Biocide Potentiation Using Cinnamic Phytochemicals and Derivatives
Abstract
1. Introduction
2. Results
2.1. Phytochemicals/Derivatives Potentiate Biocides in Growth Control
2.2. Biocide-Phytochemical/Derivative Combinations Reduced Early Sessile Bacteria
2.3. Phytochemicals/Derivatives Effects on Bacterial Surface Hydrophobicity
3. Discussion
3.1. Phytochemicals/Derivatives Combination with Lactic Acid
3.2. Phytochemicals/Derivatives Combination with Cetyltrimethy Lammonium Bromide
4. Materials and Methods
4.1. Chemicals
4.2. Microorganisms, Culture Conditions, and Test Solutions
4.3. Bacterial Susceptibility by the Checkerboard Methodology
4.4. Efficacy Against Early Sessile Cells
4.5. Bacterial Surface Hydrophobicity
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Oniciuc, E.-A.; Likotrafiti, E.; Alvarez-Molina, A.; Prieto, M.; López, M.; Alvarez-Ordóñez, A. Food processing as a risk factor for antimicrobial resistance spread along the food chain. Curr. Opin. Food Sci. 2019, 30, 21–26. [Google Scholar] [CrossRef]
- Touat, M.; Opatowski, M.; Brun-Buisson, C.; Cosker, K.; Guillemot, D.; Salomon, J.; Tuppin, P.; de Lagasnerie, G.; Watier, L. A payer perspective of the hospital inpatient additional care costs of antimicrobial resistance in france: A matched case–control study. Appl. Health Econ. Health Policy 2019, 17, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Cassini, A.; Hogberg, L.D.; Plachouras, D.; Quattrocchi, A.; Hoxha, A.; Simonsen, G.S.; Colomb-Cotinat, M.; Kretzschmar, M.E.; Devleesschauwer, B.; Cecchini, M.; et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: A population-level modelling analysis. Lancet Infect. Dis. 2019, 19, 56–66. [Google Scholar] [CrossRef]
- European Comission. EU Action on Antimicrobial Resistance. Available online: https://ec.europa.eu/health/amr/antimicrobial-resistance_en (accessed on 17 October 2019).
- ECDC/EMEA. ECDC/EMEA Joint Technical Report: The Bacterial Challenge: Time to React. Available online: https://www.ecdc.europa.eu/en/publications-data/ecdcemea-joint-technical-report-bacterial-challenge-time-react (accessed on 30 October 2019).
- Scallan, E.; Hoekstra, R.M.; Angulo, F.J.; Tauxe, R.V.; Widdowson, M.-A.; Roy, S.L.; Jones, J.L.; Griffin, P.M. Foodborne illness acquired in the United States--major pathogens. Emerg. Infect. Dis. 2011, 17, 7–15. [Google Scholar] [CrossRef]
- Simões, M.; Simões, L.C.; Vieira, M.J. A review of current and emergent biofilm control strategies. LWT—Food Sci. Technol. 2010, 43, 573–583. [Google Scholar]
- Campana, R.; Casettari, L.; Fagioli, L.; Cespi, M.; Bonacucina, G.; Baffone, W. Activity of essential oil-based microemulsions against Staphylococcus aureus biofilms developed on stainless steel surface in different culture media and growth conditions. Int. J. Food Microbiol. 2017, 241, 132–140. [Google Scholar] [CrossRef]
- Humayoun, S.B.; Hiott, L.M.; Gupta, S.K.; Barrett, J.B.; Woodley, T.A.; Johnston, J.J.; Jackson, C.R.; Frye, J.G. An assay for determining the susceptibility of Salmonella isolates to commercial and household biocides. PloS ONE 2018, 13, 1–24. [Google Scholar] [CrossRef]
- Beier, R.C.; Harvey, R.B.; Poole, T.L.; Hume, M.E.; Crippen, T.L.; Highfield, L.D.; Alali, W.Q.; Andrews, K.; Anderson, R.C.; Nisbet, D.J. Interactions of organic acids with vancomycin-resistant Enterococcus faecium isolated from community wastewater in Texas. J. Appl. Microbiol. 2019, 126, 480–488. [Google Scholar] [CrossRef]
- Gutiérrez-del-Río, I.; Fernández, J.; Lombó, F. Plant nutraceuticals as antimicrobial agents in food preservation: Terpenoids, polyphenols and thiols. Int. J. Antimicrob. Ag. 2018, 52, 309–315. [Google Scholar] [CrossRef]
- Abriouel, H.; Lavilla Lerma, L.; Perez Montoro, B.; Alonso, E.; Knapp, C.W.; Caballero Gomez, N.; Galvez, A.; Benomar, N. Efficacy of “HLE”-a multidrug efflux-pump inhibitor-as a disinfectant against surface bacteria. Env. Res. 2018, 165, 133–139. [Google Scholar] [CrossRef]
- Costa, S.S.; Ntokou, E.; Martins, A.; Viveiros, M.; Pournaras, S.; Couto, I.; Amaral, L. Identification of the plasmid-encoded qacA efflux pump gene in meticillin-resistant Staphylococcus aureus (MRSA) strain HPV107, a representative of the MRSA Iberian clone. Int. J. Antimicrob. Ag. 2010, 36, 557–561. [Google Scholar] [CrossRef] [PubMed]
- Fraise, A.P. Biocide abuse and antimicrobial resistance--a cause for concern? J. Antimicrob. Chemother. 2002, 49, 11–12. [Google Scholar] [CrossRef] [PubMed]
- Bridier, A.; Briandet, R.; Thomas, V.; Dubois-Brissonnet, F. Resistance of bacterial biofilms to disinfectants: A review. Biofouling 2011, 27, 1017–1032. [Google Scholar] [CrossRef] [PubMed]
- Chotigarpa, R.; Na Lampang, K.; Pikulkaew, S.; Okonogi, S.; Ajariyakhajorn, K.; Mektrirat, R. Inhibitory effects and killing kinetics of lactic acid rice gel against pathogenic bacteria causing bovine mastitis. Sci. Pharm. 2018, 86, 29. [Google Scholar] [CrossRef] [PubMed]
- Espadale, E.; Pinchbeck, G.; Williams, N.J.; Timofte, D.; McIntyre, K.M.; Schmidt, V.M. Are the hands of veterinary staff a reservoir for antimicrobial-resistant bacteria? A randomized study to evaluate two hand hygiene rubs in a veterinary hospital. Microb. Drug Resist. 2018, 24, 1607–1616. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Gurtler, J.B.; Sokorai, K.J.B. Tomato type and post-treatment water rinse affect efficacy of acid washes against Salmonella enterica inoculated on stem scars of tomatoes and product quality. Int. J. Food Microbiol. 2018, 280, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Bjorland, J.; Sunde, M.; Waage, S. Plasmid-borne smr gene causes resistance to quaternary ammonium compounds in bovine Staphylococcus aureus. J. Clin. Microbiol. 2001, 39, 3999–4004. [Google Scholar] [CrossRef]
- Chaidez, C.; Lopez, J.; Castro-del Campo, N. Quaternary ammonium compounds: An alternative disinfection method for fresh produce wash water. J. Water Health 2007, 5, 329–333. [Google Scholar] [CrossRef]
- Hegstad, K.; Langsrud, S.; Lunestad, B.; Scheie, A.; Sunde, M.; Yazdankhah, S. Does the wide use of quaternary ammonium compounds enhance the selection and spread of antimicrobial resistance and thus threaten our health? Microb. Drug Resist. 2010, 16, 91–104. [Google Scholar] [CrossRef]
- Gyawali, R.; Ibrahim, S.A. Synergistic effect of copper and lactic acid against Salmonella and Escherichia coli O157:H7: A review. Emir. J. Food Agric. 2012, 24, 1–11. [Google Scholar]
- Beier, R.C.; Harvey, R.B.; Hernandez, C.A.; Hume, M.E.; Andrews, K.; Droleskey, R.E.; Davidson, M.K.; Bodeis-Jones, S.; Young, S.; Duke, S.E.; et al. Interactions of organic acids with Campylobacter coli from swine. PloS ONE 2018, 13, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Castillo, A.; Rodríguez-García, M.O. Bacterial hazards in fresh and fresh-cut produce: Sources and control. In Preharvest and Postharvest Food Safety: Contemporary Issues and Future Directions; Beier, R.C., Pillai, S.D., Phillips, T.D., Eds.; Blackwell publishing: Hoboken, NJ, USA, 2008; Volume 4, pp. 43–58. [Google Scholar]
- Al-Adham, I.; Haddadin, R.; Collier, P. Types of microbicidal and microbistatic agents. In Russell, Hugo and Ayliffe’s: Principles and Practice of Disinfection, Preservation and Sterilization; Fraise, A., Maillard, J.Y., Sattar, S., Eds.; Blackwell Publishing: Hoboken, NJ, USA, 2012; Volume 2, pp. 5–70. [Google Scholar]
- Brul, S.; Coote, P. Preservative agents in foods. Mode of action and microbial resistance mechanisms. Int. J. Food Microbiol. 1999, 50, 1–17. [Google Scholar] [CrossRef]
- Rajkovic, A.; Smigic, N.; Devlieghere, F. Contemporary strategies in combating microbial contamination in food chain. Int. J. Food Microbiol. 2010, 141, 29–42. [Google Scholar] [CrossRef] [PubMed]
- McDonnell, G.; Russell, A.D. Antiseptics and disinfectants: Activity, action, and resistance. Clin. Microbiol. Rev. 1999, 12, 147–179. [Google Scholar] [CrossRef] [PubMed]
- Simões, M.; Pereira, M.O.; Vieira, M.J. Action of a cationic surfactant on the activity and removal of bacterial biofilms formed under different flow regimes. Water Res. 2005, 39, 478–486. [Google Scholar] [CrossRef]
- Di Nica, V.; Gallet, J.; Villa, S.; Mezzanotte, V. Toxicity of quaternary ammonium compounds (QACs) as single compounds and mixtures to aquatic non-target microorganisms: Experimental data and predictive models. Ecotoxicol. Env. Saf. 2017, 142, 567–577. [Google Scholar] [CrossRef]
- Gilbert, P.; Allison, D.G.; McBain, A.J. Biofilms in vitro and in vivo: Do singular mechanisms imply cross-resistance? J. Appl. Microbiol. 2002, 92, 98–110. [Google Scholar] [CrossRef]
- Maillard, J.Y. Bacterial target sites for biocide action. J. Appl. Microbiol. 2002, 92, 16–27. [Google Scholar] [CrossRef]
- Malheiro, J.F.; Gomes, I.; Borges, A.; Bastos, M.M.S.M.; Maillard, J.Y.; Borges, F.; Simões, M. Phytochemical profiling as a solution to palliate disinfectant limitations. Biofouling 2016, 32, 1007–1016. [Google Scholar] [CrossRef]
- Abreu, A.C.; Saavedra, M.J.; Simões, L.C.; Simões, M. Combinatorial approaches with selected phytochemicals to increase antibiotic efficacy against Staphylococcus aureus biofilms. Biofouling 2016, 32, 1103–1114. [Google Scholar] [CrossRef]
- Borges, A.; Abreu, A.C.; Dias, C.; Saavedra, M.J.; Borges, F.; Simões, M. New perspectives on the use of phytochemicals as an emergent strategy to control bacterial infections including biofilms. Molecules 2016, 21, 877. [Google Scholar] [CrossRef] [PubMed]
- Bassole, I.H.; Juliani, H.R. Essential oils in combination and their antimicrobial properties. Molecules 2012, 17, 3989–4006. [Google Scholar] [CrossRef] [PubMed]
- Ferro, T.A.F.; Souza, E.B.; Suarez, M.A.M.; Rodrigues, J.F.S.; Pereira, D.M.S.; Mendes, S.J.F.; Gonzaga, L.F.; Machado, M.C.A.M.; Bomfim, M.R.Q.; Calixto, J.B.; et al. Topical application of cinnamaldehyde promotes faster healing of skin wounds infected with Pseudomonas aeruginosa. Molecules 2019, 24, 1627. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, R.; Coppo, E.; Marchese, A.; Daglia, M.; Sobarzo-Sánchez, E.; Nabavi, S.F.; Nabavi, S.M. Phytochemicals for human disease: An update on plant-derived compounds antibacterial activity. Microbiol. Res. 2017, 196, 44–68. [Google Scholar] [CrossRef]
- Khameneh, B.; Iranshahy, M.; Soheili, V.; Fazly Bazzaz, B.S. Review on plant antimicrobials: A mechanistic viewpoint. Antimicrob. Resist. Infect. Control. 2019, 8, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.C.; Cadena, M.B.; Townley, H.E.; Fricker, M.D.; Thompson, I.P. Effective delivery of volatile biocides employing mesoporous silicates for treating biofilms. J. R. Soc. Interface 2017, 14, 1–10. [Google Scholar] [CrossRef]
- Anwar, A.; Siddiqui, R.; Shah, M.R.; Khan, N.A. Gold nanoparticle-conjugated cinnamic acid exhibits antiacanthamoebic and antibacterial properties. Antimicrob. Agents Chemother. 2018, 62, 1–7. [Google Scholar] [CrossRef]
- Letsididi, K.S.; Lou, Z.; Letsididi, R.; Mohammed, K.; Maguy, B.L. Antimicrobial and antibiofilm effects of trans-cinnamic acid nanoemulsion and its potential application on lettuce. LWT 2018, 94, 25–32. [Google Scholar] [CrossRef]
- Hassan, Y.I.; Lahaye, L.; Gong, M.M.; Peng, J.; Gong, J.; Liu, S.; Gay, C.G.; Yang, C. Innovative drugs, chemicals, and enzymes within the animal production chain. Vet. Res. 2018, 49, 1–17. [Google Scholar] [CrossRef]
- Abreu, A.C.; McBain, A.J.; Simões, M. Plants as sources of new antimicrobials and resistance-modifying agents. Nat. Prod. Rep. 2012, 29, 1007–1021. [Google Scholar] [CrossRef]
- Andrade, M.; Benfeito, S.; Soares, P.; Magalhães e Silva, D.; Loureiro, J.; Borges, A.; Borges, F.; Simões, M. Fine-tuning of the hydrophobicity of caffeic acid: Studies on the antimicrobial activity against Staphylococcus aureus and Escherichia coli. Rsc. Adv. 2015, 5, 53915–53925. [Google Scholar] [CrossRef]
- Maillard, J.Y. Mechanisms of bacterial resistance to microbicides. In Russell, Hugo and Ayliffe’s: Principles and Practice of Disinfection, Preservation and Sterilization; Fraise, A., Maillard, J.Y., Sattar, S., Eds.; Blackwell Publishing: Hoboken, NJ, USA, 2012; Volume 6, pp. 108–120. [Google Scholar]
- Chapman, J.S. Disinfectant resistance mechanisms, cross-resistance, and co-resistance. Int. Biodeter. Biodegr. 2003, 51, 271–276. [Google Scholar] [CrossRef]
- Allegranzi, B.; Pittet, D. Hand hygiene. Russell, Hugo and Ayliffe’s: Principles and Practice of Disinfection, Preservation and Sterilization; Fraise, A., Maillard, J.Y., Sattar, S., Eds.; Blackwell Publishing: Hoboken, NJ, USA, 2012; Volume 19, pp. 418–444. [Google Scholar]
- Malheiro, J.F.; Maillard, J.Y.; Borges, F.; Simões, M. Evaluation of cinnamaldehyde and cinnamic acid derivatives in microbial growth control. Int. Biodeter. Biodegr. 2018, 141, 71–78. [Google Scholar] [CrossRef]
- Abreu, A.C.; Serra, S.C.; Borges, A.; Saavedra, M.J.; Salgado, A.J.; Simões, M. Evaluation of the best method to assess antibiotic potentiation by phytochemicals against Staphylococcus aureus. Diagn. Micr. Infec. Dis. 2014, 79, 125–134. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bremer, P.J.; Monk, I.; Butler, R. Inactivation of Listeria monocytogenes/Flavobacterium spp. biofilms using chlorine: Impact of substrate, pH, time and concentration. Lett. Appl. Microbiol. 2002, 35, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Maillard, J.-Y.; McDonnell, G. Selection and use of disinfectants. Practice. 2012, 34, 292–299. [Google Scholar] [CrossRef]
- Mah, T.F.; O’Toole, G.A. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 2001, 9, 34–39. [Google Scholar] [CrossRef]
- Fraise, A.P. Choosing disinfectants. J. Hosp. Infect. 1999, 43, 255–264. [Google Scholar] [CrossRef]
- Padrón, J.A.; Carrasco, R.; Pellón, R.F. Molecular descriptor based on a molar refractivity partition using Randic-type graph-theoretical invariant. J. Pharm. Pharmaceut. Sci. 2002, 5, 258–266. [Google Scholar]
- Hansch, C.; Steinmetz, W.E.; Leo, A.J.; Mekapati, S.B.; Kurup, A.; Hoekman, D. On the role of polarizability in chemical−biological interactions. J. Chem. Inf. Comput. Sci. 2003, 43, 120–125. [Google Scholar] [CrossRef]
- Habicht, J.; Brune, K. Inhibition of prostaglandin E2 release by salicylates, benzoates and phenols: A quantitative structure-activity study. J. Pharm Pharm. 1983, 35, 718–723. [Google Scholar] [CrossRef]
- Dambolena, J.S.; Zygadlo, J.A.; Rubinstein, H.R. Antifumonisin activity of natural phenolic compounds: A structure–property–activity relationship study. Int. J. Food Microbiol. 2011, 145, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Rastija, V.; Nikolić, S.; Medić-Šarić, M. Molecular modeling of wine polyphenols. J. Math. Chem. 2009, 46, 820–833. [Google Scholar] [CrossRef]
- Campos, F.M.; Couto, J.A.; Figueiredo, A.R.; Tóth, I.V.; Rangel, A.O.S.S.; Hogg, T.A. Cell membrane damage induced by phenolic acids on wine lactic acid bacteria. Int. J. Food Microbiol. 2009, 135, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Johnston, M.D.; Hanlon, G.W.; Denyer, S.P.; Lambert, R.J. Membrane damage to bacteria caused by single and combined biocides. J. Appl. Microbiol. 2003, 94, 1015–1023. [Google Scholar] [CrossRef]
- Virto, R.; Sanz, D.; Álvarez, I.; Condón, S.; Raso, J. Application of the Weibull model to describe inactivation of Listeria monocytogenes and Escherichia coli by citric and lactic acid at different temperatures. J. Sci. Food Agr. 2006, 86, 865–870. [Google Scholar] [CrossRef]
- Corry, J.E.L.; James, C.; James, S.J.; Hinton, M. Salmonella, Campylobacter and Escherichia coli 0157:H7 decontamination techniques for the future. Int. J. Food Microbiol. 1995, 28, 187–196. [Google Scholar] [CrossRef]
- Sánchez-Maldonado, A.F.; Schieber, A.; Gänzle, M.G. Structure–function relationships of the antibacterial activity of phenolic acids and their metabolism by lactic acid bacteria. J. Appl. Microbiol. 2011, 111, 1176–1184. [Google Scholar] [CrossRef]
- Yilmaz, S.; Sova, M.; Ergun, S. Antimicrobial activity of trans-cinnamic acid and commonly used antibiotics against important fish pathogens and nonpathogenic isolates. J. Appl. Microbiol. 2018, 125, 1714–1727. [Google Scholar] [CrossRef]
- Ali, S.M.; Khan, A.A.; Ahmed, I.; Musaddiq, M.; Ahmed, K.S.; Polasa, H.; Rao, L.V.; Habibullah, C.M.; Sechi, L.A.; Ahmed, N. Antimicrobial activities of eugenol and cinnamaldehyde against the human gastric pathogen Helicobacter pylori. Ann. Clin. Microbiol. Antimicrob. 2005, 4, 1–7. [Google Scholar] [CrossRef]
- Kim, S.A.; Rhee, M.S. Marked synergistic bactericidal effects and mode of action of medium-chain fatty acids in combination with organic acids against Escherichia coli O157:H7. Appl. Env. Microbiol. 2013, 79, 6552–6560. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Chang, T.; Yang, H.; Cui, M. Antibacterial mechanism of lactic acid on physiological and morphological properties of Salmonella Enteritidis, Escherichia coli and Listeria monocytogenes. Food Control. 2015, 47, 231–236. [Google Scholar] [CrossRef]
- Boomsma, B.; Bikker, E.; Lansdaal, E.; Stuut, P. L-Lactic acid—A safe antimicrobial for home- and personal care formulations. Sofw. J. 2015, 141, 1–5. [Google Scholar]
- Keeton, J.T.; Eddy, S.M. Chemical methods for decontamination of meat and poultry. In Preharvest and Postharvest Food Safety: Contemporary Issues and Future Directions; Beier, R.C., Pillai, S.D., Phillips, T.D., Eds.; Blackwell Publishing: Hoboken, NJ, USA, 2008; Volume 24, pp. 317–336. [Google Scholar]
- Alakomi, H.L.; Skyttä, E.; Saarela, M.; Mattila-Sandholm, T.; Latva-Kala, K.; Helander, I.M. Lactic acid permeabilizes Gram-negative bacteria by disrupting the outer membrane. Appl. Env. Microbiol. 2000, 66, 2001–2005. [Google Scholar] [CrossRef]
- Sikkema, J.; de Bont, J.A.; Poolman, B. Mechanisms of membrane toxicity of hydrocarbons. Microbiol. Rev. 1995, 59, 201–222. [Google Scholar]
- Gverzdys, T.; Kramer, G.; Nodwell, J.R. Tetrodecamycin: An unusual and interesting tetronate antibiotic. Bioorgan. Med. Chem. 2016, 24, 6269–6275. [Google Scholar] [CrossRef]
- Jackson, P.A.; Widen, J.C.; Harki, D.A.; Brummond, K.M. Covalent modifiers: A chemical perspective on the reactivity of α,β-unsaturated carbonyls with thiols via hetero-Michael addition reactions. J. Med. Chem. 2017, 60, 839–885. [Google Scholar] [CrossRef]
- Azeredo, L.; Pacheco, A.P.; Lopes, I.; Oliveira, R.; Vieira, M.J. Monitoring cell detachment by surfactants in a parallel plate flow chamber. Water Sci. Technol. 2003, 47, 77–82. [Google Scholar] [CrossRef]
- Rodrigues, A.; Nogueira, R.; Melo, L.F.; Brito, A.G. Effect of low concentrations of synthetic surfactants on polycyclic aromatic hydrocarbons (PAH) biodegradation. Int. Biodeter. Biodegr. 2013, 83, 48–55. [Google Scholar] [CrossRef]
- Nakata, K.; Tsuchido, T.; Matsumura, Y. Antimicrobial cationic surfactant, cetyltrimethylammonium bromide, induces superoxide stress in Escherichia coli cells. J. Appl. Microbiol. 2011, 110, 568–579. [Google Scholar] [CrossRef]
- Yakabe, L.E.; Parker, S.R.; Kluepfel, D.A. Cationic surfactants: Potential surface disinfectants to manage Agrobacterium tumefaciens biovar 1 contamination of grafting tools. Plant. Dis. 2011, 96, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Rajagopal, K.; Singh, P.K.; Kumar, R.; Siddiqui, K.F. CTAB-mediated, single-step preparation of competent Escherichia coli, Bifidobacterium sp. and Kluyveromyces lactis cells. Meta Gene 2014, 2, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Weber, D.J.; Rutala, W.A.; Sickbert-Bennett, E.E. Outbreaks associated with contaminated antiseptics and disinfectants. Antimicrob. Agents Chemother. 2007, 51, 4217–4224. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M. Chemistry, antimicrobial mechanisms, and antibiotic activities of cinnamaldehyde against pathogenic bacteria in animal feeds and human foods. J. Agr. Food Chem. 2017, 65, 10406–10423. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Salinas, S.; Elizondo-Castillo, H.; Arruebo, M.; Mendoza, G.; Irusta, S. Evaluation of the antimicrobial activity and a cytotoxicity of different components of natural origin present in essential oils. Molecules 2018, 23, 1399. [Google Scholar] [CrossRef] [PubMed]
- Gill, A.O.; Holley, R.A. Mechanisms of bactericidal action of cinnamaldehyde against Listeria monocytogenes and of eugenol against L. monocytogenes and Lactobacillus sakei. Appl. Env. Microbiol. 2004, 70, 5750–5755. [Google Scholar] [CrossRef]
- Minatel, I.O.; Borges, C.; Ferreira, M.I.; Gomez Gomez, H.; Chen, O.; Pace, G.; Lima, P. Phenolic compounds: Functional properties, impact of processing and bioavailability. In Phenolic Compounds: Biological Activity; Soto-Hernandez, M., Palma-Tenango, M., Garcia-Mateos, M.d.R., Eds.; IntechOpen: London, UK, 2017. [Google Scholar]
- Singh, I. Antimicrobials in higher plants: Classification, mode of action and bioactivities. Chem. Biol. Lett. 2017, 4, 48–62. [Google Scholar]
- Rutala, W.A.; Weber, D.J.; HICPAC. Guideline for Disinfection and Sterilization in Healthcare Facilities; Centers for Disease Control (CDC): Atlanta, GA, USA, 2008. [Google Scholar]
- BS EN 1276:2009. Chemical Disinfectants and Antiseptics—Quantitative suspension test for the Evaluation of Bactericidal Activity of Chemical Disinfectants and Antiseptics Used in Food, Industrial, Domestic and Institutional Areas—Test Method and Requirements (Phase 2, Step 1); Kenya Bureau of Standards: Nairobi, Kenya, 2010. [Google Scholar]
- Chan, B.C.; Ip, M.; Lau, C.B.; Lui, S.L.; Jolivalt, C.; Ganem-Elbaz, C.; Litaudon, M.; Reiner, N.E.; Gong, H.; See, R.H.; et al. Synergistic effects of baicalein with ciprofloxacin against NorA over-expressed methicillin-resistant Staphylococcus aureus (MRSA) and inhibition of MRSA pyruvate kinase. J. Ethnopharmacol. 2011, 137, 767–773. [Google Scholar] [CrossRef]
- Ferreira, C.; Pereira, A.M.; Pereira, M.C.; Melo, L.F.; Simões, M. Physiological changes induced by the quaternary ammonium compound benzyldimethyldodecylammonium chloride on Pseudomonas fluorescens. J. Antimicrob. Chemother. 2011, 66, 1036–1043. [Google Scholar] [CrossRef]
- Simões, L.C.; Simões, M.; Oliveira, R.; Vieira, M.J. Potential of the adhesion of bacteria isolated from drinking water to materials. J. Basic Microbiol. 2007, 47, 174–183. [Google Scholar] [CrossRef]
- Van Oss, C.J.; Chaudhury, M.K.; Good, R.J. Monopolar surfaces. Adv. Colloid. Interface. Sci. 1987, 28, 35–64. [Google Scholar] [CrossRef]
- Van Oss, C.J.; Good, R.J.; Chaudhury, M.K. Additive and nonadditive surface tension components and the interpretation of contact angles. Langmuir 1988, 4, 884–891. [Google Scholar] [CrossRef]
- Van Oss, C.J.; Ju, L.; Chaudhury, M.K.; Good, R.J. Estimation of the polar parameters of the surface tension of liquids by contact angle measurements on gels. J. Colloid. Interf. Sci. 1989, 128, 313–319. [Google Scholar] [CrossRef]
Sample Availability: Samples of all the compounds (except α-fluorocinnamic acid) are available from the authors. |
 ) and biocide (lactic acid or CTAB;
) and biocide (lactic acid or CTAB;  ) alone and in combination (
) alone and in combination ( ). Bacteria were exposed to the phytochemicals/derivatives concentrations presented in Table 1. Values are mean ± SD. The statistical significance is represented (* p < 0.05; ** p < 0.01; *** p < 0.001).
). Bacteria were exposed to the phytochemicals/derivatives concentrations presented in Table 1. Values are mean ± SD. The statistical significance is represented (* p < 0.05; ** p < 0.01; *** p < 0.001).
 ) and biocide (lactic acid or CTAB;
) and biocide (lactic acid or CTAB;  ) alone and in combination (
) alone and in combination ( ). Bacteria were exposed to the phytochemicals/derivatives concentrations presented in Table 1. Values are mean ± SD. The statistical significance is represented (* p < 0.05; ** p < 0.01; *** p < 0.001).
). Bacteria were exposed to the phytochemicals/derivatives concentrations presented in Table 1. Values are mean ± SD. The statistical significance is represented (* p < 0.05; ** p < 0.01; *** p < 0.001).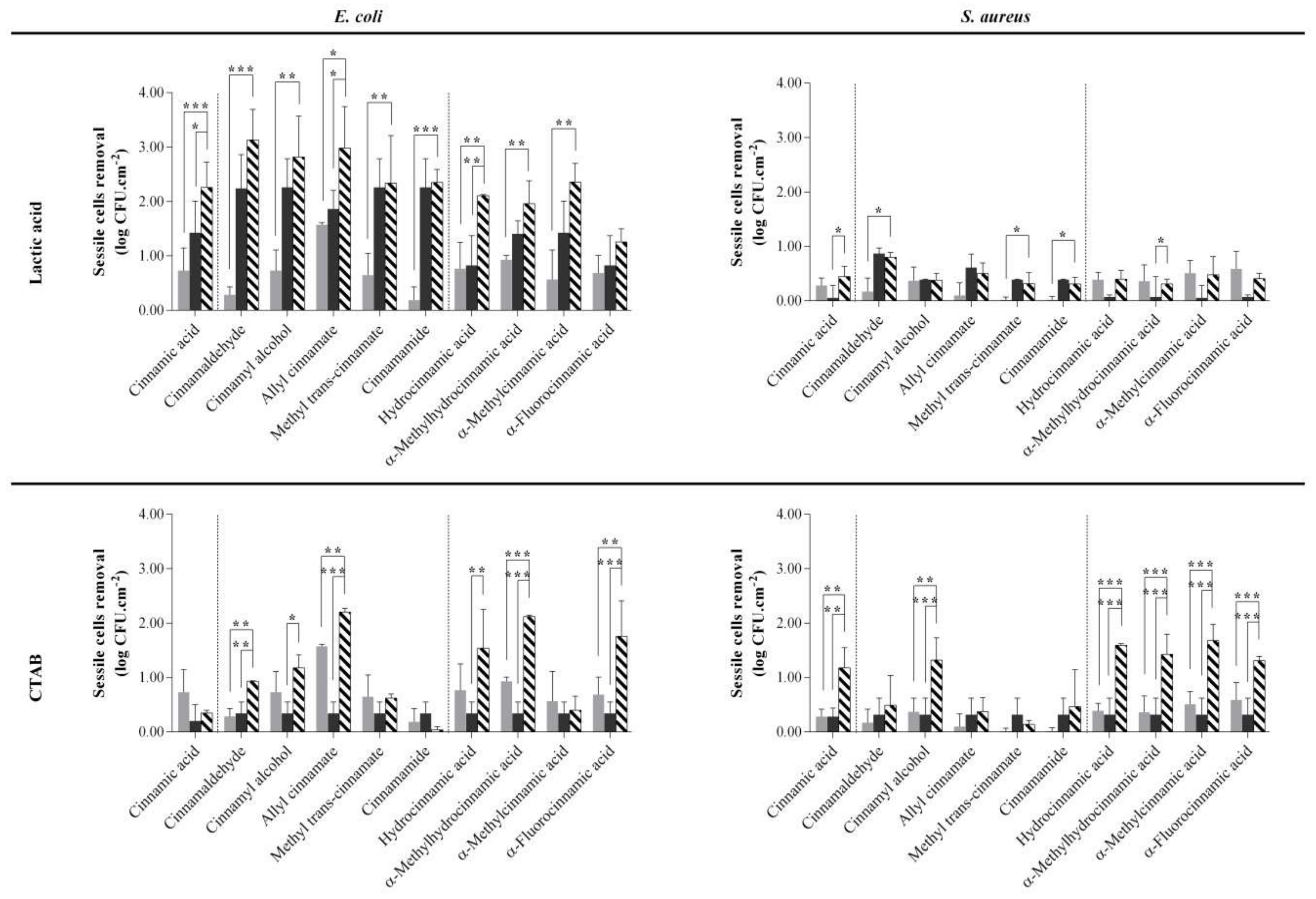
| Phytochemical or Derivative (mM) | Combination with | ||||
|---|---|---|---|---|---|
| LA (mM) | CTAB (mM) | ||||
| Concentration (mM) | Bacterium (FICI) | Concentration (mM) | Bacterium (FICI) | ||
| Cinnamic Acid | 5 | 20 | E. coli (0.8) S. aureus (0.9) | 0.01 | - |
| Cinnamaldehyde | 0.5 | 300 | - | 0.015 | S. aureus (0.9) |
| Cinnamyl alcohol | 5 | 40 | - | 0.015 | - |
| Allyl cinnamate | 5 | 200 | E. coli (1) | 0.015 | - |
| Methyl trans-cinnamate | 5 | 40 | - | 0.015 | E. coli (0.8) |
| Cinnamamide | 5 | 40 | - | 0.015 | - |
| Hydrocinnamic acid | 8 | 15 | E. coli (0.9) S. aureus (0.9) | 0.015 | - |
| α-Methylhydrocinnamic acid | 5 | 30 | S. aureus (1) | 0.015 | - |
| α-Methylcinnamic acid | 3 | 20 | E. coli (0.8) S. aureus (0.7) | 0.015 | - |
| α-Fluorocinnamic acid | 5 | 15 | E. coli (0.8) S. aureus (0.7) E. hirae (1) | 0.015 | - |
| Phytochemical or Derivative | Brand | CAS Number | Price per 1 g (€) a | |
|---|---|---|---|---|
 | ||||
| Cinnamic Acid | Merck | 140-10-3 | 4.86 | |
| Cinnamaldehyde |  | Sigma Aldrich | 14371-10-9 | 0.05 |
| Cinnamyl Alcohol |  | Acros Organics | 104-54-1 | 0.15 |
| Allyl Cinnamate |  | Sigma Aldrich | 1866-31-5 | 0.49 |
| Methyl Trans-Cinnamate |  | Merck | 1754-62-7 | 0.12 |
| Cinnamamide |  | Alfa Aesar | 621-79-4 | 7.22 |
| Hydrocinnamic Acid |  | Acros Organics | 501-52-0 | 0.31 |
| α-Methylhydrocinnamic Acid |  | Acros Organics | 1009-67-2 | 12.02 |
| α-Methylcinnamic Acid |  | Acros Organics | 1199-77-5 | 2.64 |
| α-Fluorocinnamic Acid |  | Sigma Aldrich | 350-90-3 | 91.90 |
| Biocide | Brand | CAS number | Price per 1 g (€) a | |
| CTAB | Acros Organics | 57-09-0 | 0.26 | |
| Lactic Acid | Fluka | 50-21-5 | 0.14 | |
| Surface Tension Parameters (mJ m−2) | Hydrophobicity (mJ m−2) | |||||||||||||||
| E. coli | Control (water) | 33.43 | ± | 1.98 | 13.74 | ± | 3.65 | 1.03 | ± | 0.52 | 48.78 | ± | 3.08 | 28.98 | ± | 4.49 |
| Control (DMSO) | 31.99 | ± | 1.36 | 15.78 | ± | 1.99 | 1.26 | ± | 0.32 | 50.11 | ± | 4.16 | 29.91 | ± | 4.98 | |
| Cinnamic acid | 29.67 | ± | 3.33 | 21.63 | ± | 1.76 | 2.45 | ± | 0.34 | 47.81 | ± | 1.49 | 24.65 | ± | 1.64 | |
| Cinnamaldehyde | 32.96 | ± | 0.61 | 12.78 | ± | 2.02 | 0.92 | ± | 0.27 | 45.02 | ± | 3.13 | 24.84 | ± | 3.73 | |
| Hydrocinnamic acid | 30.48 | ± | 1.04 | 19.14 | ± | 2.34 | 1.95 | ± | 0.49 | 47.63 | ± | 1.14 | 25.69 | ± | 2.90 | |
| α-Methylhydrocinnamic acid | 28.55 | ± | 1.19 | 24.40 | ± | 1.86 ** | 3.00 | ± | 0.45 * | 49.87 | ± | 0.68 | 25.75 | ± | 0.80 | |
| α-Methylcinnamic acid | 21.91 | ± | 4.42 *** | 31.60 | ± | 4.80 *** | 5.51 | ± | 1.76 *** | 46.42 | ± | 1.86 | 19.08 | ± | 4.13 ** | |
| S. aureus | Control (water) | 35.26 | ± | 1.18 | 18.01 | ± | 2.09 | 1.71 | ± | 0.52 | 48.68 | ± | 4.23 | 25.80 | ± | 5.62 |
| Control (DMSO) | 36.24 | ± | 1.19 | 17.56 | ± | 0.68 | 1.58 | ± | 0.09 | 48.92 | ± | 4.14 | 25.80 | ± | 4.73 | |
| Cinnamic acid | 34.79 | ± | 1.73 | 19.35 | ± | 0.75 | 1.83 | ± | 0.14 | 51.07 | ± | 0.66 | 27.94 | ± | 1.31 | |
| Cinnamaldehyde | 35.70 | ± | 0.52 | 17.25 | ± | 3.06 | 1.51 | ± | 0.51 | 50.19 | ± | 2.08 | 27.76 | ± | 3.06 | |
| Hydrocinnamic acid | 34.36 | ± | 1.55 | 19.60 | ± | 1.25 | 1.89 | ± | 0.14 | 50.79 | ± | 3.03 | 27.62 | ± | 3.05 | |
| α-Methylhydrocinnamic acid | 34.49 | ± | 2.20 | 16.93 | ± | 0.90 | 1.34 | ± | 0.09 | 53.59 | ± | 2.47 | 32.40 | ± | 3.09 | |
| α-Methylcinnamic acid | 35.82 | ± | 1.04 | 16.68 | ± | 1.09 | 1.32 | ± | 0.18 | 52.98 | ± | 1.21 | 31.34 | ± | 2.18 | |
| E. hirae | Control (water) | 35.65 | ± | 1.77 | 13.20 | ± | 2.62 | 0.86 | ± | 0.33 | 52.45 | ± | 2.79 | 32.88 | ± | 3.80 |
| Control (DMSO) | 33.93 | ± | 0.59 | 17.72 | ± | 2.40 | 1.52 | ± | 0.52 | 53.02 | ± | 3.10 | 31.58 | ± | 4.83 | |
| Cinnamic acid | 32.26 | ± | 2.25 | 20.96 | ± | 1.62 | 2.15 | ± | 0.34 | 51.38 | ± | 1.27 | 28.31 | ± | 1.34 | |
| Cinnamaldehyde | 33.02 | ± | 1.72 | 20.03 | ± | 3.58 | 2.00 | ± | 0.75 | 51.64 | ± | 2.64 | 28.94 | ± | 4.11 | |
| Hydrocinnamic acid | 30.61 | ± | 1.70 | 22.87 | ± | 2.29 | 2.61 | ± | 0.46 | 50.25 | ± | 1.73 | 26.48 | ± | 1.76 | |
| α-Methylhydrocinnamic acid | 33.15 | ± | 0.50 | 18.76 | ± | 2.37 | 1.69 | ± | 0.50 | 53.41 | ± | 3.69 | 31.70 | ± | 5.47 | |
| α-Methylcinnamic acid | 34.33 | ± | 2.72 | 17.22 | ± | 2.81 | 1.41 | ± | 0.40 | 53.17 | ± | 2.43 | 31.75 | ± | 3.02 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malheiro, J.F.; Maillard, J.-Y.; Borges, F.; Simões, M. Biocide Potentiation Using Cinnamic Phytochemicals and Derivatives. Molecules 2019, 24, 3918. https://doi.org/10.3390/molecules24213918
Malheiro JF, Maillard J-Y, Borges F, Simões M. Biocide Potentiation Using Cinnamic Phytochemicals and Derivatives. Molecules. 2019; 24(21):3918. https://doi.org/10.3390/molecules24213918
Chicago/Turabian StyleMalheiro, Joana F., Jean-Yves Maillard, Fernanda Borges, and Manuel Simões. 2019. "Biocide Potentiation Using Cinnamic Phytochemicals and Derivatives" Molecules 24, no. 21: 3918. https://doi.org/10.3390/molecules24213918
APA StyleMalheiro, J. F., Maillard, J.-Y., Borges, F., & Simões, M. (2019). Biocide Potentiation Using Cinnamic Phytochemicals and Derivatives. Molecules, 24(21), 3918. https://doi.org/10.3390/molecules24213918







