Effectiveness of the Diabetes Prevention Program for Obesity Treatment in Real World Clinical Practice in a Middle-Income Country in Latin America
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participating Clinics
2.3. Training for Health Providers
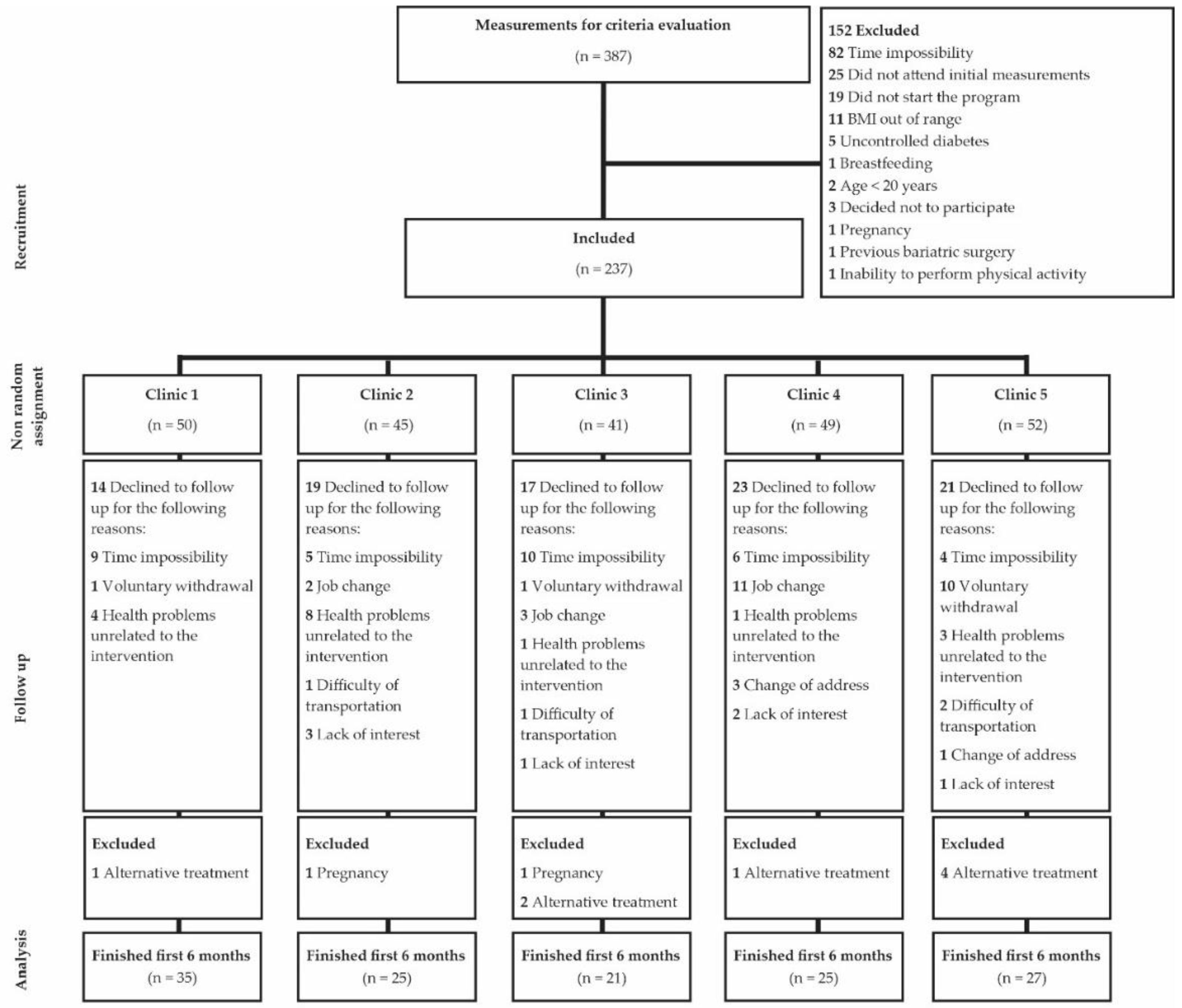
2.4. Recruitment
2.5. Inclusion Criteria
2.6. Study Intervention
2.7. Behavior Change Protocol
2.8. Individual Consultations for Nutrition Counseling
2.9. Study Measures
2.10. Statistical Analysis
3. Results
3.1. Participants, Baseline Characteristics, and Attendance at Scheduled Visits
3.2. Change in Primary Outcome at 6 Months
Changes in Secondary Variables at 6 Months
3.3. Follow-Up of Participants at 12 Months
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in diabetes since 1980: A pooled analysis of 751 population-based studies with 4·4 million participants. Lancet 2016, 387, 1513–1530. [Google Scholar] [CrossRef]
- World Health Organization. 10 Facts on Diabetes; World Health Organization: Geneva, Switzerland, 2016; Available online: https://www.who.int/features/factfiles/diabetes/en/ (accessed on 30 December 2018).
- Islam, S.M.; Purnat, T.D.; Phuong, N.T.; Mwingira, U.; Schacht, K.; Fröschl, G. Non-Communicable Diseases (NCDs) in developing countries: A symposium report. Commentary. Glob. Health 2014, 10, 81. [Google Scholar] [CrossRef] [PubMed]
- King, H.; Aubert, R.E.; Herman, W.H. Global burden of diabetes, 1995–2025: Prevalence, numerical estimates, and projections. Diabetes Care 1998, 21, 1414–1431. [Google Scholar] [CrossRef]
- International Diabetes Federation. IDF Diabetes Atlas, 4th ed.; International Diabetes Federation: Brussels, Belgium, 2009. [Google Scholar]
- Hu, F.B.; Manson, J.E.; Stampfer, M.J.; Colditz, G.; Liu, S.; Solomon, C.G.; Willett, W.C. Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N. Engl. J. Med. 2001, 345, 790–797. [Google Scholar] [CrossRef] [PubMed]
- The Lancet Diabetes Endocrinology. Obesity prevention in Latin America: Now is the time. Lancet Diabetes Endocrinol. 2014, 2, 263. [Google Scholar] [CrossRef]
- NCD Risk Factor Collaboration (NCD-RisC). Trends in adult body-mass index in 200 countries from 1975 to 2014: A pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet 2016, 387, 1377–1396. [Google Scholar] [CrossRef]
- Hoffman, D.J. Upper limits in developing countries: Warning against too much in lands of too little. J. Am. Coll. Nutr. 2004, 23, 610S–615S. [Google Scholar] [CrossRef] [PubMed]
- Kain, J.; Hernández, C.S.; Pineda, D.; de Moraes, A.F.; Antiporta, D.; Collese, T.; Costa de Oliveira, F.E.; González, L.; Miranda, J.J.; Rivera, J. Obesity Prevention in Latin America. Curr. Obes. Rep. 2014, 3, 150–155. [Google Scholar] [CrossRef]
- Uauy, R.; Albala, C.; Kain, J. Obesity trends in Latin America: Transiting from under to overweight. J. Nutr. 2001, 131, 893S–899S. [Google Scholar] [CrossRef] [PubMed]
- The Economist. Obesity in Latin America: Battle of the Bulge. Available online: http://www.economist.com/news/americas/21582273-waistlinesgrow-sodoes-fight-against-junk-food-batt e-bulge (accessed on 5 August 2018).
- Barquera, S.; Hernandez-Barrera, L.; Tolentino, M.L.; Espinosa, J.; Ng, S.W.; Rivera, J.A.; Popkin, B.M. Energy intake from beverages is increasing among Mexican adolescents and adults. J. Nutr. 2008, 138, 2454–2461. [Google Scholar] [CrossRef] [PubMed]
- Alegre-Díaz, J.; Herrington, W.; López-Cervantes, M.; Gnatiuc, L.; Ramírez, R.; Hill, M.; Baigen, T.; McCarthy, M.I.; Lewington, S.; Collins, R.; et al. Diabetes and Cause-Specific Mortality in Mexico City. N. Engl. J. Med. 2016, 375, 1961–1971. [Google Scholar] [CrossRef] [PubMed]
- Barquera, S.; Campos-Nonato, I.; Aguilar-Salinas, C.; Lopez-Ridaura, R.; Arredondo, A.; Rivera-Dommarco, J. Diabetes in Mexico: Cost and management of diabetes and its complications and challenges for health policy. Glob. Health 2013, 9, 3. [Google Scholar] [CrossRef] [PubMed]
- The Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N. Engl. J. Med. 2002, 346, 393–403. [Google Scholar] [CrossRef] [PubMed]
- The Look AHEAD Research Group; Pi-Sunyer, X.; Blackburn, G.; Brancati, F.L.; Bray, G.A.; Bright, R.; Clark, J.M.; Curtis, J.M.; Espeland, M.A.; Foreyt, J.P.; et al. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: One-year results of the look AHEAD trial. Diabetes Care 2007, 30, 1374–1383. [Google Scholar] [CrossRef] [PubMed]
- Whittemore, R. A systematic review of the translational research on the Diabetes Prevention Program. Transl. Behav. Med. 2011, 1, 480–491. [Google Scholar] [CrossRef] [PubMed]
- Albright, A.L.; Gregg, E.W. Preventing type 2 diabetes in communities across the U.S.: The National Diabetes Prevention Program. Am. J. Prev. Med. 2013, S346–S351. [Google Scholar] [CrossRef]
- Ely, E.K.; Gruss, S.M.; Luman, E.T.; Gregg, E.W.; Ali, M.K.; Nhim, K.; Rolka, D.B.; Albright, A.L. A National Effort to Prevent Type 2 Diabetes: Participant-Level Evaluation of CDC’s National Diabetes Prevention Program. Diabetes Care 2017, 40, 1331–1341. [Google Scholar] [CrossRef]
- Heisler, M.; Kaselitz, E.; Rana, G.; Piette, J.D. Diabetes prevention interventions in Latin American countries: A scoping review. Curr. Diabetes Rep. 2016, 16, 80. [Google Scholar] [CrossRef]
- Díaz-Zavala, R.G.; Armenta-Guirado, B.I.; Martínez-Contreras, T.J.; Candia-Plata, M.C.; Esparza-Romero, J.; Martínez-Mir, R.; Haby, M.M.; Valencia, E.M. Translational study of obesity management using the Diabetes Prevention Program “Group Lifestyle Balance” in primary care clinics and public hospitals from Mexico: Study protocol. Rev. Esp. Nutr. Hum. Diet. 2017, 21, 369–383. [Google Scholar] [CrossRef]
- Jekel, J.F.; Katz, D.L.; Elmore, J.G.; Wild, D.M. Jekel’s Epidemiology, Biostatistics, and Preventive Medicine; WB Saunders Elsevier: Philadelphia, PA, USA, 2007; p. 200. [Google Scholar]
- Kramer, M.K.; Kriska, A.M.; Venditti, E.M.; Miller, R.G.; Brooks, M.M.; Burke, L.E.; Siminerio, L.M.; Solano, F.X.; Orchard, T.J. Translating the Diabetes Prevention Program: A comprehensive model for prevention training and program delivery. Am. J. Prev. Med. 2009, 7, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Ware, J.H. Interpreting incomplete data in studies of diet and weight loss. N. Engl. J. Med. 2003, 348, 2136–2137. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M.; Cleeman, J.I.; Daniels, S.R.; Donato, K.A.; Eckel, R.H.; Franklin, B.A.; Gordon, D.J.; Krauss, R.M.; Savage, P.J.; Smith, S.C., Jr.; et al. Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005, 112, 2735–2752. [Google Scholar] [CrossRef] [PubMed]
- Stefan, N.; Haring, H.U.; Schulze, M.B. Metabolically healthy obesity: The low-hanging fruit in obesity treatment? Lancet Diabetes Endocrinol. 2018, 6, 249–258. [Google Scholar] [CrossRef]
- Katula, J.A.; Vitolings, M.Z.; Rosenberger, E.L.; Blackwell, C.S.; Morgan, T.M.; Lawlor, M.S.; Goff, D.C., Jr. One-year results of a community-based translation of the diabetes prevention program. Healthy-Living Partnerships to Prevent Diabetes (HELP PD) project. Diabetes Care 2011, 34, 1451–1457. [Google Scholar] [CrossRef] [PubMed]
- Boltri, J.M.; Davis-Smith, M.; Okosun, I.S.; Seale, J.P.; Foster, B. Translation of the National Institutes of Health diabetes prevention program in african american churches. J. Natl. Med. Assoc. 2011, 103, 194–202. [Google Scholar] [CrossRef]
- Ackermann, R.T.; Finch, E.A.; Brizendine, E.; Zhou, H.; Marrero, D.G. Translating the diabetes prevention program into the community. The DEPLOY pilot study. Am. J. Prev. Med. 2008, 35, 357–363. [Google Scholar] [CrossRef]
- Aziz, Z.; Absetz, P.; Oldroyd, J.; Pronk, N.P.; Oldenburg, B. A systematic review of realworld diabetes prevention programs: Learnings from the last 15 years. Implement. Sci. 2015, 10, 172. [Google Scholar] [CrossRef]
- Nieto-Martínez, R.; González-Rivas, J.P.; Aschner, P.; Barengo, N.C.; Mechanick, J.I. Transculturalizing diabetes prevention in Latin America. Ann. Glob. Health 2017, 83, 432–443. [Google Scholar] [CrossRef]
- Armenta-Guirado, B.I.; Díaz-Zavala, R.G.; Valencia, J.M.E.; Quizán-Plata, T. Manejo de la obesidad en el primer nivel de atención con un programa intensivo de cambio de estilo de vida. Nutr. Hosp. 2015, 32, 1526–1534. [Google Scholar] [CrossRef]
- Tsai, A.; Remmert, J.; Butryn, M.; Wadden, T. Treatment of obesity in primary care. Med. Clin. N. Am. 2018, 102, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Hartmann-Boyce, J.; Johns, D.; Jebb, S.; Summerbell, C.; Aveyard, P. Behavioural Weight Management Review Group. Behavioural weight management programmes for adults assessed by trials conducted in everyday contexts: Systematic review and metaanalysis. Obes. Rev. 2014, 15, 920–932. [Google Scholar] [CrossRef] [PubMed]
- Fortin, M.; Dionne, J.; Pinho, G.; Gignac, J.; Almirall, J.; Lapointe, L. Randomized controlled trials: Do they have external validity for patients with multiple comorbidities? Ann. Fam. Med. 2006, 4, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Garfield, S.; Malozowski, S.; Chin, M.; Narayan, V.; Glasgow, R.; Green, L.W.; Hiss, R.G.; Krumholz, H.M. Diabetes Mellitus Interagency Coordinating Committee (DIMCC) Translation Conference Working Group. Considerations for diabetes translational research in real-world settings. Diabetes Care 2003, 26, 2670–2674. [Google Scholar] [CrossRef] [PubMed]
- Admundson, H.A.; Butcher, M.K.; Gohdes, D.; Hall, T.O.; Harwell, T.S.; Helgerson, S.D.; Vanderwood, K.K. The Montana Cardiovascular Disease and Prevention Program Workgroup. Translating the diabetes prevention program into practice in the general community: Findings from the Montana cardiovascular disease and diabetes prevention program. Diabetes Educ. 2009, 35, 209–223. [Google Scholar] [CrossRef]
- Seidel, M.C.; Powell, R.O.; Zgibor, J.C.; Siminerio, L.M.; Piatt, G.A. Translating the diabetes prevention program into an urban medically underserved community. Diabetes Care 2008, 31, 684–689. [Google Scholar] [CrossRef]
- Ali, M.L.; Echouffo-Tcheugui, J.; Williamson, D.F. How effective were lifestyle interventions in real-world settings that were modeled on the Diabetes Prevention Program? Health Aff. 2012, 31, 67–75. [Google Scholar] [CrossRef]
- Diabetes Prevention Program Reserach Group. The 10-year cost-effectiveness of lifestyle intervention or metformin for diabetes prevention: An intent-to-treat analysis of the DPP/DPPOS. Diabetes Care 2012, 35, 723–730. [Google Scholar] [CrossRef]
- Ackermann, R.T.; Marrero, D.G. Adapting the Diabetes Prevention Program lifestyle intervention for delivery in the community: The YMCA model. Diabetes Educ. 2007, 33, 69–78. [Google Scholar] [CrossRef]
- Johns, D.J.; Hartmann-Boyce, J.; Jebb, S.A.; Aveyard, P. Behavioural Weight Management Review Group. Weight change among people randomized to minimal intervention control groups in weight loss trials. Obesity (Silver Spring) 2016, 24, 772–780. [Google Scholar] [CrossRef]

| Hours | Topics |
|---|---|
| 3.5 months (weekly) | Session 1. Welcome to the Lifestyle Balance Program® Session 2. Be a fat and calorie detective Session 2.1 Reading a nutrition label Session 2.2 Cooking demonstration and food weighing * Session 3. Move those muscles Session 4. Food groups and portion sizes * Session 5. Healthy eating and calorie balance tilting Session 6. Take control of what’s around you Session 7. How to design your own menu (Mexican System for Food Equivalents) * Session 8. Problem solving Session 9. Four key points to eating out healthily and the slippery slope of lifestyle change Session 10. Make social cues work for you and activity plan kickoff Session 11. You can manage stress Session 12. How to feel motivated |
| 3.5–6 months (biweekly) | Session 13. Obesity risks * Session 14. Diabetes prevention * Session 15.1 Heart health and cholesterol * Session 15.2 Heart health and hypertension * Session 16. Relationship between obesity and cancer * |
| 6–12 months (monthly) | Session 17. Getting ready for long-term self-control and adjust your thoughts for long-term self-control Session 18. More volume, less calories and conscious eating Session 19. Strengthen your exercise program Session 20. Stretching: the truth about flexibility Session 21. Rise for your health Session 22. Looking at the past and looking at the future |
| Variable | Clinic 1 n = 35 | Clinic 2 n = 25 | Clinic 3 n = 21 | Clinic 4 n = 25 | Clinic 5 n = 27 |
|---|---|---|---|---|---|
| Female, n (%) | 26 (74.2) | 18 (72.0) | 18 (85.7) | 20 (80.0) | 24 (88.9) |
| Age, mean ± SD, year | 43.5 ± 11.7 | 44.8 ± 9.84 | 48.1 ± 8.05 | 47.2 ± 8.10 | 46.4 ± 9.94 |
| Education, n (%) | |||||
| Elementary school | 2 (5.71) | 10 (40.0) | 4 (19.1) | 5 (20.0) | 3 (11.1) |
| High school | 15 (42.9) | 7 (28.0) | 7 (33.3) | 8 (32.0) | 7 (25.9) |
| College/University | 14 (40.0) | 6 (24.0) | 9 (42.9) | 9 (36.0) | 15 (55.6) |
| Postgraduate | 4 (11.4) | 2 (8.00) | 1 (4.76) | 3 (12.0) | 2 (7.41) |
| Monthly income, n (%) a | |||||
| <U.S. $296 | 11 (31.4) | 8 (32.0) | 1 (4.76) | 7 (28.0) | 3 (11.1) |
| U.S. $296 to $592 | 4 (11.4) | 6 (24.0) | 6 (28.6) | 9 (36.0) | 11 (40.7) |
| U.S. $592 to $1,185 | 11 (31.4) | 4 (16.0) | 12 (57.1) | 7 (28.0) | 7 (25.9) |
| U.S. $1,185 to $1,777 | 6 (17.1) | 4 (16.0) | 1 (4.76) | 0 (0.00) | 1 (3.70) |
| ≥U.S. $1,777 | 3 (8.57) | 3 (12.0) | 1 (4.76) | 2 (8.00) | 5 (18.5) |
| Marital status, n (%) | |||||
| Single | 10 (28.6) | 4 (16.0) | 2 (9.52) | 5 (20.0) | 9 (33.3) |
| Married | 21 (60.0) | 19 (76.0) | 17 (81.0) | 16 (64.0) | 16 (59.3) |
| Divorced | 3 (8.57) | 2 (8.00) | 2 (9.52) | 4 (16.0) | 0 (0.00) |
| Widowed | 1 (2.86) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 2 (7.41) |
| Diseases by self-report, n (%) | |||||
| Type 2 diabetes | 2 (5.71) | 5 (20.0) | 5 (23.8) | 4 (16.0) | 4 (14.8) |
| Hypertension | 6 (17.1) | 6 (24.0) | 8 (38.1) | 6 (24.0) | 6 (22.2) |
| Abnormal lipids | 2 (5.71) | 3 (12.0) | 0 (0.00) | 2 (8.00) | 1 (3.70) |
| Hypothyroidism | 4 (11.4) 3 | 1 (4.00) 3 | 7 (33.3) 1,2,4 | 1 (4.00) 3 | 5 (18.5) |
| Depression | 1 (2.86) | 2 (8.00) | 2 (9.52) | 1 (4.00) | 0 (0.00) |
| Hypoglycemic drugs, n (%) b | 1 (2.86) | 5 (20.0) | 5 (23.8) | 4 (16.0) | 4 (14.8) |
| Height, mean ± SD, m | 1.63 ± 0.08 | 1.63 ± 0.07 | 1.60 ± 0.07 | 1.63 ± 0.07 | 1.61 ± 0.07 |
| Weight, mean ± SD, kg | 92.6 ± 19.6 | 91.7 ± 11.8 | 86.8 ± 13.2 | 94.7 ± 21.2 | 85.8 ± 13.0 |
| Body mass index, mean ± SD, kg/m2 | 34.7 ± 5.20 | 34.6 ± 3.98 | 34.2 ± 6.11 | 35.4 ± 6.54 | 33.2 ± 5.17 |
| Waist circumference, mean ± SD, cm | 107 ± 13.0 | 109 ± 10.6 | 105 ± 12.4 | 110 ± 16.5 | 103 ± 10.5 |
| Body fat percentage, mean ± SD c | 44.6 ± 5.42 | 44.7 ± 5.78 | 45.2 ± 5.57 | 44.8 ± 5.32 | 45.3 ± 6.24 |
| Systolic blood pressure, mean ± SD, mmHg | 122 ± 12.3 | 119 ± 11.8 | 130 ± 18.3 | 125 ± 16.6 | 119 ± 11.1 |
| Diastolic blood pressure, mean ± SD, mmHg | 77.3 ± 6.81 | 74.8 ± 9.98 | 78.0 ± 12.2 | 75.8 ± 11.7 | 75.7 ± 7.75 |
| Fasting glucose, mean ± SD, mg/dL | 86.3 ± 32.2 | 97.9 ± 42.6 | 86.4 ± 15.4 | 91.2 ± 24.9 | 84.7 ± 26.2 |
| Fasting insulin, median (P25, P75), µU/mL c | 6.63 (5.11, 11.8) | 6.06 (4.39, 11.9) | 6.07 (4.49, 8.60) | 5.27 (3.24, 8.17) | 4.93 (4.06, 8.46) |
| HOMA-IR, median (P25, P75) d | 1.31 (1.01, 2.39) | 1.21 (0.81, 2.97) | 1.15 (0.86, 2.37) | 1.06 (0.60, 1.86) | 0.95 (0.71, 1.91) |
| Triglycerides, mean ± SD, mg/dL | 138 ± 81.9 | 161 ± 71.6 | 177 ± 156 | 164 ± 68.4 | 135 ± 74.6 |
| Total cholesterol, mean ± SD, mg/dL | 169 ± 27.5 4 | 194 ± 50.2 | 181 ± 57.4 | 197 ± 34.0 1 | 190 ± 46.9 |
| High-density lipoprotein-cholesterol (HDL-cholesterol), mean ± SD, mg/dL | 53.0 ± 10.5 3 | 50.6 ± 17.4 | 42.2 ± 12.9 1 | 45.9 ± 11.3 | 43.6 ± 11.8 |
| Low-density lipoprotein-cholesterol (LDL-cholesterol), mean ± SD, mg/dL | 89.3 ± 28.1 4,5 | 111 ± 44.3 | 103 ± 36.5 | 119 ± 31.5 1 | 119 ± 45.7 1 |
| Aspartate aminotransferase, mean ± SD, U/L | 14.9 ± 5.78 | 16.3 ± 3.39 | 18.9 ± 6.98 | 18.2 ± 8.57 | 20.6 ± 9.99 d |
| Alanine aminotransferase, mean ± SD, U/L | 14.7 ± 4.20 5 | 17.1 ± 4.54 | 15.6 ± 4.64 | 17.8 ± 6.54 | 19.9 ± 7.58 1 |
| Metabolic syndrome, n (%) e | 12 (34.3) | 15 (60.0) | 13 (61.9) | 15 (60.0) | 10 (37.0) |
| Metabolically healthy, n (%) f | 23 (65.8) | 10 (40.0) | 8 (38.1) | 10 (40.0) | 16 (59.3) |
| Metabolically unhealthy, n (%) g | 12 (34.3) | 15 (60.0) | 13 (61.9) | 15 (60.0) | 11 (40.7) |
| Variable | Baseline Mean ± SD | 6 Months Mean ± SD | Difference to 6 Months Mean ± SD | pa Value | pb Value |
|---|---|---|---|---|---|
| BMI (kg/m2) | <0.001 | ||||
| Clinic 1 | 34.7 ± 5.20 | 31.8 ± 5.34 | −2.97 ± 2.65 2,3,5 | <0.0001 | |
| Clinic 2 | 34.6 ± 3.98 | 33.4 ± 4.26 | −1.28 ± 1.51 1 | <0.001 | |
| Clinic 3 | 34.2 ± 6.12 | 33.1 ± 6.11 | −1.07 ± 1.87 1 | 0.015 | |
| Clinic 4 | 35.4 ± 6.54 | 33.5 ± 6.02 | −1.90 ± 1.89 | <0.0001 | |
| Clinic 5 | 33.2 ± 5.17 | 32.0 ± 4.96 | −1.26 ± 1.55 1 | <0.001 | |
| Waist circumference (cm) | 0.023 | ||||
| Clinic 1 | 107 ± 13.0 | 98.0 ± 13.3 | −9.44 ± 6.86 3 | <0.0001 | |
| Clinic 2 | 109 ± 10.6 | 103 ± 9.40 | −5.81 ± 5.86 | <0.0001 | |
| Clinic 3 | 105 ± 12.4 | 101 ± 11.7 | −3.76 ± 5.91 1 | 0.009 | |
| Clinic 4 | 110 ± 16.5 | 103 ± 15.9 | −7.44 ± 5.59 | <0.0001 | |
| Clinic 5 | 103 ± 10.5 | 96.6 ± 9.40 | −6.81 ± 6.90 | <0.0001 | |
| Body fat percentage c | 0.006 | ||||
| Clinic 1 | 44.5 ± 5.41 | 40.5 ± 7.62 | −4.03 ± 4.23 3,4,5 | <0.0001 | |
| Clinic 2 | 44.5 ± 5.84 | 42.5 ± 6.30 | −2.00 ± 2.15 | <0.001 | |
| Clinic 3 | 45.1 ± 5.57 | 43.8 ± 5.56 | −1.40 ± 2.35 1 | 0.015 | |
| Clinic 4 | 44.8 ± 5.32 | 43.1 ± 4.79 | −1.69 ± 2.18 1 | <0.0001 | |
| Clinic 5 | 45.3 ± 6.24 | 43.6 ± 5.99 | −1.75 ± 2.31 1 | <0.001 | |
| Systolic blood pressure (mmHg) | 0.509 | ||||
| Clinic 1 | 122 ± 12.2 | 120 ± 12.2 | −2.83 ± 13.2 | 0.213 | |
| Clinic 2 | 119 ± 11.8 | 113 ± 12.4 | −6.04 ± 8.96 | <0.01 | |
| Clinic 3 | 130 ± 18.3 | 123 ± 16.0 | −6.67 ± 10.9 | 0.011 | |
| Clinic 4 | 125 ± 16.6 | 117 ± 13.7 | −8.04 ± 15.7 | 0.014 | |
| Clinic 5 | 119 ± 11.1 | 116 ± 14.5 | −3.19 ± 12.3 | 0.190 | |
| Diastolic blood pressure (mmHg) | 0.739 | ||||
| Clinic 1 | 77.3 ± 6.81 | 72.8 ± 9.30 | −4.54 ± 9.14 | 0.006 | |
| Clinic 2 | 74.8 ± 9.98 | 70.2 ± 10.2 | −4.60 ± 9.29 | 0.021 | |
| Clinic 3 | 78.0 ± 12.2 | 75.0 ± 7.59 | −3.10 ± 8.61 | 0.115 | |
| Clinic 4 | 75.8 ± 11.7 | 73.2 ± 9.27 | −2.56 ± 8.52 | 0.146 | |
| Clinic 5 | 75.7 ± 7.75 | 71.9 ± 9.83 | −3.81 ± 8.95 | 0.036 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Armenta-Guirado, B.; Martínez-Contreras, T.; Candia-Plata, M.C.; Esparza-Romero, J.; Martínez-Mir, R.; Haby, M.M.; Valencia, M.E.; Díaz-Zavala, R.G. Effectiveness of the Diabetes Prevention Program for Obesity Treatment in Real World Clinical Practice in a Middle-Income Country in Latin America. Nutrients 2019, 11, 2324. https://doi.org/10.3390/nu11102324
Armenta-Guirado B, Martínez-Contreras T, Candia-Plata MC, Esparza-Romero J, Martínez-Mir R, Haby MM, Valencia ME, Díaz-Zavala RG. Effectiveness of the Diabetes Prevention Program for Obesity Treatment in Real World Clinical Practice in a Middle-Income Country in Latin America. Nutrients. 2019; 11(10):2324. https://doi.org/10.3390/nu11102324
Chicago/Turabian StyleArmenta-Guirado, Brianda, Teresita Martínez-Contreras, Maria C. Candia-Plata, Julián Esparza-Romero, Raúl Martínez-Mir, Michelle M. Haby, Mauro E. Valencia, and Rolando G. Díaz-Zavala. 2019. "Effectiveness of the Diabetes Prevention Program for Obesity Treatment in Real World Clinical Practice in a Middle-Income Country in Latin America" Nutrients 11, no. 10: 2324. https://doi.org/10.3390/nu11102324
APA StyleArmenta-Guirado, B., Martínez-Contreras, T., Candia-Plata, M. C., Esparza-Romero, J., Martínez-Mir, R., Haby, M. M., Valencia, M. E., & Díaz-Zavala, R. G. (2019). Effectiveness of the Diabetes Prevention Program for Obesity Treatment in Real World Clinical Practice in a Middle-Income Country in Latin America. Nutrients, 11(10), 2324. https://doi.org/10.3390/nu11102324






