The Pathogenic Factors from Oral Streptococci for Systemic Diseases
Abstract
:1. Introduction
2. Pathogenic Factors Involved in Adhesion, Colonization, Internalization and Invasion
2.1. Cell Wall-Anchored Polypeptides
2.2. Cell Wall-Anchorless Polypeptides
2.3. Proteases
3. Pathogenic Factors Associated with Biofilm Formation
3.1. Bis-(3’-5’)-Cyclic Dimeric Guanosine Monophosphate (Cyclic di-GMP) as a Bacterial Second Messenger
3.2. Extracellular DNA (eDNA)
3.3. DNA Binding Protein
3.4. Membrane Vesicle
4. Effects of Oral Streptococci on Systemic Diseases
5. Relationship Between Oral Streptococci and Autoimmune Diseases
6. Conclusions
Funding
Conflicts of Interest
Abbreviations
| LPS | Lipopolysaccharide |
| HSP | heat shock protein |
| GAS | group A Streptococcus |
| SpeB | Streptococcal pyrogenic exotoxin B |
| TNF | tumor necrosis factor |
| MMP | matrix metalloproteinase |
| RGD | Arg-Gly-Asp |
| EPS | extracellular polymeric substances |
| QS | quorum sensing |
| cyclic-di-GMP | bis-(3’-5’)-cyclic dimeric guanosine monophosphate |
| HLP | histone-like DNA binding protein |
| PAMPs | pathogen-associated molecular patterns |
| PRRs | pattern recognition receptors |
| LTA | lipoteichoic acid |
| NETs | neutrophil extracellular traps |
| CBP | collagen binding protein |
| DMFT | decayed, missing, and filled teeth |
| PA | 190-kDa protein antigen |
| PBC | primary biliary cirrhosis |
References
- Dewhirst, F.E.; Chen, T.; Izard, J.; Paster, B.J.; Tanner, A.C.; Yu, W.H.; Lakshmanan, A.; Wade, W.G. The human oral microbiome. J. Bacteriol. 2010, 192, 5002–5017. [Google Scholar] [CrossRef] [PubMed]
- Zbinden, A.; Bostanci, N.; Belibasakis, G.N. The novel species streptococcus tigurinus and its association with oral infection. Virulence 2015, 6, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef] [PubMed]
- Struzycka, I. The oral microbiome in dental caries. Pol. J. Microbiol. 2014, 63, 127–135. [Google Scholar] [PubMed]
- Sitkiewicz, I. How to become a killer, or is it all accidental? Virulence strategies in oral streptococci. Mol. Oral Microbiol. 2018, 33, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Whiley, R.A.; Beighton, D. Current classification of the oral streptococci. Oral Microbiol. Immunol. 1998, 13, 195–216. [Google Scholar] [CrossRef] [PubMed]
- Krzysciak, W.; Pluskwa, K.K.; Jurczak, A.; Koscielniak, D. The pathogenicity of the streptococcus genus. Eur. J. Clin. Microbiol. Infect. Dis. 2013, 32, 1361–1376. [Google Scholar] [CrossRef] [PubMed]
- Richards, V.P.; Palmer, S.R.; Pavinski Bitar, P.D.; Qin, X.; Weinstock, G.M.; Highlander, S.K.; Town, C.D.; Burne, R.A.; Stanhope, M.J. Phylogenomics and the dynamic genome evolution of the genus streptococcus. Genome Biol. Evol. 2014, 6, 741–753. [Google Scholar] [CrossRef] [PubMed]
- Frandsen, E.V.; Pedrazzoli, V.; Kilian, M. Ecology of viridans streptococci in the oral cavity and pharynx. Oral Microbiol. Immunol. 1991, 6, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Abranches, J.; Zeng, L.; Kajfasz, J.K.; Palmer, S.R.; Chakraborty, B.; Wen, Z.T.; Richards, V.P.; Brady, L.J.; Lemos, J.A. Biology of oral streptococci. Microbiol. Spectr. 2018, 6. [Google Scholar] [CrossRef]
- Kim, S.L.; Gordon, S.M.; Shrestha, N.K. Distribution of streptococcal groups causing infective endocarditis: A descriptive study. Diagn Microbiol. Infect. Dis. 2018, 91, 269–272. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.G.; Spatafora, G.A. Gene regulation in s. Mutans: Complex control in a complex environment. J. Dent. Res. 2012, 91, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Ng, K.W.; Mukhopadhyay, A. Streptococcus constellatus bacteremia causing septic shock following tooth extraction: A case report. Cases J. 2009, 2, 6493. [Google Scholar] [CrossRef] [PubMed]
- Tran, M.P.; Caldwell-McMillan, M.; Khalife, W.; Young, V.B. Streptococcus intermedius causing infective endocarditis and abscesses: A report of three cases and review of the literature. BMC Infect. Dis. 2008, 8, 154. [Google Scholar] [CrossRef] [PubMed]
- Neumayr, A.; Kubitz, R.; Bode, J.G.; Bilk, B.; Haussinger, D. Multiple liver abscesses with isolation of streptococcus intermedius related to a pyogenic dental infection in an immuno-competent patient. Eur J. Med. Res. 2010, 15, 319–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whiley, R.A.; Beighton, D.; Winstanley, T.G.; Fraser, H.Y.; Hardie, J.M. Streptococcus intermedius, streptococcus constellatus, and streptococcus anginosus (the streptococcus milleri group): Association with different body sites and clinical infections. J. Clin. Microbiol. 1992, 30, 243–244. [Google Scholar]
- Fisher, L.E.; Russell, R.R. The isolation and characterization of milleri group streptococci from dental periapical abscesses. J. Dent. Res. 1993, 72, 1191–1193. [Google Scholar] [CrossRef]
- Socransky, S.S.; Haffajee, A.D.; Cugini, M.A.; Smith, C.; Kent, R.L., Jr. Microbial complexes in subgingival plaque. J. Clin. Periodontol 1998, 25, 134–144. [Google Scholar] [CrossRef]
- Burton, J.P.; Drummond, B.K.; Chilcott, C.N.; Tagg, J.R.; Thomson, W.M.; Hale, J.D.; Wescombe, P.A. Influence of the probiotic streptococcus salivarius strain m18 on indices of dental health in children: A randomized double-blind, placebo-controlled trial. J. Med. Microbiol. 2013, 62, 875–884. [Google Scholar] [CrossRef]
- Nobbs, A.H.; Jenkinson, H.F.; Everett, D.B. Generic determinants of streptococcus colonization and infection. Infect. Genet. Evol. 2015, 33, 361–370. [Google Scholar] [CrossRef]
- Cunningham, M.W. Pathogenesis of group a streptococcal infections and their sequelae. Adv. Exp. Med. Biol 2008, 609, 29–42. [Google Scholar] [PubMed]
- Brady, L.J.; Maddocks, S.E.; Larson, M.R.; Forsgren, N.; Persson, K.; Deivanayagam, C.C.; Jenkinson, H.F. The changing faces of streptococcus antigen i/ii polypeptide family adhesins. Mol. Microbiol. 2010, 77, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Nobbs, A.H.; Lamont, R.J.; Jenkinson, H.F. Streptococcus adherence and colonization. Microbiol. Mol. Biol Rev. 2009, 73, 407–450. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Green, N.M.; Sitkiewicz, I.; Lefebvre, R.B.; Musser, J.M. Identification and characterization of an antigen i/ii family protein produced by group a streptococcus. Infect. Immun. 2006, 74, 4200–4213. [Google Scholar] [CrossRef] [PubMed]
- Christie, J.; McNab, R.; Jenkinson, H.F. Expression of fibronectin-binding protein fbpa modulates adhesion in streptococcus gordonii. Microbiology 2002, 148, 1615–1625. [Google Scholar] [CrossRef] [PubMed]
- Walker, M.J.; Barnett, T.C.; McArthur, J.D.; Cole, J.N.; Gillen, C.M.; Henningham, A.; Sriprakash, K.S.; Sanderson-Smith, M.L.; Nizet, V. Disease manifestations and pathogenic mechanisms of group a streptococcus. Clin. Microbiol. Rev. 2014, 27, 264–301. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M.; Terao, Y.; Kawabata, S. Pleiotropic virulence factor-streptococcus pyogenes fibronectin-binding proteins. Cell Microbiol. 2013, 15, 503–511. [Google Scholar] [CrossRef]
- Aviles-Reyes, A.; Miller, J.H.; Lemos, J.A.; Abranches, J. Collagen-binding proteins of streptococcus mutans and related streptococci. Mol. Oral Microbiol. 2017, 32, 89–106. [Google Scholar] [CrossRef]
- Kahn, F.; Hurley, S.; Shannon, O. Platelets promote bacterial dissemination in a mouse model of streptococcal sepsis. Microbes Infect. 2013, 15, 669–676. [Google Scholar] [CrossRef]
- Xiong, Y.Q.; Bensing, B.A.; Bayer, A.S.; Chambers, H.F.; Sullam, P.M. Role of the serine-rich surface glycoprotein gspb of streptococcus gordonii in the pathogenesis of infective endocarditis. Microb. Pathog. 2008, 45, 297–301. [Google Scholar] [CrossRef]
- Kimura, K.R.; Nakata, M.; Sumitomo, T.; Kreikemeyer, B.; Podbielski, A.; Terao, Y.; Kawabata, S. Involvement of t6 pili in biofilm formation by serotype m6 streptococcus pyogenes. J. Bacteriol. 2012, 194, 804–812. [Google Scholar] [CrossRef] [PubMed]
- Pezzicoli, A.; Santi, I.; Lauer, P.; Rosini, R.; Rinaudo, D.; Grandi, G.; Telford, J.L.; Soriani, M. Pilus backbone contributes to group b streptococcus paracellular translocation through epithelial cells. J. Infect. Dis. 2008, 198, 890–898. [Google Scholar] [CrossRef] [PubMed]
- Maisey, H.C.; Hensler, M.; Nizet, V.; Doran, K.S. Group b streptococcal pilus proteins contribute to adherence to and invasion of brain microvascular endothelial cells. J. Bacteriol. 2007, 189, 1464–1467. [Google Scholar] [CrossRef] [PubMed]
- Barocchi, M.A.; Ries, J.; Zogaj, X.; Hemsley, C.; Albiger, B.; Kanth, A.; Dahlberg, S.; Fernebro, J.; Moschioni, M.; Masignani, V.; et al. A pneumococcal pilus influences virulence and host inflammatory responses. Proc. Natl. Acad. Sci. USA 2006, 103, 2857–2862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maisey, H.C.; Quach, D.; Hensler, M.E.; Liu, G.Y.; Gallo, R.L.; Nizet, V.; Doran, K.S. A group b streptococcal pilus protein promotes phagocyte resistance and systemic virulence. FASEB J. 2008, 22, 1715–1724. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Park, S.E.; Yadav, P.; Paoletti, L.C.; Wessels, M.R. Regulation and function of pilus island 1 in group b streptococcus. J. Bacteriol. 2012, 194, 2479–2490. [Google Scholar] [CrossRef] [PubMed]
- Gianfaldoni, C.; Censini, S.; Hilleringmann, M.; Moschioni, M.; Facciotti, C.; Pansegrau, W.; Masignani, V.; Covacci, A.; Rappuoli, R.; Barocchi, M.A.; et al. Streptococcus pneumoniae pilus subunits protect mice against lethal challenge. Infect. Immun. 2007, 75, 1059–1062. [Google Scholar] [CrossRef] [PubMed]
- Nilson, B.H.; Frick, I.M.; Akesson, P.; Forsen, S.; Bjorck, L.; Akerstrom, B.; Wikstrom, M. Structure and stability of protein h and the m1 protein from streptococcus pyogenes. Implications for other surface proteins of gram-positive bacteria. Biochemistry 1995, 34, 13688–13698. [Google Scholar] [CrossRef]
- McNamara, C.; Zinkernagel, A.S.; Macheboeuf, P.; Cunningham, M.W.; Nizet, V.; Ghosh, P. Coiled-coil irregularities and instabilities in group a streptococcus m1 are required for virulence. Science 2008, 319, 1405–1408. [Google Scholar] [CrossRef]
- Smeesters, P.R.; McMillan, D.J.; Sriprakash, K.S. The streptococcal m protein: A highly versatile molecule. Trends Microbiol. 2010, 18, 275–282. [Google Scholar] [CrossRef]
- Pahlman, L.I.; Olin, A.I.; Darenberg, J.; Morgelin, M.; Kotb, M.; Herwald, H.; Norrby-Teglund, A. Soluble m1 protein of streptococcus pyogenes triggers potent t cell activation. Cell Microbiol. 2008, 10, 404–414. [Google Scholar] [CrossRef] [PubMed]
- Henningham, A.; Chiarot, E.; Gillen, C.M.; Cole, J.N.; Rohde, M.; Fulde, M.; Ramachandran, V.; Cork, A.J.; Hartas, J.; Magor, G.; et al. Conserved anchorless surface proteins as group a streptococcal vaccine candidates. J. Mol. Med. 2012, 90, 1197–1207. [Google Scholar] [CrossRef] [PubMed]
- Antikainen, J.; Kuparinen, V.; Lahteenmaki, K.; Korhonen, T.K. Enolases from gram-positive bacterial pathogens and commensal lactobacilli share functional similarity in virulence-associated traits. FEMS Immunol. Med. Microbiol. 2007, 51, 526–534. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.C.; Garbe, J.; Collin, M. Cysteine proteinase speb from streptococcus pyogenes-a potent modifier of immunologically important host and bacterial proteins. Biol Chem. 2011, 392, 1077–1088. [Google Scholar] [CrossRef] [PubMed]
- Tamura, F.; Nakagawa, R.; Akuta, T.; Okamoto, S.; Hamada, S.; Maeda, H.; Kawabata, S.; Akaike, T. Proapoptotic effect of proteolytic activation of matrix metalloproteinases by streptococcus pyogenes thiol proteinase (streptococcus pyrogenic exotoxin b). Infect. Immun. 2004, 72, 4836–4847. [Google Scholar] [CrossRef] [PubMed]
- Barnett, T.C.; Liebl, D.; Seymour, L.M.; Gillen, C.M.; Lim, J.Y.; Larock, C.N.; Davies, M.R.; Schulz, B.L.; Nizet, V.; Teasdale, R.D.; et al. The globally disseminated m1t1 clone of group a streptococcus evades autophagy for intracellular replication. Cell Host Microbe 2013, 14, 675–682. [Google Scholar] [CrossRef]
- Morefield, G.; Touhey, G.; Lu, F.; Dunham, A.; HogenEsch, H. Development of a recombinant fusion protein vaccine formulation to protect against streptococcus pyogenes. Vaccine 2014, 32, 3810–3815. [Google Scholar] [CrossRef]
- Brown, C.K.; Gu, Z.Y.; Matsuka, Y.V.; Purushothaman, S.S.; Winter, L.A.; Cleary, P.P.; Olmsted, S.B.; Ohlendorf, D.H.; Earhart, C.A. Structure of the streptococcal cell wall c5a peptidase. Proc. Natl. Acad. Sci. USA 2005, 102, 18391–18396. [Google Scholar] [CrossRef]
- Sagar, V.; Bergmann, R.; Nerlich, A.; McMillan, D.J.; Nitsche Schmitz, D.P.; Chhatwal, G.S. Variability in the distribution of genes encoding virulence factors and putative extracellular proteins of streptococcus pyogenes in india, a region with high streptococcal disease burden, and implication for development of a regional multisubunit vaccine. Clin. Vaccine Immunol. 2012, 19, 1818–1825. [Google Scholar] [CrossRef]
- Tamura, G.S.; Hull, J.R.; Oberg, M.D.; Castner, D.G. High-affinity interaction between fibronectin and the group b streptococcal c5a peptidase is unaffected by a naturally occurring four-amino-acid deletion that eliminates peptidase activity. Infect. Immun. 2006, 74, 5739–5746. [Google Scholar] [CrossRef]
- Cheng, Q.; Stafslien, D.; Purushothaman, S.S.; Cleary, P. The group b streptococcal c5a peptidase is both a specific protease and an invasin. Infect. Immun. 2002, 70, 2408–2413. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.H.; Lau, P.C.; Lee, J.H.; Ellen, R.P.; Cvitkovitch, D.G. Natural genetic transformation of streptococcus mutans growing in biofilms. J. Bacteriol. 2001, 183, 897–908. [Google Scholar] [CrossRef] [PubMed]
- Jakobsen, T.H.; Tolker-Nielsen, T.; Givskov, M. Bacterial biofilm control by perturbation of bacterial signaling processes. Int J. Mol. Sci. 2017, 18, 1970. [Google Scholar] [CrossRef] [PubMed]
- McDougald, D.; Rice, S.A.; Barraud, N.; Steinberg, P.D.; Kjelleberg, S. Should we stay or should we go: Mechanisms and ecological consequences for biofilm dispersal. Nat. Rev. Microbiol. 2011, 10, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Romling, U.; Galperin, M.Y.; Gomelsky, M. Cyclic di-gmp: The first 25 years of a universal bacterial second messenger. Microbiol. Mol. Biol Rev. 2013, 77, 1–52. [Google Scholar] [CrossRef] [PubMed]
- Okshevsky, M.; Regina, V.R.; Meyer, R.L. Extracellular DNA as a target for biofilm control. Curr. Opin. Biotechnol. 2015, 33, 73–80. [Google Scholar] [CrossRef]
- Whitchurch, C.B.; Tolker-Nielsen, T.; Ragas, P.C.; Mattick, J.S. Extracellular DNA required for bacterial biofilm formation. Science 2002, 295, 1487. [Google Scholar] [CrossRef]
- Jakubovics, N.S.; Burgess, J.G. Extracellular DNA in oral microbial biofilms. Microbes Infect. 2015, 17, 531–537. [Google Scholar] [CrossRef]
- Nur, A.; Hirota, K.; Yumoto, H.; Hirao, K.; Liu, D.; Takahashi, K.; Murakami, K.; Matsuo, T.; Shu, R.; Miyake, Y. Effects of extracellular DNA and DNA-binding protein on the development of a streptococcus intermedius biofilm. J. Appl. Microbiol. 2013, 115, 260–270. [Google Scholar] [CrossRef]
- Jung, C.J.; Hsu, R.B.; Shun, C.T.; Hsu, C.C.; Chia, J.S. Atla mediates extracellular DNA release, which contributes to streptococcus mutans biofilm formation in an experimental rat model of infective endocarditis. Infect. Immun. 2017, 85, e00252-17. [Google Scholar] [CrossRef]
- Liu, D.; Yumoto, H.; Hirota, K.; Murakami, K.; Takahashi, K.; Hirao, K.; Matsuo, T.; Ohkura, K.; Nagamune, H.; Miyake, Y. Histone-like DNA binding protein of streptococcus intermedius induces the expression of pro-inflammatory cytokines in human monocytes via activation of erk1/2 and jnk pathways. Cell Microbiol. 2008, 10, 262–276. [Google Scholar] [CrossRef]
- Liu, D.; Yumoto, H.; Murakami, K.; Hirota, K.; Ono, T.; Nagamune, H.; Kayama, S.; Matsuo, T.; Miyake, Y. The essentiality and involvement of streptococcus intermedius histone-like DNA-binding protein in bacterial viability and normal growth. Mol. Microbiol. 2008, 68, 1268–1282. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.; Wolf, J.M.; Prados-Rosales, R.; Casadevall, A. Through the wall: Extracellular vesicles in gram-positive bacteria, mycobacteria and fungi. Nat. Rev. Microbiol. 2015, 13, 620–630. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Lee, J.; Park, J.; Gho, Y.S. Gram-negative and gram-positive bacterial extracellular vesicles. Semin Cell Dev. Biol 2015, 40, 97–104. [Google Scholar] [CrossRef]
- Toyofuku, M.; Tashiro, Y.; Hasegawa, Y.; Kurosawa, M.; Nomura, N. Bacterial membrane vesicles, an overlooked environmental colloid: Biology, environmental perspectives and applications. Adv. Colloid Interface Sci. 2015, 226, 65–77. [Google Scholar] [CrossRef]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil extracellular traps kill bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Sun, L.; Liu, W.; Guo, L.; Liu, Z.; Wei, X.; Ling, J. A nuclease from streptococcus mutans facilitates biofilm dispersal and escape from killing by neutrophil extracellular traps. Front. Cell Infect. Microbiol. 2017, 7, 97. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, T.J. The pathogenesis of streptococcal infections: From tooth decay to meningitis. Nat. Rev. Microbiol. 2003, 1, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Nakano, K.; Hokamura, K.; Taniguchi, N.; Wada, K.; Kudo, C.; Nomura, R.; Kojima, A.; Naka, S.; Muranaka, Y.; Thura, M.; et al. The collagen-binding protein of streptococcus mutans is involved in haemorrhagic stroke. Nat. Commun 2011, 2, 485. [Google Scholar] [CrossRef] [PubMed]
- Miyatani, F.; Kuriyama, N.; Watanabe, I.; Nomura, R.; Nakano, K.; Matsui, D.; Ozaki, E.; Koyama, T.; Nishigaki, M.; Yamamoto, T.; et al. Relationship between cnm-positive streptococcus mutans and cerebral microbleeds in humans. Oral Dis 2015, 21, 886–893. [Google Scholar] [CrossRef] [PubMed]
- Kojima, A.; Nakano, K.; Wada, K.; Takahashi, H.; Katayama, K.; Yoneda, M.; Higurashi, T.; Nomura, R.; Hokamura, K.; Muranaka, Y.; et al. Infection of specific strains of streptococcus mutans, oral bacteria, confers a risk of ulcerative colitis. Sci. Rep. 2012, 2, 332. [Google Scholar] [CrossRef] [PubMed]
- Misaki, T.; Naka, S.; Hatakeyama, R.; Fukunaga, A.; Nomura, R.; Isozaki, T.; Nakano, K. Presence of streptococcus mutans strains harbouring the cnm gene correlates with dental caries status and iga nephropathy conditions. Sci. Rep. 2016, 6, 36455. [Google Scholar] [CrossRef] [PubMed]
- Otsugu, M.; Nomura, R.; Matayoshi, S.; Teramoto, N.; Nakano, K. Contribution of streptococcus mutans strains with collagen-binding proteins in the presence of serum to the pathogenesis of infective endocarditis. Infect. Immun. 2017, 85, e00401-17. [Google Scholar] [CrossRef]
- Tsuneyama, K.; Harada, K.; Kono, N.; Hiramatsu, K.; Zen, Y.; Sudo, Y.; Gershwin, M.E.; Ikemoto, M.; Arai, H.; Nakanuma, Y. Scavenger cells with gram-positive bacterial lipoteichoic acid infiltrate around the damaged interlobular bile ducts of primary biliary cirrhosis. J. Hepatol. 2001, 35, 156–163. [Google Scholar] [CrossRef]
- Haruta, I.; Hashimoto, E.; Kato, Y.; Kikuchi, K.; Kato, H.; Yagi, J.; Uchiyama, T.; Kobayash, M.; Shiratori, K. Lipoteichoic acid may affect the pathogenesis of bile duct damage in primary biliary cirrhosis. Autoimmunity 2006, 39, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Haruta, I.; Kikuchi, K.; Hashimoto, E.; Kato, H.; Hirota, K.; Kobayashi, M.; Miyake, Y.; Uchiyama, T.; Yagi, J.; Shiratori, K. A possible role of histone-like DNA-binding protein of streptococcus intermedius in the pathogenesis of bile duct damage in primary biliary cirrhosis. Clin. Immunol. 2008, 127, 245–251. [Google Scholar] [CrossRef]
- Haruta, I.; Kikuchi, K.; Hashimoto, E.; Nakamura, M.; Miyakawa, H.; Hirota, K.; Shibata, N.; Kato, H.; Arimura, Y.; Kato, Y.; et al. Long-term bacterial exposure can trigger nonsuppurative destructive cholangitis associated with multifocal epithelial inflammation. Lab. Invest. 2010, 90, 577–588. [Google Scholar] [CrossRef] [Green Version]
- Haruta, I.; Kikuchi, K.; Nakamura, M.; Hirota, K.; Kato, H.; Miyakawa, H.; Shibata, N.; Miyake, Y.; Hashimoto, E.; Shiratori, K.; et al. Involvement of commensal bacteria may lead to dysregulated inflammatory and autoimmune responses in a mouse model for chronic nonsuppurative destructive cholangitis. J. Clin. Immunol. 2012, 32, 1026–1037. [Google Scholar] [CrossRef]
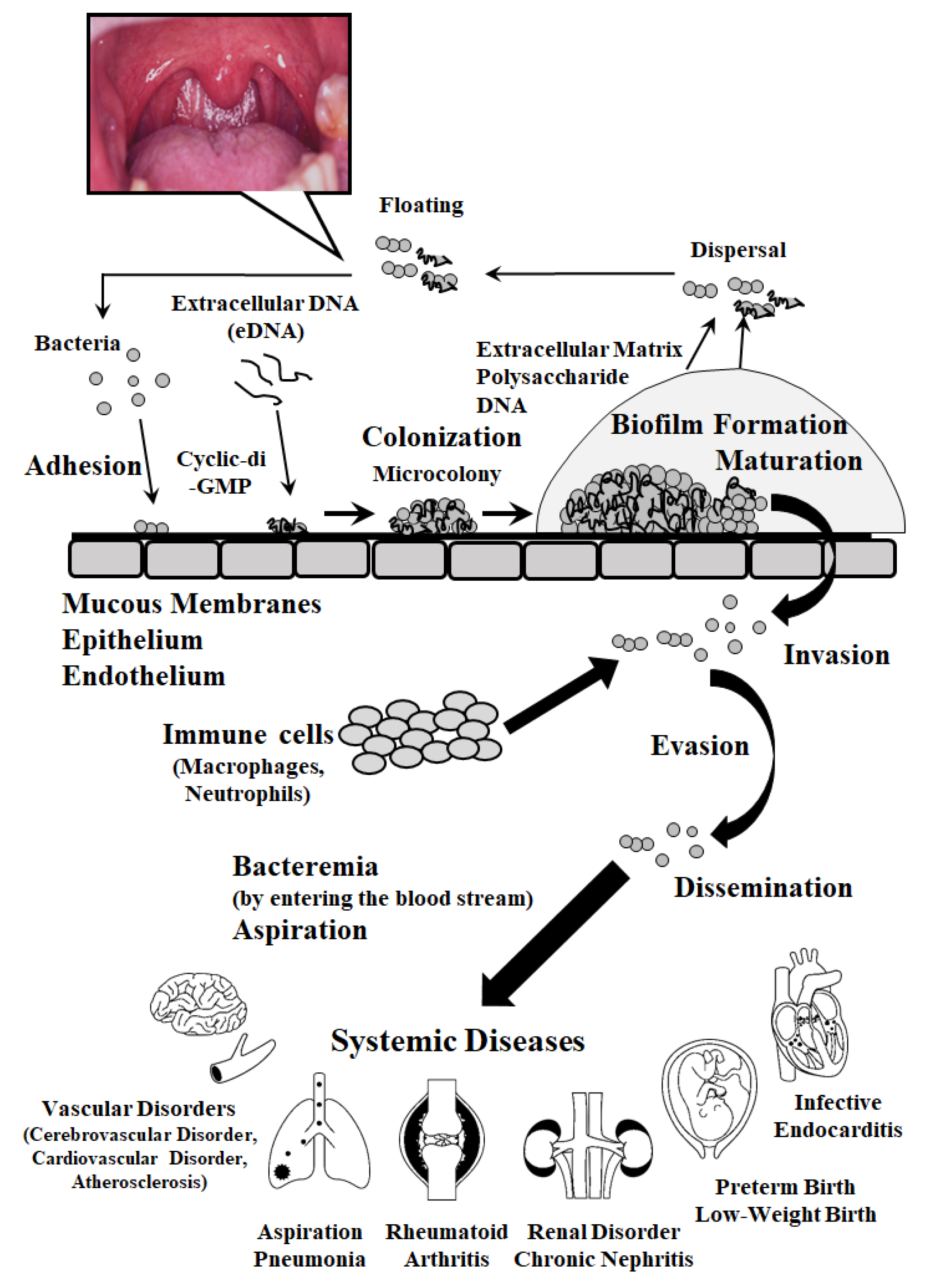



| Bacteremia and Sepsis |
| Infective Endocarditis, Pericarditis |
| Heart Valve Disease |
| Aortic Aneurysm |
| Deep-seated purulent Abscess (Brain, Tonsillar, Abdominal, Spleen or Liver Abscess) |
| Pleural Empyema |
| Meningitis |
| Cerebrovascular Disease (Cerebral Hemorrhage etc.) |
| Gastrointestinal Diseases (Exacerbation and Chronicity of Enteritis) |
| Kidney Diseases (IgA Nephropathy) |
| Pneumonia |
| Pharyngitis, Tonsillitis |
| Sinusitis |
| Premature Birth, Neonatal Infections, Puerperal Sepsis |
| Urinary Tract Infection |
| Central Nerve System Infections |
| Arthritis, Necrotizing Fasciitis |
| Pyarthrosis |
| Toxic Shock Syndrome |
| Osteomyelitis |
| Vulvovaginitis |
| Peritonitis |
| Impetigo, Cellulitis, Pyoderma |
| Otitis Media |
| Conjunctivitis |
| Scarlet Fever |
| Pathogenic Factors | References |
|---|---|
| Factors for adhesion, colonization, and evasion from host immune defense | |
| Antigen I/II | [22,23,24] |
| Fibronectin-binding proteins | [23,25,26,27] |
| Collagen-binding proteins | [20,28] |
| Laminin-binding proteins | |
| Fibrinogen-binding proteins | |
| Platelet-binding proteins | |
| Serine-rich repeat proteins | [23,29,30] |
| Pili | [20,23,31,32,33,34,35,36,37] |
| Major surface adhesins (M protein) | [38,39,40,41] |
| Enolase | [23,42,43] |
| Proteases | |
| SpeB | [44,45,46,47] |
| C5a peptidase | [26,48,49,50,51] |
| Capsule | |
| Lipoteichoic acid as pathogen-associated molecular pattern (PAMP) |
| Pathogenic Factors | Roles |
|---|---|
| 1. DNA binding proteins | Binding to eDNA strand Present in the biofilm matrix and on the surface of bacterial cells Involvement in transformation ability |
| 2. Toxins | Cross-linked with eDNA Secreted virulence factor Insoluble nuclear-protein complex formation |
| 3. Pili | Binding to eDNA Involvement in motility Involvement in the structure of biofilm |
| 4. Polysaccharides | Co-localization with eDNA |
| 5. Membrane Vesicles | Interaction with eDNA Involvement in the secretion and transport of DNA, toxins and cell membrane components such as lipoproteins to outside of bacterial cells |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yumoto, H.; Hirota, K.; Hirao, K.; Ninomiya, M.; Murakami, K.; Fujii, H.; Miyake, Y. The Pathogenic Factors from Oral Streptococci for Systemic Diseases. Int. J. Mol. Sci. 2019, 20, 4571. https://doi.org/10.3390/ijms20184571
Yumoto H, Hirota K, Hirao K, Ninomiya M, Murakami K, Fujii H, Miyake Y. The Pathogenic Factors from Oral Streptococci for Systemic Diseases. International Journal of Molecular Sciences. 2019; 20(18):4571. https://doi.org/10.3390/ijms20184571
Chicago/Turabian StyleYumoto, Hiromichi, Katsuhiko Hirota, Kouji Hirao, Masami Ninomiya, Keiji Murakami, Hideki Fujii, and Yoichiro Miyake. 2019. "The Pathogenic Factors from Oral Streptococci for Systemic Diseases" International Journal of Molecular Sciences 20, no. 18: 4571. https://doi.org/10.3390/ijms20184571
APA StyleYumoto, H., Hirota, K., Hirao, K., Ninomiya, M., Murakami, K., Fujii, H., & Miyake, Y. (2019). The Pathogenic Factors from Oral Streptococci for Systemic Diseases. International Journal of Molecular Sciences, 20(18), 4571. https://doi.org/10.3390/ijms20184571




