The Protective Effect of Brazilian Propolis against Glycation Stress in Mouse Skeletal Muscle
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Treatment
2.2. Anti-Glycation Assay
2.3. Measurement of MGO-Derived AGE Content
2.4. Measurement of Glyoxalase 1 Activity
2.5. Real-Time RT-PCR Analysis
2.6. Statistics
3. Results
3.1. Anti-Glycation Effects of Brazilian Propolis In Vitro
3.2. The Effect of Brazilian Propolis on Body Weight, Food and Fluid Intake, and Muscle Weight
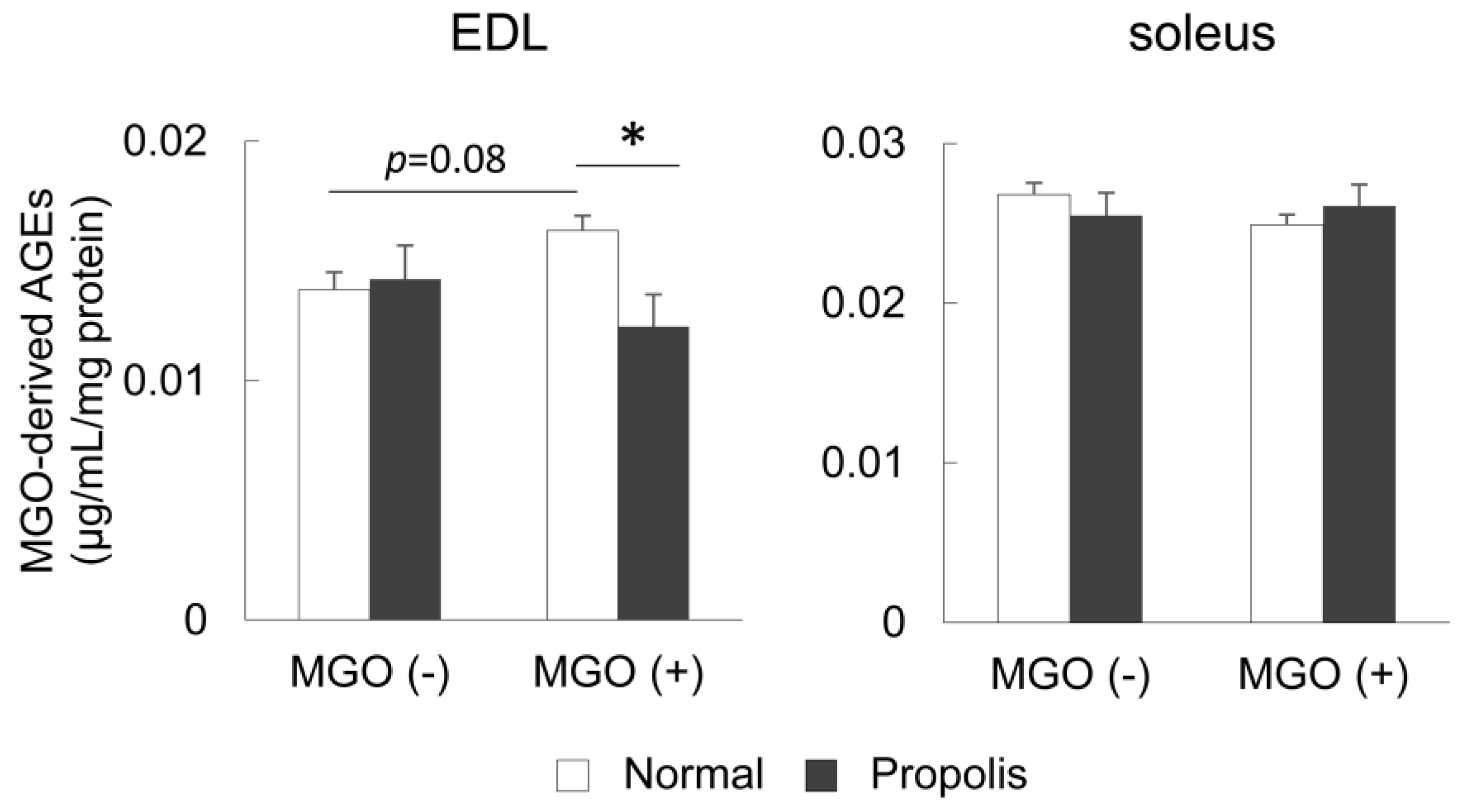
3.3. Brazilian Propolis Suppressed the Accumulation of MGO-Derived AGEs in the Skeletal Muscle In Vivo
3.4. Brazilian Propolis Enhanced Glyoxalase 1 Activity in The Skeletal Muscle
3.5. Brazilian Propolis Suppressed MGO-Induced mRNA Expression of Inflammatory-Related Molecules in The Skeletal Muscle
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Argiles, J.M.; Campos, N.; Lopez-Pedrosa, J.M.; Rueda, R.; Rodriguez-Manas, L. Skeletal muscle regulates metabolism via interorgan crosstalk: Roles in health and disease. J. Am. Med. Dir. Assoc. 2016, 17, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, L.; Volpato, S. Muscle dysfunction in type 2 diabetes: A major threat to patient’s mobility and independence. Acta Diabetol. 2016, 53, 879–889. [Google Scholar] [CrossRef]
- Lin, J.A.; Wu, C.H.; Lu, C.C.; Hsia, S.M.; Yen, G.C. Glycative stress from advanced glycation end products (ages) and dicarbonyls: An emerging biological factor in cancer onset and progression. Mol. Nutr. Food Res. 2016, 60, 1850–1864. [Google Scholar] [CrossRef] [PubMed]
- Dalal, M.; Ferrucci, L.; Sun, K.; Beck, J.; Fried, L.P.; Semba, R.D. Elevated serum advanced glycation end products and poor grip strength in older community-dwelling women. J. Gerontol. A Biol. Sci. Med. Sci. 2009, 64, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Semba, R.D.; Bandinelli, S.; Sun, K.; Guralnik, J.M.; Ferrucci, L. Relationship of an advanced glycation end product, plasma carboxymethyl-lysine, with slow walking speed in older adults: The inchianti study. Eur. J. Appl. Physiol. 2010, 108, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Momma, H.; Niu, K.; Kobayashi, Y.; Guan, L.; Sato, M.; Guo, H.; Chujo, M.; Otomo, A.; Yufei, C.; Tadaura, H.; et al. Skin advanced glycation end product accumulation and muscle strength among adult men. Eur. J. Appl. Physiol. 2011, 111, 1545–1552. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.; Kubo, A.; Sugioka, Y.; Mitsui, R.; Fukuhara, N.; Nihei, F.; Takeda, Y. Relationship between advanced glycation end-product accumulation and low skeletal muscle mass in japanese men and women. Geriatr. Gerontol. Int. 2017, 17, 785–790. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.C.; Shiu, S.W.; Wong, Y.; Tam, X. Serum advanced glycation end products (ages) are associated with insulin resistance. Diabetes Metab. Res. Rev. 2011, 27, 488–492. [Google Scholar] [CrossRef] [PubMed]
- Mori, H.; Kuroda, A.; Ishizu, M.; Ohishi, M.; Takashi, Y.; Otsuka, Y.; Taniguchi, S.; Tamaki, M.; Kurahashi, K.; Yoshida, S.; et al. Association of accumulated advanced glycation end-products with a high prevalence of sarcopenia and dynapenia in patients with type 2 diabetes. J. Diabetes Investig. 2019, 10, 1332–1340. [Google Scholar] [CrossRef] [PubMed]
- Egawa, T.; Ohno, Y.; Yokoyama, S.; Goto, A.; Ito, R.; Hayashi, T.; Goto, K. The effect of advanced glycation end products on cellular signaling molecules in skeletal muscle. J. Phys. Fit. Sports Med. 2018, 7, 229–238. [Google Scholar] [CrossRef]
- Brunvand, L.; Heier, M.; Brunborg, C.; Hanssen, K.F.; Fugelseth, D.; Stensaeth, K.H.; Dahl-Jorgensen, K.; Margeirsdottir, H.D. Advanced glycation end products in children with type 1 diabetes and early reduced diastolic heart function. BMC Cardiovasc. Disord. 2017, 17, 133. [Google Scholar] [CrossRef] [PubMed]
- Chiarelli, F.; de Martino, M.; Mezzetti, A.; Catino, M.; Morgese, G.; Cuccurullo, F.; Verrotti, A. Advanced glycation end products in children and adolescents with diabetes: Relation to glycemic control and early microvascular complications. J. Pediatr. 1999, 134, 486–491. [Google Scholar] [CrossRef]
- Chiarelli, F.; Catino, M.; Tumini, S.; Cipollone, F.; Mezzetti, A.; Vanelli, M.; Verrotti, A. Advanced glycation end products in adolescents and young adults with diabetic angiopathy. Pediatr. Nephrol. 2000, 14, 841–846. [Google Scholar] [CrossRef] [PubMed]
- Egawa, T.; Tsuda, S.; Goto, A.; Ohno, Y.; Yokoyama, S.; Goto, K.; Hayashi, T. Potential involvement of dietary advanced glycation end products in impairment of skeletal muscle growth and muscle contractile function in mice. Br. J. Nutr. 2017, 117, 21–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cassese, A.; Esposito, I.; Fiory, F.; Barbagallo, A.P.; Paturzo, F.; Mirra, P.; Ulianich, L.; Giacco, F.; Iadicicco, C.; Lombardi, A.; et al. In skeletal muscle advanced glycation end products (ages) inhibit insulin action and induce the formation of multimolecular complexes including the receptor for ages. J. Biol. Chem. 2008, 283, 36088–36099. [Google Scholar] [CrossRef] [PubMed]
- Pinto-Junior, D.C.; Silva, K.S.; Michalani, M.L.; Yonamine, C.Y.; Esteves, J.V.; Fabre, N.T.; Thieme, K.; Catanozi, S.; Okamoto, M.M.; Seraphim, P.M.; et al. Advanced glycation end products-induced insulin resistance involves repression of skeletal muscle glut4 expression. Sci. Rep. 2018, 8, 8109. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, R.; Yan, S.F.; Schmidt, A.M. Receptor for age (rage): Signaling mechanisms in the pathogenesis of diabetes and its complications. Ann. N. Y. Acad. Sci. 2011, 1243, 88–102. [Google Scholar] [CrossRef] [PubMed]
- Chuah, Y.K.; Basir, R.; Talib, H.; Tie, T.H.; Nordin, N. Receptor for advanced glycation end products and its involvement in inflammatory diseases. Int. J. Inflam. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Riuzzi, F.; Sorci, G.; Sagheddu, R.; Chiappalupi, S.; Salvadori, L.; Donato, R. Rage in the pathophysiology of skeletal muscle. J. Cachexia Sarcopenia Muscle 2018, 9, 1213–1234. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, Z.A.; Armour, C.L.; Phipps, S.; Sukkar, M.B. Rage and tlrs: Relatives, friends or neighbours? Mol. Immunol. 2013, 56, 739–744. [Google Scholar] [CrossRef]
- Liu, Y.; Liang, C.; Liu, X.; Liao, B.; Pan, X.; Ren, Y.; Fan, M.; Li, M.; He, Z.; Wu, J.; et al. Ages increased migration and inflammatory responses of adventitial fibroblasts via rage, mapk and nf-kappab pathways. Atherosclerosis 2010, 208, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Ohtsu, A.; Shibutani, Y.; Seno, K.; Iwata, H.; Kuwayama, T.; Shirasuna, K. Advanced glycation end products and lipopolysaccharides stimulate interleukin-6 secretion via the rage/tlr4-nf-kappab-ros pathways and resveratrol attenuates these inflammatory responses in mouse macrophages. Exp. Ther. Med. 2017, 14, 4363–4370. [Google Scholar] [PubMed]
- Seno, K.; Sase, S.; Ozeki, A.; Takahashi, H.; Ohkuchi, A.; Suzuki, H.; Matsubara, S.; Iwata, H.; Kuwayama, T.; Shirasuna, K. Advanced glycation end products regulate interleukin-1beta production in human placenta. J. Reprod. Dev. 2017, 63, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Costamagna, D.; Costelli, P.; Sampaolesi, M.; Penna, F. Role of inflammation in muscle homeostasis and myogenesis. Mediat. Inflamm. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Chen, K.; Whaley-Connell, A.T.; Stump, C.S.; Ibdah, J.A.; Sowers, J.R. Skeletal muscle insulin resistance: Role of inflammatory cytokines and reactive oxygen species. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 294, R673–R680. [Google Scholar] [CrossRef] [PubMed]
- Pasupuleti, V.R.; Sammugam, L.; Ramesh, N.; Gan, S.H. Honey, propolis, and royal jelly: A comprehensive review of their biological actions and health benefits. Oxid. Med. Cell. Longev. 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- Marcucci, M.C.; Ferreres, F.; Custodio, A.R.; Ferreira, M.M.; Bankova, V.S.; Garcia-Viguera, C.; Bretz, W.A. Evaluation of phenolic compounds in brazilian propolis from different geographic regions. Z. Naturforsch. C 2000, 55, 76–81. [Google Scholar] [CrossRef]
- Righi, A.A.; Negri, G.; Salatino, A. Comparative chemistry of propolis from eight brazilian localities. Evid. Based Complement. Alternat. Med. 2013, 2013. [Google Scholar] [CrossRef]
- Kocot, J.; Kielczykowska, M.; Luchowska-Kocot, D.; Kurzepa, J.; Musik, I. Antioxidant potential of propolis, bee pollen, and royal jelly: Possible medical application. Oxid. Med. Cell. Longev. 2018, 2018. [Google Scholar] [CrossRef]
- Yeh, W.J.; Hsia, S.M.; Lee, W.H.; Wu, C.H. Polyphenols with antiglycation activity and mechanisms of action: A review of recent findings. J. Food Drug. Anal. 2017, 25, 84–92. [Google Scholar] [CrossRef]
- Boisard, S.; Le Ray, A.M.; Gatto, J.; Aumond, M.C.; Blanchard, P.; Derbre, S.; Flurin, C.; Richomme, P. Chemical composition, antioxidant and anti-ages activities of a french poplar type propolis. J. Agric. Food Chem. 2014, 62, 1344–1351. [Google Scholar] [CrossRef] [PubMed]
- Sahebi, U.; Divsalar, A. Synergistic and inhibitory effects of propolis and aspirin on structural changes of human hemoglobin resulting from glycation: An in vitro study. J. Iran. Chem. Soc. 2016, 13, 2001–2011. [Google Scholar] [CrossRef]
- Boisard, S.; Shahali, Y.; Aumond, M.-C.; Derbré, S.; Blanchard, P.; Dadar, M.; Le Ray, A.-M.; Richomme, P. Anti-age activity of poplar-type propolis: Mechanism of action of main phenolic compounds. International J. Food Sci. Technol. 2019. [Google Scholar] [CrossRef]
- Aoi, W.; Hosogi, S.; Niisato, N.; Yokoyama, N.; Hayata, H.; Miyazaki, H.; Kusuzaki, K.; Fukuda, T.; Fukui, M.; Nakamura, N.; et al. Improvement of insulin resistance, blood pressure and interstitial ph in early developmental stage of insulin resistance in oletf rats by intake of propolis extracts. Biochem. Biophys. Res. Commun. 2013, 432, 650–653. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, P.; Chen, H.; Sang, S. Dietary quercetin inhibits methylglyoxal-induced advanced glycation end products formation in mice. FASEB J. 2016, 30, 692. [Google Scholar]
- Goto, A.; Ohno, Y.; Ikuta, A.; Suzuki, M.; Ohira, T.; Egawa, T.; Sugiura, T.; Yoshioka, T.; Ohira, Y.; Goto, K. Up-regulation of adiponectin expression in antigravitational soleus muscle in response to unloading followed by reloading, and functional overloading in mice. PLoS ONE 2013, 8, e81929. [Google Scholar] [CrossRef] [PubMed]
- Ishioka, Y.; Yagi, M.; Ogura, M.; Yonei, Y. Polyphenol content of various vegetables: Relationship to antiglycation activity. Glycative Stress Res. 2015, 2, 41–51. [Google Scholar]
- Chinchansure, A.A.; Korwar, A.M.; Kulkarni, M.J.; Joshi, S.P. Recent development of plant products with anti-glycation activity: A review. RSC Adv. 2015, 5, 31113–31138. [Google Scholar] [CrossRef]
- Snow, L.M.; Fugere, N.A.; Thompson, L.V. Advanced glycation end-product accumulation and associated protein modification in type ii skeletal muscle with aging. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2007, 62, 1204–1210. [Google Scholar] [CrossRef]
- van Wessel, T.; de Haan, A.; van der Laarse, W.J.; Jaspers, R.T. The muscle fiber type-fiber size paradox: Hypertrophy or oxidative metabolism? Eur. J. Appl. Physiol. 2010, 110, 665–694. [Google Scholar] [CrossRef]
- Lewis, S.E.; Kelly, F.J.; Goldspink, D.F. Pre- and post-natal growth and protein turnover in smooth muscle, heart and slow- and fast-twitch skeletal muscles of the rat. Biochem. J. 1984, 217, 517–526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baillie, A.G.; Garlick, P.J. Responses of protein synthesis in different skeletal muscles to fasting and insulin in rats. Am. J. Physiol. 1991, 260, E891–E896. [Google Scholar] [CrossRef] [PubMed]
- Ueda, M.; Hayashibara, K.; Ashida, H. Propolis extract promotes translocation of glucose transporter 4 and glucose uptake through both pi3k- and ampk-dependent pathways in skeletal muscle. Biofactors 2013, 39, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Kwon, T.D.; Lee, M.W.; Kim, K.H. The effect of exercise training and water extract from propolis intake on the antioxidant enzymes activity of skeletal muscle and liver in rat. J. Exerc. Nutr. Biochem. 2014, 18, 9–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacob, A.; Parolia, A.; Pau, A.; Davamani Amalraj, F. The effects of malaysian propolis and brazilian red propolis on connective tissue fibroblasts in the wound healing process. BMC Complement. Altern. Med. 2015, 15, 294. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, R.; Yan, S.F.; Schmidt, A.M. Methylglyoxal comes of age. Cell 2006, 124, 258–260. [Google Scholar] [CrossRef] [PubMed]
- Wetzels, S.; Wouters, K.; Schalkwijk, C.G.; Vanmierlo, T.; Hendriks, J.J. Methylglyoxal-derived advanced glycation endproducts in multiple sclerosis. Int. J. Mol. Sci. 2017, 18, 421. [Google Scholar] [CrossRef] [PubMed]
- Rabbani, N.; Thornalley, P.J. Dicarbonyl proteome and genome damage in metabolic and vascular disease. Biochem. Soc. Trans. 2014, 42, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Londhe, P.; Guttridge, D.C. Inflammation induced loss of skeletal muscle. Bone 2015, 80, 131–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dalle, S.; Rossmeislova, L.; Koppo, K. The role of inflammation in age-related sarcopenia. Front. Physiol. 2017, 8, 1045. [Google Scholar] [CrossRef]
- Chen, Y.J.; Sheu, M.L.; Tsai, K.S.; Yang, R.S.; Liu, S.H. Advanced glycation end products induce peroxisome proliferator-activated receptor gamma down-regulation-related inflammatory signals in human chondrocytes via toll-like receptor-4 and receptor for advanced glycation end products. PLoS ONE 2013, 8, e66611. [Google Scholar]
- Ansorge, S.; Reinhold, D.; Lendeckel, U. Propolis and some of its constituents down-regulate DNA synthesis and inflammatory cytokine production but induce tgf-beta1 production of human immune cells. Z. Naturforsch. C 2003, 58, 580–589. [Google Scholar] [CrossRef]
- Bachiega, T.F.; Orsatti, C.L.; Pagliarone, A.C.; Sforcin, J.M. The effects of propolis and its isolated compounds on cytokine production by murine macrophages. Phytother. Res. 2012, 26, 1308–1313. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Wang, W.X.; Chen, H.J.; He, Z.C.; Jia, A.Q. The inhibition of advanced glycation end-products by five fractions and three main flavonoids from camellia nitidissima chi flowers. J. Food Drug. Anal. 2018, 26, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Silvan, J.M.; Assar, S.H.; Srey, C.; Dolores Del Castillo, M.; Ames, J.M. Control of the maillard reaction by ferulic acid. Food Chem. 2011, 128, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; Chiba, S.; Yoshizaki, F. Effect of natural flavonoids, stilbenes and caffeic acid oligomers on protein glycation. Biomed. Rep. 2014, 2, 628–632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paulino, N.; Abreu, S.R.; Uto, Y.; Koyama, D.; Nagasawa, H.; Hori, H.; Dirsch, V.M.; Vollmar, A.M.; Scremin, A.; Bretz, W.A. Anti-inflammatory effects of a bioavailable compound, artepillin c, in brazilian propolis. Eur. J. Pharmacol. 2008, 587, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Frandsen, J.R.; Narayanasamy, P. Neuroprotection through flavonoid: Enhancement of the glyoxalase pathway. Redox Biol. 2018, 14, 465–473. [Google Scholar] [CrossRef] [PubMed]



| Components | AIN-93G (per 100 g) | Brazilian Propolis Powder (per 100 g) |
|---|---|---|
| Carbohydrate | 63.0 g | 4.2 g |
| Protein | 20.0 g | 0.7 g |
| Fat | 7.0 g | 47.0 g |
| Mineral | 3.5 g | 0.4 g |
| Vitamin | 1.0 g | |
| Calories | 400 kcal | 758 kcal |
| Normal | Propolis | MGO | MGO + Propolis | ANOVA | |
|---|---|---|---|---|---|
| Initial body weight (g) | 17.7 ± 0.8 | 17.7 ± 0.5 | 17.6 ± 0.5 | 17.7 ± 0.4 | ― |
| Final body weight (g) | 41.1 ± 0.7 | 41.3 ± 0.8 | 38.5 ± 0.8 | 40.4 ± 0.5 | p = 0.070 |
| Food intake (g/day/mouse) | 3.8 ± 0.6 † | 3.7 ± 0.4 † | 3.7 ± 0.4 † | 3.4 ± 0.4 | p = 0.0004 |
| Fluid intake (g/day/mouse) | 3.2 ± 0.4 | 2.8 ± 0.3 * | 2.9 ± 0.3 * | 2.7 ± 0.3 * | p = 0.0004 |
| EDL weight/tibia (mg/mm) | 0.65 ± 0.03 | 0.67 ± 0.02 | 0.62 ± 0.02 | 0.62 ± 0.01 | Propolis (p = 0.69) MGO (p = 0.039) |
| Soleus weight/tibia (mg/mm) | 0.55 ± 0.01 | 0.59 ± 0.01 | 0.53 ± 0.02 | 0.56 ± 0.02 | Propolis (p = 0.054) MGO (p = 0.086) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Egawa, T.; Ohno, Y.; Yokoyama, S.; Yokokawa, T.; Tsuda, S.; Goto, K.; Hayashi, T. The Protective Effect of Brazilian Propolis against Glycation Stress in Mouse Skeletal Muscle. Foods 2019, 8, 439. https://doi.org/10.3390/foods8100439
Egawa T, Ohno Y, Yokoyama S, Yokokawa T, Tsuda S, Goto K, Hayashi T. The Protective Effect of Brazilian Propolis against Glycation Stress in Mouse Skeletal Muscle. Foods. 2019; 8(10):439. https://doi.org/10.3390/foods8100439
Chicago/Turabian StyleEgawa, Tatsuro, Yoshitaka Ohno, Shingo Yokoyama, Takumi Yokokawa, Satoshi Tsuda, Katsumasa Goto, and Tatsuya Hayashi. 2019. "The Protective Effect of Brazilian Propolis against Glycation Stress in Mouse Skeletal Muscle" Foods 8, no. 10: 439. https://doi.org/10.3390/foods8100439
APA StyleEgawa, T., Ohno, Y., Yokoyama, S., Yokokawa, T., Tsuda, S., Goto, K., & Hayashi, T. (2019). The Protective Effect of Brazilian Propolis against Glycation Stress in Mouse Skeletal Muscle. Foods, 8(10), 439. https://doi.org/10.3390/foods8100439





