Clumps of Mesenchymal Stem Cell/Extracellular Matrix Complexes Generated with Xeno-Free Conditions Facilitate Bone Regeneration via Direct and Indirect Osteogenesis
Abstract
:1. Introduction
2. Results
2.1. Generation of 3D-Culture C-MSCs in Xeno-Free Conditions
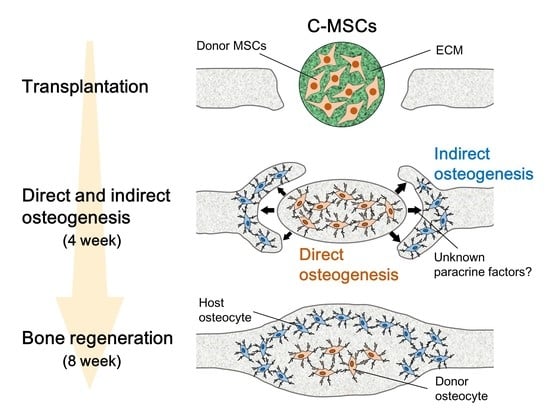
2.2. Implantation of C-MSCs Generated in Xeno-Free Conditions with No Artificial Scaffold Can Induce Mouse Calvarial Bone Regeneration
2.3. Transplanted Human Donor and Mouse Host Cells Contribute to Bone Reconstruction Induced by Transplantation of C-MSCs
2.4. Transplantation of C-MSCs Induces Bone Regeneration via Direct and Indirect Osteogenesis in a SCID Mouse Calvarial Defect Model
2.5. Transplantation of Decell-C-MSCs Fails to Induce Bone Regeneration
3. Discussion
4. Materials and Methods
4.1. C-MSC Preparation and Culture
4.2. Flow Cytometric Analysis of MSC Markers
4.3. Decellularization of C-MSCs
4.4. Histological and Immunofluorescence Analysis of C-MSCs and Decell-C-MSCs
4.5. Surgical Procedures
4.6. Micro-CT Analysis
4.7. Tissue Preparation and Histological Analysis
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jackson, W.M.; Nesti, L.J.; Tuan, R.S. Concise review: Clinical translation of wound healing therapies based on mesenchymal stem cells. Stem Cells Transl. Med. 2012, 1, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Liew, A.; O’Brien, T. Therapeutic potential for mesenchymal stem cell transplantation in critical limb ischemia. Stem Cell Res. Ther. 2012, 3, 28. [Google Scholar] [CrossRef] [PubMed]
- Samsonraj, R.M.; Raghunath, M.; Nurcombe, V.; Hui, J.H.; van Wijnen, A.J.; Cool, S.M. Concise review: Multifaceted characterization of human mesenchymal stem cells for use in regenerative medicine. Stem Cells Transl. Med. 2017, 6, 2173–2185. [Google Scholar] [CrossRef] [PubMed]
- Colnot, C. Cell sources for bone tissue engineering: Insights from basic science. Tissue Eng. Part B Rev. 2011, 17, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Watson, L.; Elliman, S.J.; Coleman, C.M. From isolation to implantation: A concise review of mesenchymal stem cell therapy in bone fracture repair. Stem Cell Res. Ther. 2014, 5, 51. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.; Roy, M.; Bandyopadhyay, A. Recent advances in bone tissue engineering scaffolds. Trends Biotechnol. 2012, 30, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Kittaka, M.; Kajiya, M.; Shiba, H.; Takewaki, M.; Takeshita, K.; Khung, R.; Fujita, T.; Iwata, T.; Nguyen, T.Q.; Ouhara, K.; et al. Clumps of a mesenchymal stromal cell/extracellular matrix complex can be a novel tissue engineering therapy for bone regeneration. Cytotherapy 2015, 17, 860–873. [Google Scholar] [CrossRef]
- Takewaki, M.; Kajiya, M.; Takeda, K.; Sasaki, S.; Motoike, S.; Komatsu, N.; Matsuda, S.; Ouhara, K.; Mizuno, N.; Fujita, T.; et al. Msc/ecm cellular complexes induce periodontal tissue regeneration. J. Dent. Res. 2017, 96, 984–991. [Google Scholar] [CrossRef]
- Takeshita, K.; Motoike, S.; Kajiya, M.; Komatsu, N.; Takewaki, M.; Ouhara, K.; Iwata, T.; Takeda, K.; Mizuno, N.; Fujita, T.; et al. Xenotransplantation of interferon-gamma-pretreated clumps of a human mesenchymal stem cell/extracellular matrix complex induces mouse calvarial bone regeneration. Stem Cell Res. Ther. 2017, 8, 101. [Google Scholar] [CrossRef]
- Cimino, M.; Goncalves, R.M.; Barrias, C.C.; Martins, M.C.L. Xeno-free strategies for safe human mesenchymal stem/stromal cell expansion: Supplements and coatings. Stem Cells Int. 2017, 2017, 6597815. [Google Scholar] [CrossRef] [PubMed]
- Halme, D.G.; Kessler, D.A. Fda regulation of stem-cell-based therapies. N. Engl. J. Med. 2006, 355, 1730–1735. [Google Scholar] [CrossRef] [PubMed]
- Bakopoulou, A.; Apatzidou, D.; Aggelidou, E.; Gousopoulou, E.; Leyhausen, G.; Volk, J.; Kritis, A.; Koidis, P.; Geurtsen, W. Isolation and prolonged expansion of oral mesenchymal stem cells under clinical-grade, gmp-compliant conditions differentially affects “stemness” properties. Stem Cell Res. Ther. 2017, 8, 247. [Google Scholar] [CrossRef] [PubMed]
- Shih, D.T.; Burnouf, T. Preparation, quality criteria, and properties of human blood platelet lysate supplements for ex vivo stem cell expansion. New Biotechnol. 2015, 32, 199–211. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, S.K.; Dooner, M.S.; Weier, H.U.; Frenkel, B.; Lian, J.B.; Stein, G.S.; Quesenberry, P.J. Cells capable of bone production engraft from whole bone marrow transplants in nonablated mice. J. Exp. Med. 1999, 189, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Granero-Molto, F.; Weis, J.A.; Miga, M.I.; Landis, B.; Myers, T.J.; O’Rear, L.; Longobardi, L.; Jansen, E.D.; Mortlock, D.P.; Spagnoli, A. Regenerative effects of transplanted mesenchymal stem cells in fracture healing. Stem Cells 2009, 27, 1887–1898. [Google Scholar] [CrossRef]
- Tasso, R.; Fais, F.; Reverberi, D.; Tortelli, F.; Cancedda, R. The recruitment of two consecutive and different waves of host stem/progenitor cells during the development of tissue-engineered bone in a murine model. Biomaterials 2010, 31, 2121–2129. [Google Scholar] [CrossRef]
- Lopes, D.; Martins-Cruz, C.; Oliveira, M.B.; Mano, J.F. Bone physiology as inspiration for tissue regenerative therapies. Biomaterials 2018, 185, 240–275. [Google Scholar] [CrossRef]
- Shao, J.; Zhang, W.; Yang, T. Using mesenchymal stem cells as a therapy for bone regeneration and repairing. Biol. Res. 2015, 48, 62. [Google Scholar] [CrossRef]
- Linero, I.; Chaparro, O. Paracrine effect of mesenchymal stem cells derived from human adipose tissue in bone regeneration. PLoS ONE 2014, 9, e107001. [Google Scholar] [CrossRef]
- Li, F.; Wang, X.; Niyibizi, C. Bone marrow stromal cells contribute to bone formation following infusion into femoral cavities of a mouse model of osteogenesis imperfecta. Bone 2010, 47, 546–555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Y.; Fan, W.; Prasadam, I.; Crawford, R.; Xiao, Y. Implantation of osteogenic differentiated donor mesenchymal stem cells causes recruitment of host cells. J. Tissue Eng. Regen. Med. 2015, 9, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Chen, X.; Dong, F.; Li, W.; Ren, X.; Zhang, Y.; Shi, Y. Concise review: Mesenchymal stem cells and translational medicine: Emerging issues. Stem Cells Transl. Med. 2012, 1, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Huang, R.; Fan, W.; Prasadam, I.; Crawford, R.; Xiao, Y. Mesenchymal stromal cells regulate the cell mobility and the immune response during osteogenesis through secretion of vascular endothelial growth factor a. J. Tissue Eng. Regen. Med. 2018, 12, e566–e578. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; You, X.; Xing, W.; Zhang, Z.; Zou, W. Paracrine and endocrine actions of bone-the functions of secretory proteins from osteoblasts, osteocytes, and osteoclasts. Bone Res. 2018, 6, 16. [Google Scholar] [CrossRef] [PubMed]
- Sobacchi, C.; Erreni, M.; Strina, D.; Palagano, E.; Villa, A.; Menale, C. 3d bone biomimetic scaffolds for basic and translational studies with mesenchymal stem cells. Int. J. Mol. Sci. 2018, 19, 3150. [Google Scholar] [CrossRef]
- Przekora, A. Current trends in fabrication of biomaterials for bone and cartilage regeneration: Materials modifications and biophysical stimulations. Int. J. Mol. Sci. 2019, 20, 435. [Google Scholar] [CrossRef]
- Leeming, D.J.; Henriksen, K.; Byrjalsen, I.; Qvist, P.; Madsen, S.H.; Garnero, P.; Karsdal, M.A. Is bone quality associated with collagen age? Osteoporos. Int. 2009, 20, 1461–1470. [Google Scholar] [CrossRef]
- Rauh, J.; Milan, F.; Gunther, K.P.; Stiehler, M. Bioreactor systems for bone tissue engineering. Tissue Eng. Part B Rev. 2011, 17, 263–280. [Google Scholar] [CrossRef]
- Lin, H.; Lozito, T.P.; Alexander, P.G.; Gottardi, R.; Tuan, R.S. Stem cell-based microphysiological osteochondral system to model tissue response to interleukin-1beta. Mol. Pharm. 2014, 11, 2203–2212. [Google Scholar] [CrossRef]
- Pirosa, A.; Gottardi, R.; Alexander, P.G.; Tuan, R.S. Engineering in-vitro stem cell-based vascularized bone models for drug screening and predictive toxicology. Stem Cell Res. Ther. 2018, 9, 112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lalu, M.M.; McIntyre, L.; Pugliese, C.; Fergusson, D.; Winston, B.W.; Marshall, J.C.; Granton, J.; Stewart, D.J.; Canadian Critical Care Trials Group. Safety of cell therapy with mesenchymal stromal cells (safecell): A systematic review and meta-analysis of clinical trials. PLoS ONE 2012, 7, e47559. [Google Scholar] [CrossRef] [PubMed]
- Motoike, S.; Kajiya, M.; Komatsu, N.; Takewaki, M.; Horikoshi, S.; Matsuda, S.; Ouhara, K.; Iwata, T.; Takeda, K.; Fujita, T.; et al. Cryopreserved clumps of mesenchymal stem cell/extracellular matrix complexes retain osteogenic capacity and induce bone regeneration. Stem Cell Res. Ther. 2018, 9, 73. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, N.; Kajiya, M.; Motoike, S.; Takewaki, M.; Horikoshi, S.; Iwata, T.; Ouhara, K.; Takeda, K.; Matsuda, S.; Fujita, T.; et al. Type i collagen deposition via osteoinduction ameliorates yap/taz activity in 3d floating culture clumps of mesenchymal stem cell/extracellular matrix complexes. Stem Cell Res. Ther. 2018, 9, 342. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Hoshiba, T.; Kawazoe, N.; Chen, G. Comparison of decellularization techniques for preparation of extracellular matrix scaffolds derived from three-dimensional cell culture. J. Biomed. Mater. Res. A 2012, 100, 2507–2516. [Google Scholar] [CrossRef] [PubMed]
- Bouxsein, M.L.; Boyd, S.K.; Christiansen, B.A.; Guldberg, R.E.; Jepsen, K.J.; Muller, R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J. Bone Miner. Res. 2010, 25, 1468–1486. [Google Scholar] [CrossRef] [PubMed]





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Motoike, S.; Kajiya, M.; Komatsu, N.; Horikoshi, S.; Ogawa, T.; Sone, H.; Matsuda, S.; Ouhara, K.; Iwata, T.; Mizuno, N.; et al. Clumps of Mesenchymal Stem Cell/Extracellular Matrix Complexes Generated with Xeno-Free Conditions Facilitate Bone Regeneration via Direct and Indirect Osteogenesis. Int. J. Mol. Sci. 2019, 20, 3970. https://doi.org/10.3390/ijms20163970
Motoike S, Kajiya M, Komatsu N, Horikoshi S, Ogawa T, Sone H, Matsuda S, Ouhara K, Iwata T, Mizuno N, et al. Clumps of Mesenchymal Stem Cell/Extracellular Matrix Complexes Generated with Xeno-Free Conditions Facilitate Bone Regeneration via Direct and Indirect Osteogenesis. International Journal of Molecular Sciences. 2019; 20(16):3970. https://doi.org/10.3390/ijms20163970
Chicago/Turabian StyleMotoike, Souta, Mikihito Kajiya, Nao Komatsu, Susumu Horikoshi, Tomoya Ogawa, Hisakatsu Sone, Shinji Matsuda, Kazuhisa Ouhara, Tomoyuki Iwata, Noriyoshi Mizuno, and et al. 2019. "Clumps of Mesenchymal Stem Cell/Extracellular Matrix Complexes Generated with Xeno-Free Conditions Facilitate Bone Regeneration via Direct and Indirect Osteogenesis" International Journal of Molecular Sciences 20, no. 16: 3970. https://doi.org/10.3390/ijms20163970
APA StyleMotoike, S., Kajiya, M., Komatsu, N., Horikoshi, S., Ogawa, T., Sone, H., Matsuda, S., Ouhara, K., Iwata, T., Mizuno, N., Fujita, T., Ikeya, M., & Kurihara, H. (2019). Clumps of Mesenchymal Stem Cell/Extracellular Matrix Complexes Generated with Xeno-Free Conditions Facilitate Bone Regeneration via Direct and Indirect Osteogenesis. International Journal of Molecular Sciences, 20(16), 3970. https://doi.org/10.3390/ijms20163970