Supercritical Fluid Extract of Angelica sinensis and Zingiber officinale Roscoe Ameliorates TNBS-Induced Colitis in Rats
Abstract
1. Introduction
2. Results
2.1. Qualitative Analysis of AZ-SFE Based on GC/MS Analysis
2.2. Optimization of Extraction Process of AZ-SFE by Orthogonal Experimental Design
2.3. Effects of AZ-SFE and Major Components on Cell Viability of RAW264.7 Cells
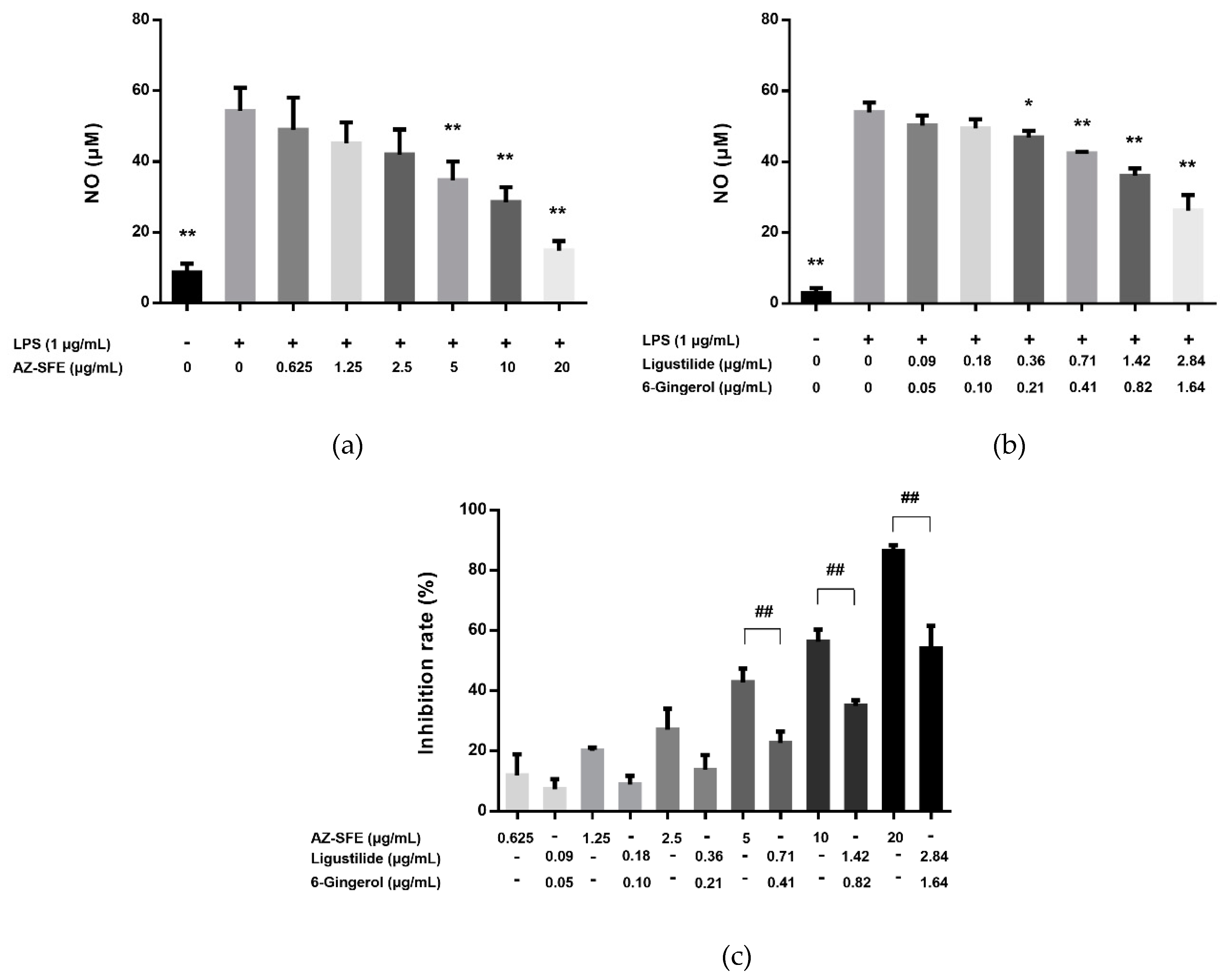
2.4. Effects of AZ-SFE and Major Components on NO Production in LPS-Induced RAW264.7 Cells
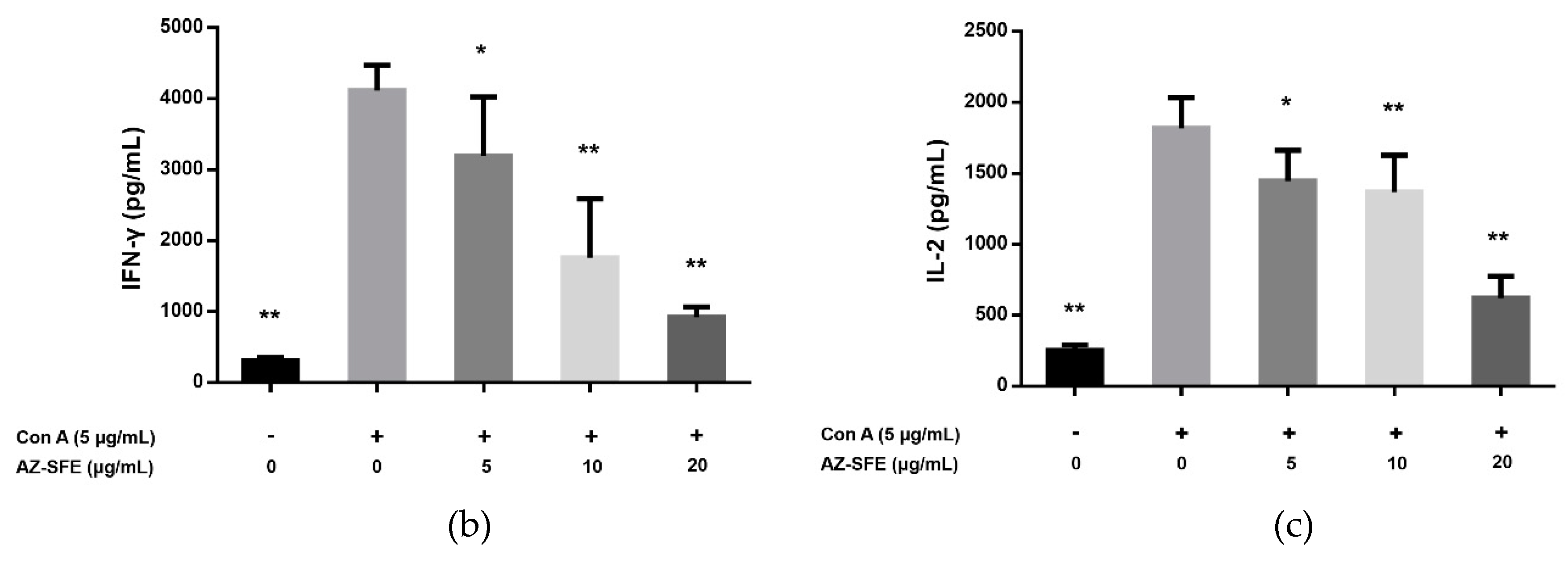
2.5. Effects of AZ-SFE on Splenocyte Proliferation and Cytokine Secretion
2.6. Effects of AZ-SFE on Body Weight and Disease Activity Index
2.7. Effects of AZ-SFE on Colon Length and Macroscopic Score
2.8. Effects of AZ-SFE on Histopathology Improvement
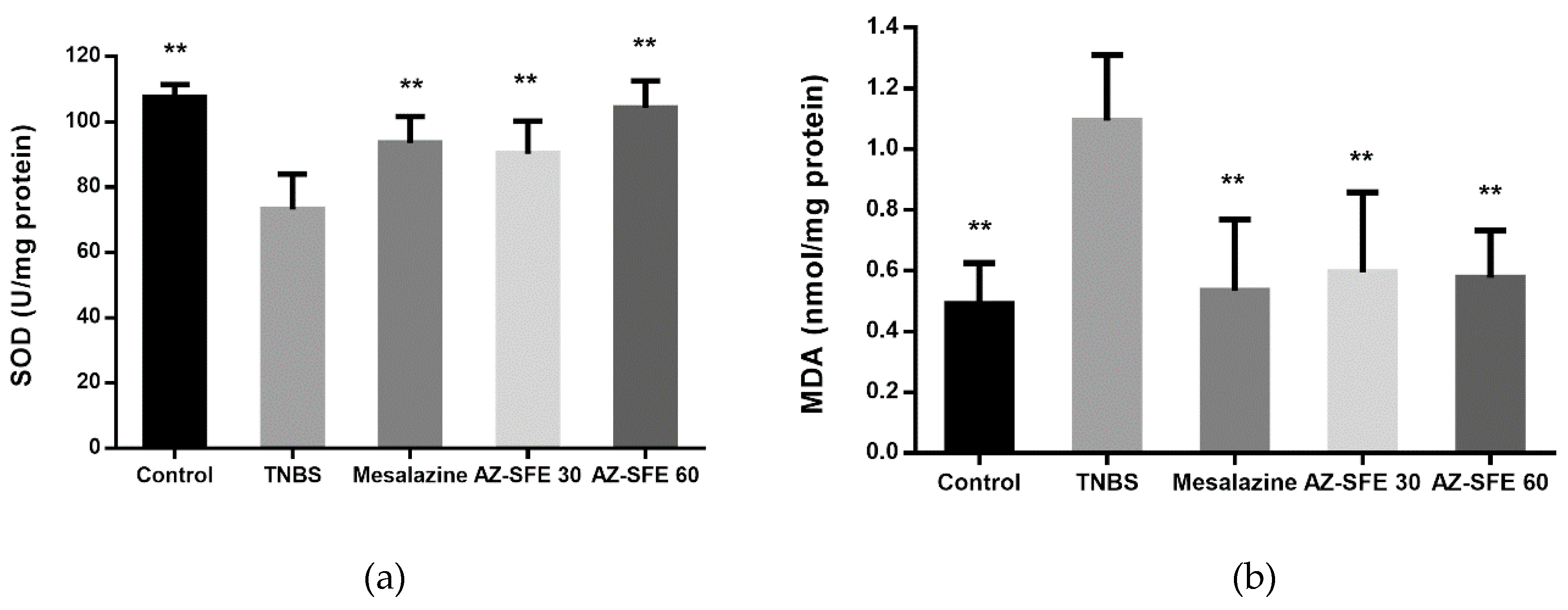
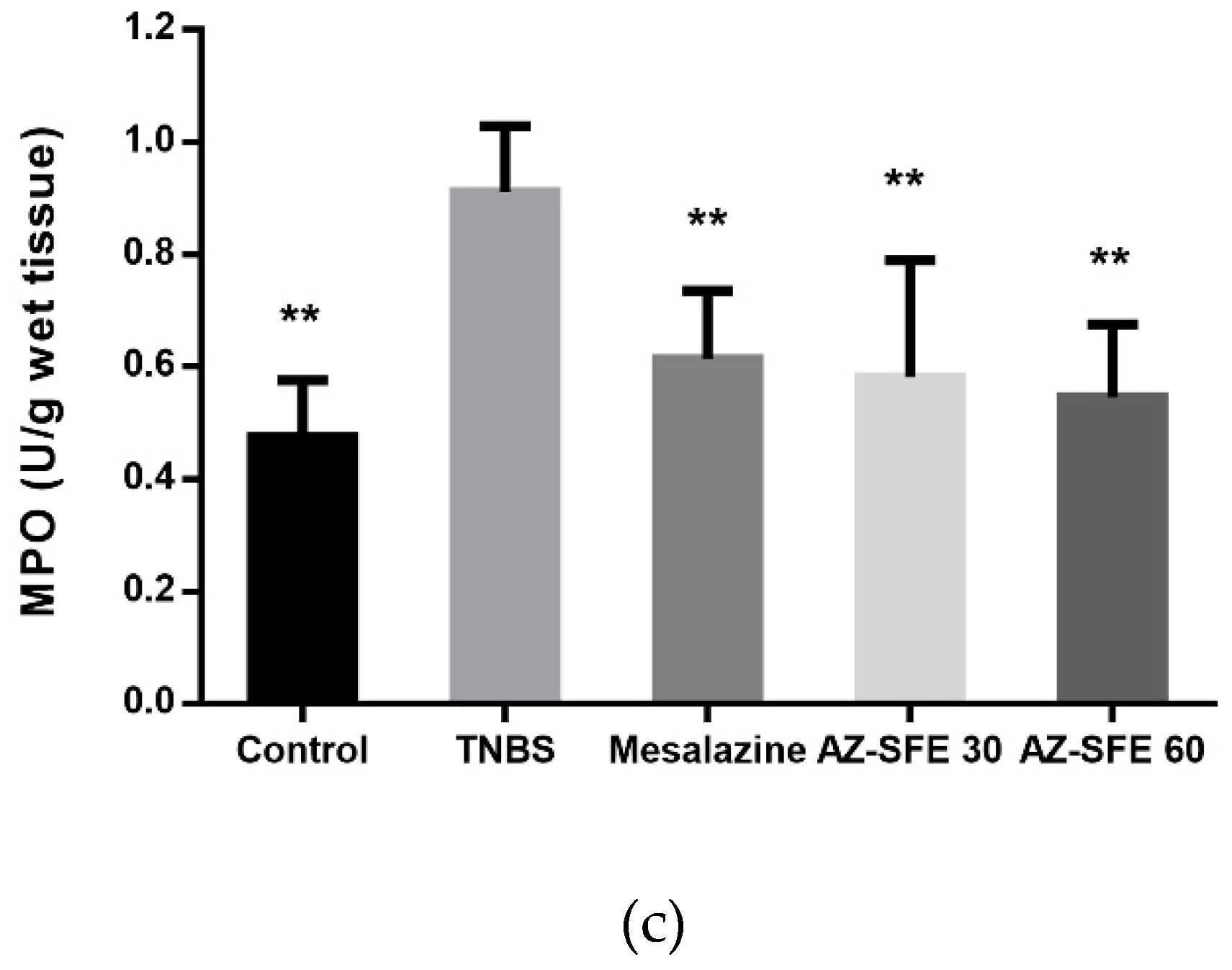
2.9. Effects of AZ-SFE on superoxide dismutase (SOD), malonic dialdehyde (MDA) and myeloperoxidase (MPO)
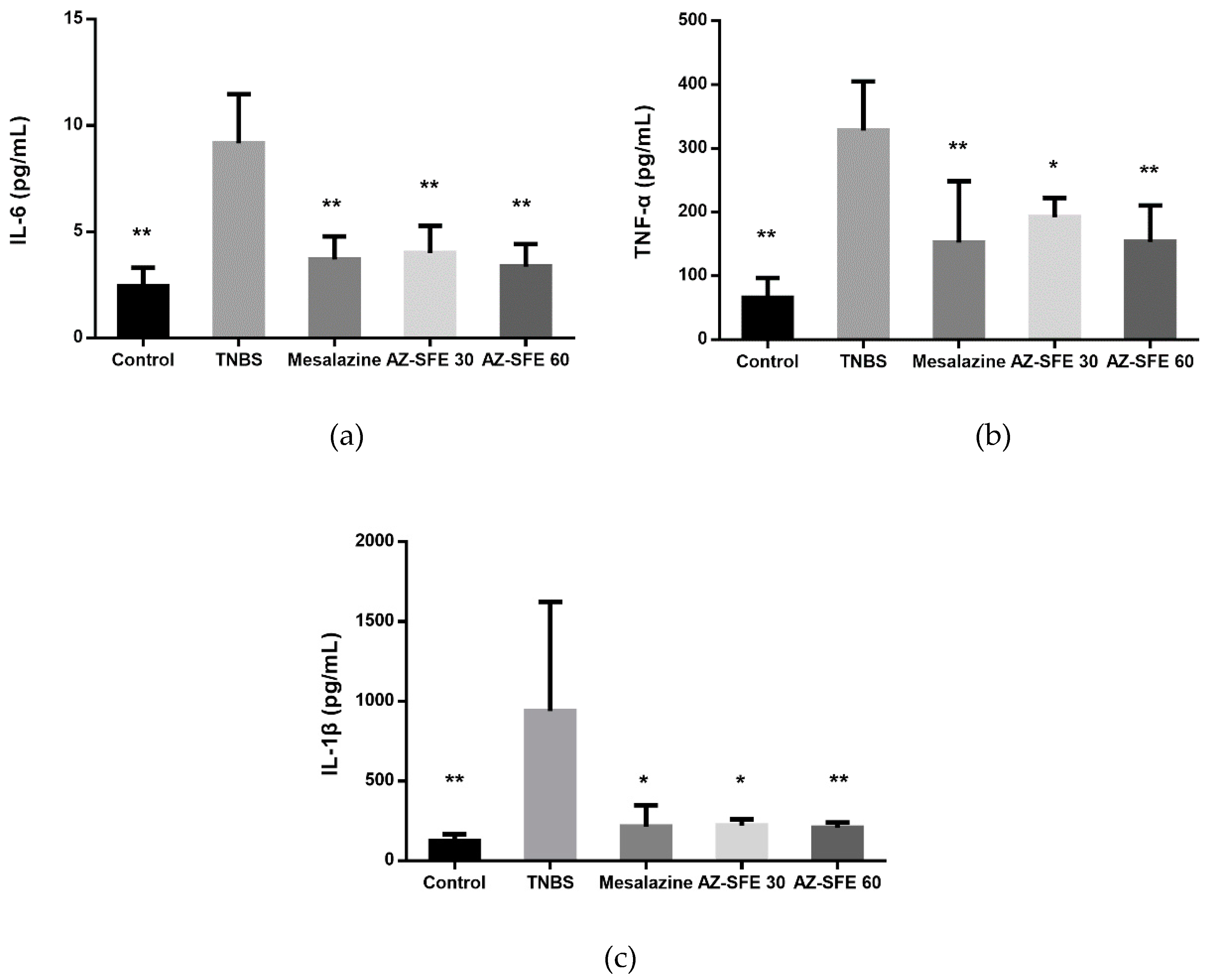
2.10. Effects of AZ-SFE on Inflammatory Cytokines in Serum
2.11. Effects of AZ-SFE on Serum Hepcidin and Serum Iron
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Plant Material and Preparation of AZ-SFE
4.3. GC/MS Analysis of AZ-SFE
4.4. HPLC Analysis of AZ-SFE
4.5. Optimization of Extraction Process of AZ-SFE
4.6. Cell Culture and MTT Cell Viability Assay
4.7. Measurement of NO Production in RAW264.7 Cells
4.8. Experimental Animals
4.9. Induction of Experimental Colitis and Intervention with AZ-SFE
4.10. Splenocyte Proliferation and Cytokine Detection
4.11. Evaluation of DAI
4.12. Evaluation of Macroscopic Damage
4.13. Histological Analysis
4.14. Measurement of SOD, MDA and MPO in Colonic Tissue
4.15. Measurement of Pro-inflammatory Cytokines in Serum
4.16. Measurement of Hepcidin and Serum Iron
4.17. Statistic Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AZ-SFE | Supercritical fluid extract of Angelica sinensis and Zingiber officinale Roscoe |
| Con A | Concanavalin A |
| CD | Crohn’s disease |
| DAI | Disease activity index |
| FBS | Fetal bovine serum |
| H&E | Hematoxylin and eosin |
| IBD | Inflammatory bowel disease |
| LPS | Lipopolysaccharide |
| MDA | Malonic dialdehyde |
| MPO | Myeloperoxidase |
| SOD | Superoxide dismutase |
| TNBS | 2, 4, 6-Trinitrobenzenesulfonic acid |
| UC | Ulcerative colitis |
References
- De Mattos, B.R.; Garcia, M.P.; Nogueira, J.B.; Paiatto, L.N.; Albuquerque, C.G.; Souza, C.L.; Fernandes, L.G.; Tamashiro, W.M.; Simioni, P.U. Inflammatory Bowel Disease: An Overview of Immune Mechanisms and Biological Treatments. Mediat. Inflamm. 2015, 2015, 493012. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Z.; Li, Y.Y. Inflammatory bowel disease: Pathogenesis. World J. Gastroenterol. 2014, 20, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Szandruk, M.; Merwid-La, A.; Szelag, A. The impact of mangiferin from Belamcanda chinensis on experimental colitis in rats. Inflammopharmacology 2018, 26, 571–581. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.H.; Yu, J.P.; Liu, Y.F.; Teng, X.J.; Ming, M.; Lv, P.; An, P.; Liu, S.Q.; Yu, H.G. Effects of Ginkgo biloba Extract on Inflammatory Mediators (SOD, MDA, TNF-α, NF-κBp65, IL-6) in TNBS-Induced Colitis in Rats. Mediat. Inflamm. 2006, 2006, 92642. [Google Scholar] [CrossRef] [PubMed]
- Grivennikov, S.I. Inflammation and colorectal cancer: Colitis-associated neoplasia. Semin. Immunopathol. 2013, 35, 229–244. [Google Scholar] [CrossRef] [PubMed]
- Ungaro, R.; Mehandru, S.; Allen, P.B.; Peyrin-Biroulet, L.; Colombel, J.F. Ulcerative colitis. Lancet 2017, 389, 1756–1770. [Google Scholar] [CrossRef]
- E Silva, F.G.D.; Paiatto, L.N.; Yamada, A.T.; Netto, F.M.; Simioni, P.U.; Tamashiro, W.M.S.C. Intake of Protein Hydrolysates and Phenolic Fractions Isolated from Flaxseed Ameliorates TNBS-Induced Colitis. Mol. Nutr. Food Res. 2018, 62, 1800088. [Google Scholar] [CrossRef] [PubMed]
- Recio, M.C.; Andújar, I.; Ríos, J.L. Anti-Inflammatory Agents from Plants: Progress and Potential. Curr. Med. Chem. 2012, 19, 2088–2103. [Google Scholar] [CrossRef]
- Sodagari, H.R.; Farzaei, M.H.; Bahramsoltani, R.; Abdolghaffari, A.H.; Mahmoudi, M.; Rezaei, N. Dietary anthocyanins as a complementary medicinal approach for management of inflammatory bowel disease. Expert Rev. Gastroenterol. Hepatol. 2015, 9, 807–820. [Google Scholar] [CrossRef]
- Chang, K.W.; Kuo, C.Y. 6-Gingerol modulates proinflammatory responses in dextran sodium sulfate (DSS)-treated Caco-2 cells and experimental colitis in mice through adenosine monophosphate-activated protein kinase (AMPK) activation. Food Funct. 2015, 6, 3334–3341. [Google Scholar] [CrossRef]
- Wang, T. Waitai Miyao Fang, 1st ed.; China Medical Science Press: Beijing, China, 2011; pp. 443–444. [Google Scholar]
- Edris, A.E. Pharmaceutical and Therapeutic Potentials of Essential Oils and Their Individual Volatile Constituents: A Review. Phytother. Res. 2007, 21, 308–323. [Google Scholar] [CrossRef] [PubMed]
- Shaaban, H.A.E.; El-Ghorab, A.H.; Shibamoto, T. Bioactivity of essential oils and their volatile aroma components: Review. J. Essent. Oil Res. 2012, 24, 203–212. [Google Scholar] [CrossRef]
- Zhong, L.J.; Hua, Y.L.; Ji, P.; Yao, W.L.; Zhang, W.Q.; Li, J.; Wei, Y.M. Evaluation of the Anti-inflammatory Effects of Volatile Oils from Processed Products of Angelica sinensis radix by GC–MS-Based Metabolomics. J. Ethnopharmacol. 2016, 191, 195–205. [Google Scholar] [CrossRef] [PubMed]
- de Melo, G.A.N.; Grespan, R.; Fonseca, J.P.; Farinha, T.O.; Da Silva, E.L.; Romero, A.L.; Bersani-Amado, C.A.; Cuman, R.K.N. Inhibitory effects of ginger (Zingiber officinale Roscoe) essential oil on leukocyte migration in vivo and in vitro. J. Nat. Med. 2011, 65, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Butt, M.S.; Sultan, M.T. Ginger and its Health Claims: Molecular Aspects. Crit. Rev. Food Sci. Nutr. 2011, 51, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Ma, N.; Gao, Y.F.; Sun, L.L.; Zhang, J.G. Therapeutic Effects of 6-Gingerol, 8-Gingerol, and 10-Gingerol on Dextran Sulfate Sodium-Induced Acute Ulcerative Colitis in Rats. Phytother. Res. 2017, 31, 1427–1432. [Google Scholar] [CrossRef] [PubMed]
- Ajayi, B.; Adedara, I.; Farombi, E. Protective mechanisms of 6-gingerol in dextran sulfate sodium-induced chronic ulcerative colitis in mice. Hum. Exp. Toxicol. 2018, 37, 1054–1068. [Google Scholar] [CrossRef] [PubMed]
- Nikkhah-Bodaghi, M.; Maleki, I.; Agah, S.; Hekmatdoost, A. Zingiber officinale and oxidative stress in patients with ulcerative colitis: A randomized, placebo-controlled, clinical trial. Complement. Med. 2019, 43, 1–6. [Google Scholar] [CrossRef]
- Zhao, B.; Kang, Q.; Peng, Y.; Xie, Y.; Chen, C.; Li, B.; Wu, Q. Effect of Angelica sinensis Root Extract on Cancer Prevention in Different Stages of an AOM/DSS Mouse Model. Int. J. Mol. Sci. 2017, 18, 1750. [Google Scholar] [CrossRef]
- Mesomo, M.C.; Scheer, A.D.P.; Perez, E.; Ndiaye, P.M.; Corazza, M.L. Ginger (Zingiber officinale R.) extracts obtained using supercritical CO2 and compressed propane: Kinetics and antioxidant activity evaluation. J. Supercrit. Fluids 2012, 71, 102–109. [Google Scholar] [CrossRef]
- Randhawa, P.K.; Singh, K.; Singh, N.; Jaggi, A.S. A Review on Chemical-Induced Inflammatory Bowel Disease Models in Rodents. Korean J. Physiol. Pharmacol. 2014, 18, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Cota, D.; Mishra, S.; Shengule, S. Beneficial role of Terminalia arjuna hydro-alcoholic extract in colitis and its possible mechanism. J. Ethnopharmacol. 2019, 230, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Debnath, T.; Kim, D.H.; Lim, B.O. Natural Products as a Source of Anti-Inflammatory Agents Associated with Inflammatory Bowel Disease. Molecules 2013, 18, 7253–7270. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.F.; Wahlquist, M.L.; He, G.Q.; Yang, M.; Li, D. Natural products and anti-inflammatory activity. Asia Pac. J. Clin. Nutr. 2006, 15, 143–152. [Google Scholar] [PubMed]
- Shin, J.S.; Cho, E.J.; Choi, H.E.; Seo, J.H.; An, H.J.; Park, H.J.; Cho, Y.W.; Lee, K.T. Anti-inflammatory effect of a standardized triterpenoid-rich fraction isolated from Rubus coreanus on dextran sodium sulfate-induced acute colitis in mice and LPS-induced macrophages. J. Ethnopharmacol. 2014, 158, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Guan, Q.; Moreno, S.; Qing, G.; Weiss, C.R.; Lu, L.; Bernstein, C.N.; Warrington, R.J.; Ma, Y.; Peng, Z. The role and potential therapeutic application of myeloid-derived suppressor cells in TNBS-induced colitis. J. Leukoc. Biol. 2013, 94, 803–811. [Google Scholar] [CrossRef]
- Şengül, N.; Işık, S.; Aslım, B.; Uçar, G.; Demirbağ, A.E. The Effect of Exopolysaccharide-Producing Probiotic Strains on Gut Oxidative Damage in Experimental Colitis. Dig. Dis. Sci. 2011, 56, 707–714. [Google Scholar] [CrossRef]
- de Faria, F.M.; Luiz-Ferreira, A.; Socca, E.A.R.; De Almeida, A.C.A.; Dunder, R.J.; Manzo, L.P.; Da Silva, M.A.; Vilegas, W.; Rozza, A.L.; Pellizzon, C.H.; et al. Effects of Rhizophora mangle on Experimental Colitis Induced by TNBS in Rats. Evid. Based Complement. Altern. Med. 2012, 2012, 753971. [Google Scholar] [CrossRef]
- Li, Y.; Shen, L.; Luo, H.S. Luteolin ameliorates dextran sulfate sodium-induced colitis in mice possibly through activation of the Nrf2 signaling pathway. Int. Immunopharmacol. 2016, 40, 24–31. [Google Scholar] [CrossRef]
- Xing, J.F.; Sun, J.Y.; You, H.S.; Lv, J.; Sun, J.N.; Dong, Y.L. Anti-Inflammatory Effect of 3,4-Oxo-isopropylidene-shikimic Acid on Acetic Acid-Induced Colitis in Rats. Inflammation 2012, 35, 1872–1879. [Google Scholar] [CrossRef]
- Rashidian, A.; Muhammadnejad, A.; Dehpou, A.; Mehr, S.E.; Akhavan, M.M.; Shirkoohi, R.; Chamanara, M.; Mousavi, S.; Rezaya, S. Atorvastatin attenuates TNBS-induced rat colitis: The involvement of the TLR4/NF-kB signaling pathway. Inflammopharmacology 2016, 24, 109–118. [Google Scholar] [CrossRef]
- Mao, T.Y.; Shi, R.; Zhao, W.H.; Guo, Y.; Gao, K.L.; Chen, C.; Xie, T.H.; Li, J.X. Qingchang Wenzhong Decoction Ameliorates Dextran Sulphate Sodium-Induced Ulcerative Colitis in Rats by Downregulating the IP10/CXCR3 Axis-Mediated Inflammatory Response. Evid. Based Complement. Altern. Med. 2016, 2016, 4312538. [Google Scholar] [CrossRef]
- Ishiguro, Y. Mucosal proinflammatory cytokine production correlates with endoscopic activity of ulcerative colitis. J. Gastroenterol. 1999, 34, 66–74. [Google Scholar] [CrossRef]
- Stevens, C.; Walz, G.; Singaram, C.; Lipman, M.L.; Zanker, B.; Muggia, A.; Antonioli, D.; Peppercorn, M.A.; Strom, T.B. Tumor Necrosis Factor-α, Interleukin- 1β, and Interleukin-6 Expression in Inflammatory Bowel Disease. Dig. Dis. Sci. 1992, 37, 818–826. [Google Scholar] [CrossRef]
- Zhang, P.; Niu, F.L.; Liu, W.Z.; Shi, Y.; Lu, L.G. Anti-inflammatory mechanism of oxymatrine in dextran sulfate sodium-induced colitis of rats. World J. Gastroenterol. 2005, 11, 4912–4915. [Google Scholar] [CrossRef]
- Yang, X.L.; Guo, T.K.; Wang, Y.H.; Liu, X.; Wang, X.X.; Li, W.; Zhao, X.; Wang, L.P.; Yan, S.; Wu, D.; et al. Ginsenoside Rd attenuates the inflammatory response via modulating p38 and JNK signaling pathways in rats with TNBS-induced relapsing colitis. Int. Immunopharmacol. 2012, 12, 408–414. [Google Scholar] [CrossRef]
- Coccia, M.; Harrison, O.J.; Schiering, C.; Asquith, M.J.; Becher, B.; Powrie, F.; Maloy, K.J. IL-1β mediates chronic intestinal inflammation by promoting the accumulation of IL-17A secreting innate lymphoid cells and CD4+ Th17 cells. J. Exp. Med. 2012, 209, 1595–1609. [Google Scholar] [CrossRef]
- Wessling-Resnick, M. Iron homeostasis and the inflammatory response. Annu. Rev. Nutr. 2010, 30, 105–122. [Google Scholar] [CrossRef]
- Weiss, G.; Gasche, C. Pathogenesis and treatment of anemia in inflammatory bowel disease. Haematologica 2010, 95, 175–178. [Google Scholar] [CrossRef]
- Shanmugam, N.K.N.; Ellenbogen, S.; Trebicka, E.; Wang, L.; Mukhopadhyay, S.; Lacy-Hulbert, A.; Gallini, C.A.; Garrett, W.S.; Cherayil, B.J. Tumor Necrosis Factor a Inhibits Expression of the Iron Regulating Hormone Hepcidin in Murine Models of Innate Colitis. PLoS ONE 2012, 7, e38136. [Google Scholar] [CrossRef]
- Oustamanolakis, P.; Koutroubakis, I.E.; Messaritakis, I.; Malliaraki, N.; Sfiridaki, A.; Kouroumalis, E.A. Serum hepcidin and prohepcidin concentrations in inflammatory bowel disease. Eur. J. Gastroenterol. Hepatol. 2011, 23, 262–268. [Google Scholar] [CrossRef]
- Mecklenburg, I.; Reznik, D.; Fasler-Kan, E.; Drewe, J.; Beglinger, C.; Hruz, P.; Swiss IBD Cohort Study Group. Serum hepcidin concentrations correlate with ferritin in patients with inflammatory bowel disease. J. Crohn’s Colitis 2014, 8, 1392–1397. [Google Scholar] [CrossRef]
- Arnold, J.; Sangwaiya, A.; Bhatkal, B.; Geoghegan, F.; Busbridge, M. Hepcidin and inflammatory bowel disease: Dual role in host defence and iron homoeostasis. Eur. J. Gastroenterol. Hepatol. 2009, 21, 425–429. [Google Scholar] [CrossRef]
- Gotardo, É.M.; Ribeiro, G.de.; Clemente, T.R.; Moscato, C.H.; Tomé, R.B.; Rocha, T.; Jr, J.P.; Ribeiro, M.L.; Gambero, A. Hepcidin expression in colon during trinitrobenzene sulfonic acid-induced colitis in rats. World J. Gastroenterol. 2014, 20, 4345–4352. [Google Scholar] [CrossRef]
- Wang, L.J.; Johnson, E.E.; Shi, H.N.; Walker, W.A.; Wessling-Resnick, M.; Cherayil, B.J. Attenuated inflammatory responses in hemochromatosis reveal a role for iron in the regulation of macrophage cytokine translation. J. Immunol. 2008, 181, 2723–2731. [Google Scholar] [CrossRef]
- Toblli, J.E.; Cao, G.; Angerosa, M. Ferrous sulfate, but not iron polymaltose complex, aggravates local and systemic inflammation and oxidative stress in dextran sodium sulfate-induced colitis in rats. Drug Des. Devel. Ther. 2015, 9, 2585–2597. [Google Scholar] [CrossRef]
- Baykalir, B.G.; Aksit, D.; Dogru, M.S.; Yay, A.H.; Aksit, H.; Seyrek, K.; Atessahin, A. Lycopene Ameliorates Experimental Colitis in Rats via Reducing Apoptosis and Oxidative Stress. Int J. Vitam Nutr. Res. 2016, 86, 27–35. [Google Scholar] [CrossRef]
- Yue, Y.; Wu, S.; Li, Z.; Li, J.; Li, X.; Xiang, J.; Ding, H. Wild jujube polysaccharides protect against experimental inflammatory bowel disease by enabling enhanced intestinal barrier function. Food Funct. 2015, 6, 2568–2577. [Google Scholar] [CrossRef]
- McGuckin, M.A.; Eri, R.; Simms, L.A.; Florin, T.H.J.; Radford-Smith, G. Intestinal Barrier Dysfunction in Inflammatory Bowel Diseases. Inflamm. Bowel Dis. 2009, 15, 100–113. [Google Scholar] [CrossRef]
- Chinese Pharmacopoeia Commission. The Pharmacopoeia of the People’s Republic of China; 2015 ed. 1st part; China Medical Science Press: Beijing, China, 2015; p. 101. [Google Scholar]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Liu, J.; Huang, H.; Huang, Z.; Ma, Y.; Zhang, L.; He, Y.; Li, D.; Liu, W.; Goodin, S.; Zhang, K.; et al. Eriocitrin in combination with resveratrol ameliorates LPS-induced inflammation in RAW264.7 cells and relieves TPA-induced mouse ear edema. J. Funct. Foods. 2019, 56, 321–332. [Google Scholar] [CrossRef]
- Zhou, Y.; Ruan, Z.; Zhou, X.; Huang, X.; Li, H.; Wang, L.; Zhang, C.; Liu, S.; Deng, Z.; Wu, G.; et al. A diet with lactosucrose supplementation ameliorates trinitrobenzene sulfonic acid-induced colitis in rats. Food Funct. 2015, 6, 162–172. [Google Scholar] [CrossRef]
- Han, F.; Zhang, H.; Xia, X.; Xiong, H.; Song, D.; Zong, X.; Wang, Y. Porcine β-defensin 2 attenuates inflammation and mucosal lesions in dextran sodium sulfate-induced colitis. J. Immunol. 2015, 194, 1882–1893. [Google Scholar] [CrossRef]
- Luk, H.H.; Ko, J.K.; Fung, H.S.; Cho, C.H. Delineation of the protective action of zinc sulfate on ulcerative colitis in rats. Eur. J. Pharmacol. 2002, 443, 197–204. [Google Scholar] [CrossRef]
- Darsigny, M.; St-Jean, S.; Boudreau, F. Cux1 transcription factor is induced in inflammatory bowel disease and protects against experimental colitis. Inflamm. Bowel Dis. 2010, 16, 1739–1750. [Google Scholar] [CrossRef]







| No. | Retention Time (min) | Compound Name | Molecular Formula |
|---|---|---|---|
| 1 | 13.211 | Decanal | C10H20O |
| 2 | 17.137 | α-Curcumene | C15H22 |
| 3 | 17.202 | β-Copaene | C15H24 |
| 4 | 17.305 | Zingiberene | C15H24 |
| 5 | 17.422 | α-Farnesene | C15H24 |
| 6 | 17.480 | β-Bisabolene | C15H24 |
| 7 | 17.706 | β-Sesquiphellandrene | C15H24 |
| 8 | 18.114 | Hedycaryol | C15H26O |
| 9 | 19.129 | Zingiberenol | C15H26O |
| 10 | 19.770 | Zingerone | C11H14O3 |
| 11 | 19.930 | N-Butylphthalide | C12H14O2 |
| 12 | 20.003 | β-Eudesmol | C15H26O |
| 13 | 20.378 | N-Butylidenephthalide | C12H12O2 |
| 14 | 20.824 | Dehydronerolidol | C15H24O |
| 15 | 21.54 | Senkyunolide | C12H16O2 |
| 16 | 22.060 | Z-Ligustilide | C12H14O2 |
| 17 | 23.657 | E-Ligustilide | C12H14O2 |
| 18 | 28.392 | Hexadecanoic acid | C16H32O2 |
| 19 | 29.718 | Senkyunolide H | C12H16O4 |
| 20 | 33.626 | Linoleic acid | C18H32O2 |
| 21 | 35.346 | Panaxynone | C17H22O |
| 22 | 36.219 | 6-Paradol | C17H26O3 |
| 23 | 37.934 | 6-Shogaol | C17H24O3 |
| 24 | 38.917 | 6-Gingerdione | C17H24O4 |
| 25 | 40.638 | 6-Gingerol | C17H26O4 |
| 26 | 42.513 | 6-Gingerol monoacetate | C19H28O5 |
| 27 | 43.613 | 8-Shogaol | C19H30O4 |
| 28 | 44.435 | 6-Gingerdiol 3,5-diacetate | C21H32O6 |
| 29 | 44.758 | 8-Gingerdione | C19H28O4 |
| 30 | 46.518 | 6-Dehydrogingerdione | C17H22O4 |
| 31 | 46.731 | 8-Gingerol | C19H30O4 |
| 32 | 49.500 | 10-Shogaol | C21H32O3 |
| 33 | 50.353 | 10-Gingerdione | C21H32O4 |
| 34 | 55.218 | 10-Dehydrogingerdione | C21H30O4 |
| Run | Factor | Evaluation Index | ||||||
|---|---|---|---|---|---|---|---|---|
| A | B | C | D | Yield (%) | Ligustilide Content (%) | 6-Gingerol Content (%) | Score | |
| 1 | 1 | 1 | 1 | 1 | 1.74 | 15.51 | 8.03 | 76.23 |
| 2 | 1 | 2 | 2 | 2 | 1.84 | 15.14 | 8.99 | 79.34 |
| 3 | 1 | 3 | 3 | 3 | 2.20 | 15.19 | 8.91 | 85.35 |
| 4 | 2 | 1 | 2 | 3 | 1.88 | 15.04 | 9.58 | 80.96 |
| 5 | 2 | 2 | 3 | 1 | 2.35 | 14.25 | 7.74 | 83.77 |
| 6 | 2 | 3 | 1 | 2 | 2.92 | 14.05 | 8.50 | 94.65 |
| 7 | 3 | 1 | 3 | 2 | 1.68 | 13.68 | 9.70 | 75.17 |
| 8 | 3 | 2 | 1 | 3 | 2.06 | 15.54 | 8.66 | 83.04 |
| 9 | 3 | 3 | 2 | 1 | 2.29 | 12.58 | 9.57 | 83.23 |
| K1 | 80.31 | 77.45 | 84.64 | 81.08 | ||||
| K2 | 86.46 | 82.05 | 81.18 | 83.05 | ||||
| K3 | 80.48 | 87.74 | 81.43 | 83.12 | ||||
| R | 6.15 | 10.29 | 3.46 | 2.04 | ||||
| Factor | DF | Anova SS | Mean Square | F | P |
|---|---|---|---|---|---|
| Pressure | 2 | 73.65 | 36.83 | 9.12 | 0.0988 |
| Temperature | 2 | 159.43 | 79.71 | 19.75 | 0.0482 * |
| Time | 2 | 22.36 | 11.18 | 2.77 | 0.2652 |
| Error | 2 | 8.07 | 4.04 |
| Level | Factor | ||
|---|---|---|---|
| Pressure (MPa) | Temperature (°C) | Time (h) | |
| A | B | C | |
| 1 | 20 | 35 | 1 |
| 2 | 30 | 45 | 2 |
| 3 | 40 | 55 | 3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Yu, L.; Mo, N.; Lan, H.; Zhang, Y.; Liu, X.; Wu, Q. Supercritical Fluid Extract of Angelica sinensis and Zingiber officinale Roscoe Ameliorates TNBS-Induced Colitis in Rats. Int. J. Mol. Sci. 2019, 20, 3816. https://doi.org/10.3390/ijms20153816
Liu J, Yu L, Mo N, Lan H, Zhang Y, Liu X, Wu Q. Supercritical Fluid Extract of Angelica sinensis and Zingiber officinale Roscoe Ameliorates TNBS-Induced Colitis in Rats. International Journal of Molecular Sciences. 2019; 20(15):3816. https://doi.org/10.3390/ijms20153816
Chicago/Turabian StyleLiu, Jia, Ling Yu, Nuolan Mo, Hai Lan, Yan Zhang, Xin Liu, and Qing Wu. 2019. "Supercritical Fluid Extract of Angelica sinensis and Zingiber officinale Roscoe Ameliorates TNBS-Induced Colitis in Rats" International Journal of Molecular Sciences 20, no. 15: 3816. https://doi.org/10.3390/ijms20153816
APA StyleLiu, J., Yu, L., Mo, N., Lan, H., Zhang, Y., Liu, X., & Wu, Q. (2019). Supercritical Fluid Extract of Angelica sinensis and Zingiber officinale Roscoe Ameliorates TNBS-Induced Colitis in Rats. International Journal of Molecular Sciences, 20(15), 3816. https://doi.org/10.3390/ijms20153816




