai, Y.F.; Shen, S.C.; Kuo, K.N.; Chen, C.Y.; Liu, K.; et al. Association between dietary fiber intake and physical performance in older adults: A nationwide study in Taiwan. PLoS One. 2013, 8, e80209. [Google Scholar] [CrossRef]
- Rezar, V.; Pajk, T.; Marinsek Logar, R.; Jese Janezic, V.; Salobir, K.; Oresnik, A.; Salobir, J. Wheat bran and oat bran effectively reduce oxidative stress induced by high-fat diets in pigs. Ann. Nutr. Metab. 2003, 47, 78–84. [Google Scholar] [CrossRef]
- Wang, C.P.; Hazuda, H.P. Better glycemic control is associated with maintenance of lower-extremity function over time in Mexican American and European American older adults with diabetes. Diabetes Care 2011, 34, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Schwenk, M.; Mohler, J.; Wendel, C.; D’Huyvetter, K.; Fain, M.; Taylor-Piliae, R.; Najafi, B. Wearable sensor-based in-home assessment of gait, balance, and physical activity for discrimination of frailty status: Baseline results of the Arizona frailty cohort study. Gerontology. 2015, 61, 258–267. [Google Scholar] [CrossRef]
- Momma, H.; Niu, K.; Kobayashi, Y.; Guan, L.; Sato, M.; Guo, H.; Chujo, M.; Otomo, A.; Yufei, C.; Tadaura, H.; et al. Skin advanced glycation end product accumulation and muscle strength among adult men. Eur. J. Appl. Physiol. 2011, 111, 1545–1552. [Google Scholar] [CrossRef] [PubMed]
- Palomera-Ávalos, V.; Griñán-Ferré, C.; Izquierdo, V.; Camins, A.; Sanfeliu, C.; Pallàs, M. Metabolic Stress Induces Cognitive Disturbances and Inflammation in Aged Mice: Protective Role of Resveratrol. Rejuvenation Res. 2017, 20, 202–217. [Google Scholar] [CrossRef]
- Biessels, G.J.; Staekenborg, S.; Brunner, E.; Brayne, C.; Scheltens, P. Risk of dementia in diabetes mellitus: A systematic review. Lancet Neurol. 2006, 5, 64–74. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Di Domenico, F.; Barone, E. Elevated risk of type 2 diabetes for development of Alzheimer disease: A key role for oxidative stress in brain. Biochim. Biophys. Acta. 2014, 1842, 1693–1706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tseng, C.H. Pioglitazone reduces dementia risk in patients with type 2 diabetes mellitus: A retrospective cohort analysis. J. Clin. Med. 2018, 7, 306. [Google Scholar] [CrossRef] [PubMed]
- Calsolaro, V.; Edison, P. Novel GLP-1 (Glucagon-Like Peptide-1) analogues and insulin in the treatment for Alzheimer’s disease and other neurodegenerative diseases. CNS Drugs 2015, 29, 1023–1039. [Google Scholar] [CrossRef] [PubMed]
- Hansen, H.H.; Fabricius, K.; Barkholt, P.; Niehoff, M.L.; Morley, J.E.; Jelsing, J.; Pyke, C.; Knudsen, L.B.; Farr, S.A.; Vrang, N. The GLP-1 receptor agonist liraglutide improves memory function and increases hippocampal CA1 neuronal numbers in a senescence-accelerated mouse model of Alzheimer’s disease. J. Alzheimers Dis. 2015, 46, 877–888. [Google Scholar] [CrossRef] [PubMed]
- Saji, N.; Niida, S.; Murotani, K.; Hisada, T.; Tsuduki, T.; Sugimoto, T.; Kimura, A.; Toba, K.; Sakurai, T. Analysis of the relationship between the gut microbiome and dementia: A cross-sectional study conducted in Japan. Sci. Rep. 2019, 9, 1008. [Google Scholar] [CrossRef] [PubMed]
- Mitsuoka, T. Intestinal flora and aging. Nutr. Rev. 1992, 50, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ames, N.P.; Tun, H.M.; Tosh, S.M.; Jones, P.J.; Khafipour, E. High molecular weight barley β-glucan alters gut microbiota toward reduced cardiovascular disease risk. Front Microbiol. 2016, 7, 129. [Google Scholar] [CrossRef]
- Idehen, E.; Tang, Y.; Sang, S. Bioactive phytochemicals in barley. J. Food Drug Anal. 2017, 25, 148–161. [Google Scholar] [CrossRef]
- Huazano-García, A.; Shin, H.; López, M.G. Modulation of gut microbiota of overweight mice by agavins and their association with body weight loss. Nutrients. 2017, 9, 821. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.; Lin, D.; Zhao, Y.; Li, W.; Yang, X. Effects of dietary fiber supplementation on fatty acid metabolism and intestinal microbiota diversity in C57BL/6J mice fed with a high-fat diet. J. Agric. Food Chem. 2018, 66, 12706–12718. [Google Scholar] [CrossRef] [PubMed]
- Kovatcheva-Datchary, P.; Nilsson, A.; Akrami, R.; Lee, Y.S.; De Vadder, F.; Arora, T.; Hallen, A.; Martens, E.; Björck, I.; Bäckhed, F. Dietary Fiber-Induced Improvement in Glucose Metabolism Is Associated with Increased Abundance of Prevotella. Cell Metab. 2015, 22, 971–982. [Google Scholar] [CrossRef] [PubMed] [Green Version]








| Standard Diet AIN-93G | Rice Diet | Barley Diet | Mixed Barley Diet (Rice Diet: Barley Diet = 1:4) | |
|---|---|---|---|---|
| Milk casein | 200 | 157.5 | 130.0 | 135.5 |
| L-Cysteine | 3 | 3 | 3 | 3 |
| Corn starch | 397.486 | 0.0 | 0.0 | 0.0 |
| Pregelatinized corn starch | 132 | 0.0 | 0.0 | 0.0 |
| Sucrose | 100 | 100 | 100 | 100 |
| Soybean oil | 70 | 65.8 | 57.8 | 59.4 |
| Lard | 50 | 50 | 50 | |
| Cellulose | 50 | 45.8 | 0.0 | 9.2 |
| Pregelatinized rice | - | 530.5 | - | 106.1 |
| Pregelatinized barley | - | - | 611.8 | 489.4 |
| AIN-93 mineral mix | 35 | 35 | 35 | 35 |
| AIN-93 vitamin mix | 10 | 10 | 10 | 10 |
| Choline bitartrate | 2.5 | 2.5 | 2.5 | 2.5 |
| tert-butylhydroquinone | 0.014 | 0.014 | 0.014 | 0.014 |
| Plasma Cholesterols | Rice Group (mg/dL) | Barley Group (mg/dL) | p value |
|---|---|---|---|
| Total LDL-Cho | 26.7 ± 2.1 | 21.1 ± 1.9 | 0.059 |
| large + medium LDL-Cho | 9.4 ± 2.1 | 5.7 ± 0.5 | 0.002 |
| small + very small LDL-Cho (high-risk) | 17.2 ± 2.1 | 15.4 ± 1.9 | 0.522 |
| Total HDL-Cho | 114.4 ± 5.6 | 118.2 ± 4.0 | 0.582 |
| very large + large HDL-Cho | 50.9 ± 4.4 | 50.0 ± 3.2 | 0.871 |
| medium + small + very small HDL-Cho (low risk) | 63.5 ± 1.7 | 68.2 ± 1.5 | 0.045 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shimizu, C.; Wakita, Y.; Kihara, M.; Kobayashi, N.; Tsuchiya, Y.; Nabeshima, T. Association of Lifelong Intake of Barley Diet with Healthy Aging: Changes in Physical and Cognitive Functions and Intestinal Microbiome in Senescence-Accelerated Mouse-Prone 8 (SAMP8). Nutrients 2019, 11, 1770. https://doi.org/10.3390/nu11081770
Shimizu C, Wakita Y, Kihara M, Kobayashi N, Tsuchiya Y, Nabeshima T. Association of Lifelong Intake of Barley Diet with Healthy Aging: Changes in Physical and Cognitive Functions and Intestinal Microbiome in Senescence-Accelerated Mouse-Prone 8 (SAMP8). Nutrients. 2019; 11(8):1770. https://doi.org/10.3390/nu11081770
Chicago/Turabian StyleShimizu, Chikako, Yoshihisa Wakita, Makoto Kihara, Naoyuki Kobayashi, Youichi Tsuchiya, and Toshitaka Nabeshima. 2019. "Association of Lifelong Intake of Barley Diet with Healthy Aging: Changes in Physical and Cognitive Functions and Intestinal Microbiome in Senescence-Accelerated Mouse-Prone 8 (SAMP8)" Nutrients 11, no. 8: 1770. https://doi.org/10.3390/nu11081770
APA StyleShimizu, C., Wakita, Y., Kihara, M., Kobayashi, N., Tsuchiya, Y., & Nabeshima, T. (2019). Association of Lifelong Intake of Barley Diet with Healthy Aging: Changes in Physical and Cognitive Functions and Intestinal Microbiome in Senescence-Accelerated Mouse-Prone 8 (SAMP8). Nutrients, 11(8), 1770. https://doi.org/10.3390/nu11081770





