1. Introduction
Fruits and vegetables are rich sources of nutrients that contain phytochemicals (also known as bioactive compounds), which are recognised for their nutraceutical effects and health benefits [
1]. The cultivated carrot (
Daucus carota L.) is one of the most important vegetable plants in the world because of its high yield potential and use as fresh or processed product. With an annual world production (carrots and turnips) of >428 million tons and a total growing area of about 11.5 million hectares [
2], carrots rank among the top 10 vegetable crops in the world [
3]. They play a major role in human nutrition, because of their high dietary value and good storage attributes [
4,
5]. Phytochemicals contribute to the dietary value of carrots and comprise mainly four types; namely, phenolic compounds, carotenoids, polyacetylenes, and ascorbic acid. This review article comprehensively describes the occurrence, biosynthesis, factors affecting concentration, and health benefits of phytochemicals found in
Daucus carota.
2. Methods
The literature for this review paper was retrieved from Google Scholar by using the following key words: occurrence of phenolics or phenols or phenolic acids, carotenoids, polyacetylenes, and ascorbic acid or vitamin C in carrot; biosynthesis of phenolics, or phenols or phenolic acids or hydroxycinnamic acids or chlorogenic acids, carotenoids, polyacetylenes, and ascorbic acid or vitamin C, in carrot; factors affecting the concentration of phenolics or phenols or phenolic acids, carotenoids, polyacetylenes, and ascorbic acid or vitamin C in carrot; nutritional importance or nutritional benefits of phenolics or phenols or phenolic acids, carotenoids, polyacetylenes, and ascorbic acid or vitamin C in carrot; health effects of carrot phenolics, carotenoids, polyacetylenes, and ascorbic acid/vitamin C after carrot consumption. The key words: structures of phenols or phenolic acids, carotenoids, polyacetylenes, and ascorbic acid or vitamin C in carrot, were searched in the NCBI website and redrawn in MS word using PNG format. Two hundred and fifty-five (255) articles including original research papers, books, and book chapters were downloaded, of which one hundred and thirty articles (130) most relevant to the topic were selected for writing the review article. The rejected research papers were too old or irrelevant. Literature was summarised according to the four types of phytochemicals found in carrots; namely, phenolics, carotenoids, polyacetylenes, and ascorbic acid. Under each phytochemical, the literature was summarised according to occurrence, biosynthesis, factors affecting concentrations, and resulting health benefits.
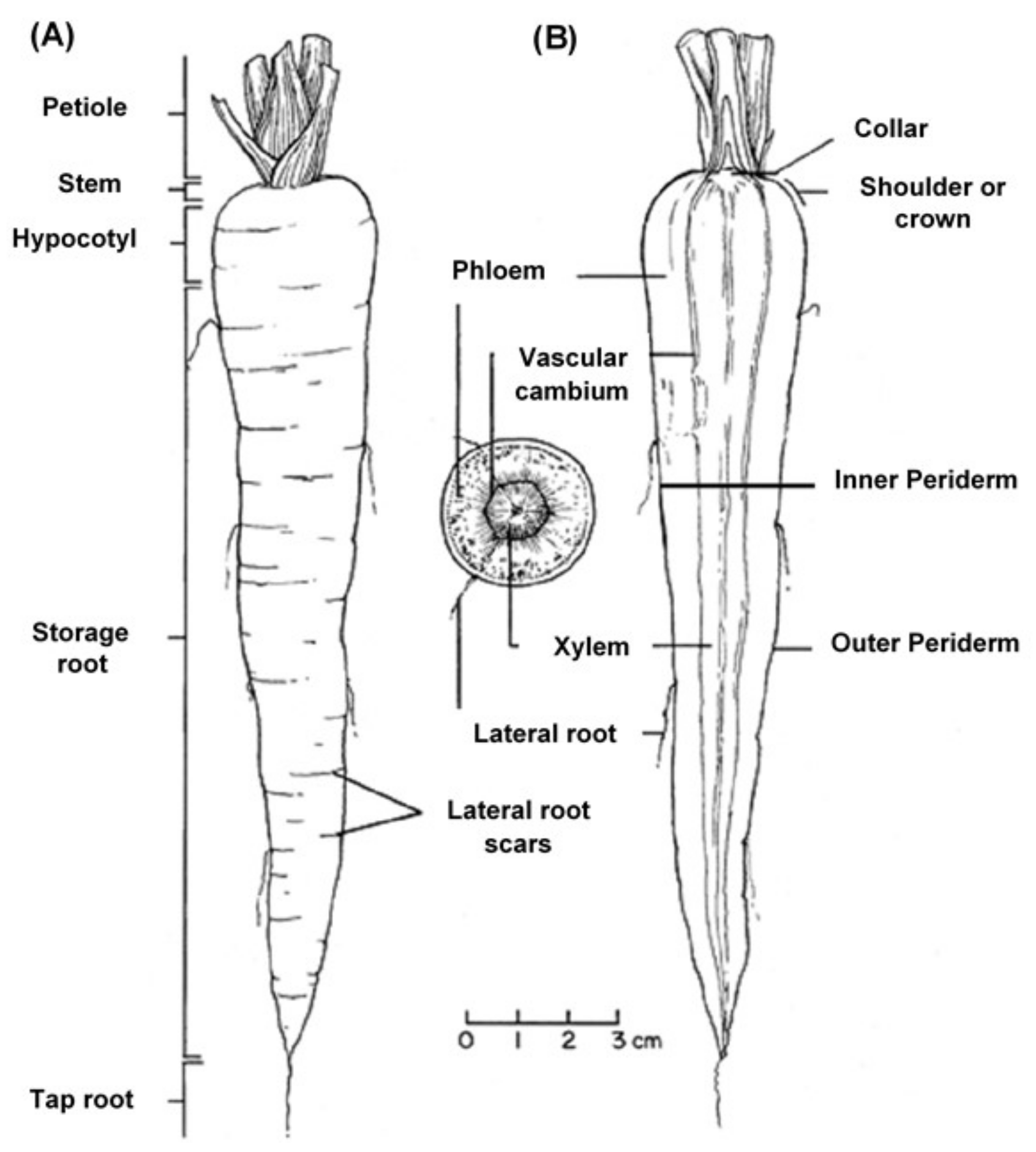
The Carrot Plant
The edible carrot,
Daucus carota, is the most important root vegetable plant grown worldwide and it is part of the
Apiaceae family [
6]. The carrot consists mainly of two parts, the stem and the root, and most of the root consists of the peel (periderm), a pulpy outer cortex (phloem), and an inner core (xylem).
Figure 1 shows the carrot root’s anatomy. Cultivated carrots have orange, reddish, purple, black, or yellow roots. The most commonly eaten part of the carrot plant is the root, though the stems and leaves are eaten as well, so the present review is in regard to the root, unless otherwise indicated.
3. Phenolic Compounds
Phenolic compounds constitute one of the most ubiquitous groups of plant metabolites and are an integral part of both human and animal diets. The role of polyphenols in the prevention of degenerative diseases, like cancer, cardiovascular diseases, and neurodegenerative diseases has been reported. Interest in food phenolics has increased greatly over the past two decades, owing to their antioxidant capacity and their function as a defence against oxidative stress caused by excess reactive oxygen species [
7].
Phenolic compounds are secondary plant metabolites, mainly composed of an aromatic ring bearing one or more hydroxyl groups, playing a crucial role in counteracting various type of stress (ultraviolet irradiation, aggression by pathogens, parasites, and plant predators), and contributing to the organoleptic properties of plants and plant-derived food [
8,
9,
10,
11]. Phenolic compounds can be divided into different subgroups, such as phenolic acids, flavonoids, tannins, lignans, stilbenoids, and curcuminoids. It has been reported that carrots are rich in phenolic acids, such as
p-hydroxybenzoic, caffeic, and chlorogenic, as well as in anthocyanins, a class of flavonoids (
Figure 2) [
12].
Isocoumarins and phenolic acids are the potentially bitter compounds found in carrot peels. Czepa and Hofmann [
13] reported that the bitter taste in carrots is caused by terpenoids and water-soluble phenolics. Therefore, their presences can be used as biological markers to assess the quality of fruits and vegetables during postharvest operations [
14].
3.1. Occurrence of Phenolic Compounds
Phenolic compounds are present in high concentrations in the root periderm tissues of carrots. Carrot roots contain hydroxycinnamic acids and derivatives [
15]. Sharma et al. [
14] reported that chlorogenic acid was the main hydroxycinnamic acid identified in different carrot tissues, accounting for 42.2% to 61.8% of total phenolics. The concentrations of phenolic compounds in different carrot root tissues decrease from the peel (periderm) to the xylem (
Figure 1). The peel is only 11% of the total fresh weight of carrot but contains 54.1% of the total phenolic compounds, followed by the phloem (39.5%) and xylem (6.4%). However, the concentration depends on the cultivar, the extraction method, the manner in which the results are expressed, and the post-harvest and processing circumstances [
15,
16]. Carrots of different colours displayed a high variation in antioxidant properties [
17]. The results consistently indicated that among different carrot colours, purple exhibited the highest antioxidant capacity due to its higher phenolic compound concentration [
4]. It has been reported that carrot contains 27 ± 1.7 µg/g gallic acid equivalents of phenolic compounds, as determined with Folin–Ciocalteu reagent [
18].
3.2. Biosynthesis of Phenolic Compounds
Phenolic compounds are formed biosynthetically from either the shikimic acid pathway or the acetate pathway. Plant-derived phenylpropanoids, with a C6–C3 skeleton, compose the largest group of secondary metabolites produced by higher plants. They are parent molecules for the biosynthesis of numerous structurally and functionally diverse plant polyphenols (simple phenolic acids and esters, glycosylated derivatives of primary phenylpropanoids, flavonoids, isoflavonoids, stilbenes, coumarins, curcuminoids, lignans, etc.), which play multiple essential roles in plant physiology [
19]. The phenylpropanoid, chlorogenic acid, a derivative of hydroxycinnamic acid, was reported to be the most common phenolic compound in carrots [
14] and it is biosynthesised via the shikimic acid pathway [
20,
21].
The synthesis starts with an aldol condensation reaction between phosphoenol pyruvate (PEP) and erythrose 4-phosphate, and culminates through various stages in the formation of prephenic acid. Prephenic acid serves as a branch point in the pathway and rearranges either via decarboxylative 1,4-dehydration to yield phenylpyruvic acid, or decarboxalative 1,4-dehydrogenation to yield
p-hydroxyphenylpyruvic acid. Subsequent transamination with pyridoxal phosphate yields the amino acids phenylalanine and tyrosine (C6–C3 structures), respectively. Phenylalanine ammonia lyase (PAL) and tyrosine ammonia lyase (TAL) are key enzymes of the phenylpropanoid pathway which catalyse the conversion of phenylalanine to cinnamic acid and of tyrosine to 4-hydroxycinnamic acid (p-coumaric acid), respectively. Cinnamic acid is further transformed, through the catalytic action of different enzymes; e.g., cinnamate 2-hydroxylase followed by 4-coumaroyl CoA-ligase to produce 4-hydroxycinnamic acid (p-coumaric acid), which is converted into caffeic acid and finally chlorogenic acid (
Figure 3). 4-Coumaroyl CoA probably represents the most important branch point within the central phenylpropanoid biosynthesis in plants [
20,
22,
23].
3.3. Factors Affecting Phenolics Concentration
Phenolic compounds are affected by multiple factors, such as the cultivar, storage conditions and temperature, fertilizer application, processing procedures, and various biotic and abiotic stress factors [
24].
3.3.1. Cultivar
Alasalvar et al. [
16] reported that fresh purple genotypes have 2.9 times higher phenolic concentrations (102 ± 3.8 mg/100 g) than orange genotypes (34.8 ± 1.9 mg/100 g).
3.3.2. Fertilizer Application
The application of different doses of fertiliser and fertiliser varieties in different growth systems (conventional and organic) and in different locations significantly affected phenolic content [
25]. For example, a deficiency of boron increases the accumulation of phenolics, and the application of nitrogen fertiliser could change phenolic concentration in carrot roots [
26].
3.3.3. Storage Conditions and Temperature
Storage conditions and temperature affect the concentration of phenolic compounds, particularly that of chlorogenic acid. In a recent study by Kamiloglu et al. [
27] with black carrots, it was found that after 20 weeks of storage, the preserved anthocyanins in samples stored at 4 °C (53.4%–81.0%) were higher than samples stored at 25 °C (7.8%–69.3%). Simões et al. [
28] investigated the effect of controlled atmosphere on baby carrots and reported that controlled atmosphere of 5 kPa O
2 and 5 kPa CO
2 significantly increased chlorogenic acid content. In general, plants subjected to postharvest abiotic stresses synthesize secondary metabolites, such as phenolic compounds. Wounding stress, water loss, peel removal, and UV light affect the biosynthesis of phenolic compounds in carrots [
29,
30,
31,
32,
33]. Wounding stress induced an increase of ∼287% in total phenolic content (PC) in carrots stored for 48 h at 20 °C, while this increase was higher (∼349%) in the wounded tissue treated with low-oxygen stress [
34]. Wounding stress (23.5 cm
2/g) produced approximately 2.5 times more soluble phenols in carrots than in undamaged carrots. It mostly stimulates the synthesis of chlorogenic acid (5-CQA) and 3,5-dicaffeoylquinic acid, which enhances the antioxidant capacity of carrots [
35].
3.3.4. Processing Procedures
Ma et al. [
36] studied the effects of different processing units on fresh carrot juice and concluded that blanching and enzyme liquefaction can increase total phenolic content, while pasteurization decreases total phenolic content compared to fresh carrot juice. Another study reported that un-blanched frozen and blanched (soaking carrots in water at 95 °C for three minutes) frozen treatments non-significantly affected phenolic concentration in carrots, even after seven days of storage at 4 °C [
37]. The processing of black carrots into jam and marmalade led to a decrease in total phenolic content of 89.2% to 90.5%, antioxidant capacity by 83.3% to 91.3%, and phenolic acids by 49.5 to 96.7%. The reasons are that cell structures are destroyed during processing of black carrots into jam and raw material is exposed to both enzymatic and non-enzymatic oxidation, resulting in the loss of phenolic compounds [
27].
3.4. The Health Benefits of Phenolic Compounds
It has been estimated that the average dietary intake of polyphenols is 1058 mg per day for males and 780 mg per day for females, comprised as follows: 50% hydroxycinnamic acids, 20% to 25% flavonoids, and 1% anthocyanins [
38]. The dietary intake of phenolic compounds has been related to favourable health impacts, particularly due to their antioxidant activity, such as anti-aging, anti-inflammatory, and antiproliferative effects. Additionally, these compounds contribute to the maintenance of normal blood glucose and cholesterol levels, as well as to the normal functioning of the nervous system. Due to their antioxidant properties, the risk of cardiovascular diseases is minimised, and polyphenols possess anti-ageing properties, as well as anti-carcinogenic properties, by functioning as free-radical scavengers [
39]. Polyphenols also potentially protect against diabetes and Alzheimer’s disease [
38]. They enhance bile secretion, decrease cholesterol and lipid levels in the blood, and exhibit antimicrobial properties towards
Staphylococcus aureus [
40].
Preclinical and epidemiological studies suggest that polyphenols might be helpful in reversing neurodegenerative pathogenic actions and ageing in neurocognitive development. However, there is no evidence for the role of polyphenols in the improvement of neurological health. Their potential roles are due to their capability to interrelate with intracellular neuronal and glial signalling, affect peripheral and cerebrovascular blood flow, and lessen neural injury and damage caused by neurotoxins and the inflammation of neurons [
41]. Carrot genotypes exhibited diverse antioxidant capacities; in low rainfall seasons, the white genotypes contain higher quantities of phenolic compounds [
4].
The anthocyanins of black carrot are effective for the risk reduction of different types of cancer. The growth of HT-29 and HL-60 cancer cells were inhibited by 80% when 2.0 mg/mL of the lyophilised powder of aqueous black carrot was ingested [
42,
43,
44]. Ethanol extracts of black carrot anthocyanins were used in the treatment of human colon, breast, and prostate cancers and were found to have antioxidant and anti-proliferative properties against different cancer cell lines [
45]. Cancer types resistant to chemotherapy can be treated using black carrot extract alone or together with anticancer drugs.
The bioactive compounds of black carrot were also found to reduce cardiovascular diseases by decreasing the blood cholesterol and glucose levels; additionally, cholesterol production in liver is reduced by the inhibition of 3-hydroxy-3-methylglutaryl-coenzyme A reductase activity. Lymphocyte activation, the inhibition of cell proliferation, anti-inflammatory effects, a reduction of the body mass index, a reduction in triglyceride level and blood pressure, and a reduction in the binding activity of bile acid are other distinguishable properties of bioactive compounds of black carrot that help in the prevention of cardiovascular diseases [
46]. Phenolic compounds of black carrots are also helpful in reducing the risk of diabetes [
44].
4. Carotenoids
Carotenoids are a group of isoprenoid molecules present in all photosynthetic plants, including carrots. Some non-photosynthetic fungi and bacteria also possess carotenoids. Carotenoids are acyclic or have five or six C rings on one or both ends of the molecule [
14]. Several conjugated double bonds of a polyene chain that function as a chromophore are responsible for the yellow, orange, and red colours of carotenoids [
47,
48]. There are two types of carotenoids present in carrot; namely, carotenes and xanthophylls. The major carotenoids (
Figure 4A) in carrot roots are β-carotene (75%); α-carotene (23%); lutein (1.9%); and β-cryptoxanthin, lycopene, and zeaxanthin [
49].
4.1. Occurrence of Carotenoids
Carotenoids are named after carrot, because carrot accumulates an enormous number of carotenoids in its roots. Orange and purple carrots have higher concentrations of carotenoids in the phloem than in the xylem [
50]. Beta-carotene makes up 80% of total carotenoids contained in domestic carrot roots [
51]. Generally, a carrot contains 16 to 38 mg/100 g carotenoids [
52].
4.2. The Biosynthesis of Carotenoids
Carotenoids are formed in plastids from isoprenoid precursors via the methylerythritol 4-phosphate (MEP) pathway [
53]. In the first step, 15-cis-phytoene (colourless carotenoid) is produced by the catalytic action of phytoene synthase (PSY). This compound is desaturated, isomerised, and converted into reddish all-trans lycopene via the catalytic actions of phytoene desaturase (PDS), 15-cis-γ-carotene isomerase (ZISO), γ-carotene desaturase (ZDS), and carotenoid (pro-lycopene) isomerase (CRTISO).
During cyclization of lycopene, the pathway splits into two branches to yield two orange carotenes; namely, beta (β) and epsilom (ε). Beta-carotene (with two β-rings on two ends of the lycopene molecule) is formed via lycopene β-cyclase (LCYB) and α-carotenes (one β-ring on one end and one ε-ring on the other) via lycopene β-cyclase (LCYE). Zeaxanthin is produced by the hydroxylation of β-carotene by carotenoid β-hydroxylase (CHYB) enzymes, particularly of the nonheme diiron (BCH) type; meanwhile, yellowish xanthophyll lutein is formed by hydroxylation of α-carotene catalysed by β and ε-hydroxylase (CHYB and CHYE) enzymes, primarily of the cytochrome P450 (CYP97) type [
48,
49,
54]. A schematic biosynthetic pathway for carotenoids is depicted in
Figure 5.
4.3. Factors Affecting Carotenoids’ Concentrations
Carotenoids are influenced by two main factors; i.e., inherited characteristics and the environment [
17,
55,
56].
4.3.1. Cultivar
A seven to eleven-fold difference in β-carotene concentration was observed in cultivars with different genetic makeups [
57]. There is controversy in the literature about the highest number of carotenoids present in different carrot genotypes. In previous studies, Alasalvar et al. [
58] found 2.3 times more β-carotenes in purple carrots than orange varieties. Similar results were presented by Seljåsen et al. [
57]. However, according to recent studies, higher contents of α and β-carotene are present in orange carrots, lutein in yellow carrots, lycopene in red carrots, anthocyanins in the roots of purple carrots, and phenolic compounds in black carrots [
44,
59,
60,
61]. Nicolle et al. [
62] studied the effect of genetic variability on carotenoids in carrots of different colours (genotypes) and found that the range of carotenoids in yellow and purple carrots is 469 to 605 μg/100 g, while 10 times more carotenoids are present in orange carrots. The highest carotenoids’ content, particularly β-carotene (170 mg/kg), is present in dark orange carrots, whereas purple carrots have the lowest β-carotene content (3.2 mg/kg). Suggestions for retaining carotenoid concentrations include the usage of cultivars known to have higher ranges of the useful compounds, and those which might be more suited to the local weather and geographical location. Gene expression partly describes the differences in carotenoids’ accumulation in the secondary phloem and xylem of the fleshy roots of carrots [
50].
4.3.2. Environment
Environmental conditions during growth and packaging alter the level of carotenoids, sugars, and volatiles [
55,
63]; however, results may vary when research is conducted under different conditions. Other researchers, Martín-Diana et al. [
64] and Rico et al. [
65], emphasise that crops grown in sandy soil tend to build up fewer provitamin A carotenoids than those grown in clay soils.
4.3.3. Storage Conditions and Temperature
Retail storage of carrots often takes place at a temperature range of 18 to 22 °C. Carrots can be subjected to these temperatures for a few days. Storage’s effect on carrot β-carotene is inconsistent based on different temperature levels. According to Imsic et al. [
66], α and β-carotene concentrations increased up to 35% and 25% after three days of storage and up to 42% and 34% after ten days of storage in Nantes carrots stored at 2 °C and 90% relative humidity. Significant increases in β-carotene were observed in both Nevis and Kingston cultivars stored at 20 °C for seven days. Longer storage periods of 21 days at 20 °C have a negative effect on α and β-carotene [
66]. After four weeks of storage, β-carotene was enhanced from 8% to 23% at 4 °C, compared to the levels at harvesting time [
57,
67]. Negi and Roy [
68] reported that β-carotene contents were reduced after eight days of storage at different temperatures, by 46% (7.5 to 8.5 °C), 51% (17 to 21 °C), and 70% (22 to 37.5 °C). Some studies also evidenced slight variations in α or β-carotene when carrots were stored at 0 °C, even for six months [
69].
4.4. Health Benefits of Carotenoids
The dietary intake of carotenoids, especially vitamin A, has been related to the protection of DNA, proteins, and lipids from oxidative damage; as well as to the maintenance of the normal function of the immune system, normal skin, normal mucosal membranes, and normal vision [
49,
52,
70,
71]. Digested purple carrot extract when passed through the colon mucosal cells, decreases oxidative DNA damage by 20.7%, protecting colon cells against reactive oxygen species stress [
72].
Beta-carotene, which is present in purple and orange carrots, is the most widely studied carotenoid so far, due to its significance in medical science. Dietary provitamin A carotenoids derived from plants are a major source of our vitamin A needs, and bioconversion to retinol may account for one-third of total retinol intake in developed countries. Vitamin A is essential for normal organogenesis, immune functions, tissue differentiation, and eyesight [
71]. Alpha-carotene, β-carotene, and β-cryptoxanthin obtained from carrot consumption are the carotenes that are converted into retinol in the human body.
Lutein from yellow carrot and its isomer, zeaxanthin, both accumulate in the centre of the retina (also known as the macula) of the eye. These are the only carotenoids that pass through the retinal barrier and form the macula in the eye. The macula enhances eyesight through its light-filtering characteristics. They are also powerful antioxidants and essential for healthy eyes. They protect eyes from diseases by absorbing harmful blue light that enters the eye. Lutein is also the most dominant carotenoid in brain tissue and the predominant carotenoid in the developing primate brain and retina. The amount of lutein is twice as much in paediatric brains than in adult brains, indicating its role in neural growth, and it may play a role in biological functions, including anti-oxidation, anti-inflammation, and in structural activity. It shields neural tissue, especially during infancy when the retina and brain are continuously in a state of change after birth. In adults, it is linked to cognitive health, and its supplementation enhances cognition. High ingestion (near 6 mg per day) of lutein is associated with low risk of muscular degeneration during old age, although actual intake of lutein varies between 1 and 2 mg per day in adults. It can also prevent the production of harmful free radicals, such as reactive oxygen species, via physical or chemical quenching of singlet oxygen [
73,
74,
75].
5. Polyacetylenes
Polyacetylenes are a prominent group of non-volatile bioactive phytochemicals that comprise at least two conjugated triple C–C bonds. Plants of the
Apiaceae family (to which the carrot belongs) contain aliphatic C
17-polyacetylenes of the falcarinol type [
76]. Recent studies on the biological activity of polyacetylenes have indicated their potential to improve human health due to anticancerous, antifungal, antibacterial, anti-inflammatory, and serotogenic effects. These findings suggest targeting vegetables with elevated levels of bisacetylenic oxylipins, such as falcarinol; and due to the abundant availability, high diversity of cultivars, worldwide experience, and high agricultural yields, carrot (
Daucus carota L.) genotypes are a very promising target vegetable [
3].
From more than 1400 polyacetylenes identified in plants, 12 polyacetylenes were isolated from carrot. Out of the twelve, falcarinol, falcarindiol, and falcarindiol-3-acetate are essential polyacetylenes predominately found in carrot roots (
Figure 4B). The other nine polyacetylenes that are isolated from carrot are: (E)-isofalcarinolone, falcarindiol-8-acetate, 1,2-dihydrofalcarindiol-3-acetate, (E)-falcarindiolone-8-acetate, (E)-falcarindiolone-9-acetate, 1,2-dihydrofalcarindiol, (E)-1-methoxy-falcarindiolone-8-acetate, (E)-1-methoxy-falcarindiolone-9-acetate, and panaxydiol [
3,
77].
5.1. The Occurrence of Polyacetylenes
The polyacetylenes are mainly present in the pericyclic parenchyma of the root and phloem near the secondary cambium [
78,
79]. Falcarinol is uniformly distributed in the carrot peel [
80] and it is allocated to all parts of carrot root, while falcarindiol and falcarindiol-3-acetate are more abundant inside the higher and outer segments, respectively [
13,
79].
5.2. Biosynthesis of Polyacetylenes
The crepenynate pathway is involved in the biosynthesis of falcarinol-type polyacetylenes. Acetyl-CoA and malonyl-CoA react in the presence of fatty acid synthase and Δ
9-desaturase enzymes and are converted into oleic acid. Oleic acid undergoes dehydrogenation to C
18-acetylenes—linoleic acid, crepenynic acid, and dehydrocrepenynic acid—by the catalytic action of Δ
12-desaturase, Δ
12-acetylenase, and Δ
14-desaturase enzymes, respectively. Dehydrocrepenynic acid is further transformed into C
17-acetylenes in the presence of Δ
14-acetylenase through β-oxidation [
3,
81]. A schematic biosynthetic pathway for falcarinol type polyacetylenes is depicted in
Figure 6.
5.3. Factors Affecting Polyacetylenes’ Concentration
The amount of falcarinol-type polyacetylenes in carrots is significantly affected by cultivar, geographic area (location), root size, harvesting time [
79], storage conditions [
82], industrial processing [
83], and biotic and abiotic stresses during the growing period and postharvest practices [
13,
56].
5.3.1. Cultivar and Location
Cultivars affect the polyacetylenes in carrot; for instance, cultivated orange carrots contain falcarinol, falcarindiol, and falcarindiol-3-acetate in the range of 16 to 84 mg/kg, 8 to 40 mg/kg, and 8 to 27 mg/kg of fresh weight, respectively [
3,
13,
84]. The concentration of falcarindiol polyacetylenes was observed within the range of 7 to 40.6 mg/kg of fresh weight when 27 different carrot cultivars were grown and harvested under the same growing conditions. The concentration of polyacetylenes found in the carrot may be 10–20 times more in wild type of carrots than domesticated carrots [
3,
85].
In another experiment, Kidmose et al. [
56] studied the effect of six carrot genotypes on polyacetylenes’ concentration by growing them in two different locations and found significant variations in the range of falcarinol (0.4 to 1.6 mg/100 g), falcarindiol (1.9 to 5.4 mg/100 g), and falcarindiol-3-acetate (0.9 to 1.9 mg/100 g) in fresh weights. Kjellenberg et al. [
86] reported the influence of the chemical composition of the soil on the concentrations of falcarinol-type polyacetylenes in carrots. Carrots grown in soils generally low in available phosphorus exhibited higher levels of falcarindiol if the soil was also low in available magnesium and calcium.
5.3.2. Root Size
It is reported that the concentrations of falcarindiol and falcarindiol-3-acetate decrease by increasing the root size of carrot, while the concentration of falcarinol is independent of root size. That is because falcarinol is mostly present in the phloem, while falcarindiol and falcarindiol-3-acetate are present in the root’s periderm [
56].
5.3.3. Harvesting Time
Kjellenberg [
87] indicated that the falcarinol level was slightly enriched after harvesting (with a short storage span), ultimately reaching a stabilised stage. The concentration of falcarindiol and falcarindiol-3-acetate reduced during early harvesting and increased during late harvesting dates, while falcarinol concentration did not change significantly. Similar effects were observed during storage [
79].
5.3.4. Processing and Storage
High concentrations of falcarinol, falcarindiol, and falcarindiol-3-acetate were documented in whole carrots that were refrigerated for four months at 1 °C. This indicates that polyacetylenes were produced during postharvest storage or there was little degradation in intact carrots after cold storage [
56]. According to Rawson et al. [
88], polyacetylene concentrations in carrot disks decreased at low temperatures (50 to 60 °C) and increased at high temperatures (70 to 100 °C), particularly the concentration of falcarinol. High pressure-temperature (HPT) processing enhances the retention of polyacetylenes in carrots. The highest combination which gave the maximum retention of falcarinol was 400 MPa, at 50 and 60 °C for 10 min; for falcarindiol it was 400 MPa, at 50 °C for 10 min; and for falcarindiol-3-acetate, it was 400 MPa, at 50 °C for 10 min.
Blanching and rapid freezing increased the retention rate of polyacetylenes in carrots during storage in cool conditions [
89,
90]. Kidmose et al. [
56] found an increase in falcarinol contents in frozen carrots that were blanched before freezing. Similarly, ultrasound and blanching pre-treatments affect the concentration of polyacetylenes in freeze-dried and hot-air-dried carrots. An ultrasound followed by hot-air drying results in a higher retention of polyacetylenes in dried carrot discs than blanching. Moreover, freeze-dried samples exhibit a better retention of polyacetylenes than those of hot-air-dried samples [
91]. Koidis et al. [
92] stated that peeling also affects the retention of polyacetylenes in carrots. Peeled carrots have a higher amount of polyacetylenes, but when washed after cutting, the contents decrease substantially due to leakage.
5.4. Health Benefits of Polyacetylenes
Polyacetylenes are reported to have health promoting traits, and in vitro data suggest that plant extracts containing falcarinol type polyacetylenes have anti-cancer and anti-inflammatory actions. Polyacetylenes are extremely cytotoxic against several cancer cell lines and have revealed antifungal, anti-inflammatory, and anti-platelet aggregatory characteristics [
93]. Purup et al. [
94] suggested that the hydroxyl group (–OH) at C
3 may account for these activities. Polyacetylenes of carrots are associated with health benefits [
95], and more specifically, it was found that the falcarinol-type polyacetylenes from carrot shields against cancer [
80]. More recently, Tan et al. [
96] documented that C
17-polyacetylenes inhibit the breast cancer resistance protein BCRP/ABCG2 when used as a multidrug resistance reversal agent. Kjellenberg et al. [
97] studied polyacetylenes in fresh and stored carrots and reported that falcarinol activates mammalian cell differentiation, but also showed toxic effects against human cancer cells and the possibility of allergic inflammation of the skin. Falcarinol and falcarindiol may be used as antidiabetic agents in the treatment of diabetes due to their ability to arouse basal or insulin-dependent glucose absorption in adipocytes and porcine myotube cell cultures based on different doses [
98].
Zaini et al. [
99] studied the role of the bioactive chemicals of carrot juice extract in the treatment of leukaemia and concluded that carrot juice extracts can cause apoptosis, resulting in the cell cycle arrest of cells affected by leukaemia. That is why carrot juice extracts can be the best reservoir of bioactive compounds suitable for treatment of leukaemia. In another study, it was suggested that instead of beta-carotenes or lutein, polyacetylenes are the bioactive compounds from carrot that would be effective in the treatment of leukaemia [
100]. Polyacetylenes of purple carrots are involved in anti-inflammatory bioactivity in humans. These polyacetylenes enhance cell proliferation at lower concentrations. Falcarinol is the most bioactive polyacetylene, inducing epithelial cell proliferation at the low concentration range of 0.004 to 0.4 μM [
101].
6. Ascorbic Acid
l-ascorbic acid or vitamin C (
Figure 7) is one of the most abundant water-soluble low molecular weight antioxidants found throughout the kingdom Plantae. It is known to play a central role in regulating the cellular redox potential in cells [
102,
103]. As humans and some other primates lack the ability to synthesize and store vitamin C, they depend on fresh fruits and vegetables to cover their daily requirements (75–90 mg RDA). All recent studies point toward a diet rich in vitamin C for improving human health. Troesch et al. [
104], suggest that vitamin C should be a clear target for the nutritional enhancement of horticultural crops. The accumulation of vitamin C within the same species may vary between different cultivars [
103,
105,
106], tissue types [
107], and developmental stages [
106,
107]. Regardless of this variability, vitamin C is tightly regulated through net biosynthesis, recycling, degradation/oxidation, and/or intercellular and intracellular transport.
6.1. Occurrence of Ascorbic Acid
There are many authors reporting on differences between carrot cultivars regarding the content of vitamin C [
108,
109,
110]. According to Matějková and Petříková [
111], vitamin C content in six carrot cultivars ranged from 54 mg/kg to 132 mg/kg, while concentrations as low as 21 mg/kg [
112] and high as 775 mg/kg were reported [
109]. Vitamin C may accumulate at up to 20 mM in chloroplasts, and occurs in almost all parts of the cell. Dark orange carrots contain 4 times more vitamin C than yellow, purple and orange carrots [
62].
6.2. Biosynthesis of Ascorbic Acid
In plants, four alternative pathways for ascorbic acid biosynthesis have been reported; namely, the
d-mannose/
l-galactose (
d-Man/
l-Gal) pathway, myoinositol pathway, galacturonate pathway, and
l-glucose pathway. The ten-step
d-Man/
l-Gal pathway is the most acceptable for ascorbic acid biosynthesis in carrots (
Figure 7).
d-Glucose-6-phosphate (
d-glucose-6-P), obtained from the hexokinase of
d-glucose, is converted into its furanosyl derivative
d-fructose-6-phosphate (
d-fructose-6-P) in the presence of phosphoglucose isomerase (PGI). Phosphomannose isomerase (PMI) converts
d-fructose-6-P into
d-mannose-6-phosphate (
d-mannose-6-P).
d-Mannose-6-P subsequently rearranges (phosphate moves from C6 to C1) due to the catalytic action of phosphomannose mutase (PMM) to yield
d-mannose-1-phosphate (
d-Mannose-1-P).
d-Mannose-1-P is converted into glucose diphosphate
d-mannose (GDP-
d-mannose) in the presence of GDP-
d-mannose pyrophosphorylase (GMP). GDP-
d-Mannose undergoes a reversible reaction catalysed by GDP-
d-mannose-3′,5′-epimerase (GME), and the unstable intermediate GDP-
l-glucose is then readily converted into its isomer, GDP-
l-galactose. GDP-
l-galactose phosphorylase (GGP) converts GDP-
l-galactose into
l-galactose-1-phosphate (
l-galactose-1-P), followed by dephosphorylation via the catalytic action of
l-galactose-1-P phosphatase (GPP) to afford
l-galactose.
l-Galactose is converted into 1-galactono-1,4-lactone in the presence of
l-galactose dehydrogenase (GalDH), which is dehydrogenized by
l-galactono-1,4-lactone dehydrogenase (GalLDH) to yield
l-ascorbic acid [
113].
6.3. Factors Affecting Ascorbic Acid Concentration
Numerous factors affect the concentration of ascorbic acid in carrots, such as cultivar, carbon dioxide, temperature, processing, and storage.
6.3.1. Cultivar
Nicolle et al. [
62] studied the effect of genetic variability on vitamin C in 20 different carrot genotypes and observed significant differences. The concentration of vitamin C was highest in dark orange (four times), yellow (3.7 times), and white (2.3 times) carrot cultivars compared to the orange carrot cultivar. It has been reported that boron deficiency during the growth of carrots enhances ascorbic acid contents from 45% to 70% [
26]. Ascorbic acid oxidase affects the stability of vitamin C in carrots. It converts
l-ascorbic acid (active form of vitamin C) into dehydro-
l-ascorbic acid via oxidation. Its affinity for
l-ascorbic acid varies from 50 to 244 μM for different carrot genotypes [
114,
115]. Leong et al. [
116] used a pulsing electric field to reduce ascorbic acid oxidase activity (thermo-stability) in Nantes, Solar Yellow, White Belgian, Nutri Red, and Purple Haze cultivars and calculated the catalytic activity of ascorbic acid through the Michaelis–Menten enzyme kinetic model. The range of V
max values for studied genotypes was 9.54 to 34.71 μmol/min. The V
max values for catalytic activity of ascorbic acid in white and yellow carrots were significantly (
p < 0.05) higher than purple, red, and Nantes carrot genotypes. A pulsing electric field treatment of 0.8 kV/cm and 30 KJ/kg reduced variability of the thermo-stability of ascorbic acid oxidase in the puree of all studied genotypes.
6.3.2. Elevated Carbon Dioxide
Carbon dioxide is important for photosynthesis in plants and affects vitamin C concentration. For example, recently Wu et al. [
117] studied the consequence of elevated CO
2 (3000 μmol/mol) on vitamin C accumulation in carrots. They concluded that elevated levels of CO
2 significantly affected vitamin C accumulation due to the change in the transcript profile of 12 genes responsible for biosynthesis of vitamin C.
6.3.3. Storage and Temperature
Vitamin C is sensitive to adverse handling. The level of vitamin C in baby carrots was reduced during cold storage in high and moderate O
2 conditions; however, under a low O
2 atmosphere, baby carrots retained the highest amount of vitamin C [
27]. Frozen storage lessened vitamin C concentration by 4.1% [
118]. The effect of prolonged storage was considerable losses of vitamin C, 15% to 49% [
111,
119]. After eight days of storage, vitamin C concentrations decreased by 38% at 7.5 to 8.5 °C and by 70% at 22 to 37.5 °C. The maximum decrease in vitamin C contents was observed during local storage at 25 to 28 °C [
57]. Leong and Oey [
114] reported that the thermal treatment (>80 °C, 10 min) before matrix destruction effectively inactivates ascorbic acid oxidase activity and protects
l-ascorbic acid against enzymatic oxidation, consequently enhancing vitamin C concentration and stability in carrots.
6.3.4. Processing
Thermal processing of carrots decreases the vitamin C concentration, while chemical preservatives, such as potassium bisulphate, aid in preserving vitamin C [
120]. Conventional blanching enhances vitamin C contents from 37.5% to 85%, while ultrasound at temperatures above 60 °C has a negative effect on vitamin C [
121]. Patras et al. [
37] reported that blanched frozen samples of carrots have a higher vitamin C content compared to un-blanched frozen samples. Microwave assisted freeze dried carrots retain higher contents of
l-ascorbic acid [
122]. Vishwanathan et al. [
123] investigated the effect of infrared assisted dry blanching and hybrid drying on carrots and concluded that infrared blanched-hybrid dried slices of carrots have higher (39%) vitamin C concentrations than water blanched-hot air-dried samples of carrots. Fast diffusion of air into the sample surface due to infrared heating and the removal of moisture at the same time makes the drying process faster.
6.4. The Health Benefits of Ascorbic Acid
Vitamin C (l-ascorbic acid) plays an important role in the biosynthesis of collagen, is essential for the synthesis of carnitine and catecholamines, and is also involved in the metabolism of cholesterol to bile acids. Vitamin C in an aqueous solution readily scavenges reactive oxygen and nitrogen species, and is part of the antioxidant network of the body. It plays a vital role in Fe absorption from the gut by reducing Fe3+ to Fe2+ and maintains the structure of Fe-binding proteins.
Vitamin C is involved in the regulation of hypoxia-inducible factor 1α (HIF 1α, a transcription factor that activates genes that control several mechanisms at the cell level, like cell survival, the development of new blood vessels, Fe transport, and glycolysis), which induces cellular responses to hypoxic conditions. It can aid in the treatment of neurodegenerative diseases like Alzheimer’s disease, Huntington’s disease, ischemic stroke, and Parkinson’s disease [
124,
125,
126]. At high concentrations, it acts as a prodrug, and transports a high flux of H
2O
2 to cancer cells and plays a role in the treatment of cancer [
127].
Scurvy, characterised by symptoms related to connective tissue defects, can be prevented with an adequate intake of
l-ascorbic acid. Vitamin C maintains healthy skin, gums, and blood vessels. It also aids in the reduction of plasma cholesterol, the vitality of the immune system, and the elimination of reactive oxygen species. Leong and Oey [
114] and Dias [
128], also described detailed evidence on health benefits of vitamin C regarding its assistance against cancer, arteriosclerosis, and other cardiovascular diseases.
7. Conclusions and Future Challenges
It is evident from the present review that there is an abundant diversity of carrot cultivars grown successfully worldwide, delivering high agricultural yields. Due to the rich source of phytochemicals present in carrots, they serves as a multi-nutritional food source. The biological activities of some of the phytochemicals found in carrots; namely, phenolic compounds (particularly chlorogenic acid), carotenoids, polyacetylenes, and ascorbic acid (vitamin C), have indicated their potential to improve human health due to their anticancer, antioxidant, anti-inflammatory, antibacterial, plasma lipid modification, and serotogenic effects.
However, the concentration and nature of phytochemicals are affected by several factors, such as carrot genotype (colour differences), environmental conditions, and the preparation and storage of carrot products. Experiments addressing these factors are of great importance to improve the quality of carrots, and to develop genotypes enriched for selected beneficial phytochemicals.
Large quantities of carrots are annually discarded in different parts of the world because they do not meet market standards. Additionally, the carrot-processing industry (puree and juice) gives rise to a number of waste products, such as carrot peel, that can be recovered and used as a source of bioactive compounds. Thus, a series of valuable by-products, such as carotenoids, phenolic compounds, fractions of dietary fibre, and bioethanol, can be obtained from food-processing wastes and discarded carrots [
6,
129]. In addition, carrots can be processed for the production of anthocyanin-rich concentrate for pigment industry, while the resulting pomace can be extracted to obtain high-value-added phenolic compounds that can be used as functional food ingredients [
130].
Author Contributions
T.A. conceptualized the idea and wrote the introduction. M.C. prepared all figures using the TIF format. Q.I., A.B., R.M.S.T., and M.A. wrote phenolic compound, carotenoids, polyacetylenes, and ascorbic acid sections, respectively. S.A. and A.A. wrote the abstract, conclusion, and future challenges sections. All authors read, revised, and approved the review article for publication.
Funding
Author Ariño thanks the Government of Aragón and FEDER 2014–2020 (grant Grupo A06_17R) for support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tiwari, U.; Cummins, E. Factors influencing levels of phytochemicals in selected fruit and vegetables during pre-and post-harvest food processing operations. Food Res. Int. 2013, 50, 497–506. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations Carrots and Turnips. Available online: http://www.fao.org/faostat/en/#data/QC/visualize (accessed on 10 July 2019).
- Dawid, C.; Dunemann, F.; Schwab, W.; Nothnagel, T.; Hofmann, T. Bioactive C 17-Polyacetylenes in Carrots (Daucus carota L.): Current Knowledge and Future Perspectives. J. Agric. Food Chem. 2015, 63, 9211–9222. [Google Scholar] [CrossRef] [PubMed]
- Leja, M.; Kamińska, I.; Kramer, M.; Maksylewicz-Kaul, A.; Kammerer, D.; Carle, R.; Baranski, R. The Content of Phenolic Compounds and Radical Scavenging Activity Varies with Carrot Origin and Root Color. Plant. Foods Hum. Nutr. 2013, 68, 163–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Umar, G.; Kaur, S.; Gurumayum, S.; Rasane, P. Effect of Hot Water Blanching Time and Drying Temperature on the Thin Layer Drying Kinetics of and Anthocyanin Degradation in Black Carrot (Daucus carota L.) Shreds. Food Technol. Biotechnol. 2015, 53, 324–330. [Google Scholar]
- Nguyen, H.H.V.; Nguyen, L.T. Carrot processing. In Handbook of Vegetable Preservation Processing, 2nd ed.; Hui, Y.H., Evranuz, E.Ö., Eds.; CRC Press: Boca Raton, FL, USA, 2015; pp. 449–478. [Google Scholar]
- Tsao, R. Chemistry and Biochemistry of Dietary Polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef]
- Balasundram, N.; Sundram, K.; Samman, S. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chem. 2006, 99, 191–203. [Google Scholar] [CrossRef]
- Brglez Mojzer, E.; Knez Hrnčič, M.; Škerget, M.; Knez, Ž.; Bren, U. Polyphenols: Extraction methods, antioxidative action, bioavailability and anticarcinogenic effects. Molecules 2016, 21, 901. [Google Scholar] [CrossRef]
- Di Mauro, M.D.; Giardina, R.C.; Fava, G.; Mirabella, E.F.; Acquaviva, R.; Renis, M.; D’Antona, N. Polyphenolic profile and antioxidant activity of olive mill wastewater from two Sicilian olive cultivars: Cerasuola and Nocellara etnea. Eur. Food Res. Technol. 2017, 243, 1895–1903. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef]
- Gonçalves, E.M.; Pinheiro, J.; Abreu, M.; Brandão, T.R.S.; Silva, C.L.M. Carrot (Daucus carota L.) peroxidase inactivation, phenolic content and physical changes kinetics due to blanching. J. Food Eng. 2013, 97, 574–581. [Google Scholar] [CrossRef]
- Czepa, A.; Hofmann, T. Quantitative Studies and Sensory Analyses on the Influence of Cultivar, Spatial Tissue Distribution, and Industrial Processing on the Bitter Off-Taste of Carrots (Daucus carota L.) and Carrot Products. J. Agric. Food Chem. 2004, 52, 4508–4514. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.D.; Karki, S.; Thakur, N.S.; Attri, S. Chemical composition, functional properties and processing of carrot—A review. J. Food Sci. Technol. 2012, 49, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Hamauzu, Y. Phenolic compounds and their antioxidant properties in different tissues of carrots (Daucus carota L.). J. Food Agric. Environ. 2004, 2, 95–100. [Google Scholar]
- Alasalvar, C.; Al-Farsi, M.; Quantick, P.; Shahidi, F.; Wiktorowicz, R. Effect of chill storage and modified atmosphere packaging (MAP) on antioxidant activity, anthocyanins, carotenoids, phenolics and sensory quality of ready-to-eat shredded orange and purple carrots. Food Chem. 2005, 89, 69–76. [Google Scholar] [CrossRef]
- Gajewski, M.; Szymczak, P.; Elkner, K.; Dąbrowska, A.; Kret, A.; Danilcenko, H. Some Aspects of Nutritive and Biological Value of Carrot Cultivars with Orange, Yellow and Purple-Coloured Roots. Veg. Crop. Res. Bull. 2007, 67, 149–161. [Google Scholar] [CrossRef] [Green Version]
- Oviasogie, P.; Okoro, D.; Ndiokwere, C. Determination of total phenolic amount of some edible fruits and vegetables. African J. Biotechnol. 2009, 8, 2819–2820. [Google Scholar]
- Korkina, L.; Kostyuk, V.; De Luca, C.; Pastore, S. Plant phenylpropanoids as emerging anti-inflammatory agents. Mini-Rev. Med. Chem. 2011, 11, 823–835. [Google Scholar] [CrossRef]
- El-Seedi, H.R.; El-Said, A.M.A.; Khalifa, S.A.M.; Göransson, U.; Bohlin, L.; Borg-Karlson, A.-K.; Verpoorte, R. Biosynthesis, Natural Sources, Dietary Intake, Pharmacokinetic Properties, and Biological Activities of Hydroxycinnamic Acids. J. Agric. Food Chem. 2012, 60, 10877–10895. [Google Scholar] [CrossRef]
- Bartley, G.E.; Avena-Bustillos, R.J.; Du, W.-X.; Hidalgo, M.; Cain, B.; Breksa, A.P., III. Transcriptional regulation of chlorogenic acid biosynthesis in carrot root slices exposed to UV-B light. Plant. Gene 2016, 7, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Vogt, T. Phenylpropanoid biosynthesis. Mol. Plant. 2010, 3, 2–20. [Google Scholar] [CrossRef]
- Gross, G.G. Phenolic acids. In Secondary Plant Products; Conn, E.E., Ed.; In the series The Biochemistry of Plants — A Comprehensive Treatise; Academic Press: New York, NY, USA, 1981; Volume 7, pp. 301–316. [Google Scholar]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed]
- Søltoft, M.; Nielsen, J.; Holst Laursen, K.; Husted, S.; Halekoh, U.; Knuthsen, P. Effects of Organic and Conventional Growth Systems on the Content of Flavonoids in Onions and Phenolic Acids in Carrots and Potatoes. J. Agric. Food Chem. 2010, 58, 10323–10329. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.P.; Beloy, J.; McInerney, J.K.; Day, L. Impact of boron, calcium and genetic factors on vitamin C, carotenoids, phenolic acids, anthocyanins and antioxidant capacity of carrots (Daucus carota). Food Chem. 2012, 132, 1161–1170. [Google Scholar] [CrossRef] [PubMed]
- Kamiloglu, S.; Pasli, A.A.; Ozcelik, B.; Van Camp, J.; Capanoglu, E. Colour retention, anthocyanin stability and antioxidant capacity in black carrot (Daucus carota) jams and marmalades: Effect of processing, storage conditions and in vitro gastrointestinal digestion. J. Funct. Foods 2015, 13, 1–10. [Google Scholar] [CrossRef]
- Simões, A.D.N.; Allende, A.; Tudela, J.A.; Puschmann, R.; Gil, M.I. Optimum controlled atmospheres minimise respiration rate and quality losses while increase phenolic compounds of baby carrots. LWT Food Sci. Technol. 2011, 44, 277–283. [Google Scholar] [CrossRef]
- Becerra-Moreno, A.; Redondo-Gil, M.; Benavides, J.; Nair, V.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Combined effect of water loss and wounding stress on gene activation of metabolic pathways associated with phenolic biosynthesis in carrot. Front. Plant. Sci. 2015, 6, 837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alegria, C.; Gonçalves, E.M.; Moldão-Martins, M.; Cisneros-Zevallos, L.; Abreu, M. Peel removal improves quality without antioxidant loss, through wound-induced phenolic biosynthesis in shredded carrot. Postharvest Biol. Technol. 2016, 120, 232–239. [Google Scholar] [CrossRef]
- Del Rosario Cuéllar-Villarreal, M.; Ortega-Hernández, E.; Becerra-Moreno, A.; Welti-Chanes, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Effects of ultrasound treatment and storage time on the extractability and biosynthesis of nutraceuticals in carrot (Daucus carota). Postharvest Biol. Technol. 2016, 119, 18–26. [Google Scholar] [CrossRef]
- Carolina Formica-Oliveira, A.; Benito Martínez-Hernández, G.; Díaz-López, V.; Artés, F.; Artés-Hernández, F. Effects of UV-B and UV-C combination on phenolic compounds biosynthesis in fresh-cut carrots. Postharvest Biol. Technol. 2017, 127, 99–104. [Google Scholar] [CrossRef]
- Surjadinata, B.; Jacobo-Velázquez, D.; Cisneros-Zevallos, L. UVA, UVB and UVC Light Enhances the Biosynthesis of Phenolic Antioxidants in Fresh-Cut Carrot through a Synergistic Effect with Wounding. Molecules 2017, 22, 668. [Google Scholar] [CrossRef]
- Jacobo-Velázquez, D.A.; Martínez-Hernández, G.B.; del C. Rodríguez, S.; Cao, C.-M.; Cisneros-Zevallos, L. Plants as Biofactories: Physiological Role of Reactive Oxygen Species on the Accumulation of Phenolic Antioxidants in Carrot Tissue under Wounding and Hyperoxia Stress. J. Agric. Food Chem. 2011, 59, 6583–6593. [Google Scholar] [CrossRef] [PubMed]
- Surjadinata, B.B.; Cisneros-Zevallos, L. Biosynthesis of phenolic antioxidants in carrot tissue increases with wounding intensity. Food Chem. 2012, 134, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Tian, C.; Luob, J.; Zhouc, R.; Sun, X.; Ma, J. Influence of technical processing units on polyphenols and antioxidant capacity of carrot (Daucus carrota L.) juice. Food Chem. 2013, 141, 1637–1644. [Google Scholar] [CrossRef] [PubMed]
- Patras, A.; Tiwari, B.K.; Brunton, N.P. Influence of blanching and low temperature preservation strategies on antioxidant activity and phytochemical content of carrots, green beans and broccoli. LWT Food Sci. Technol. 2011, 44, 299–306. [Google Scholar] [CrossRef]
- Soto-Vaca, A.; Gutierrez, A.; Losso, J.N.; Xu, Z.; Finley, J.W. Evolution of Phenolic Compounds from Color and Flavor Problems to Health Benefits. J. Agric. Food Chem. 2012, 60, 6658–6677. [Google Scholar] [CrossRef]
- Stan, S.D.; Kar, S.; Stoner, G.D.; Singh, S.V. Bioactive food components and cancer risk reduction. J. Cell. Biochem. 2008, 104, 339–356. [Google Scholar] [CrossRef]
- Ghasemzadeh, A.; Ghasemzadeh, N. Flavonoids and phenolic acids: Role and biochemical activity in plants and human. J. Med. Plants Res. 2011, 5, 6697–6703. [Google Scholar] [CrossRef]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.E.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (Poly)phenolics in Human Health: Structures, Bioavailability, and Evidence of Protective Effects Against Chronic Diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef] [Green Version]
- Jing, P.; Bomser, J.A.; Schwartz, S.J.; He, J.; Magnuson, B.A.; Giusti, M.M. Structure-function relationships of anthocyanins from various anthocyanin-rich extracts on the inhibition of colon cancer cell growth. J. Agric. Food Chem. 2008, 56, 9391–9398. [Google Scholar] [CrossRef]
- Netzel, M.; Netzel, G.; Kammerer, D.R.; Schieber, A.; Carle, R.; Simons, L.; Bitsch, I.; Bitsch, R.; Konczak, I. Cancer cell antiproliferation activity and metabolism of black carrot anthocyanins. Innov. Food Sci. Emerg. Technol. 2007, 8, 365–372. [Google Scholar] [CrossRef]
- Akhtar, S.; Rauf, A.; Imran, M.; Qamar, M.; Riaz, M.; Mubarak, M.S. Black carrot (Daucus carota L.), dietary and health promoting perspectives of its polyphenols: A review. Trends Food Sci. Technol. 2017, 66, 36–47. [Google Scholar] [CrossRef]
- Wang, L.S.; Stoner, G.D. Anthocyanins and their role in cancer prevention. Cancer Lett. 2008, 269, 281–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wright, O.R.L.; Netzel, G.A.; Sakzewski, A.R. A randomized, double-blind, placebo-controlled trial of the effect of dried purple carrot on body mass, lipids, blood pressure, body composition, and inflammatory markers in overweight and obese adults: The QUENCH Trial. Can. J. Physiol. Pharmacol. 2013, 91, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Águila Ruiz-Sola, M.; Rodríguez-Concepción, M. Carotenoid biosynthesis in arabidopsis: A colorful pathway. BioOne 2012, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Concepcion, M.; Stange, C. Biosynthesis of carotenoids in carrot: An underground story comes to light. Arch. Biochem. Biophys. 2013, 539, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Søltoft, M.; Bysted, A.; Madsen, K.H.; Mark, A.B.; Bügel, S.G.; Nielsen, J.; Knuthsen, P. Effects of organic and conventional growth systems on the content of carotenoids in carrot roots, and on intake and plasma status of carotenoids in humans. J. Sci. Food Agric. 2011, 91, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Perrin, F.; Hartmann, L.; Dubois-Laurent, C.; Welsch, R.; Huet, S.; Hamama, L.; Briard, M.; Peltier, D.; Gagné, S.; Geoffriau, E. Carotenoid gene expression explains the difference of carotenoid accumulation in carrot root tissues. Planta 2017, 245, 737–747. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Rensing, K.H.; Douglas, C.J.; Cheng, K.M. Chromoplasts ultrastructure and estimated carotene content in root secondary phloem of different carrot varieties. Planta 2010, 231, 549–558. [Google Scholar] [CrossRef]
- Mustafa, A.; Trevino, L.M.; Turner, C. Pressurized Hot Ethanol Extraction of Carotenoids from Carrot By-Products. Molecules 2012, 17, 1809–1818. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Concepción, M. Supply of precursors for carotenoid biosynthesis in plants. Arch. Biochem. Biophys. 2010, 504, 118–122. [Google Scholar] [CrossRef]
- Britton, G. Biosynthesis of carotenoids. In Carotenoids in Photosynthesis; Young, A., Britton, G., Eds.; Springer Science & Business Media: Berlin, Germany, 2012; pp. 96–126. [Google Scholar]
- Seljasen, R.; Bengtsson, G.B.; Hoftun, H.; Vogt, G. Sensory and chemical changes in five varieties of carrot (Daucus carota L.) in response to mechanical stress at harvest and post-harvest. J. Sci. Food Agric. 2001, 81, 436–447. [Google Scholar] [CrossRef]
- Kidmose, U.; Hansen, S.L.; Christensen, L.P.; Edelenbos, M.; Larsen, E.; Nørbaek, R. Effects of Genotype, Root Size, Storage, and Processing on Bioactive Compounds in Organically Grown Carrots (Daucus carota L.). J. Food Sci. 2004, 69, S388–S394. [Google Scholar] [CrossRef]
- Seljåsen, R.; Kristensen, H.L.; Lauridsen, C.; Wyss, G.S.; Kretzschmar, U.; Birlouez-Aragone, I.; Kahl, J. Quality of carrots as affected by pre- and postharvest factors and processing. J. Sci. Food Agric. 2013, 93, 2611–2626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alasalvar, C.; Grigor, J.M.; Zhang, D.; Quantick, P.C.; Shahidi, F. Comparison of Volatiles, Phenolics, Sugars, Antioxidant Vitamins, and Sensory Quality of Different Colored Carrot Varieties. J. Agric. Food Chem. 2001, 49, 1410–1416. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.-P.; Chou, C.-C.; Yu, R.-C. Antioxidant activity of lactic-fermented Chinese cabbage. Food Chem. 2009, 115, 912–917. [Google Scholar] [CrossRef]
- Arscott, S.A.; Tanumihardjo, S.A. Carrots of many colors provide basic nutrition and bioavailable phytochemicals acting as a functional food. Compr. Rev. Food Sci. Food Saf. 2010, 9, 223–239. [Google Scholar] [CrossRef]
- Baranski, R.; Allender, C.; Klimek-Chodacka, M. Towards better tasting and more nutritious carrots: Carotenoid and sugar content variation in carrot genetic resources. Food Res. Int. 2012, 47, 182–187. [Google Scholar] [CrossRef]
- Nicolle, C.; Simon, G.; Rock, E.; Amouroux, P.; Remesy, C. Genetic Variability In fl uences Carotenoid, Vitamin, Phenolic, and Mineral Content in White, Yellow, Purple, Orange, and Dark-orange Carrot Cultivars. J. Am. Soc. Hortic. Sci. 2004, 129, 523–529. [Google Scholar] [CrossRef]
- Gajewski, M.; Dąbrowska, A. Quality characteristics of carrot cultivars depending to long-term storage. In Spontaneous and Induced Variation for the Genetic Improvement of Horticultural Products; Nowaczyk, P., Ed.; University of Technology and Life Sciences in Bydgoszcz: Bydgoszcz, Poland, 2007; pp. 95–103. [Google Scholar]
- Martín-Diana, A.B.; Rico, D.; Frías, J.M.; Barat, J.M.; Henehan, G.T.M.; Barry-Ryan, C. Calcium for extending the shelf life of fresh whole and minimally processed fruits and vegetables: A review. Trends Food Sci. Technol. 2007, 18, 210–218. [Google Scholar] [CrossRef]
- Rico, D.; Martín-Diana, A.B.; Barat, J.M.; Barry-Ryan, C. Extending and measuring the quality of fresh-cut fruit and vegetables: A review. Trends Food Sci. Technol. 2007, 18, 373–386. [Google Scholar] [CrossRef]
- Imsic, M.; Winkler, S.; Tomkins, B.; Jones, R. Effect of Storage and Cooking on β-Carotene Isomers in Carrots (Daucus carota L. cv. ‘Stefano’). J. Agric. Food Chem. 2010, 58, 5109–5113. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.; Küchler, T.; Maaßen, A.; Busch-Stockfisch, M.; Steinhart, H. Correlations of carotene with sensory attributes in carrots under different storage conditions. Food Chem. 2008, 106, 235–240. [Google Scholar] [CrossRef]
- Negi, P.S.; Roy, S.K. Effect of low-cost storage and packaging on quality and nutritive value of fresh and dehydrated carrots. J. Sci. Food Agric. 2000, 80, 2169–2175. [Google Scholar] [CrossRef]
- Koca, N.; Karadeniz, F. Changes of bioactive compounds and anti-oxidant activity during cold storage of carrots. Int. J. Food Sci. Technol. 2008, 43, 2019–2025. [Google Scholar] [CrossRef]
- Elvira-Torales, L.I.; García-Alonso, J.; Periago-Castón, M.J. Nutritional Importance of Carotenoids and Their Effect on Liver Health: A Review. Antioxidants 2019, 8, 229. [Google Scholar] [CrossRef] [PubMed]
- Sommer, A.; Vyas, K.S. A global clinical view on vitamin A and carotenoids. Am. J. Clin. Nutr. 2012, 96, 1204S–1206S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olejnik, A.; Rychlik, J.; Kidod, M.; Czapski, J.; Kowalska, K.; Juzwa, W.; Olkowicz, M.; Dembczyski, R.; Moyer, M.P. Antioxidant effects of gastrointestinal digested purple carrot extract on the human cells of colonic mucosa. Food Chem. 2016, 190, 1069–1077. [Google Scholar] [CrossRef]
- Vílchez, C.; Forján, E.; Cuaresma, M.; Bédmar, F.; Garbayo, I.; Vega, J.M. Marine Carotenoids: Biological Functions and Commercial Applications. Mar. Drugs 2011, 9, 319–333. [Google Scholar] [CrossRef] [Green Version]
- Johnson, E.J. Role of lutein and zeaxanthin in visual and cognitive function throughout the lifespan. Nutr. Rev. 2014, 72, 605–612. [Google Scholar] [CrossRef]
- Vishwanathan, K.H.; Giwari, G.K.; Hebbar, H.U. Lutein and Preterm Infants With Decreased Concentrations of Brain Carotenoids. Food Bioprod. Process. 2014, 59, 659–665. [Google Scholar] [CrossRef]
- Christensen, P.L. Aliphatic C17-Polyacetylenes of the Falcarinol Type as Potential Health Promoting Compounds in Food Plants of the Apiaceae Family. Rec. Patents Food Nutr. Agric. 2011, 3, 64–77. [Google Scholar] [CrossRef]
- Schmiech, L.; Carole, A.; Witulski, B.; Hofmann, T. Structure determination of bisacetylenic oxylipins in carrots (Daucus carota L.) and enantioselective synthesis of falcarindiol. J. Agric. Food Chem. 2009, 57, 11030–11040. [Google Scholar] [CrossRef] [PubMed]
- Baranska, M.; Schulz, H. In Situ Simultaneous Analysis of Polyacetylenes, Carotenoids and Polysaccharides in Carrot Roots. J. Agric. Food Chem. 2005, 53, 6565–6571. [Google Scholar] [CrossRef] [PubMed]
- Kjellenberg, L.; Johansson, E.; Gustavsson, K.-E.; Olsson, M.E. Effects of Harvesting Date and Storage on the Amounts of Polyacetylenes in Carrots, Daucus carota. J. Agric. Food Chem. 2010, 58, 11703–11708. [Google Scholar] [CrossRef] [PubMed]
- Kreutzmann, S.; Christensen, L.P.; Edelenbos, M. Investigation of bitterness in carrots (Daucus carota L.) based on quantitative chemical and sensory analyses. LWT Food Sci. Technol. 2008, 41, 193–205. [Google Scholar] [CrossRef]
- Hansen, L.; Boll, P.M. Polyacetylenes in araliaceae: Their chemistry, biosynthesis and biological significance. Phytochemistry 1986, 25, 285–293. [Google Scholar] [CrossRef]
- Hansen, S.L.; Purup, S.; Christensen, L.P. Bioactivity of falcarinol and the influenceof processing and storage on its content in carrots (Daucus carota L.). J. Sci. Food Agric. 2003, 83, 1010–1017. [Google Scholar] [CrossRef]
- Minto, R.E.; Blacklock, B.J. Biosynthesis and function of polyacetylenes and allied natural products. Prog. Lipid Res. 2008, 47, 233–306. [Google Scholar] [CrossRef] [Green Version]
- Czepa, A.; Hofmann, T. Structural and Sensory Characterization of Compounds Contributing to the Bitter Off-Taste of Carrots (Daucus carota L.) and Carrot Puree. J. Agric. Food Chem. 2003, 51, 3865–3873. [Google Scholar] [CrossRef]
- Pferschy-Wenzig, E.-M.; Getzinger, V.; Kunert, O.; Woelkart, K.; Zahrl, J.; Bauer, R. Determination of falcarinol in carrot (Daucus carota L.) genotypes using liquid chromatography/mass spectrometry. Food Chem. 2009, 114, 1083–1090. [Google Scholar] [CrossRef]
- Kjellenberg, L.; Johansson, E.; Gustavsson, K.-E.; Granstedt, A.; Olsson, M.; Kjellenberg, L.; Johansson, E.; Gustavsson, K.-E.; Granstedt, A.; Olsson, M.E. Correlations between Polyacetylene Concentrations in Carrot (Daucus carota L.) and Various Soil Parameters. Foods 2016, 5, 60. [Google Scholar] [CrossRef] [PubMed]
- Kjellenberg, L. Sweet and Bitter Taste in Organic Carrot; Swedish University of Agricultural Sciences: Alnarp, Sweden, 2007. [Google Scholar]
- Rawson, A.; Brunton, N.; Tuohy, M. High pressure–temperature degradation kinetics of polyacetylenes in carrots. Food Chem. 2012, 133, 15–20. [Google Scholar] [CrossRef]
- Rawson, A.; Tiwari, B.K.; Tuohy, M.; Brunton, N. Impact of frozen storage on polyacetylene content, texture and colour in carrots disks. J. Food Eng. 2012, 108, 563–569. [Google Scholar] [CrossRef]
- Kramer, M.; Bufler, G.; Nothnagel, T.; Carle, R.; Kammerer, D.R. Effects of cultivation conditions and cold storage on the polyacetylene contents of carrot (Daucus carota L.) and parsnip (Pastinaca sativa L.). J. Hortic. Sci. Biotechnol. 2012, 87, 101–106. [Google Scholar] [CrossRef]
- Rawson, A.; Tiwari, B.K.; Tuohy, M.G.; O’Donnell, C.P.; Brunton, N. Effect of ultrasound and blanching pretreatments on polyacetylene and carotenoid content of hot air and freeze dried carrot discs. Ultrason. Sonochem. 2011, 18, 1172–1179. [Google Scholar] [CrossRef] [PubMed]
- Koidis, A.; Rawson, A.; Tuohy, M.; Brunton, N. Influence of unit operations on the levels of polyacetylenes in minimally processed carrots and parsnips: An industrial trial. Food Chem. 2012, 132, 1406–1412. [Google Scholar] [CrossRef] [PubMed]
- Baranska, M.; Roman, M.; Dobrowolski, J.; Schulz, H.; Baranski, R. Recent Advances in Raman Analysis of Plants: Alkaloids, Carotenoids, and Polyacetylenes. Curr. Anal. Chem. 2013, 9, 108–127. [Google Scholar] [CrossRef]
- Purup, S.; Larsen, E.; Christensen, L.P. Differential Effects of Falcarinol and Related Aliphatic C17-Polyacetylenes on Intestinal Cell Proliferation. J. Agric. Food Chem. 2009, 57, 8290–8296. [Google Scholar] [CrossRef]
- Christensen, L.P.; Brandt, K. Bioactive polyacetylenes in food plants of the Apiaceae family: Occurrence, bioactivity and analysis. J. Pharm. Biomed. Anal. 2006, 41, 683–693. [Google Scholar] [CrossRef]
- Tan, K.W.; Killeen, D.P.; Li, Y.; Paxton, J.W.; Birch, N.P.; Scheepens, A. Molecular and cellular pharmacology Dietary polyacetylenes of the falcarinol type are inhibitors of breast cancer resistance protein (BCRP/ABCG2). Eur. J. Pharmacol. 2014, 723, 346–352. [Google Scholar] [CrossRef]
- Kjellenberg, L.; Johansson, E.; Gustavsson, K.-E.; Olsson, M.E. Polyacetylenes in fresh and stored carrots (Daucus carota): Relations to root morphology and sugar content. J. Sci. Food Agric. 2012, 92, 1748–1754. [Google Scholar] [CrossRef] [PubMed]
- El-Houri, R.B.; Kotowska, D.; Christensen, K.B.; Bhattacharya, S.; Oksbjerg, N.; Wolber, G.; Kristiansen, K.; Christensen, L.P. Polyacetylenes from carrots (Daucus carota) improve glucose uptake in vitro in adipocytes and myotubes. Food Funct. 2015, 6, 2135–2144. [Google Scholar] [CrossRef] [PubMed]
- Zaini, R.; Clench, M.R.; Le Maitre, C.L. Bioactive Chemicals from Carrot (Daucus carota) Juice Extracts for the Treatment of Leukemia. J. Med. Food 2011, 14, 1303–1312. [Google Scholar] [CrossRef] [PubMed]
- Zaini, R.G.; Brandt, K.; Clench, M.R.; Le Maitre, C.L. Effects of bioactive compounds from carrots (Daucus carota L.), polyacetylenes, beta-carotene and lutein on human lymphoid leukaemia cells. Anticancer. Agents Med. Chem. 2012, 12, 640–652. [Google Scholar] [CrossRef] [PubMed]
- Metzger, B.T.; Barnes, D.M.; Reed, J.D. Purple carrot (Daucus carota L.) polyacetylenes decrease lipopolysaccharide-induced expression of inflammatory proteins in macrophage and endothelial cells. J. Agric. Food Chem. 2008, 56, 3554–3560. [Google Scholar] [CrossRef]
- Fotopoulos, V.; Kanellis, A.K. Altered apoplastic ascorbate redox state in tobacco plants via ascorbate oxidase overexpression results in delayed dark-induced senescence in detached leaves. Plant. Physiol. Biochem. 2013, 73, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Gest, N.; Gautier, H.; Stevens, R. Ascorbate as seen through plant evolution: The rise of a successful molecule? J. Exp. Bot. 2013, 64, 33–53. [Google Scholar] [CrossRef]
- Troesch, B.; Hoeft, B.; McBurney, M.; Eggersdorfer, M.; Weber, P. Dietary surveys indicate vitamin intakes below recommendations are common in representative Western countries. Br. J. Nutr. 2012, 108, 692–698. [Google Scholar] [CrossRef]
- Mellidou, I.; Chagné, D.; Laing, W.A.; Keulemans, J.; Davey, M.W. Allelic variation in paralogs of GDP-L-galactose phosphorylase is a major determinant of vitamin C concentrations in apple fruit. Plant. Physiol. 2012. [Google Scholar] [CrossRef]
- Mellidou, I.; Keulemans, J.; Kanellis, A.K.; Davey, M.W. Regulation of fruit ascorbic acid concentrations during ripening in high and low vitamin C tomato cultivars. BMC Plant. Biol. 2012, 12, 239. [Google Scholar] [CrossRef]
- Bulley, S.M.; Rassam, M.; Hoser, D.; Otto, W.; Schünemann, N.; Wright, M.; MacRae, E.; Gleave, A.; Laing, W. Gene expression studies in kiwifruit and gene over-expression in Arabidopsis indicates that GDP-L-galactose guanyltransferase is a major control point of vitamin C biosynthesis. J. Exp. Bot. 2009, 60, 765–778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.K.; Kader, A.A. Preharvest and postharvest factors influencing vitamin C content of horticultural crops. Postharvest Biol. Technol. 2000, 20, 207–220. [Google Scholar] [CrossRef] [Green Version]
- Lachman, J.; Orsák, M.; Pivec, V. Antioxidant contents and composition in some vegetables and their role in human nutrition. Zahrad. Horticul. Sci. 2000, 27, 65–78. [Google Scholar]
- Pokluda, R. An assessment of the nutritional value of vegetables using an ascorbate-nitrate index. Veg. Crop. Res. Bull. 2006, 64, 29–37. [Google Scholar]
- Matějková, J.; Petříková, K. Variation in Content of Carotenoids and Vitamin C in Carrots. Not. Sci. Biol. 2010, 2, 88–91. [Google Scholar] [CrossRef] [Green Version]
- Smirnoff, N.; Wheeler, G.L. Ascorbic Acid in Plants: Biosynthesis and Function. Crit. Rev. Biochem. Mol. Biol. 2000, 35, 291–314. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.-L.; Xu, Z.-S.; Wang, F.; Li, M.-Y.; Tan, G.-F.; Xiong, A.-S. Regulation of ascorbic acid biosynthesis and recycling during root development in carrot (Daucus carota L.). Plant. Physiol. Biochem. 2015, 94, 10–18. [Google Scholar] [CrossRef]
- Leong, S.Y.; Oey, I. Effects of processing on anthocyanins, carotenoids and vitamin C in summer fruits and vegetables. Food Chem. 2012, 133, 1577–1587. [Google Scholar] [CrossRef]
- Leong, S.Y.; Oey, I. Effect of pulsed electric field treatment on enzyme kinetics and thermostability of endogenous ascorbic acid oxidase in carrots (Daucus carota cv. Nantes). Food Chem. 2014, 146, 538–547. [Google Scholar] [CrossRef]
- Leong, S.Y.; Oey, I.; Burritt, D.J. A Novel Strategy Using Pulsed Electric Fields to Modify the Thermostability of Ascorbic Acid Oxidase in Different Carrot Cultivars. Food Bioprocess. Technol. 2015, 8, 811–823. [Google Scholar] [CrossRef]
- Wu, X.-J.; Sun, S.; Xing, G.-M.; Wang, G.-L.; Wang, F.; Xu, Z.-S.; Tian, Y.-S.; Hou, X.-L.; Xiong, A.-S. Elevated Carbon Dioxide Altered Morphological and Anatomical Characteristics, Ascorbic Acid Accumulation, and Related Gene Expression during Taproot Development in Carrots. Front. Plant. Sci. 2017, 7, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Cortés, C.; Esteve, M.J.; Frígola, A.; Torregrosa, F. Changes in carotenoids including geometrical isomers and ascorbic acid content in orange–carrot juice during frozen storage. Eur. Food Res. Technol. 2005, 221, 125–131. [Google Scholar] [CrossRef]
- Singh, G.; Kawatra, A.; Sehgal, S. Nutritional composition of selected green leafy vegetables, herbs and carrots. Plant. Foods Hum. Nutr. 2001, 56, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Aggarwal, P. Effect of thermal processing and chemical preservatives on the physicochemical and phytochemical parameters of carrot juice. Asian J. Dairy Food Res. 2016, 35, 71–75. [Google Scholar] [CrossRef]
- Gamboa-Santos, J.; Cristina Soria, A.; Pérez-Mateos, M.; Carrasco, J.A.; Montilla, A.; Villamiel, M. Vitamin C content and sensorial properties of dehydrated carrots blanched conventionally or by ultrasound. Food Chem. 2013, 136, 782–788. [Google Scholar] [CrossRef] [Green Version]
- Yan, W.-Q.; Zhang, M.; Huang, L.-L.; Tang, J.; Mujumdar, A.S.; Sun, J.-C. Studies on different combined microwave drying of carrot pieces. Int. J. Food Sci. Technol. 2010, 45, 2141–2148. [Google Scholar] [CrossRef]
- Vishwanathan, K.H.; Giwari, G.K.; Hebbar, H.U. Infrared assisted dry-blanching and hybrid drying of carrot. Food Bioprod. Process. 2013, 91, 89–94. [Google Scholar] [CrossRef]
- Duarte, T.L.; Lunec, J. Review: When is an antioxidant not an antioxidant? A review of novel actions and reactions of vitamin C. Free Radic. Res. 2005, 39, 671–686. [Google Scholar] [CrossRef]
- Li, Y.; Schellhorn, H.E. New developments and novel therapeutic perspectives for vitamin C. J. Nutr. 2007, 137, 2171–2184. [Google Scholar] [CrossRef]
- Harrison, F.E.; May, J.M. Vitamin C function in the brain: Vital role of the ascorbate transporter SVCT2. Free Radic. Biol. Med. 2009, 46, 719–730. [Google Scholar] [CrossRef]
- Du, J.; Cullen, J.J.; Buettner, G.R. Ascorbic acid: Chemistry, biology and the treatment of cancer. Biochim. Biophys. Acta Rev. Cancer 2012, 1826, 443–457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Da Silva Dias, J.C. Nutritional and Health Benefits of Carrots and Their Seed Extracts. Food Nutr. Sci. 2014, 5, 2147–2156. [Google Scholar] [Green Version]
- Clementz, A.; Torresi, P.A.; Molli, J.S.; Cardell, D.; Mammarella, E.; Yori, J.C. Novel method for valorization of by-products from carrot discards. LWT Food Sci. Technol. 2019, 100, 374–380. [Google Scholar] [CrossRef]
- Kumar, M.; Dahuja, A.; Sachdev, A.; Kaur, C.; Varghese, E.; Saha, S.; Sairam, K.V.S.S. Valorisation of black carrot pomace: Microwave assisted extraction of bioactive phytoceuticals and antioxidant activity using Box–Behnken design. J. Food Sci. Technol. 2019, 56, 995–1007. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).