B-Cell Activating Factor Enhances Hepatocyte-Driven Angiogenesis via B-Cell CLL/Lymphoma 10/Nuclear Factor-KappaB Signaling during Liver Regeneration
Abstract
:1. Introduction
2. Results
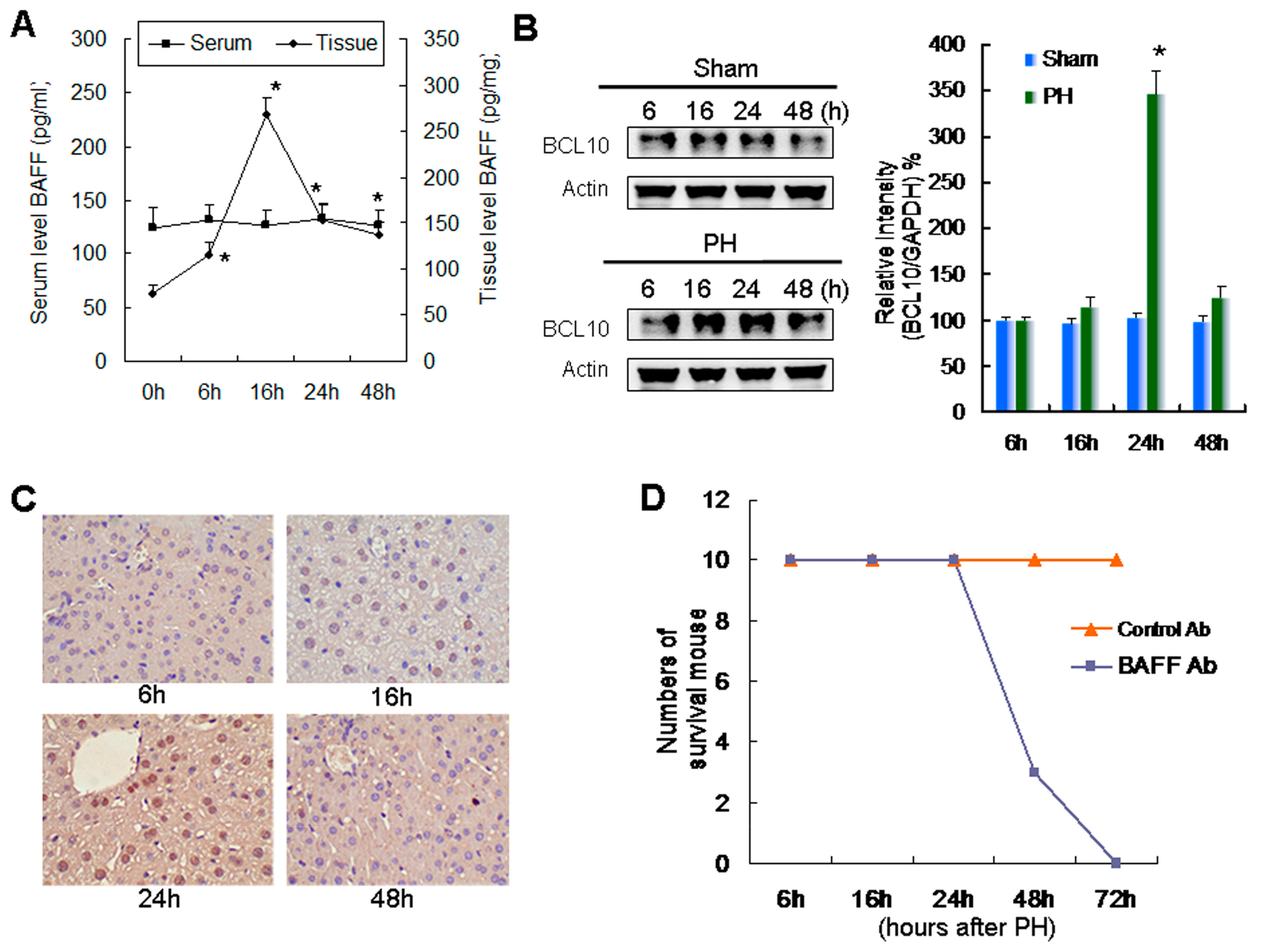
2.1. Relationship between Increased Expression of BAFF and BCL10 and Survival during Liver Regeneration
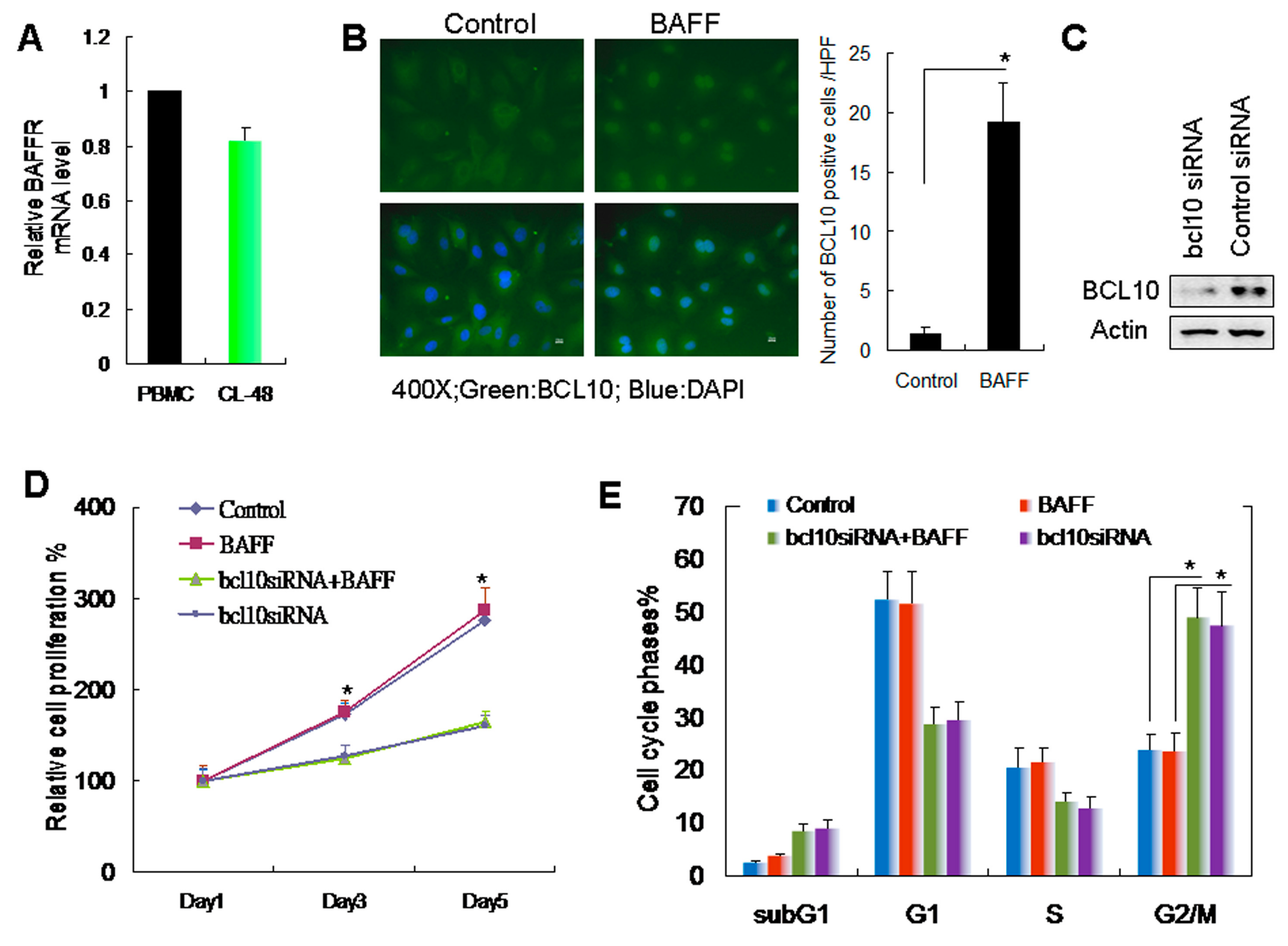
2.2. BAFF/BCL10 Signaling Plays an Important Role in Hepatocyte Proliferation
2.3. BAFF Promoted Hepatocyte-Mediated Angiogenesis
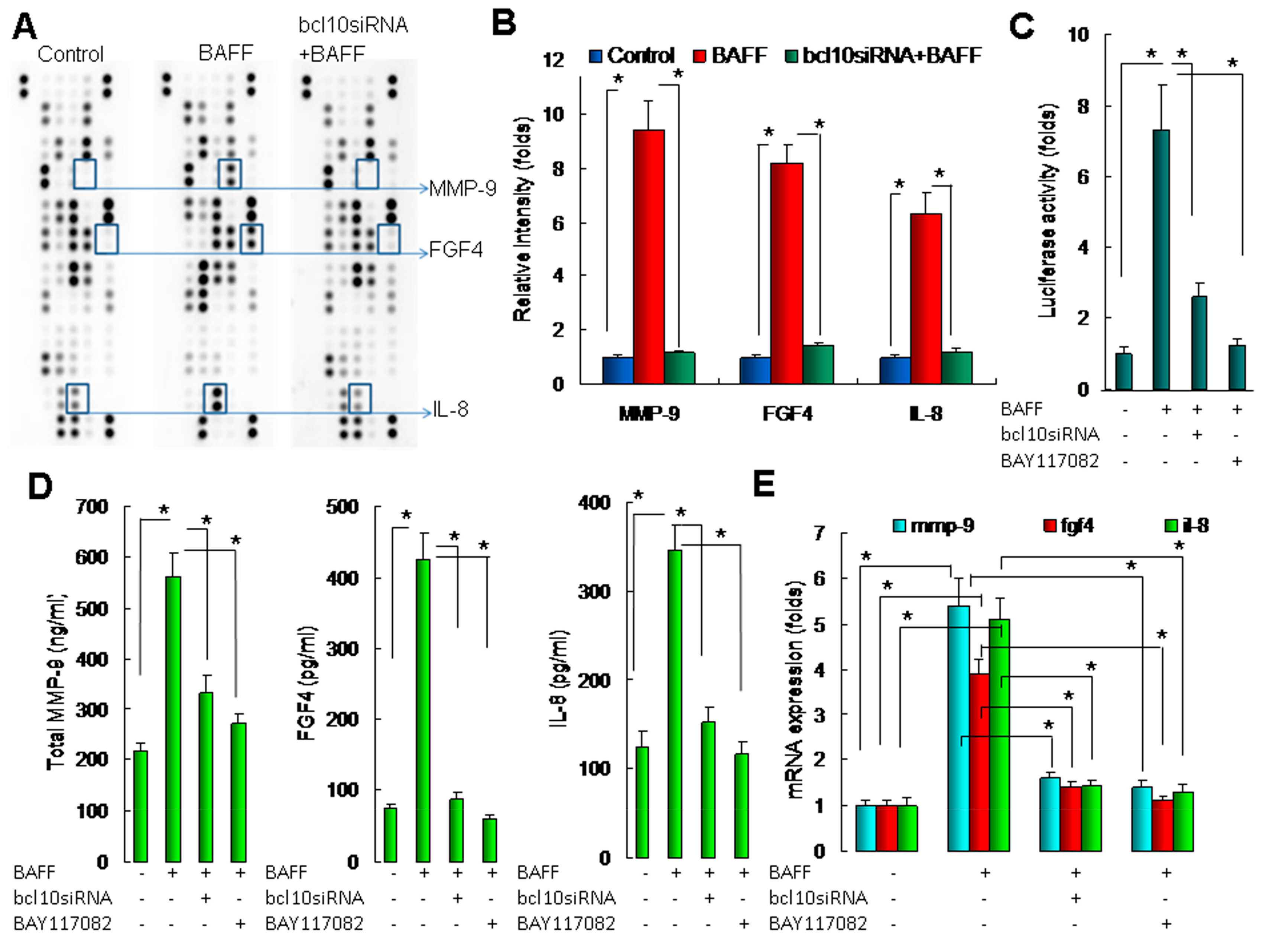
2.4. BAFF/BCL10/NF-κB Signaling in Hepatocytes Enhanced the Expression of Angiogenesis-Related Factors
2.5. Downregulation of BAFF Reduced Angiogenesis and Hepatocyte Proliferation in a Liver Regeneration Model
3. Discussion
4. Materials and Methods
4.1. Animals and Grouping
4.2. Surgical Procedures
4.3. Tissue Processing
4.4. Immunohistochemical Staining and Quantification
4.5. BAFF, Matrix Metalloproteinase (MMP)-9, Fibroblast Growth Factor 4 (FGF4), and Interleukin-8 (IL-8) Determination
4.6. Culture of CL-48 Hepatocytes and Human Umbilical Vein Endothelial Cells (HUVECs)
4.7. BAFF Protein and Chemical Inhibitors
4.8. Preparation of Conditioned Medium (CM)
4.9. Cell Growth Determination
4.10. Filamentous Actin (F-actin) Fluorescence Staining
4.11. HUVEC Monolayer Permeability Assays
4.12. HUVEC Migration Assays
4.13. HUVEC Tube Formation Assays
4.14. HUVEC Proliferation Tests
4.15. Protein Array Analysis
4.16. RNA Interference
4.17. Real-Time Quantitative Reverse Transcription Polymerase Chain Reaction (RT-PCR)
4.18. NF-κB Promoter Reporter Assays
4.19. Western Blotting
4.20. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Higgins, G.; Anderson, R.E.; Higgins, G.M.; Anderson, R.M. Experimental pathology of the liver: Restoration of the liver of the white rat following partial surgical removal. Arch. Pathol. 1931, 12, 186–202. [Google Scholar]
- Fausto, N.; Campbell, J.S.; Riehle, K.J. Liver regeneration. Hepatology 2006, 43, S45–S53. [Google Scholar] [CrossRef]
- Yamada, Y.; Kirillova, I.; Peschon, J.J.; Fausto, N. Initiation of liver growth by tumor necrosis factor: Deficient liver regeneration in mice lacking type I tumor necrosis factor receptor. Proc. Natl. Acad. Sci. USA 1997, 94, 1441–1446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, B.S.; Nolan, D.J.; Butler, J.M.; James, D.; Babazadeh, A.O.; Rosenwaks, Z.; Mittal, V.; Kobayashi, H.; Shido, K.; Lyden, D.; et al. Inductive angiocrine signals from sinusoidal endothelium are required for liver regeneration. Nature 2010, 468, 310–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michalopoulos, G.K. Hepatostat: Liver regeneration and normal liver tissue maintenance. Hepatology 2017, 65, 1384–1392. [Google Scholar] [CrossRef] [PubMed]
- Kron, P.; Linecker, M.; Limani, P.; Schlegel, A.; Kambakamba, P.; Lehn, J.M.; Nicolau, C.; Graf, R.; Humar, B.; Clavien, P.A. Hypoxia-driven Hif2a coordinates mouse liver regeneration by coupling parenchymal growth to vascular expansion. Hepatology 2016, 64, 2198–2209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, J.; Srivastava, K.; Wieland, M.; Runge, A.; Mogler, C.; Besemfelder, E.; Terhardt, D.; Vogel, M.J.; Cao, L.; Korn, C.; et al. Endothelial cell-derived angiopoietin-2 controls liver regeneration as a spatiotemporal rheostat. Science 2014, 343, 416–419. [Google Scholar] [CrossRef] [PubMed]
- Karpusas, M.; Cachero, T.G.; Qian, F.; Boriak-Sjodin, A.; Mullen, C.; Strauch, K.; Hsu, Y.M.; Kalled, S.L. Crystal structure of extracellular human BAFF, a TNF family member that stimulates B lymphocytes. J. Mol. Biol. 2002, 315, 1145–1154. [Google Scholar] [CrossRef] [PubMed]
- Kuo, S.H.; Tsai, H.J.; Lin, C.W.; Yeh, K.H.; Lee, H.W.; Wei, M.F.; Shun, C.T.; Wu, M.S.; Hsu, P.N.; Chen, L.T.; et al. The B-cell-activating factor signalling pathway is associated with Helicobacter pylori independence in gastric mucosa-associated lymphoid tissue lymphoma without t(11;18)(q21;q21). J. Pathol. 2017, 241, 420–433. [Google Scholar] [CrossRef] [PubMed]
- Kuo, S.H.; Yeh, P.Y.; Chen, L.T.; Wu, M.S.; Lin, C.W.; Yeh, K.H.; Tzeng, Y.S.; Chen, J.Y.; Hsu, P.N.; Lin, J.T.; et al. Overexpression of B cell-activating factor of TNF family (BAFF) is associated with Helicobacter pylori-independent growth of gastric diffuse large B-cell lymphoma with histologic evidence of MALT lymphoma. Blood 2008, 112, 2927–2934. [Google Scholar] [CrossRef]
- Kuo, S.H.; Chou, C.H.; Cheng, A.L.; Wang, C.W.; Chen, Y.H.; Chen, R.J. Expression of BCL10 in cervical cancer has a role in the regulation of cell growth through the activation of NF-κB-dependent cyclin D1 signaling. Gynecol. Onco. 2012, 126, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Wang, M.; Guo, Y.; Ju, S. The correlation between non-Hodgkin lymphoma and expression levels of B-cell activating factor and its receptors. Adv. Clin. Exp. Med. 2016, 25, 837–844. [Google Scholar] [CrossRef] [PubMed]
- Puri, P.; Sanyal, A.J. The BAFFling problem of B cell-activating factor in nonalcoholic fatty liver disease. Hepatol. Int. 2013, 7, 309–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawasaki, K.; Abe, M.; Tada, F.; Tokumoto, Y.; Chen, S.; Miyake, T.; Furukawa, S.; Matsuura, B.; Hiasa, Y.; Onji, M. Blockade of B-cell-activating factor signaling enhances hepatic steatosis induced by a high-fat diet and improves insulin sensitivity. Lab. Invest. 2013, 93, 311–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiu, W.; David, D.; Zhou, B.; Chu, P.G.; Zhang, B.; Wu, M.; Xiao, J.; Han, T.; Zhu, Z.; Wang, T.; et al. Down-regulation of growth arrest DNA damage-inducible gene 45beta expression is associated with human hepatocellular carcinoma. Am. J. Pathol. 2003, 162, 1961–1974. [Google Scholar] [CrossRef]
- Klein, B.; Tarte, K.; Jourdan, M.; Mathouk, K.; Moreaux, J.; Jourdan, E.; Legouffe, E.; de Vos, J.; Rossi, J.F. Survival and proliferation factors of normal and malignant plasma cells. Int. J. Hematol. 2003, 78, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Vogel, C.F.; Wu, D.; Matsumura, F. Non-genomic action of TCDD to induce inflammatory responses in HepG2 human hepatoma cells and in liver of C57BL/6J mice. Biol. Chem. 2010, 391, 1205–1219. [Google Scholar] [CrossRef]
- Patke, A.; Mecklenbräuker, I.; Tarakhovsky, A. Survival signaling in resting B cells. Curr. Opin. Immunol. 2004, 16, 251–255. [Google Scholar] [CrossRef]
- Yu, M.; Chen, Y.; He, Y.; Podd, A.; Fu, G.; Wright, J.A.; Kleiman, E.; Khan, W.N.; Wen, R.; Wang, D. Critical role of B cell lymphoma 10 in BAFF-regulated NF-κB activation and survival of anergic B cells. J. Immunol. 2012, 189, 5185–5193. [Google Scholar] [CrossRef]
- Wang, W.; Du, Z.; Yan, J.; Ma, D.; Shi, M.; Zhang, M.; Peng, C.; Li, H. Mesenchymal stem cells promote liver regeneration and prolong survival in small-for-size liver grafts: Involvement of C-Jun N-terminal kinase, cyclin D1, and NF-κB. PLoS ONE 2014, 9, e112532. [Google Scholar]
- Fragioudaki, M.; Tsirakis, G.; Pappa, C.A.; Aristeidou, I.; Tsioutis, C.; Alegakis, A.; Kyriakou, D.S.; Stathopoulos, E.N.; Alexandrakis, M.G. Serum BAFF levels are related to angiogenesis and prognosis in patients with multiple myeloma. Leuk. Res. 2012, 36, 1004–1008. [Google Scholar] [CrossRef] [PubMed]
- Bolkun, L.; Lemancewicz, D.; Jablonska, E.; Kulczynska, A.; Bolkun-Skornicka, U.; Kloczko, J.; Dzieciol, J. BAFF and APRIL as TNF superfamily molecules and angiogenesis parallel progression of human multiple myeloma. Ann. Hematol. 2014, 93, 635–644. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.H.; Lee, J.; Lee, J.W.; Choi, W.S.; Moon, E.Y. B cell activating factor-dependent expression of vascular endothelial growth factor in MH7A human synoviocytes stimulated with tumor necrosis factor-α. Int. Immunopharmacol. 2013, 17, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Uda, Y.; Hirano, T.; Son, G.; Iimuro, Y.; Uyama, N.; Yamanaka, J.; Mori, A.; Arii, S.; Fujimoto, J. Angiogenesis is crucial for liver regeneration after partial hepatectomy. Surgery 2013, 153, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Melgar-Lesmes, P.; Edelman, E.R. Monocyte-endothelial cell interactions in the regulation of vascular sprouting and liver regeneration in mouse. J. Hepatol. 2015, 63, 917–925. [Google Scholar] [CrossRef] [Green Version]
- Vu, T.H.; Shipley, J.M.; Bergers, G.; Berger, J.E.; Helms, J.A.; Hanahan, D.; Shapiro, S.D.; Senior, R.M.; Werb, Z. MMP-9/gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes. Cell 1998, 93, 411–422. [Google Scholar] [CrossRef]
- Kollet, O.; Shivtiel, S.; Chen, Y.Q.; Suriawinata, J.; Thung, S.N.; Dabeva, M.D.; Kahn, J.; Spiegel, A.; Dar, A.; Samira, S.; et al. HGF, SDF-1, and MMP-9 are involved in stress-induced human CD34+ stem cell recruitment to the liver. J. Clin. Invest. 2003, 112, 160–169. [Google Scholar] [CrossRef]
- Zhou, B.; Fan, Y.; Rao, J.; Xu, Z.; Liu, Y.; Lu, L.; Li, G. Matrix metalloproteinases-9 deficiency impairs liver regeneration through epidermal growth factor receptor signaling in partial hepatectomy mice. J. Surg. Res. 2015, 197, 201–209. [Google Scholar] [CrossRef]
- Strieter, R.M.; Kunkel, S.L.; Elner, V.M.; Martonyi, C.L.; Koch, A.E.; Polverini, P.J.; Elner, S.G. Interleukin-8. A corneal factor that induces neovascularization. Am. J. Pathol. 1992, 141, 1279–1284. [Google Scholar]
- Koch, A.E.; Polverini, P.J.; Kunkel, S.L.; Harlow, L.A.; Di Pietro, L.A.; Elner, V.M.; Elner, S.G.; Strieter, R.M. Interleukin-8 as a macrophage-derived mediator of angiogenesis. Science 1992, 258, 1798–1801. [Google Scholar] [CrossRef]
- Ueda, T.; Sakabe, T.; Oka, M.; Maeda, Y.; Nishida, M.; Murakami, F.; Maekawa, T. Levels of interleukin (IL)-6, IL-8, and IL-1 receptor antagonist in the hepatic vein following liver surgery. Hepatogastroenterology 2000, 47, 1048–1051. [Google Scholar] [PubMed]
- Yokoo, T.; Kitamura, M. Dual regulation of IL-1 beta-mediated matrix metalloproteinase-9 expression in mesangial cells by NF-kappa B and AP-1. Am. J. Physiol. 1996, 270, F123–F130. [Google Scholar] [CrossRef] [PubMed]
- Mukaida, N.; Mahe, Y.; Matsushima, K. Cooperative interaction of nuclear factor-kappa B- and cis-regulatory enhancer binding protein-like factor binding elements in activating the interleukin-8 gene by pro-inflammatory cytokines. J. Biol. Chem. 1990, 265, 21128–21133. [Google Scholar] [PubMed]
- Poisson, J.; Lemoinne, S.; Boulanger, C.; Durand, F.; Moreau, R.; Valla, D.; Rautou, P.E. Liver sinusoidal endothelial cells: Physiology and role in liver diseases. J. Hepatol. 2017, 66, 212–227. [Google Scholar] [CrossRef] [PubMed]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chou, C.-H.; Ho, C.-M.; Lai, S.-L.; Chen, C.-N.; Wu, Y.-M.; Shun, C.-T.; Wen, W.-F.; Lai, H.-S. B-Cell Activating Factor Enhances Hepatocyte-Driven Angiogenesis via B-Cell CLL/Lymphoma 10/Nuclear Factor-KappaB Signaling during Liver Regeneration. Int. J. Mol. Sci. 2019, 20, 5022. https://doi.org/10.3390/ijms20205022
Chou C-H, Ho C-M, Lai S-L, Chen C-N, Wu Y-M, Shun C-T, Wen W-F, Lai H-S. B-Cell Activating Factor Enhances Hepatocyte-Driven Angiogenesis via B-Cell CLL/Lymphoma 10/Nuclear Factor-KappaB Signaling during Liver Regeneration. International Journal of Molecular Sciences. 2019; 20(20):5022. https://doi.org/10.3390/ijms20205022
Chicago/Turabian StyleChou, Chia-Hung, Cheng-Maw Ho, Shou-Lun Lai, Chiung-Nien Chen, Yao-Ming Wu, Chia-Tung Shun, Wen-Fen Wen, and Hong-Shiee Lai. 2019. "B-Cell Activating Factor Enhances Hepatocyte-Driven Angiogenesis via B-Cell CLL/Lymphoma 10/Nuclear Factor-KappaB Signaling during Liver Regeneration" International Journal of Molecular Sciences 20, no. 20: 5022. https://doi.org/10.3390/ijms20205022
APA StyleChou, C.-H., Ho, C.-M., Lai, S.-L., Chen, C.-N., Wu, Y.-M., Shun, C.-T., Wen, W.-F., & Lai, H.-S. (2019). B-Cell Activating Factor Enhances Hepatocyte-Driven Angiogenesis via B-Cell CLL/Lymphoma 10/Nuclear Factor-KappaB Signaling during Liver Regeneration. International Journal of Molecular Sciences, 20(20), 5022. https://doi.org/10.3390/ijms20205022





