A Novel Anti-Inflammatory Role of Omega-3 PUFAs in Prevention and Treatment of Atherosclerosis and Vascular Cognitive Impairment and Dementia
Abstract
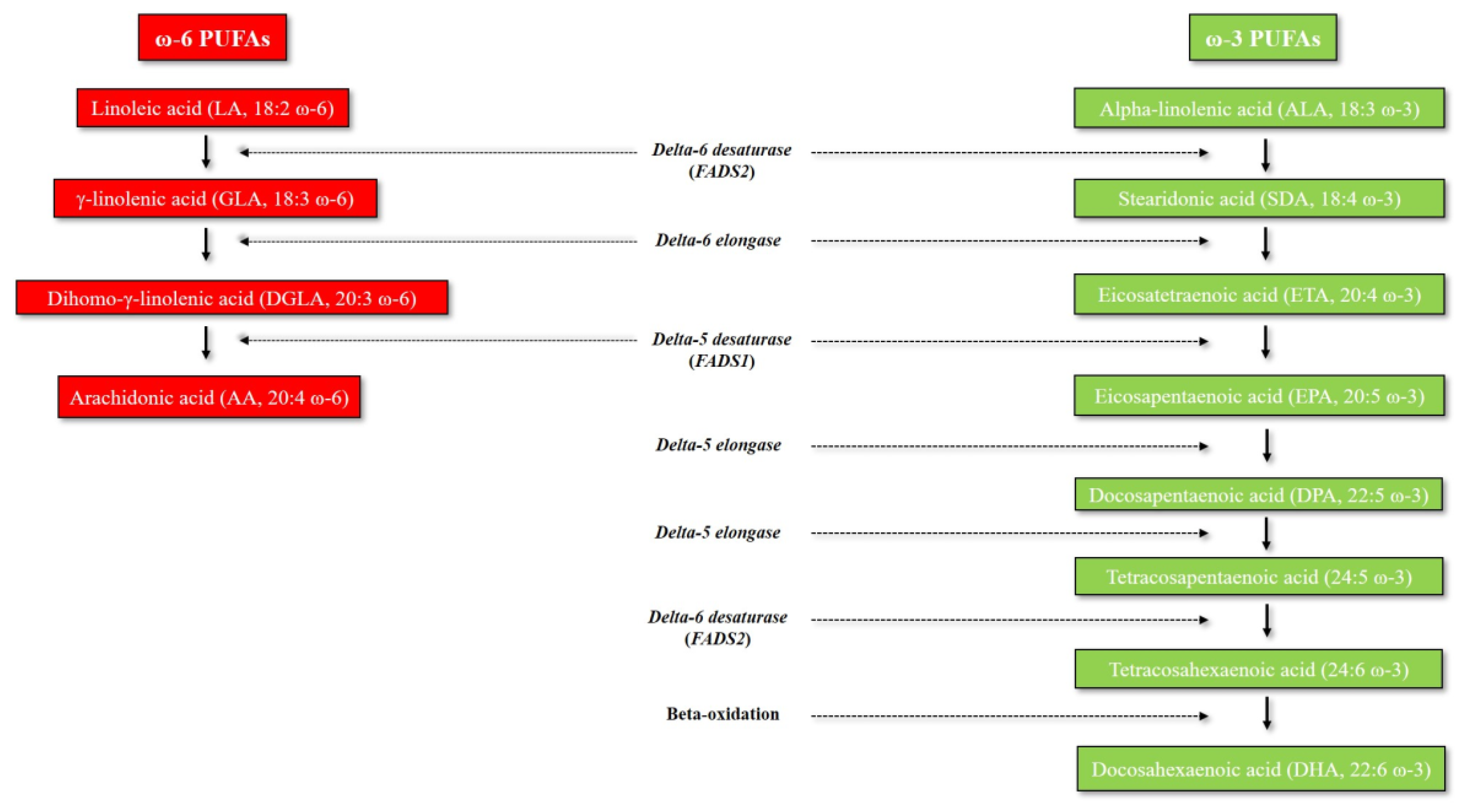
:1. Introduction to Omega-3 and Omega-6 PUFAs: An Overview of Their Metabolic Pathways
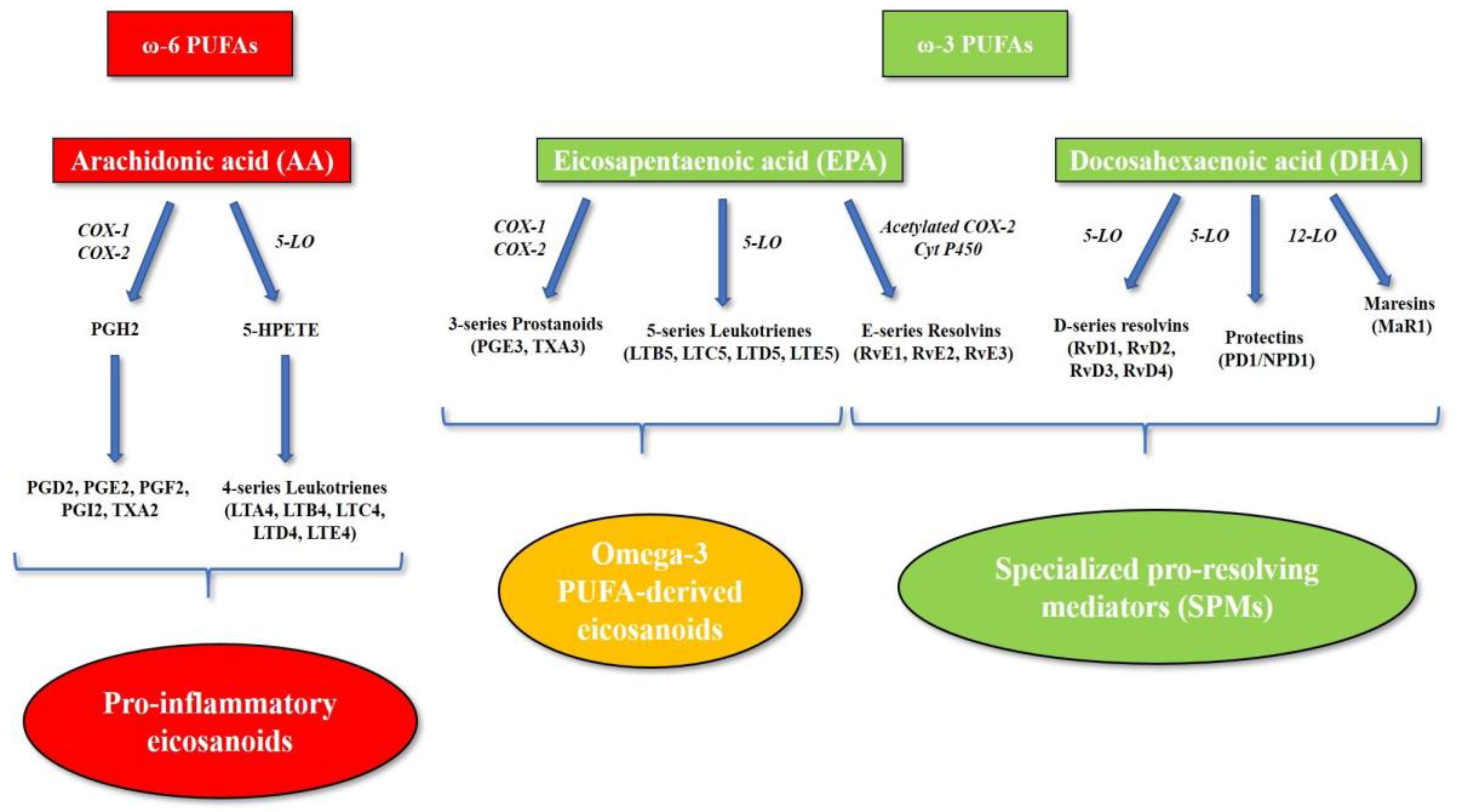
2. Role of Omega-3 and Omega-6 PUFAs in Systemic Inflammation
3. Pathogenesis of Atherosclerosis and Vascular Cognitive Impairment and Dementia: Role of Inflammation
4. The Role of Omega-3 and Omega-6 PUFAs in Atherosclerosis, Cardiovascular Disease and Vascular Inflammation
5. The Role of Linoleic Acid (LA) in Atherosclerosis and Cardiovascular Disease: Evidence and Controversies
6. Omega-3 PUFA Supplementation for Primary and Secondary Prevention of Atherosclerosis and Cardiovascular Disease: Lessons from Clinical Trials
7. Role of Omega-3 and Omega-6 PUFAs in Neuronal Cells
8. Role of Omega-3 and Omega-6 PUFAs in Alzheimer’s Disease and Vascular Cognitive Impairment and Dementia
9. Omega-3 PUFA Supplementation for Prevention of Alzheimer’s Disease and Vascular Cognitive Impairment and Dementia
10. Novel Personalized Strategies to Prevent Atherosclerosis and Vascular Cognitive Impairment and Dementia: Focus on AA/EPA Ratio and Omega-3 PUFAs
11. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Khandelwal, S.; Kelly, L.; Malik, R.; Prabhakaran, D.; Reddy, S. Impact of omega-6 fatty acids on cardiovascular outcomes: A review. J. Prev. Cardiol. 2013, 2, 325–336. [Google Scholar]
- Ander, B.P.; Dupasquier, C.M.; Prociuk, M.A.; Pierce, G.N. Polyunsaturated fatty acids and their effects on cardiovascular disease. Exp. Clin. Cardiol. 2003, 8, 164–172. [Google Scholar] [PubMed]
- Tortosa-Caparrós, E.; Navas-Carrillo, D.; Marín, F.; Orenes-Piñero, E. Anti-inflammatory effects of omega 3 and omega 6 polyunsaturated fatty acids in cardiovascular disease and metabolic syndrome. Crit. Rev. Food Sci. Nutr. 2017, 57, 3421–3429. [Google Scholar] [CrossRef] [PubMed]
- Schunck, W.H.; Konkel, A.; Fischer, R.; Weylandt, K.H. Therapeutic potential of omega-3 fatty acid-derived epoxyeicosanoids in cardiovascular and inflammatory diseases. Pharmacol. Ther. 2018, 183, 177–204. [Google Scholar] [CrossRef]
- Calder, P.C.; Yaqoob, P. Understanding omega-3 polyunsaturated fatty acids. Postgrad. Med. 2009, 121, 148–157. [Google Scholar] [CrossRef]
- Libby, P.; Ridker, P.M. Novel inflammatory markers of coronary risk: Theory versus practice. Circulation 1999, 100, 1148–1150. [Google Scholar] [CrossRef]
- Lanzmann-Petithory, D. Alpha-linolenic acid and cardiovascular diseases. J. Nutr. Health Aging 2001, 5, 179–183. [Google Scholar]
- Abedi, E.; Sahari, M.A. Long-chain polyunsaturated fatty acid sources and evaluation of their nutritional and functional properties. Food Sci. Nutr. 2014, 2, 443–463. [Google Scholar] [CrossRef]
- Tosi, F.; Sartori, F.; Guarini, P.; Olivieri, O.; Martinelli, N. Delta-5 and delta-6 desaturases: Crucial enzymes in polyunsaturated fatty acid-related pathways with pleiotropic influences in health and disease. Adv. Exp. Med. Biol. 2014, 824, 61–81. [Google Scholar] [CrossRef]
- Flower, R.J. Prostaglandins, bioassay and inflammation. Br. J. Pharmacol. 2006, 147 (Suppl. 1), S182–S192. [Google Scholar] [CrossRef]
- Samuelsson, B.; Dahlén, S.E.; Lindgren, J.A.; Rouzer, C.A.; Serhan, C.N. Leukotrienes and lipoxins: Structures, biosynthesis, and biological effects. Science 1987, 237, 1171–1176. [Google Scholar] [CrossRef]
- Funk, C.D. Prostaglandins and leukotrienes: Advances in eicosanoid biology. Science 2001, 294, 1871–1875. [Google Scholar] [CrossRef]
- Powell, W.S.; Rokach, J. Biosynthesis, biological effects, and receptors of hydroxyeicosatetraenoic acids (HETEs) and oxoeicosatetraenoic acids (oxo-ETEs) derived from arachidonic acid. Biochim. Biophys. Acta 2015, 1851, 340–355. [Google Scholar] [CrossRef]
- Fischer, S.; Weber, P.C. Thromboxane A3 (TXA3) is formed in human platelets after dietary eicosapentaenoic acid (C20:5 omega 3). Biochem. Biophys. Res. Commun. 1983, 116, 1091–1099. [Google Scholar] [CrossRef]
- Heidel, J.R.; Taylor, S.M.; Laegreid, W.W.; Silflow, R.M.; Liggitt, H.D.; Leid, R.W. In vivo chemotaxis of bovine neutrophils induced by 5-lipoxygenase metabolites of arachidonic and eicosapentaenoic acid. Am. J. Pathol. 1989, 134, 671–676. [Google Scholar]
- Hawkes, J.S.; James, M.J.; Cleland, L.G. Biological activity of prostaglandin E3 with regard to oedema formation in mice. Agents Actions 1992, 35, 85–87. [Google Scholar] [CrossRef]
- Moreno, J.J. Differential effects of arachidonic and eicosapentaenoic Acid-derived eicosanoids on polymorphonuclear transmigration across endothelial cell cultures. J. Pharmacol. Exp. Ther. 2009, 331, 1111–1117. [Google Scholar] [CrossRef]
- Serhan, C.N. Pro-resolving lipid mediators are leads for resolution physiology. Nature 2014, 510, 92–101. [Google Scholar] [CrossRef] [Green Version]
- Serhan, C.N.; Chiang, N. Resolution phase lipid mediators of inflammation: Agonists of resolution. Curr. Opin. Pharmacol. 2013, 13, 632–640. [Google Scholar] [CrossRef]
- Spite, M.; Clària, J.; Serhan, C.N. Resolvins, specialized proresolving lipid mediators, and their potential roles in metabolic diseases. Cell Metab. 2014, 19, 21–36. [Google Scholar] [CrossRef]
- Simopoulos, A.P. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother. 2002, 56, 365–379. [Google Scholar] [CrossRef]
- Simopoulos, A.P. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp. Biol. Med. 2008, 233, 674–688. [Google Scholar] [CrossRef]
- Simopoulos, A.P. An Increase in the Omega-6/Omega-3 Fatty Acid Ratio Increases the Risk for Obesity. Nutrients 2016, 8, 128. [Google Scholar] [CrossRef]
- Simopoulos, A.P. Evolutionary aspects of diet, the omega-6/omega-3 ratio and genetic variation: Nutritional implications for chronic diseases. Biomed. Pharmacother. 2006, 60, 502–507. [Google Scholar] [CrossRef]
- Kuipers, R.S.; Luxwolda, M.F.; Dijck-Brouwer, D.A.; Eaton, S.B.; Crawford, M.A.; Cordain, L.; Muskiet, F.A. Estimated macronutrient and fatty acid intakes from an East African Paleolithic diet. Br. J. Nutr. 2010, 104, 1666–1687. [Google Scholar] [CrossRef] [Green Version]
- Burdge, G.C.; Calder, P.C. Dietary alpha-linolenic acid and health-related outcomes: A metabolic perspective. Nutr. Res. Rev. 2006, 19, 26–52. [Google Scholar] [CrossRef]
- De Gŏmez Dumm, I.N.; Brenner, R.R. Oxidative desaturation of alpha-linoleic, linoleic, and stearic acids by human liver microsomes. Lipids 1975, 10, 315–317. [Google Scholar] [CrossRef]
- Hagve, T.A.; Christophersen, B.O. Evidence for peroxisomal retroconversion of adrenic acid (22:4(n-6)) and docosahexaenoic acids (22:6(n-3)) in isolated liver cells. Biochim. Biophys. Acta 1986, 875, 165–173. [Google Scholar] [CrossRef]
- Hagve, T.A.; Christophersen, B.O. Effect of dietary fats on arachidonic acid and eicosapentaenoic acid biosynthesis and conversion to C22 fatty acids in isolated rat liver cells. Biochim. Biophys. Acta 1984, 796, 205–217. [Google Scholar] [CrossRef]
- DiNicolantonio, J.J.; O’Keefe, J.H. Importance of maintaining a low omega-6/omega-3 ratio for reducing inflammation. Open Heart 2018, 5, e000946. [Google Scholar] [CrossRef]
- Alber, J.; Alladi, S.; Bae, H.J.; Barton, D.A.; Beckett, L.A.; Bell, J.M.; Berman, S.E.; Biessels, G.J.; Black, S.E.; Bos, I.; et al. White matter hyperintensities in vascular contributions to cognitive impairment and dementia (VCID): Knowledge gaps and opportunities. Alzheimers Dement. 2019, 5, 107–117. [Google Scholar] [CrossRef]
- Corriveau, R.A.; Koroshetz, W.J.; Gladman, J.T.; Jeon, S.; Babcock, D.; Bennett, D.A.; Carmichael, S.T.; Dickinson, S.L.; Dickson, D.W.; Emr, M.; et al. Alzheimer’s Disease-Related Dementias Summit 2016: National research priorities. Neurology 2017, 89, 2381–2391. [Google Scholar] [CrossRef]
- Hansson, G.K. Inflammation, atherosclerosis, and coronary artery disease. N. Engl. J. Med. 2005, 352, 1685–1695. [Google Scholar] [CrossRef]
- Libby, P. Inflammation in atherosclerosis. Arterioscler Thromb. Vasc. Biol. 2012, 32, 2045–2051. [Google Scholar] [CrossRef]
- Ridker, P.M.; Cushman, M.; Stampfer, M.J.; Tracy, R.P.; Hennekens, C.H. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N. Engl. J. Med. 1997, 336, 973–979. [Google Scholar] [CrossRef]
- Ridker, P.M.; Hennekens, C.H.; Buring, J.E.; Rifai, N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N. Engl. J. Med. 2000, 342, 836–843. [Google Scholar] [CrossRef]
- Lusis, A.J. Atherosclerosis. Nature 2000, 407, 233–241. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; de Ferranti, S.; Després, J.P.; Fullerton, H.J.; Howard, V.J.; et al. Heart disease and stroke statistics--2015 update: A report from the American Heart Association. Circulation 2015, 131, e29–e322. [Google Scholar] [CrossRef]
- Hansson, G.K.; Libby, P. The immune response in atherosclerosis: A double-edged sword. Nat. Rev. Immunol. 2006, 6, 508–519. [Google Scholar] [CrossRef]
- Libby, P. Inflammation in atherosclerosis. Nature 2002, 420, 868–874. [Google Scholar] [CrossRef]
- Williams, K.J.; Tabas, I. The response-to-retention hypothesis of early atherogenesis. Arterioscler. Thromb. Vasc. Biol. 1995, 15, 551–561. [Google Scholar] [CrossRef]
- Tabas, I.; García-Cardeña, G.; Owens, G.K. Recent insights into the cellular biology of atherosclerosis. J. Cell Biol. 2015, 209, 13–22. [Google Scholar] [CrossRef]
- Hansson, G.K.; Hermansson, A. The immune system in atherosclerosis. Nat. Immunol. 2011, 12, 204–212. [Google Scholar] [CrossRef]
- Spann, N.J.; Garmire, L.X.; McDonald, J.G.; Myers, D.S.; Milne, S.B.; Shibata, N.; Reichart, D.; Fox, J.N.; Shaked, I.; Heudobler, D.; et al. Regulated accumulation of desmosterol integrates macrophage lipid metabolism and inflammatory responses. Cell 2012, 151, 138–152. [Google Scholar] [CrossRef]
- Crowther, M.A. Pathogenesis of atherosclerosis. Hematology Am Soc Hematol Educ Program. 2005, 436–441. [Google Scholar] [CrossRef]
- Glagov, S.; Weisenberg, E.; Zarins, C.K.; Stankunavicius, R.; Kolettis, G.J. Compensatory enlargement of human atherosclerotic coronary arteries. N. Engl. J. Med. 1987, 316, 1371–1375. [Google Scholar] [CrossRef]
- Bennett, M.R.; Sinha, S.; Owens, G.K. Vascular Smooth Muscle Cells in Atherosclerosis. Circ. Res. 2016, 118, 692–702. [Google Scholar] [CrossRef]
- Geovanini, G.R.; Libby, P. Atherosclerosis and inflammation: Overview and updates. Clin. Sci. 2018, 132, 1243–1252. [Google Scholar] [CrossRef]
- Packard, R.R.; Libby, P. Inflammation in atherosclerosis: From vascular biology to biomarker discovery and risk prediction. Clin. Chem. 2008, 54, 24–38. [Google Scholar] [CrossRef]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef]
- Everett, B.M.; Pradhan, A.D.; Solomon, D.H.; Paynter, N.; Macfadyen, J.; Zaharris, E.; Gupta, M.; Clearfield, M.; Libby, P.; Hasan, A.A.; et al. Rationale and design of the Cardiovascular Inflammation Reduction Trial: A test of the inflammatory hypothesis of atherothrombosis. Am. Heart J. 2013, 166, 199–207.e15. [Google Scholar] [CrossRef] [Green Version]
- Bäck, M.; Hansson, G.K. Omega-3 fatty acids, cardiovascular risk, and the resolution of inflammation. FASEB J. 2019, 33, 1536–1539. [Google Scholar] [CrossRef]
- Fredman, G.; Hellmann, J.; Proto, J.D.; Kuriakose, G.; Colas, R.A.; Dorweiler, B.; Connolly, E.S.; Solomon, R.; Jones, D.M.; Heyer, E.J.; et al. An imbalance between specialized pro-resolving lipid mediators and pro-inflammatory leukotrienes promotes instability of atherosclerotic plaques. Nat. Commun. 2016, 7, 12859. [Google Scholar] [CrossRef]
- Thul, S.; Labat, C.; Temmar, M.; Benetos, A.; Bäck, M. Low salivary resolvin D1 to leukotriene B. Eur. J. Prev. Cardiol. 2017, 24, 903–906. [Google Scholar] [CrossRef]
- Laguna-Fernandez, A.; Checa, A.; Carracedo, M.; Artiach, G.; Petri, M.H.; Baumgartner, R.; Forteza, M.J.; Jiang, X.; Andonova, T.; Walker, M.E.; et al. ERV1/ChemR23 Signaling Protects Against Atherosclerosis by Modifying Oxidized Low-Density Lipoprotein Uptake and Phagocytosis in Macrophages. Circulation 2018, 138, 1693–1705. [Google Scholar] [CrossRef]
- Liu, G.; Gong, Y.; Zhang, R.; Piao, L.; Li, X.; Liu, Q.; Yan, S.; Shen, Y.; Guo, S.; Zhu, M.; et al. Resolvin E1 attenuates injury-induced vascular neointimal formation by inhibition of inflammatory responses and vascular smooth muscle cell migration. FASEB J. 2018, 32, 5413–5425. [Google Scholar] [CrossRef]
- Yamano, T.; Kubo, T.; Shiono, Y.; Shimamura, K.; Orii, M.; Tanimoto, T.; Matsuo, Y.; Ino, Y.; Kitabata, H.; Yamaguchi, T.; et al. Impact of eicosapentaenoic acid treatment on the fibrous cap thickness in patients with coronary atherosclerotic plaque: An optical coherence tomography study. J. Atheroscler. Thromb. 2015, 22, 52–61. [Google Scholar] [CrossRef]
- Umemoto, N.; Ishii, H.; Kamoi, D.; Aoyama, T.; Sakakibara, T.; Takahashi, H.; Tanaka, A.; Yasuda, Y.; Suzuki, S.; Matsubara, T.; et al. Reverse association of omega-3/omega-6 polyunsaturated fatty acids ratios with carotid atherosclerosis in patients on hemodialysis. Atherosclerosis 2016, 249, 65–69. [Google Scholar] [CrossRef]
- Nagahara, Y.; Motoyama, S.; Sarai, M.; Ito, H.; Kawai, H.; Takakuwa, Y.; Miyagi, M.; Shibata, D.; Takahashi, H.; Naruse, H.; et al. Eicosapentaenoic acid to arachidonic acid (EPA/AA) ratio as an associated factor of high risk plaque on coronary computed tomography in patients without coronary artery disease. Atherosclerosis 2016, 250, 30–37. [Google Scholar] [CrossRef]
- Farzaneh-Far, R.; Harris, W.S.; Garg, S.; Na, B.; Whooley, M.A. Inverse association of erythrocyte n-3 fatty acid levels with inflammatory biomarkers in patients with stable coronary artery disease: The Heart and Soul Study. Atherosclerosis 2009, 205, 538–543. [Google Scholar] [CrossRef] [Green Version]
- Grenon, S.M.; Conte, M.S.; Nosova, E.; Alley, H.; Chong, K.; Harris, W.S.; Vittinghoff, E.; Owens, C.D. Association between n-3 polyunsaturated fatty acid content of red blood cells and inflammatory biomarkers in patients with peripheral artery disease. J. Vasc. Surg. 2013, 58, 1283–1290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Massaro, M.; Habib, A.; Lubrano, L.; Del Turco, S.; Lazzerini, G.; Bourcier, T.; Weksler, B.B.; De Caterina, R. The omega-3 fatty acid docosahexaenoate attenuates endothelial cyclooxygenase-2 induction through both NADP(H) oxidase and PKC epsilon inhibition. Proc. Natl. Acad. Sci. USA 2006, 103, 15184–15189. [Google Scholar] [CrossRef] [PubMed]
- Schönbeck, U.; Sukhova, G.K.; Graber, P.; Coulter, S.; Libby, P. Augmented expression of cyclooxygenase-2 in human atherosclerotic lesions. Am. J. Pathol. 1999, 155, 1281–1291. [Google Scholar] [CrossRef]
- Wang, S.; Wu, D.; Matthan, N.R.; Lamon-Fava, S.; Lecker, J.L.; Lichtenstein, A.H. Reduction in dietary omega-6 polyunsaturated fatty acids: Eicosapentaenoic acid plus docosahexaenoic acid ratio minimizes atherosclerotic lesion formation and inflammatory response in the LDL receptor null mouse. Atherosclerosis 2009, 204, 147–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, L.G.; Cao, J.; Mao, Q.X.; Lu, X.C.; Zhou, X.L.; Fan, L. Influence of omega-3 polyunsaturated fatty acid-supplementation on platelet aggregation in humans: A meta-analysis of randomized controlled trials. Atherosclerosis 2013, 226, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Freese, R.; Mutanen, M.; Valsta, L.M.; Salminen, I. Comparison of the effects of two diets rich in monounsaturated fatty acids differing in their linoleic/alpha-linolenic acid ratio on platelet aggregation. Thromb. Haemost. 1994, 71, 73–77. [Google Scholar]
- Von Schacky, C. Prophylaxis of atherosclerosis with marine omega-3 fatty acids. A comprehensive strategy. Ann. Intern. Med. 1987, 107, 890–899. [Google Scholar] [CrossRef]
- Chowdhury, R.; Warnakula, S.; Kunutsor, S.; Crowe, F.; Ward, H.A.; Johnson, L.; Franco, O.H.; Butterworth, A.S.; Forouhi, N.G.; Thompson, S.G.; et al. Association of dietary, circulating, and supplement fatty acids with coronary risk: A systematic review and meta-analysis. Ann. Intern. Med. 2014, 160, 398–406. [Google Scholar] [CrossRef]
- Stanley, J.C.; Elsom, R.L.; Calder, P.C.; Griffin, B.A.; Harris, W.S.; Jebb, S.A.; Lovegrove, J.A.; Moore, C.S.; Riemersma, R.A.; Sanders, T.A. UK Food Standards Agency Workshop Report: The effects of the dietary n-6:n-3 fatty acid ratio on cardiovascular health. Br. J. Nutr. 2007, 98, 1305–1310. [Google Scholar] [CrossRef]
- Harris, W.S.; Poston, W.C.; Haddock, C.K. Tissue n-3 and n-6 fatty acids and risk for coronary heart disease events. Atherosclerosis 2007, 193, 1–10. [Google Scholar] [CrossRef]
- Harris, W.S.; Assaad, B.; Poston, W.C. Tissue omega-6/omega-3 fatty acid ratio and risk for coronary artery disease. Am. J. Cardiol. 2006, 98, 19i–26i. [Google Scholar] [CrossRef] [PubMed]
- Sanders, T.A.; Lewis, F.; Slaughter, S.; Griffin, B.A.; Griffin, M.; Davies, I.; Millward, D.J.; Cooper, J.A.; Miller, G.J. Effect of varying the ratio of n-6 to n-3 fatty acids by increasing the dietary intake of alpha-linolenic acid, eicosapentaenoic and docosahexaenoic acid, or both on fibrinogen and clotting factors VII and XII in persons aged 45–70 y: The OPTILIP study. Am. J. Clin. Nutr. 2006, 84, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Griffin, M.D.; Sanders, T.A.; Davies, I.G.; Morgan, L.M.; Millward, D.J.; Lewis, F.; Slaughter, S.; Cooper, J.A.; Miller, G.J.; Griffin, B.A. Effects of altering the ratio of dietary n-6 to n-3 fatty acids on insulin sensitivity, lipoprotein size, and postprandial lipemia in men and postmenopausal women aged 45–70 y: The OPTILIP Study. Am. J. Clin. Nutr. 2006, 84, 1290–1298. [Google Scholar] [CrossRef] [PubMed]
- Goyens, P.L.; Spilker, M.E.; Zock, P.L.; Katan, M.B.; Mensink, R.P. Conversion of alpha-linolenic acid in humans is influenced by the absolute amounts of alpha-linolenic acid and linoleic acid in the diet and not by their ratio. Am. J. Clin. Nutr. 2006, 84, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Griffin, B.A. How relevant is the ratio of dietary n-6 to n-3 polyunsaturated fatty acids to cardiovascular disease risk? Evidence from the OPTILIP study. Curr. Opin. Lipidol. 2008, 19, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Harris, W.S.; Von Schacky, C. The Omega-3 Index: A new risk factor for death from coronary heart disease? Prev. Med. 2004, 39, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Von Schacky, C. Omega-3 index and cardiovascular health. Nutrients 2014, 6, 799–814. [Google Scholar] [CrossRef] [PubMed]
- Harris, W.S.; Tintle, N.L.; Etherton, M.R.; Vasan, R.S. Erythrocyte long-chain omega-3 fatty acid levels are inversely associated with mortality and with incident cardiovascular disease: The Framingham Heart Study. J. Clin. Lipidol. 2018, 12, 718–727.e716. [Google Scholar] [CrossRef] [PubMed]
- Guyenet, S.J.; Carlson, S.E. Increase in adipose tissue linoleic acid of US adults in the last half century. Adv. Nutr. 2015, 6, 660–664. [Google Scholar] [CrossRef]
- Hodgson, J.M.; Wahlqvist, M.L.; Boxall, J.A.; Balazs, N.D. Can linoleic acid contribute to coronary artery disease? Am. J. Clin. Nutr. 1993, 58, 228–234. [Google Scholar] [CrossRef]
- Marchix, J.; Choque, B.; Kouba, M.; Fautrel, A.; Catheline, D.; Legrand, P. Excessive dietary linoleic acid induces proinflammatory markers in rats. J. Nutr. Biochem. 2015, 26, 1434–1441. [Google Scholar] [CrossRef] [PubMed]
- Hennig, B.; Shasby, D.M.; Spector, A.A. Exposure to fatty acid increases human low density lipoprotein transfer across cultured endothelial monolayers. Circ. Res. 1985, 57, 776–780. [Google Scholar] [CrossRef] [PubMed]
- Schwertner, H.A.; Mosser, E.L. Comparison of lipid fatty acids on a concentration basis vs weight percentage basis in patients with and without coronary artery disease or diabetes. Clin. Chem. 1993, 39, 659–663. [Google Scholar] [PubMed]
- Jira, W.; Spiteller, G.; Carson, W.; Schramm, A. Strong increase in hydroxy fatty acids derived from linoleic acid in human low density lipoproteins of atherosclerotic patients. Chem. Phys. Lipids 1998, 91, 1–11. [Google Scholar] [CrossRef]
- Reaven, P.; Parthasarathy, S.; Grasse, B.J.; Miller, E.; Steinberg, D.; Witztum, J.L. Effects of oleate-rich and linoleate-rich diets on the susceptibility of low density lipoprotein to oxidative modification in mildly hypercholesterolemic subjects. J. Clin. Investig. 1993, 91, 668–676. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, D. The LDL modification hypothesis of atherogenesis: An update. J. Lipid Res. 2009, 50, S376–S381. [Google Scholar] [CrossRef] [PubMed]
- Parthasarathy, S.; Litvinov, D.; Selvarajan, K.; Garelnabi, M. Lipid peroxidation and decomposition--conflicting roles in plaque vulnerability and stability. Biochim. Biophys. Acta 2008, 1781, 221–231. [Google Scholar] [CrossRef]
- DiNicolantonio, J.J.; O’Keefe, J.H. Omega-6 vegetable oils as a driver of coronary heart disease: The oxidized linoleic acid hypothesis. Open Heart 2018, 5, e000898. [Google Scholar] [CrossRef]
- Simopoulos, A.P.; Leaf, A.; Salem, N. Essentiality of and recommended dietary intakes for omega-6 and omega-3 fatty acids. Ann. Nutr. Metab. 1999, 43, 127–130. [Google Scholar] [CrossRef]
- Calder, P.C. Fatty acids and inflammation: The cutting edge between food and pharma. Eur. J. Pharmacol. 2011, 668 (Suppl. 1), S50–S58. [Google Scholar] [CrossRef]
- Ramsden, C.E.; Ringel, A.; Feldstein, A.E.; Taha, A.Y.; MacIntosh, B.A.; Hibbeln, J.R.; Majchrzak-Hong, S.F.; Faurot, K.R.; Rapoport, S.I.; Cheon, Y.; et al. Lowering dietary linoleic acid reduces bioactive oxidized linoleic acid metabolites in humans. Prostaglandins Leukot. Essent. Fatty Acids 2012, 87, 135–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarkkinen, E.S.; Agren, J.J.; Ahola, I.; Ovaskainen, M.L.; Uusitupa, M.I. Fatty acid composition of serum cholesterol esters, and erythrocyte and platelet membranes as indicators of long-term adherence to fat-modified diets. Am. J. Clin. Nutr. 1994, 59, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Hussein, N.; Ah-Sing, E.; Wilkinson, P.; Leach, C.; Griffin, B.A.; Millward, D.J. Long-chain conversion of [13C] linoleic acid and alpha-linolenic acid in response to marked changes in their dietary intake in men. J. Lipid Res. 2005, 46, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Ramsden, C.E.; Hibbeln, J.R.; Majchrzak, S.F.; Davis, J.M. n-6 fatty acid-specific and mixed polyunsaturate dietary interventions have different effects on CHD risk: A meta-analysis of randomised controlled trials. Br. J. Nutr. 2010, 104, 1586–1600. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D.; Micha, R.; Wallace, S. Effects on coronary heart disease of increasing polyunsaturated fat in place of saturated fat: A systematic review and meta-analysis of randomized controlled trials. PLoS Med. 2010, 7, e1000252. [Google Scholar] [CrossRef]
- Jakobsen, M.U.; O’Reilly, E.J.; Heitmann, B.L.; Pereira, M.A.; Bälter, K.; Fraser, G.E.; Goldbourt, U.; Hallmans, G.; Knekt, P.; Liu, S.; et al. Major types of dietary fat and risk of coronary heart disease: A pooled analysis of 11 cohort studies. Am. J. Clin. Nutr. 2009, 89, 1425–1432. [Google Scholar] [CrossRef] [PubMed]
- Oh, K.; Hu, F.B.; Manson, J.E.; Stampfer, M.J.; Willett, W.C. Dietary fat intake and risk of coronary heart disease in women: 20 years of follow-up of the nurses’ health study. Am. J. Epidemiol. 2005, 161, 672–679. [Google Scholar] [CrossRef]
- Laaksonen, D.E.; Nyyssönen, K.; Niskanen, L.; Rissanen, T.H.; Salonen, J.T. Prediction of cardiovascular mortality in middle-aged men by dietary and serum linoleic and polyunsaturated fatty acids. Arch. Intern. Med. 2005, 165, 193–199. [Google Scholar] [CrossRef]
- Iso, H.; Sato, S.; Umemura, U.; Kudo, M.; Koike, K.; Kitamura, A.; Imano, H.; Okamura, T.; Naito, Y.; Shimamoto, T. Linoleic acid, other fatty acids, and the risk of stroke. Stroke 2002, 33, 2086–2093. [Google Scholar] [CrossRef]
- Farvid, M.S.; Ding, M.; Pan, A.; Sun, Q.; Chiuve, S.E.; Steffen, L.M.; Willett, W.C.; Hu, F.B. Dietary linoleic acid and risk of coronary heart disease: A systematic review and meta-analysis of prospective cohort studies. Circulation 2014, 130, 1568–1578. [Google Scholar] [CrossRef]
- Marklund, M.; Wu, J.H.Y.; Imamura, F.; Del Gobbo, L.C.; Fretts, A.; de Goede, J.; Shi, P.; Tintle, N.; Wennberg, M.; Aslibekyan, S.; et al. Biomarkers of Dietary Omega-6 Fatty Acids and Incident Cardiovascular Disease and Mortality. Circulation 2019, 139, 2422–2436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, G.H.; Fritsche, K. Effect of dietary linoleic acid on markers of inflammation in healthy persons: A systematic review of randomized controlled trials. J. Acad. Nutr. Diet. 2012, 112, 1029–1041.e15. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, M.; Origasa, H.; Matsuzaki, M.; Matsuzawa, Y.; Saito, Y.; Ishikawa, Y.; Oikawa, S.; Sasaki, J.; Hishida, H.; Itakura, H.; et al. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): A randomised open-label, blinded endpoint analysis. Lancet 2007, 369, 1090–1098. [Google Scholar] [CrossRef]
- Itakura, H.; Yokoyama, M.; Matsuzaki, M.; Saito, Y.; Origasa, H.; Ishikawa, Y.; Oikawa, S.; Sasaki, J.; Hishida, H.; Kita, T.; et al. Relationships between plasma fatty acid composition and coronary artery disease. J. Atheroscler. Thromb. 2011, 18, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, M.; Yokoyama, M.; Saito, Y.; Origasa, H.; Ishikawa, Y.; Oikawa, S.; Sasaki, J.; Hishida, H.; Itakura, H.; Kita, T.; et al. Incremental effects of eicosapentaenoic acid on cardiovascular events in statin-treated patients with coronary artery disease. Circ. J. 2009, 73, 1283–1290. [Google Scholar] [CrossRef] [PubMed]
- Kromhout, D.; Giltay, E.J.; Geleijnse, J.M. n-3 fatty acids and cardiovascular events after myocardial infarction. N. Engl. J. Med. 2010, 363, 2015–2026. [Google Scholar] [CrossRef] [PubMed]
- Galan, P.; Kesse-Guyot, E.; Czernichow, S.; Briancon, S.; Blacher, J.; Hercberg, S. Effects of B vitamins and omega 3 fatty acids on cardiovascular diseases: A randomised placebo controlled trial. BMJ 2010, 341, c6273. [Google Scholar] [CrossRef] [PubMed]
- Bosch, J.; Gerstein, H.C.; Dagenais, G.R.; Díaz, R.; Dyal, L.; Jung, H.; Maggiono, A.P.; Probstfield, J.; Ramachandran, A.; Riddle, M.C.; et al. n-3 fatty acids and cardiovascular outcomes in patients with dysglycemia. N. Engl. J. Med. 2012, 367, 309–318. [Google Scholar] [CrossRef]
- Roncaglioni, M.C.; Tombesi, M.; Avanzini, F.; Barlera, S.; Caimi, V.; Longoni, P.; Marzona, I.; Milani, V.; Silletta, M.G.; Tognoni, G.; et al. n-3 fatty acids in patients with multiple cardiovascular risk factors. N. Engl. J. Med. 2013, 368, 1800–1808. [Google Scholar] [CrossRef]
- Bowman, L.; Mafham, M.; Wallendszus, K.; Stevens, W.; Buck, G.; Barton, J.; Murphy, K.; Aung, T.; Haynes, R.; Cox, J.; et al. Effects of n-3 Fatty Acid Supplements in Diabetes Mellitus. N. Engl. J. Med. 2018, 379, 1540–1550. [Google Scholar] [CrossRef]
- Manson, J.E.; Cook, N.R.; Lee, I.M.; Christen, W.; Bassuk, S.S.; Mora, S.; Gibson, H.; Albert, C.M.; Gordon, D.; Copeland, T.; et al. Marine n-3 Fatty Acids and Prevention of Cardiovascular Disease and Cancer. N. Engl. J. Med. 2019, 380, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, D.L.; Steg, P.G.; Miller, M.; Brinton, E.A.; Jacobson, T.A.; Ketchum, S.B.; Doyle, R.T.; Juliano, R.A.; Jiao, L.; Granowitz, C.; et al. Cardiovascular Risk Reduction with Icosapent Ethyl for Hypertriglyceridemia. N. Engl. J. Med. 2019, 380, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Braeckman, R.A.; Manku, M.S.; Bays, H.E.; Stirtan, W.G.; Soni, P.N. Icosapent ethyl, a pure EPA omega-3 fatty acid: Effects on plasma and red blood cell fatty acids in patients with very high triglyceride levels (results from the MARINE study). Prostaglandins Leukot. Essent. Fatty Acids 2013, 89, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Infante, M.; Ricordi, C.; Baidal, D.A.; Alejandro, R.; Lanzoni, G.; Sears, B.; Caprio, M.; Fabbri, A. VITAL study: An incomplete picture? Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 3142–3147. [Google Scholar] [CrossRef] [PubMed]
- De Caterina, R. n-3 fatty acids in cardiovascular disease. N. Engl. J. Med. 2011, 364, 2439–2450. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.L.; Deckelbaum, R.J. Omega-3 fatty acids: Mechanisms underlying ‘protective effects’ in atherosclerosis. Curr. Opin. Lipidol. 2013, 24, 345–350. [Google Scholar] [CrossRef] [PubMed]
- See, V.H.L.; Mas, E.; Prescott, S.L.; Beilin, L.J.; Burrows, S.; Barden, A.E.; Huang, R.C.; Mori, T.A. Effects of prenatal n-3 fatty acid supplementation on offspring resolvins at birth and 12 years of age: A double-blind, randomised controlled clinical trial. Br. J. Nutr. 2017, 118, 971–980. [Google Scholar] [CrossRef] [PubMed]
- Crawford, M.A.; Casperd, N.M.; Sinclair, A.J. The long chain metabolites of linoleic avid linolenic acids in liver and brain in herbivores and carnivores. Comp. Biochem. Physiol. B 1976, 54, 395–401. [Google Scholar] [CrossRef]
- Brenna, J.T.; Diau, G.Y. The influence of dietary docosahexaenoic acid and arachidonic acid on central nervous system polyunsaturated fatty acid composition. Prostaglandins Leukot. Essent. Fatty Acids 2007, 77, 247–250. [Google Scholar] [CrossRef] [Green Version]
- DeMar, J.C.; Lee, H.J.; Ma, K.; Chang, L.; Bell, J.M.; Rapoport, S.I.; Bazinet, R.P. Brain elongation of linoleic acid is a negligible source of the arachidonate in brain phospholipids of adult rats. Biochim. Biophys. Acta 2006, 1761, 1050–1059. [Google Scholar] [CrossRef]
- Ouellet, M.; Emond, V.; Chen, C.T.; Julien, C.; Bourasset, F.; Oddo, S.; LaFerla, F.; Bazinet, R.P.; Calon, F. Diffusion of docosahexaenoic and eicosapentaenoic acids through the blood-brain barrier: An in situ cerebral perfusion study. Neurochem. Int. 2009, 55, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Molero, J.C.; Weisinger, H.S.; Sinclair, A.J. Orally administered [¹⁴C]DPA and [¹⁴C]DHA are metabolised differently to [¹⁴C]EPA in rats. Br. J. Nutr. 2013, 109, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.T.; Domenichiello, A.F.; Trépanier, M.O.; Liu, Z.; Masoodi, M.; Bazinet, R.P. The low levels of eicosapentaenoic acid in rat brain phospholipids are maintained via multiple redundant mechanisms. J. Lipid Res. 2013, 54, 2410–2422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, J.A.; Batten, S.E.; Harris, M.; Rockett, B.D.; Shaikh, S.R.; Stillwell, W.; Wassall, S.R. Docosahexaenoic and eicosapentaenoic acids segregate differently between raft and nonraft domains. Biophys. J. 2012, 103, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Crawford, M.A.; Broadhurst, C.L.; Guest, M.; Nagar, A.; Wang, Y.; Ghebremeskel, K.; Schmidt, W.F. A quantum theory for the irreplaceable role of docosahexaenoic acid in neural cell signalling throughout evolution. Prostaglandins Leukot. Essent. Fatty Acids 2013, 88, 5–13. [Google Scholar] [CrossRef]
- Eldho, N.V.; Feller, S.E.; Tristram-Nagle, S.; Polozov, I.V.; Gawrisch, K. Polyunsaturated docosahexaenoic vs docosapentaenoic acid-differences in lipid matrix properties from the loss of one double bond. J. Am. Chem. Soc. 2003, 125, 6409–6421. [Google Scholar] [CrossRef] [PubMed]
- Bourre, J.M.; Francois, M.; Youyou, A.; Dumont, O.; Piciotti, M.; Pascal, G.; Durand, G. The effects of dietary alpha-linolenic acid on the composition of nerve membranes, enzymatic activity, amplitude of electrophysiological parameters, resistance to poisons and performance of learning tasks in rats. J. Nutr. 1989, 119, 1880–1892. [Google Scholar] [CrossRef]
- Cardoso, H.D.; dos Santos Junior, E.F.; de Santana, D.F.; Gonçalves-Pimentel, C.; Angelim, M.K.; Isaac, A.R.; Lagranha, C.J.; Guedes, R.C.; Beltrão, E.I.; Morya, E.; et al. Omega-3 deficiency and neurodegeneration in the substantia nigra: Involvement of increased nitric oxide production and reduced BDNF expression. Biochim. Biophys. Acta 2014, 1840, 1902–1912. [Google Scholar] [CrossRef]
- Lim, S.Y.; Hoshiba, J.; Salem, N. An extraordinary degree of structural specificity is required in neural phospholipids for optimal brain function: N-6 docosapentaenoic acid substitution for docosahexaenoic acid leads to a loss in spatial task performance. J. Neurochem. 2005, 95, 848–857. [Google Scholar] [CrossRef]
- Ames, B.N. Delaying the mitochondrial decay of aging. Ann. N. Y. Acad. Sci. 2004, 1019, 406–411. [Google Scholar] [CrossRef]
- Perluigi, M.; Swomley, A.M.; Butterfield, D.A. Redox proteomics and the dynamic molecular landscape of the aging brain. Ageing Res. Rev. 2014, 13, 75–89. [Google Scholar] [CrossRef] [PubMed]
- Svennerholm, L.; Boström, K.; Jungbjer, B. Changes in weight and compositions of major membrane components of human brain during the span of adult human life of Swedes. Acta Neuropathol. 1997, 94, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Irvine, R.F.; Letcher, A.J.; Dawson, R.M. Fatty acid stimulation of membrane phosphatidylinositol hydrolysis by brain phosphatidylinositol phosphodiesterase. Biochem. J. 1979, 178, 497–500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McPhail, L.C.; Clayton, C.C.; Snyderman, R. A potential second messenger role for unsaturated fatty acids: Activation of Ca2+-dependent protein kinase. Science 1984, 224, 622–625. [Google Scholar] [CrossRef] [PubMed]
- Lister, M.D.; Deems, R.A.; Watanabe, Y.; Ulevitch, R.J.; Dennis, E.A. Kinetic analysis of the Ca2+-dependent, membrane-bound, macrophage phospholipase A2 and the effects of arachidonic acid. J. Biol. Chem. 1988, 263, 7506–7513. [Google Scholar] [PubMed]
- Shukla, S.D.; Halenda, S.P. Phospholipase D in cell signalling and its relationship to phospholipase C. Life Sci. 1991, 48, 851–866. [Google Scholar] [CrossRef]
- Samieri, C.; Féart, C.; Proust-Lima, C.; Peuchant, E.; Dartigues, J.F.; Amieva, H.; Barberger-Gateau, P. ω-3 fatty acids and cognitive decline: Modulation by ApoEε4 allele and depression. Neurobiol. Aging 2011, 32, 2317.e13–2317.e22. [Google Scholar] [CrossRef]
- Samieri, C.; Féart, C.; Letenneur, L.; Dartigues, J.F.; Pérès, K.; Auriacombe, S.; Peuchant, E.; Delcourt, C.; Barberger-Gateau, P. Low plasma eicosapentaenoic acid and depressive symptomatology are independent predictors of dementia risk. Am. J. Clin. Nutr. 2008, 88, 714–721. [Google Scholar] [CrossRef] [Green Version]
- Samieri, C.; Maillard, P.; Crivello, F.; Proust-Lima, C.; Peuchant, E.; Helmer, C.; Amieva, H.; Allard, M.; Dartigues, J.F.; Cunnane, S.C.; et al. Plasma long-chain omega-3 fatty acids and atrophy of the medial temporal lobe. Neurology 2012, 79, 642–650. [Google Scholar] [CrossRef] [Green Version]
- Féart, C.; Samieri, C.; Rondeau, V.; Amieva, H.; Portet, F.; Dartigues, J.F.; Scarmeas, N.; Barberger-Gateau, P. Adherence to a Mediterranean diet, cognitive decline, and risk of dementia. JAMA 2009, 302, 638–648. [Google Scholar] [CrossRef]
- Lin, P.Y.; Chiu, C.C.; Huang, S.Y.; Su, K.P. A meta-analytic review of polyunsaturated fatty acid compositions in dementia. J. Clin. Psychiatry 2012, 73, 1245–1254. [Google Scholar] [CrossRef]
- Schaefer, E.J.; Bongard, V.; Beiser, A.S.; Lamon-Fava, S.; Robins, S.J.; Au, R.; Tucker, K.L.; Kyle, D.J.; Wilson, P.W.; Wolf, P.A. Plasma phosphatidylcholine docosahexaenoic acid content and risk of dementia and Alzheimer disease: The Framingham Heart Study. Arch. Neurol. 2006, 63, 1545–1550. [Google Scholar] [CrossRef]
- Tan, Z.S.; Harris, W.S.; Beiser, A.S.; Au, R.; Himali, J.J.; Debette, S.; Pikula, A.; Decarli, C.; Wolf, P.A.; Vasan, R.S.; et al. Red blood cell ω-3 fatty acid levels and markers of accelerated brain aging. Neurology 2012, 78, 658–664. [Google Scholar] [CrossRef]
- Salem, N.; Litman, B.; Kim, H.Y.; Gawrisch, K. Mechanisms of action of docosahexaenoic acid in the nervous system. Lipids 2001, 36, 945–959. [Google Scholar] [CrossRef] [Green Version]
- Dyall, S.C. Amyloid-Beta Peptide, Oxidative Stress and Inflammation in Alzheimer’s Disease: Potential Neuroprotective Effects of Omega-3 Polyunsaturated Fatty Acids. Int. J. Alzheimers Dis. 2010, 2010, 274128. [Google Scholar] [CrossRef]
- Nguyen, L.N.; Ma, D.; Shui, G.; Wong, P.; Cazenave-Gassiot, A.; Zhang, X.; Wenk, M.R.; Goh, E.L.; Silver, D.L. Mfsd2a is a transporter for the essential omega-3 fatty acid docosahexaenoic acid. Nature 2014, 509, 503–506. [Google Scholar] [CrossRef]
- Ximenes da Silva, A.; Lavialle, F.; Gendrot, G.; Guesnet, P.; Alessandri, J.M.; Lavialle, M. Glucose transport and utilization are altered in the brain of rats deficient in n-3 polyunsaturated fatty acids. J. Neurochem. 2002, 81, 1328–1337. [Google Scholar] [CrossRef] [Green Version]
- Moriguchi, T.; Greiner, R.S.; Salem, N. Behavioral deficits associated with dietary induction of decreased brain docosahexaenoic acid concentration. J. Neurochem. 2000, 75, 2563–2573. [Google Scholar] [CrossRef]
- Haag, M. Essential fatty acids and the brain. Can. J. Psychiatry 2003, 48, 195–203. [Google Scholar] [CrossRef]
- Horrobin, D.F.; Bennett, C.N. Depression and bipolar disorder: Relationships to impaired fatty acid and phospholipid metabolism and to diabetes, cardiovascular disease, immunological abnormalities, cancer, ageing and osteoporosis. Possible candidate genes. Prostaglandins Leukot. Essent. Fatty Acids 1999, 60, 217–234. [Google Scholar] [CrossRef]
- Horrobin, D.F.; Bennett, C.N. New gene targets related to schizophrenia and other psychiatric disorders: Enzymes, binding proteins and transport proteins involved in phospholipid and fatty acid metabolism. Prostaglandins Leukot. Essent. Fatty Acids 1999, 60, 141–167. [Google Scholar] [CrossRef]
- Lynch, M.A. Long-term potentiation and memory. Physiol. Rev. 2004, 84, 87–136. [Google Scholar] [CrossRef]
- McGahon, B.M.; Martin, D.S.; Horrobin, D.F.; Lynch, M.A. Age-related changes in synaptic function: Analysis of the effect of dietary supplementation with omega-3 fatty acids. Neuroscience 1999, 94, 305–314. [Google Scholar] [CrossRef]
- Lynch, A.M.; Loane, D.J.; Minogue, A.M.; Clarke, R.M.; Kilroy, D.; Nally, R.E.; Roche, O.J.; O’Connell, F.; Lynch, M.A. Eicosapentaenoic acid confers neuroprotection in the amyloid-beta challenged aged hippocampus. Neurobiol. Aging 2007, 28, 845–855. [Google Scholar] [CrossRef]
- Martin, D.S.; Lonergan, P.E.; Boland, B.; Fogarty, M.P.; Brady, M.; Horrobin, D.F.; Campbell, V.A.; Lynch, M.A. Apoptotic changes in the aged brain are triggered by interleukin-1beta-induced activation of p38 and reversed by treatment with eicosapentaenoic acid. J. Biol. Chem. 2002, 277, 34239–34246. [Google Scholar] [CrossRef]
- Minogue, A.M.; Lynch, A.M.; Loane, D.J.; Herron, C.E.; Lynch, M.A. Modulation of amyloid-beta-induced and age-associated changes in rat hippocampus by eicosapentaenoic acid. J. Neurochem. 2007, 103, 914–926. [Google Scholar] [CrossRef]
- Kelly, L.; Grehan, B.; Chiesa, A.D.; O’Mara, S.M.; Downer, E.; Sahyoun, G.; Massey, K.A.; Nicolaou, A.; Lynch, M.A. The polyunsaturated fatty acids, EPA and DPA exert a protective effect in the hippocampus of the aged rat. Neurobiol. Aging 2011, 32, 2318.e1–2318.e15. [Google Scholar] [CrossRef]
- Serini, S.; Bizzarro, A.; Piccioni, E.; Fasano, E.; Rossi, C.; Lauria, A.; Cittadini, A.R.; Masullo, C.; Calviello, G. EPA and DHA differentially affect in vitro inflammatory cytokine release by peripheral blood mononuclear cells from Alzheimer’s patients. Curr. Alzheimer Res. 2012, 9, 913–923. [Google Scholar] [CrossRef]
- Yurko-Mauro, K.; McCarthy, D.; Rom, D.; Nelson, E.B.; Ryan, A.S.; Blackwell, A.; Salem, N.; Stedman, M.; Investigators, M. Beneficial effects of docosahexaenoic acid on cognition in age-related cognitive decline. Alzheimers Dement. 2010, 6, 456–464. [Google Scholar] [CrossRef]
- Lee, L.K.; Shahar, S.; Chin, A.V.; Yusoff, N.A. Docosahexaenoic acid-concentrated fish oil supplementation in subjects with mild cognitive impairment (MCI): A 12-month randomised, double-blind, placebo-controlled trial. Psychopharmacology 2013, 225, 605–612. [Google Scholar] [CrossRef]
- Freund-Levi, Y.; Eriksdotter-Jönhagen, M.; Cederholm, T.; Basun, H.; Faxén-Irving, G.; Garlind, A.; Vedin, I.; Vessby, B.; Wahlund, L.O.; Palmblad, J. Omega-3 fatty acid treatment in 174 patients with mild to moderate Alzheimer disease: OmegAD study: A randomized double-blind trial. Arch. Neurol. 2006, 63, 1402–1408. [Google Scholar] [CrossRef]
- Bo, Y.; Zhang, X.; Wang, Y.; You, J.; Cui, H.; Zhu, Y.; Pang, W.; Liu, W.; Jiang, Y.; Lu, Q. The n-3 Polyunsaturated Fatty Acids Supplementation Improved the Cognitive Function in the Chinese Elderly with Mild Cognitive Impairment: A Double-Blind Randomized Controlled Trial. Nutrients 2017, 9. [Google Scholar] [CrossRef]
- Hooper, C.; De Souto Barreto, P.; Coley, N.; Cantet, C.; Cesari, M.; Andrieu, S.; Vellas, B. Cognitive Changes with Omega-3 Polyunsaturated Fatty Acids in Non-Demented Older Adults with Low Omega-3 Index. J. Nutr. Health Aging 2017, 21, 988–993. [Google Scholar] [CrossRef]
- Boespflug, E.L.; McNamara, R.K.; Eliassen, J.C.; Schidler, M.D.; Krikorian, R. Fish Oil Supplementation Increases Event-Related Posterior Cingulate Activation in Older Adults with Subjective Memory Impairment. J. Nutr. Health Aging 2016, 20, 161–169. [Google Scholar] [CrossRef]
- Duffy, S.L.; Lagopoulos, J.; Cockayne, N.; Lewis, S.J.; Hickie, I.B.; Hermens, D.F.; Naismith, S.L. The effect of 12-wk ω-3 fatty acid supplementation on in vivo thalamus glutathione concentration in patients “at risk” for major depression. Nutrition 2015, 31, 1247–1254. [Google Scholar] [CrossRef]
- McNamara, R.K.; Kalt, W.; Shidler, M.D.; McDonald, J.; Summer, S.S.; Stein, A.L.; Stover, A.N.; Krikorian, R. Cognitive response to fish oil, blueberry, and combined supplementation in older adults with subjective cognitive impairment. Neurobiol. Aging 2018, 64, 147–156. [Google Scholar] [CrossRef]
- Power, R.; Prado-Cabrero, A.; Mulcahy, R.; Howard, A.; Nolan, J.M. The Role of Nutrition for the Aging Population: Implications for Cognition and Alzheimer’s Disease. Annu. Rev. Food Sci. Technol. 2019, 10, 619–639. [Google Scholar] [CrossRef]
- Sears, B. Omega-3 fatty acids and cardiovascular disease: Dose and AA/EPA ratio determine the therapeutic outcome. CellR4 2018, 6, e2531. [Google Scholar]
- Germano, M.; Meleleo, D.; Montorfano, G.; Adorni, L.; Negroni, M.; Berra, B.; Rizzo, A.M. Plasma, red blood cells phospholipids and clinical evaluation after long chain omega-3 supplementation in children with attention deficit hyperactivity disorder (ADHD). Nutr. Neurosci. 2007, 10, 1–9. [Google Scholar] [CrossRef]
- Infante, M.; Sears, B.; Rizzo, A.M.; Mariani Cerati, D.; Caprio, M.; Ricordi, C.; Fabbri, A. Omega-3 PUFAs and vitamin D co-supplementation as a safe-effective therapeutic approach for core symptoms of autism spectrum disorder: Case report and literature review. Nutr. Neurosci. 2018, 1–2. [Google Scholar] [CrossRef]
- Baidal, D.A.; Ricordi, C.; Garcia-Contreras, M.; Sonnino, A.; Fabbri, A. Combination high-dose omega-3 fatty acids and high-dose cholecalciferol in new onset type 1 diabetes: A potential role in preservation of beta-cell mass. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 3313–3318. [Google Scholar]
- Cadario, F.; Savastio, S.; Ricotti, R.; Rizzo, A.M.; Carrera, D.; Maiuri, L.; Ricordi, C. Administration of vitamin D and high dose of omega 3 to sustain remission of type 1 diabetes. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 512–515. [Google Scholar]
- Sears, B. Anti-inflammatory Diets. J. Am. Coll. Nutr. 2015, 34 (Suppl. 1), 14–21. [Google Scholar] [CrossRef]
- Sears, B. Appropriate doses of omega-3 fatty acids for therapeutic results. CellR4 2018, 6, e2578. [Google Scholar]
- Endres, S.; Ghorbani, R.; Kelley, V.E.; Georgilis, K.; Lonnemann, G.; van der Meer, J.W.; Cannon, J.G.; Rogers, T.S.; Klempner, M.S.; Weber, P.C. The effect of dietary supplementation with n-3 polyunsaturated fatty acids on the synthesis of interleukin-1 and tumor necrosis factor by mononuclear cells. N. Engl. J. Med. 1989, 320, 265–271. [Google Scholar] [CrossRef]
- Papanikolaou, Y.; Brooks, J.; Reider, C.; Fulgoni, V.L. U.S. adults are not meeting recommended levels for fish and omega-3 fatty acid intake: Results of an analysis using observational data from NHANES 2003–2008. Nutr. J. 2014, 13, 31. [Google Scholar] [CrossRef]
- Zhang, Z.; Fulgoni, V.L.; Kris-Etherton, P.M.; Mitmesser, S.H. Dietary Intakes of EPA and DHA Omega-3 Fatty Acids among US Childbearing-Age and Pregnant Women: An Analysis of NHANES 2001–2014. Nutrients 2018, 10, 416. [Google Scholar] [CrossRef]
- Elkind, M.S.; Leon, V.; Moon, Y.P.; Paik, M.C.; Sacco, R.L. High-sensitivity C-reactive protein and lipoprotein-associated phospholipase A2 stability before and after stroke and myocardial infarction. Stroke 2009, 40, 3233–3237. [Google Scholar] [CrossRef]
- Luna, J.M.; Moon, Y.P.; Liu, K.M.; Spitalnik, S.; Paik, M.C.; Cheung, K.; Sacco, R.L.; Elkind, M.S. High-sensitivity C-reactive protein and interleukin-6-dominant inflammation and ischemic stroke risk: The northern Manhattan study. Stroke 2014, 45, 979–987. [Google Scholar] [CrossRef]
- O’Donoghue, M.L.; Braunwald, E.; White, H.D.; Steen, D.P.; Lukas, M.A.; Tarka, E.; Steg, P.G.; Hochman, J.S.; Bode, C.; Maggioni, A.P.; et al. Effect of darapladib on major coronary events after an acute coronary syndrome: The SOLID-TIMI 52 randomized clinical trial. JAMA 2014, 312, 1006–1015. [Google Scholar] [CrossRef]
- White, H.D.; Held, C.; Stewart, R.; Tarka, E.; Brown, R.; Davies, R.Y.; Budaj, A.; Harrington, R.A.; Steg, P.G.; Ardissino, D.; et al. Darapladib for preventing ischemic events in stable coronary heart disease. N. Engl. J. Med. 2014, 370, 1702–1711. [Google Scholar] [CrossRef]
- Prasad, M.; Lennon, R.; Barsness, G.W.; Prasad, A.; Gulati, R.; Lerman, L.O.; Lerman, A. Chronic inhibition of lipoprotein-associated phospholipase A. Int. J. Cardiol. 2018, 253, 7–13. [Google Scholar] [CrossRef]
- Della-Morte, D.; Guadagni, F.; Palmirotta, R.; Testa, G.; Caso, V.; Paciaroni, M.; Abete, P.; Rengo, F.; Ferroni, P.; Sacco, R.L.; et al. Genetics of ischemic stroke, stroke-related risk factors, stroke precursors and treatments. Pharmacogenomics 2012, 13, 595–613. [Google Scholar] [CrossRef]

| Study Population | Study Duration | Omega-3 PUFA dose | Clinical Findings | |
|---|---|---|---|---|
| JELIS [103] | 18,645 patients with hypercholesterolemia and with/without history of CAD | Mean follow-up: 4.6 years | EPA 1800 mg/day + statin vs. statin alone | Reduction in major coronary events in the EPA group compared to control in the total study population (hazard ratio, 0.81; 95% CI, 0.69 to 0.95; p = 0.011) Reduction in major coronary events in the EPA group compared to control group among patients with history of CAD (hazard ratio, 0.81; 95% CI, 0.66 to 1.00; p = 0.048) |
| Alpha Omega Trial [106] | 4837 subjects with history of MI, who were receiving state-of-the-art antithrombotic, antihypertensive, and lipid-lowering therapy | Median follow-up: 40.8 months | Margarine enriched with EPA and DHA (400 mg of EPA and DHA/day) or ALA (2 g of ALA/day) | Rate of major CV events: hazard ratio with EPA-DHA-enriched margarine, 1.01; 95% CI, 0.87 to 1.17; p = 0.93; hazard ratio with ALA-enriched margarine, 0.91; 95% CI, 0.78 to 1.05; p = 0.20 |
| SU.FOL.OM3 [107] | 2501 patients with a history of unstable angina, MI, or ischemic stroke | Median follow-up: 4.7 years | 600 mg/day of EPA and DHA in a ratio of 2:1 vs. placebo | No significant difference in major CV events between omega-3 group and placebo group (81 vs. 76 patients, hazard ratio 1.08; 95% CI 0.79 to 1.47, p = 0.64) |
| ORIGIN [108] | 12,536 participants with diabetes, impaired glucose tolerance or impaired fasting glucose, who were at increased CV risk, defined as history of MI, angina with documented ischemia, stroke, or revascularization | Median follow-up: 6.2 years | 465 mg of EPA/day plus 375 mg of DHA/day vs. placebo | No significant difference in death from CV causes between omega-3 group and placebo group (9.1% vs. 9.3%; hazard ratio, 0.98; 95% CI, 0.87 to 1.10; p = 0.72) No significant difference between omega-3 group and placebo group in the rate of major CV events (16.5% vs. 16.3%; hazard ratio, 1.01; 95% CI, 0.93 to 1.10; p = 0.81), death from arrhythmia (4.6% vs. 4.1%; hazard ratio, 1.10; 95% CI, 0.93 to 1.30; p = 0.26), or death from any cause (15.1% vs. 15.4%; hazard ratio, 0.98; 95% CI, 0.89 to 1.07; p = 0.63) |
| Risk and Prevention Study [109] | 12,513 patients with multiple CV risk factors or clinical evidence of atherosclerotic vascular disease (defined as a history of transient ischemic attack or ischemic stroke, angina pectoris, peripheral artery disease, or previous arterial revascularization procedure) without history of previous MI | Median follow-up: 5 years | 1-g daily capsule containing not less than 85% of EPA and DHA content and in a ratio ranging between 0.9:1 and 1.5:1 vs. placebo | No significant difference in time to death from CV causes or first hospitalization for CV causes between omega-3 group and placebo group (11.7% vs. 11.9%; adjusted hazard ratio with n-3 fatty acids, 0.97; 95% CI, 0.88 to 1.08; p = 0.58) |
| ASCEND [110] | 15,480 participants with diabetes and no evidence of CVD | Mean follow-up: 7.4 years | 460 mg of EPA/day plus 380 mg of DHA/day vs. placebo | No significant difference in first serious vascular event between omega-3 group and placebo group (8.9% vs. 9.2%; rate ratio, 0.97; 95% CI, 0.87 to 1.08; p = 0.55) No significant difference in the secondary composite outcome of any serious vascular event or any revascularization procedure between omega-3 group and placebo group (11.4% vs. 11.5%; rate ratio, 1.00; 95% CI, 0.91 to 1.09) |
| VITAL [111] | 25,871 participants without history of CVD | Median follow-up: 5.3 years | 460 mg of EPA/day plus 380 mg of DHA/day vs. placebo | No significant difference in the incidence of major CV events between omega-3 group and placebo group (hazard ratio, 0.92; 95% CI, 0.80 to 1.06; p = 0.24) Omega-3 PUFA supplementation associated with a significant reduction in risk of total MI compared to placebo (hazard ratio, 0.72; 95% CI, 0.59 to 0.90) |
| REDUCE-IT [112] | 8179 patients with hypertriglyceridemia and established CVD or diabetes and other risk factors (70.7% for secondary prevention of CV events) | Median follow-up: 4.9 years | 4 g/day of icosapent ethyl, a highly purified EPA ethyl ester vs. placebo | Significant reduction in the rates of the primary endpoint (a composite of CV death, non-fatal MI, non-fatal stroke, coronary revascularization, or unstable angina) in the icosapent ethyl group compared to placebo group (hazard ratio, 0.75; 95% CI, 0.68 to 0.83; p < 0.001) Significant reduction in the rates of the key secondary endpoint (a composite of CV death, non-fatal MI, or non-fatal stroke) in the icosapent ethyl group compared to placebo group (hazard ratio, 0.74; 95% CI, 0.65 to 0.83; p < 0.001) Significant reduction in circulating levels of hsCRP in the icosapent ethyl group compared to placebo group (median observed values at the last follow-up visit: 1.8 mg/L vs. 2.8 mg/L, respectively; p < 0.001) |
| Study Population | Study Duration | Omega-3 PUFA dose | Clinical Findings | |
|---|---|---|---|---|
| Duffy et al. [165] | 51 heathy older adults (mean age = 71 years) | 12 weeks | Four 1000 mg omega-3 supplements (containing EPA 1200 mg + DHA 800 mg) daily | The participants treated with the omega-3 supplements had lower oxidative stress (measured as higher glutathione-to-creatine ratio in the thalamus) compared to the placebo group (p = 0.049) |
| Boespflug et al. [164] | 140 healthy adults aged 62–80 years with subjective memory complaints, but not meeting criteria for MCI or dementia | 24 weeks | EPA + DHA at a dose of 2.4 g/day | Dietary fish oil supplementation ameliorated working memory performance, and enhanced neuronal response to working memory challenge (defined as increased blood oxygen level dependent signal in the posterior cingulate cortex during greater working memory load) |
| Hooper et al. [163] | 183 adults aged 70 years or older with subjective memory complaints but clinically dementia-free | 36 months | Two capsules of omega-3 supplement providing a total 800 mg DHA + 225 mg EPA daily | Omega-3 PUFAs showed benefits in maintenance of executive functions in older adults at risk of dementia due to low omega-3 index |
| Bo et al. [162] | 86 adults with mean age 71 years affected by MCI | 6 months | Omega-3 PUFA supplement capsules of 480 mg DHA + 720 mg EPA/daily | Omega-3 PUFA supplementation was associated with improved total Basic Cognitive Aptitude Test scores, space imagery efficiency, processing speed, and working memory (p < 0.01) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simonetto, M.; Infante, M.; Sacco, R.L.; Rundek, T.; Della-Morte, D. A Novel Anti-Inflammatory Role of Omega-3 PUFAs in Prevention and Treatment of Atherosclerosis and Vascular Cognitive Impairment and Dementia. Nutrients 2019, 11, 2279. https://doi.org/10.3390/nu11102279
Simonetto M, Infante M, Sacco RL, Rundek T, Della-Morte D. A Novel Anti-Inflammatory Role of Omega-3 PUFAs in Prevention and Treatment of Atherosclerosis and Vascular Cognitive Impairment and Dementia. Nutrients. 2019; 11(10):2279. https://doi.org/10.3390/nu11102279
Chicago/Turabian StyleSimonetto, Marialaura, Marco Infante, Ralph L. Sacco, Tatjana Rundek, and David Della-Morte. 2019. "A Novel Anti-Inflammatory Role of Omega-3 PUFAs in Prevention and Treatment of Atherosclerosis and Vascular Cognitive Impairment and Dementia" Nutrients 11, no. 10: 2279. https://doi.org/10.3390/nu11102279
APA StyleSimonetto, M., Infante, M., Sacco, R. L., Rundek, T., & Della-Morte, D. (2019). A Novel Anti-Inflammatory Role of Omega-3 PUFAs in Prevention and Treatment of Atherosclerosis and Vascular Cognitive Impairment and Dementia. Nutrients, 11(10), 2279. https://doi.org/10.3390/nu11102279







