Biomimetic Synthesis of Nanocrystalline Hydroxyapatite Composites: Therapeutic Potential and Effects on Bone Regeneration
Abstract
:1. Introduction
2. Results
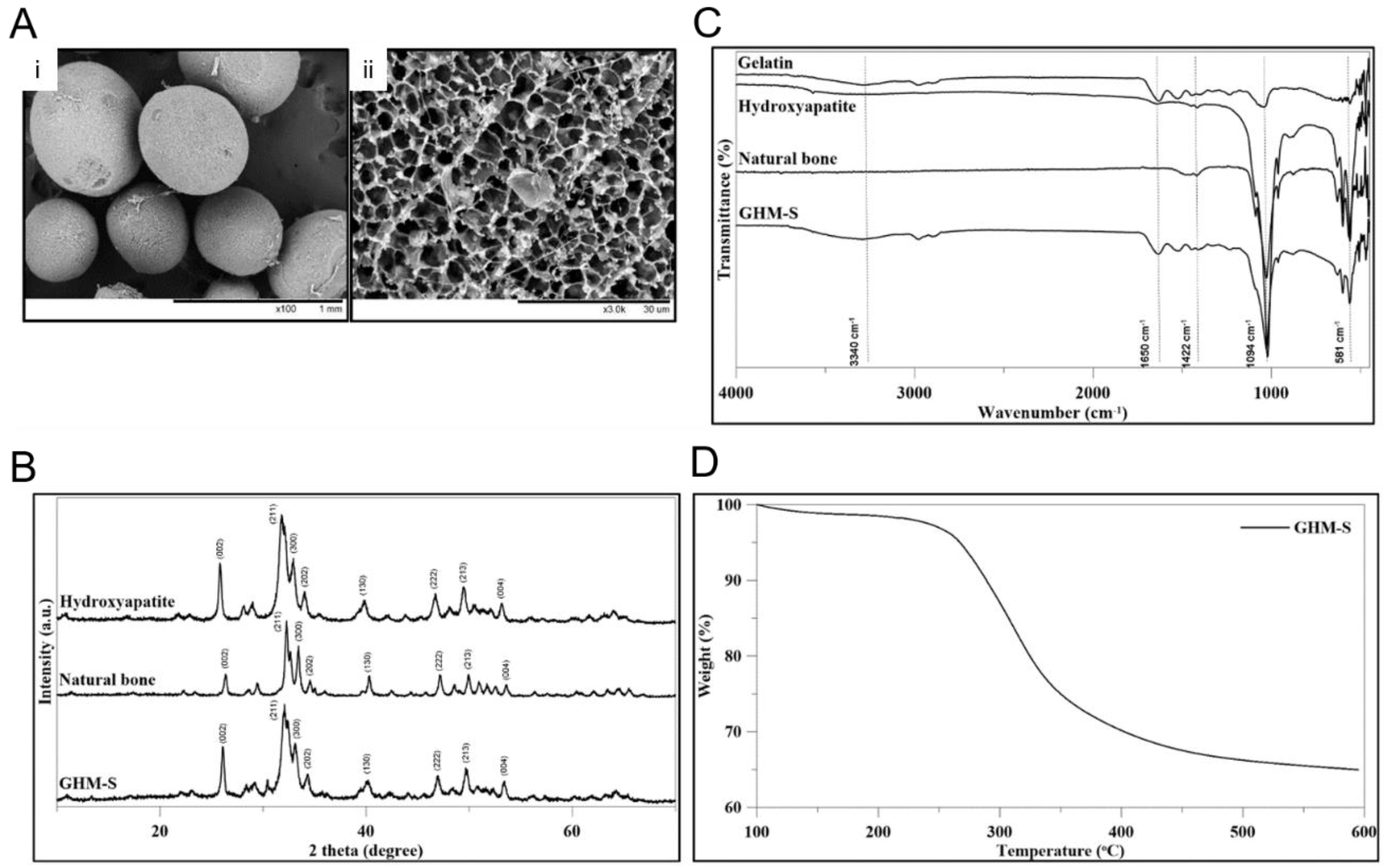
2.1. Material Characteristics and Biocompatibility of the Biomimetic Hydroxyapatite Microspheres (GHM-S)
2.2. Material Characteristics of Gelatin/Hydroxyapatite Microsphere (GHM)
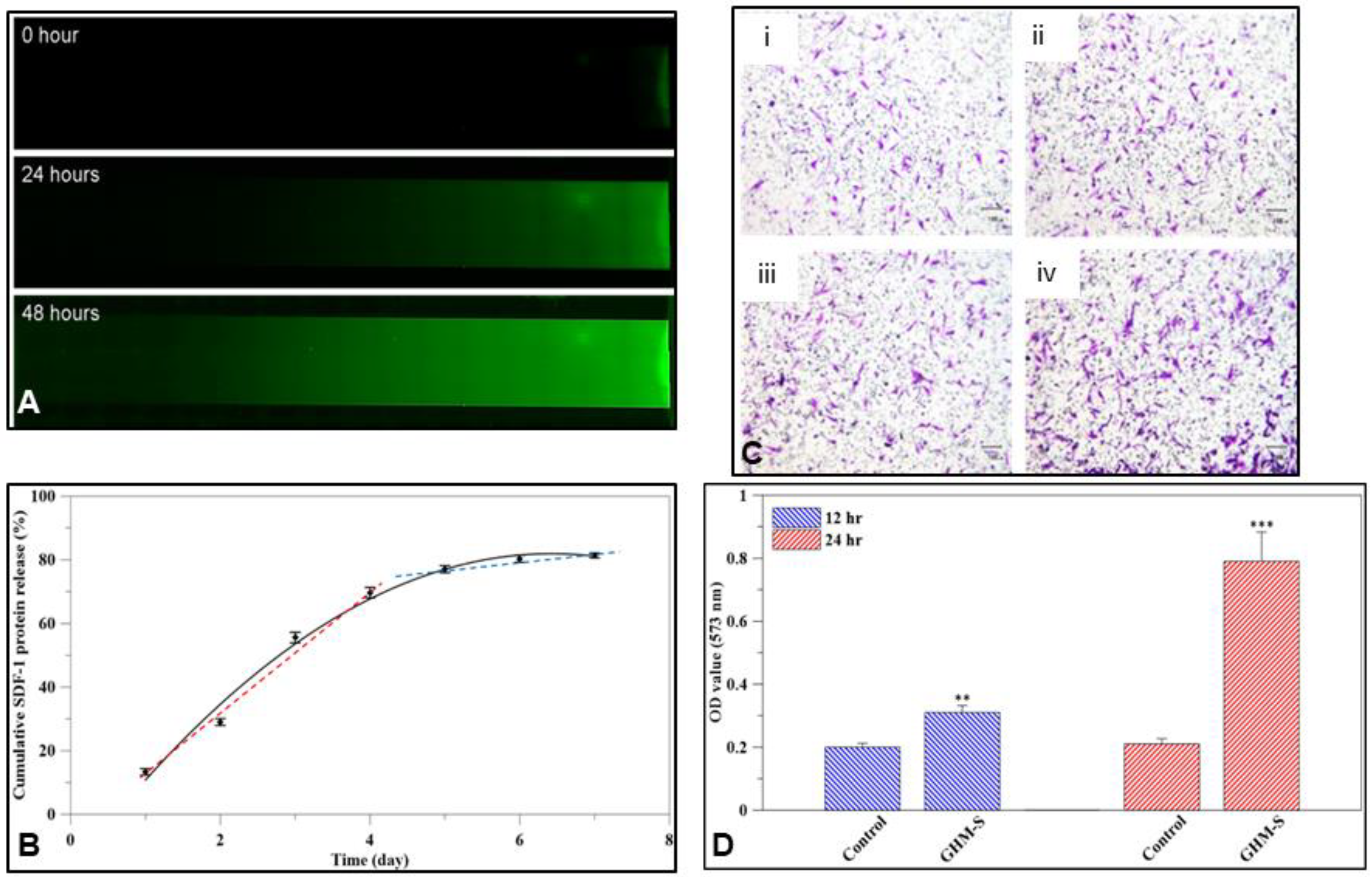
2.3. SDF-1 Releasing Profile
2.4. Gene Expression and Quantitative Analysis of Osteogenic Proteins
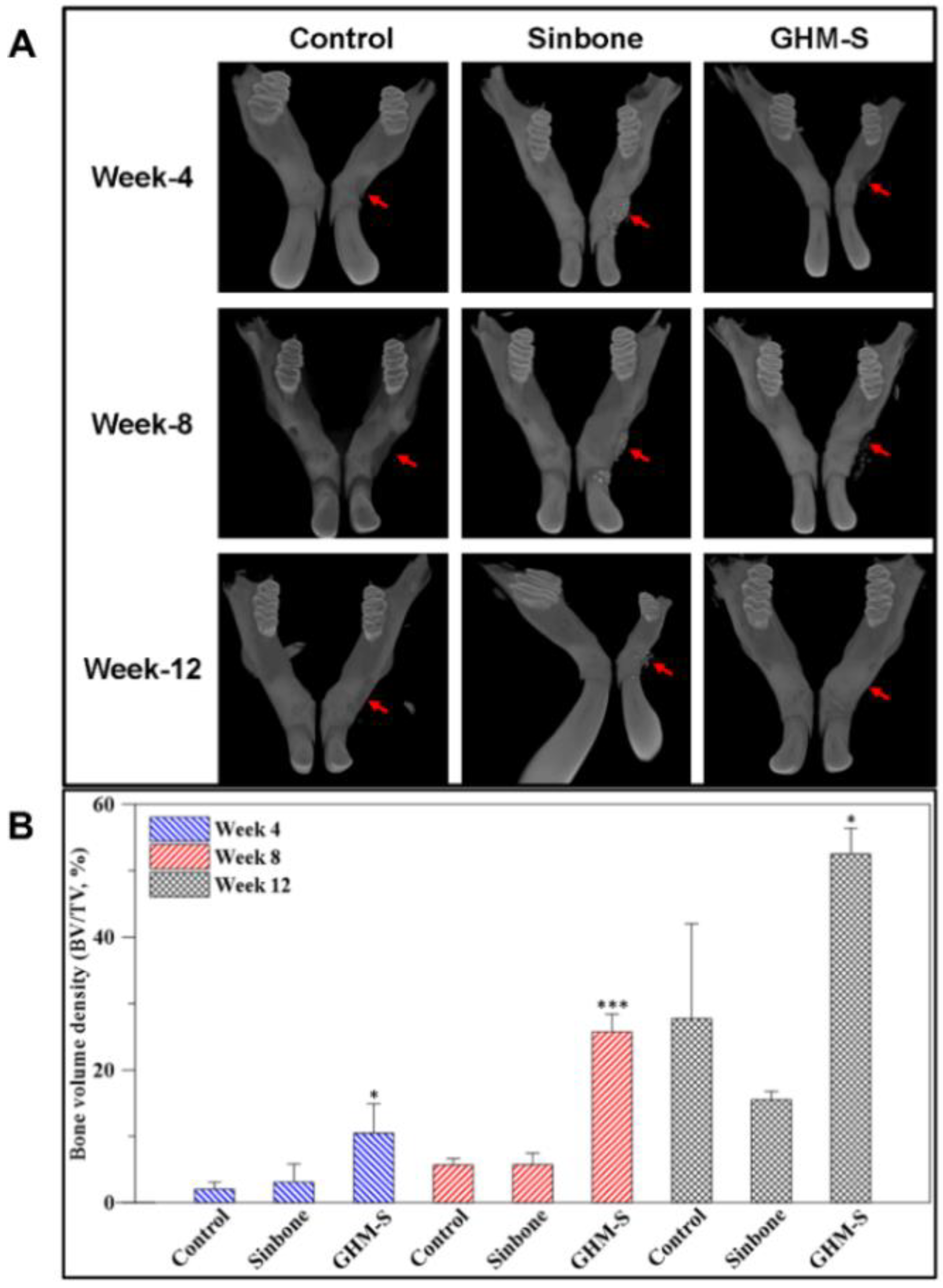
2.5. Micro-Computed Tomography (μ-CT) Analysis
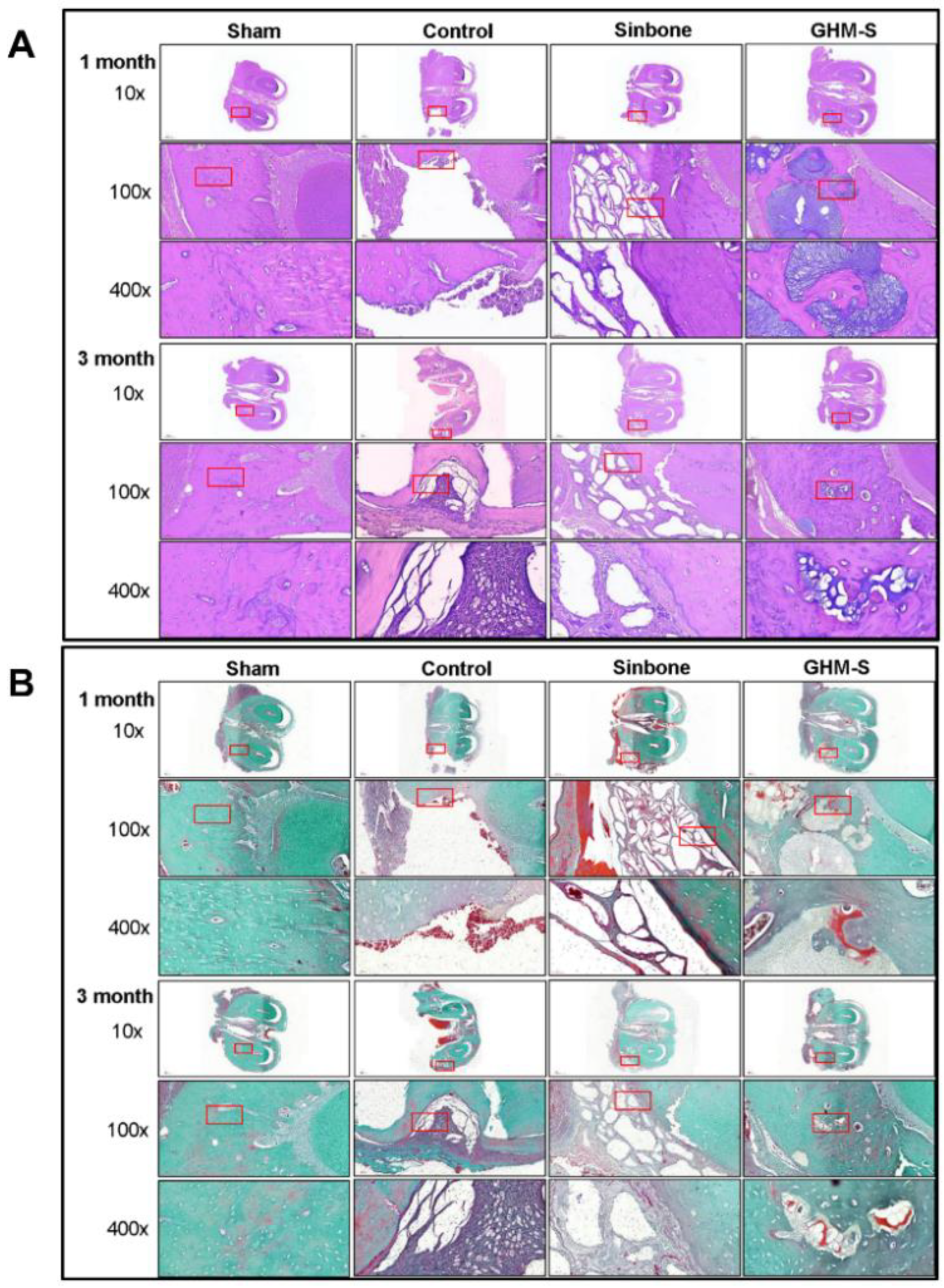
2.6. Histological and Immunohistochemical Analysis
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Fabrication of Gelatin/Hydroxyapatite Microsphere (GHM) and Biomimetic Hydroxyapatite Microspheres (GHM-S)
4.3. Morphology of Gelatin/Nano-Hydroxyapatite Microsphere (GHM)
4.4. Characterization of Gelatin/Nano-hydroxyapatite Microsphere (GHM)
4.5. Thermogravimetric Analysis (TGA) of Biomimetic Hydroxyapatite Microspheres (GHM-S)
4.6. SDF-1 Release Profile and Gradient Formation
4.7. In Vitro Study
4.8. In Vivo Study
4.9. Histological Analysis
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hernigou, P. Bone transplantation and tissue engineering. Part II: Bone graft and osteogenesis in the seventeenth, eighteenth and nineteenth centuries (Duhamel, Haller, Ollier and MacEwen). Int. Orthop. 2015, 39, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Coelho, C.C.; Grenho, L.; Gomes, P.S.; Quadros, P.A.; Fernandes, M.H. Nano-hydroxyapatite in oral care cosmetics: Characterization and cytotoxicity assessment. Sci. Rep. 2019, 9, 11050. [Google Scholar] [CrossRef] [PubMed]
- Roberts, T.T.; Rosenbaum, A.J. Bone grafts, bone substitutes and orthobiologics: The bridge between basic science and clinical advancements in fracture healing. Organogenesis 2012, 8, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, B.; Jayakrishnan, A. Self-cross-linking biopolymers as injectable in situ forming biodegradable scaffolds. Biomaterials 2005, 26, 3941–3951. [Google Scholar] [CrossRef]
- Takahashi, Y.; Yamamoto, M.; Tabata, Y. Enhanced osteoinduction by controlled release of bone morphogenetic protein-2 from biodegradable sponge composed of gelatin and beta-tricalcium phosphate. Biomaterials 2005, 26, 4856–4865. [Google Scholar] [CrossRef] [PubMed]
- Hokugo, A.; Ozeki, M.; Kawakami, O.; Sugimoto, K.; Mushimoto, K.; Morita, S.; Tabata, Y. Augmented bone regeneration activity of platelet-rich plasma by biodegradable gelatin hydrogel. Tissue Eng. 2005, 11, 1224–1233. [Google Scholar] [CrossRef]
- Young, S.; Wong, M.; Tabata, Y.; Mikos, A.G. Gelatin as a delivery vehicle for the controlled release of bioactive molecules. J. Control Release 2005, 109, 256–274. [Google Scholar] [CrossRef]
- Hou, S.; Lake, R.; Park, S.; Edwards, S.; Jones, C.; Jeong, K.J. Injectable macroporous hydrogel formed by enzymatic cross-linking of gelatin microgels. ACS Appl. Bio Mater. 2018, 1, 1430–1439. [Google Scholar] [CrossRef]
- Fisher, D.M.; Wong, J.M.; Crowley, C.; Khan, W.S. Preclinical and clinical studies on the use of growth factors for bone repair: A systematic review. Curr. Stem Cell Res. 2013, 8, 260–268. [Google Scholar] [CrossRef]
- Einhorn, T.A.; Gerstenfeld, L.C. Fracture healing: Mechanisms and interventions. Nat. Rev. Rheumatol. 2015, 11, 45–54. [Google Scholar] [CrossRef]
- EI Bialy, I.; Jiskoot, W.; Nejadnik, M.R. Formulation, delivery and stability of bone morphogenetic proteins for effective bone regeneration. Pharm. Res. 2017, 34, 1152–1170. [Google Scholar] [CrossRef] [PubMed]
- Carragee, E.J.; Chu, G.; Rohatgi, R.; Hurwitz, E.L.; Weiner, B.K.; Yoon, S.T.; Comer, G.; Kopjar, B. Cancer risk after use of recombinant bone morphogenetic protein-2 for spinal arthrodesis. J. Bone Jt. Surg. Am. 2013, 95, 1537–1545. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Liu, H.; Deng, X.; Chen, M.; Han, X.; Yan, W.; Wang, N. CXCR4 antagonist delivery on decellularized skin scaffold facilitates impaired wound healing in diabetic mice by increasing expression of SDF-1 and enhancing migration of CXCR4-positive cells. Wound Repair Regen. 2017, 25, 652–664. [Google Scholar] [CrossRef] [PubMed]
- Shafiq, M.; Kong, D.; Kim, S.H. SDF-1alpha peptide tethered polyester facilitates tissue repair by endogenous cell mobilization and recruitment. J. Biomed. Mater. Res. A 2017, 105, 2670–2684. [Google Scholar] [CrossRef]
- Zhou, W.; Guo, S.; Liu, M.; Burow, M.E.; Wang, G. Targeting CXCL12/CXCR4 axis in tumor immunotherapy. Curr. Med. Chem. 2019, 26, 3026–3041. [Google Scholar] [CrossRef]
- Kortesidis, A.; Zannettino, A.; Isenmann, S.; Shi, S.; Lapidot, T.; Gronthos, S. Stromal-derived factor-1 promotes the growth, survival, and development of human bone marrow stromal stem cells. Blood 2005, 105, 3793–3801. [Google Scholar] [CrossRef]
- Hosogane, N.; Huang, Z.; Rawlins, B.A.; Liu, X.; Boachie-Adjei, O.; Boskey, A.L.; Zhu, W. Stromal derived factor-1 regulates bone morphogenetic protein 2-induced osteogenic differentiation of primary mesenchymal stem cells. Int. J. Biochem. Cell Biol. 2010, 42, 1132–1141. [Google Scholar] [CrossRef]
- Walenkamp, A.M.E.; Lapa, C.; Herrmann, K.; Wester, H.J. CXCR4 ligands: The next big hit? J. Nucl. Med. 2017, 58 (Suppl. 2), 77S–82S. [Google Scholar] [CrossRef]
- Szalaj, U.; Świderska-Środa, A.; Chodara, A.; Gierlotka, S.; Łojkowski, W. Nanoparticle size effect on water vapour adsorption by hydroxyapatite. Nanomaterials (Basel) 2019, 9, E1005. [Google Scholar] [CrossRef]
- Mosa, I.F.; Yousef, M.; Kamel, M.; Mosa, O.F.; Helmy, Y. The protective role of CsNPs and CurNPs against DNA damage, oxidative stress, and histopathological and immunohistochemical alterations induced by hydroxyapatite nanoparticles in male rat kidney. Toxicol. Res. (Camb.) 2019, 8, 741–753. [Google Scholar] [CrossRef]
- Rios-Pimentel, F.F.; Chang, R.; Webster, T.J.; Mendez-Gonzalez, M.M.; Garcia-Rocha, M. Greater osteoblast densities due to the addition of amphiphilic peptide nanoparticles to nano hydroxyapatite coatings. Int. J. Nanomed. 2019, 14, 3265–3272. [Google Scholar] [CrossRef] [PubMed]
- Mazzone, N.; Mici, E.; Calvo, A.; Runci, M.; Crimi, S.; Lauritano, F.; Belli, E. Preliminary results of bone regeneration in oromaxillomandibular surgery using synthetic granular graft. Biomed. Res. Int. 2018, 2018, 8503427. [Google Scholar] [CrossRef] [PubMed]
- Gorojod, R.M.; Porte Alcon, S.; Dittler, M.L.; Gonzalez, M.C.; Kotler, M.L. Nanohydroxyapatite exerts cytotoxic effects and prevents cellular proliferation and migration in glioma cells. Toxicol. Sci. 2019, 169, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Oberbek, P.; Bolek, T.; Chlanda, A.; Hirano, S.; Kusnieruk, S.; Rogowska-Tylman, J.; Nechyporenko, G.; Zinchenko, V.; Swieszkowski, W.; Puzyn, T. Characterization and influence of hydroxyapatite nanopowders on living cells. Beilstein J. Nanotechnol. 2018, 9, 3079–3094. [Google Scholar] [CrossRef]
- Raucci, M.G.; D’Amora, U.; Ronca, A.; Demitri, C.; Ambrosio, L. bioactivation routes of gelatin-based scaffolds to enhance at nanoscale level bone tissue regeneration. Front. Bioeng. Biotechnol. 2019, 7, 27. [Google Scholar] [CrossRef]
- Sang, L.; Huang, J.; Luo, D.; Chen, Z.; Li, X. Bone-like nanocomposites based on self-assembled protein-based matrices with Ca2+ capturing capability. J. Mater. Sci. Mater. Med. 2010, 21, 2561–2568. [Google Scholar] [CrossRef]
- Morejon, L.; Delgado, J.A.; Antunes Ribeiro, A.; Varella de Oliveira, M.; Mendizábal, E.; García, I.; Alfonso, A.; Poh, P.; van Griensven, M.; Balmayor, E.R. Development, characterization and in vitro biological properties of scaffolds fabricated from calcium phosphate nanoparticles. Int. J. Mol. Sci. 2019, 20, E1790. [Google Scholar] [CrossRef]
- Feng, X. Chemical and biochemical basis of cell-bone matrix interaction in health and disease. Curr. Chem. Biol. 2009, 3, 189–196. [Google Scholar]
- Ji, W.; Yang, F.; Ma, J.; Bouma, M.J.; Boerman, O.C.; Chen, Z.; van den Beucken, J.J.; Jansen, J.A. Incorporation of stromal cell-derived factor-1α in PCL/gelatin electrospun membranes for guided bone regeneration. Biomaterials 2013, 34, 735–745. [Google Scholar] [CrossRef]
- Muvaffak, A.; Gurhan, I.; Hasirci, N. Prolonged cytotoxic effect of colchicine released from biodegradable microspheres. J. Biomed. Mater. Res. B Appl. Biomater. 2004, 71, 295–304. [Google Scholar] [CrossRef]
- Saravanan, M.; Bhaskar, K.; Maharajan, G.; Pillai, K.S. Development of gelatin microspheres loaded with diclofenac sodium for intra-articular administration. J. Drug Target 2011, 19, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Thevenot, P.T.; Nair, A.M.; Shen, J.; Lotfi, P.; Ko, C.Y.; Tang, L. The effect of incorporation of SDF-1alpha into PLGA scaffolds on stem cell recruitment and the inflammatory response. Biomaterials 2010, 31, 3997–4008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hattori, K.; Heissig, B.; Tashiro, K.; Honjo, T.; Tateno, M.; Shieh, J.H.; Hackett, N.R.; Quitoriano, M.S.; Crystal, R.G.; Rafii, S.; et al. Plasma elevation of stromal cell-derived factor-1 induces mobilization of mature and immature hematopoietic progenitor and stem cells. Blood 2001, 97, 3354–3360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, F.M.; Wu, L.A.; Zhang, M.; Zhang, R.; Sun, H.H. Homing of endogenous stem/progenitor cells for in situ tissue regeneration: Promises, strategies, and translational perspectives. Biomaterials 2011, 32, 3189–3209. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Yang, P.; Ge, S. Stromal cell-derived factor-1 significantly induces proliferation, migration, and collagen type I expression in a human periodontal ligament stem cell subpopulation. J. Periodontol. 2012, 83, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Li, M.; Du, L.; Yang, P.; Ge, S. Local administration of stromal cell-derived factor-1 promotes stem cell recruitment and bone regeneration in a rat periodontal bone defect model. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 53, 83–94. [Google Scholar] [CrossRef]
- Abbott, J.D.; Huang, Y.; Liu, D.; Hickey, R.; Krause, D.S.; Giordano, F.J. Stromal cell-derived factor-1alpha plays a critical role in stem cell recruitment to the heart after myocardial infarction but is not sufficient to induce homing in the absence of injury. Circulation 2004, 110, 3300–3305. [Google Scholar] [CrossRef] [Green Version]
- Chen, F.M.; Lu, H.; Wu, L.A.; Gao, L.N.; An, Y.; Zhang, J. Surface-engineering of glycidyl methacrylated dextran/gelatin microcapsules with thermo-responsive poly(N-isopropylacrylamide) gates for controlled delivery of stromal cell-derived factor-1alpha. Biomaterials 2013, 34, 6515–6527. [Google Scholar] [CrossRef]
- Mavrogenis, A.F.; Dimitriou, R.; Parvizi, J.; Babis, G.C. Biology of implant osseointegration. J. Musculoskelet. Neuronal Interact. 2009, 9, 61–71. [Google Scholar]
- Sordi, V.; Malosio, M.L.; Marchesi, F.; Mercalli, A.; Melzi, R.; Giordano, T.; Belmonte, N.; Ferrari, G.; Leone, B.E.; Bertuzzi, F.; et al. Bone marrow mesenchymal stem cells express a restricted set of functionally active chemokine receptors capable of promoting migration to pancreatic islets. Blood 2005, 106, 419–427. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.; Nakamura, Y.; Wang, X.; Hu, Q.; Suggs, L.J.; Zhang, J. Controlled release of stromal cell-derived factor-1 alpha in situ increases c-kit+ cell homing to the infarcted heart. Tissue Eng. 2007, 13, 2063–2071. [Google Scholar] [CrossRef] [PubMed]
- Hankenson, K.D.; Dishowitz, M.; Gray, C.; Schenker, M. Angiogenes bone regeneration. Injury 2011, 42, 556–561. [Google Scholar]


| Gene Name | Forward Primer (5′–3′) | Reverse Primer (5′–3′) |
|---|---|---|
| ALPP | GAGAAGCCGGGACACAGTTC | CCTCCTCAACTGGGATGATGC |
| RUNX-2 | TAGGCGCATTTCAGGTGCTT | GGTGTGGTAGTGAGTGGTGG |
| SP7 | TAGGACTGTAGGACCGGAGC | CATAGTGAACTTCCTCCTGGGG |
| SPARC | ATTGACGGGTACCTCTCCCA | GAAAAAGCGGGTGGTGCAAT |
| BGLAP | CTCACACTCCTCGCCCTATTG | GCTTGGACACAAAGGCTGCAC |
| COL1a1 | AGAGGTCGCCCTGGAGC | CAGGAACACCCTGTTCACCA |
| GAPDH | AATGGGCAGCCGTTAGGAAA | GCCCAATACGACCAAATCAGAG |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, C.-H.; Lin, Y.-W.; Lin, F.-H.; Sun, J.-S.; Chao, Y.-H.; Lin, H.-Y.; Chang, Z.-C. Biomimetic Synthesis of Nanocrystalline Hydroxyapatite Composites: Therapeutic Potential and Effects on Bone Regeneration. Int. J. Mol. Sci. 2019, 20, 6002. https://doi.org/10.3390/ijms20236002
Fang C-H, Lin Y-W, Lin F-H, Sun J-S, Chao Y-H, Lin H-Y, Chang Z-C. Biomimetic Synthesis of Nanocrystalline Hydroxyapatite Composites: Therapeutic Potential and Effects on Bone Regeneration. International Journal of Molecular Sciences. 2019; 20(23):6002. https://doi.org/10.3390/ijms20236002
Chicago/Turabian StyleFang, Chih-Hsiang, Yi-Wen Lin, Feng-Huei Lin, Jui-Sheng Sun, Yuan-Hung Chao, Hung-Ying Lin, and Zwei-Chieng Chang. 2019. "Biomimetic Synthesis of Nanocrystalline Hydroxyapatite Composites: Therapeutic Potential and Effects on Bone Regeneration" International Journal of Molecular Sciences 20, no. 23: 6002. https://doi.org/10.3390/ijms20236002
APA StyleFang, C.-H., Lin, Y.-W., Lin, F.-H., Sun, J.-S., Chao, Y.-H., Lin, H.-Y., & Chang, Z.-C. (2019). Biomimetic Synthesis of Nanocrystalline Hydroxyapatite Composites: Therapeutic Potential and Effects on Bone Regeneration. International Journal of Molecular Sciences, 20(23), 6002. https://doi.org/10.3390/ijms20236002







