Mediterranean Diet and NAFLD: What We Know and Questions That Still Need to Be Answered
Abstract
:1. Introduction
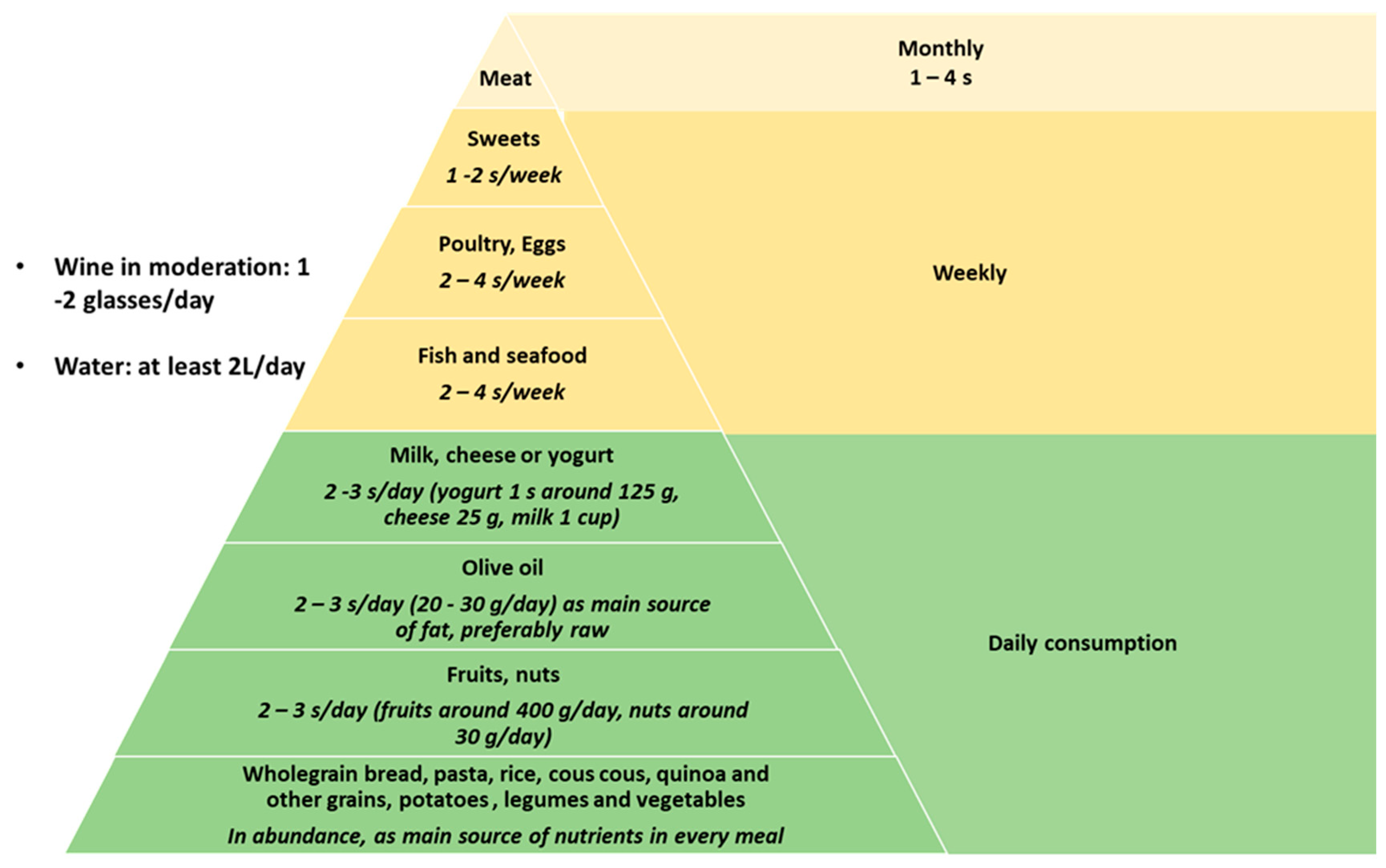
2. Mediterranean Diet Characteristics and Its Potential Effects on NAFLD
3. Current Evidence about the Effects of the Mediterranean Diet on NAFLD
4. Future Research
Author Contributions
Funding
Conflicts of Interest
References
- Ratziu, V.; Bellentani, S.; Cortez-Pinto, H.; Day, C.; Marchesini, G. A position statement on NAFLD/NASH based on the EASL 2009 special conference. J. Hepatol. 2010, 53, 372–384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Younossi, Z.; Anstee, Q.M.; Marietti, M.; Hardy, T.; Henry, L.; Eslam, M.; George, J.; Bugianesi, E. Global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2017, 15, 11. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver and European Association for the Study of Diabetes. EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. 2016, 64, 1388–1402. [Google Scholar] [CrossRef] [PubMed]
- Parikh, N.D.; Marrero, W.J.; Wang, J.; Steuer, J.; Tapper, E.B.; Konerman, M.; Singal, A.G.; Hutton, D.W.; Byon, E.; Lavieri, M.S. Projected increase in obesity and non-alcoholic-steatohepatitis-related liver transplantation waitlist additions in the United States. Hepatology 2019, 70, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Agopian, V.G.; Kaldas, F.M.; Hong, J.C.; Whittaker, M.; Holt, C.; Rana, A.; Zarrinpar, A.; Petrowsky, H.; Farmer, D.; Yersiz, H.; et al. Liver transplantation for nonalcoholic steatohepatitis: The new epidemic. Ann. Surg. 2012, 256, 624–633. [Google Scholar] [CrossRef]
- Younossi, Z.; Tacke, F.; Arrese, M.; Chander Sharma, B.; Mostafa, I.; Bugianesi, E.; Wai-Sun Wong, V.; Yilmaz, Y.; George, J.; Fan, J.; et al. Global Perspectives on Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis. Hepatology 2019, 69, 2672–2682. [Google Scholar] [CrossRef] [Green Version]
- Anstee, Q.M.; Targher, G.; Day, C.P. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 330–344. [Google Scholar] [CrossRef]
- Soderberg, C.; Stal, P.; Askling, J.; Glaumann, H.; Lindberg, G.; Marmur, J.; Hultcrantz, R. Decreased survival of subjects with elevated liver function tests during a 28-year follow-up. Hepatology 2010, 51, 595–602. [Google Scholar] [CrossRef] [Green Version]
- Adams, L.A.; Anstee, Q.M.; Tilg, H.; Targher, G. Non-alcoholic fatty liver disease and its relationship with cardiovascular disease and other extrahepatic diseases. Gut 2017, 66, 1138–1153. [Google Scholar] [CrossRef] [Green Version]
- Haigh, L.; Bremner, S.; Houghton, D.; Henderson, E.; Avery, L.; Hardy, T.; Hallsworth, K.; McPherson, S.; Anstee, Q.M. Barriers and Facilitators to Mediterranean Diet Adoption by Patients With Nonalcoholic Fatty Liver Disease in Northern Europe. Clin. Gastroenterol. Hepatol. 2019, 17, 1364–1371. [Google Scholar] [CrossRef] [Green Version]
- Gepner, Y.; Shelef, I.; Komy, O.; Cohen, N.; Schwarzfuchs, D.; Bril, N.; Rein, M.; Serfaty, D.; Kenigsbuch, S.; Zelicha, H.; et al. The beneficial effects of Mediterranean diet over low-fat diet may be mediated by decreasing hepatic fat content. J. Hepatol. 2019, 71, 379–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Properzi, C.; O’Sullivan, T.A.; Sherriff, J.L.; Ching, H.L.; Jeffrey, G.P.; Buckley, R.F.; Tibballs, J.; MacQuillan, G.C.; Garas, G.; Adams, L.A. Ad Libitum Mediterranean and Low-Fat Diets Both Significantly Reduce Hepatic Steatosis: A Randomized Controlled Trial. Hepatology 2018, 68, 1741–1754. [Google Scholar] [CrossRef] [Green Version]
- Kastorini, C.M.; Milionis, H.J.; Esposito, K.; Giugliano, D.; Goudevenos, J.A.; Panagiotakos, D.B. The effect of Mediterranean diet on metabolic syndrome and its components: A meta-analysis of 50 studies and 534,906 individuals. J. Am. Coll. Cardiol. 2011, 57, 1299–1313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kastorini, C.M.; Panagiotakos, D.B. The role of the mediterranean diet on the development of the metabolic syndrome. Front. Biosci. 2010, 2, 1320–1333. [Google Scholar]
- Esposito, K.; Giugliano, D. Mediterranean diet for primary prevention of cardiovascular disease. N. Engl. J. Med. 2013, 369, 674–675. [Google Scholar] [CrossRef]
- Esposito, K.; Kastorini, C.M.; Panagiotakos, D.B.; Giugliano, D. Mediterranean diet and metabolic syndrome: An updated systematic review. Rev. Endocr. Metab. Disord. 2013, 14, 255–263. [Google Scholar] [CrossRef]
- Buzzetti, E.; Pinzani, M.; Tsochatzis, E.A. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism 2016, 65, 1038–1048. [Google Scholar] [CrossRef]
- Bugianesi, E.; Moscatiello, S.; Ciaravella, M.F.; Marchesini, G. Insulin resistance in nonalcoholic fatty liver disease. Curr. Pharm. Des. 2010, 16, 1941–1951. [Google Scholar] [CrossRef]
- Guilherme, A.; Virbasius, J.V.; Puri, V.; Czech, M.P. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat. Rev. Mol. Cell Biol. 2008, 9, 367. [Google Scholar] [CrossRef] [Green Version]
- Cusi, K. Role of insulin resistance and lipotoxicity in non-alcoholic steatohepatitis. Clin. Liver Dis. 2009, 13, 545–563. [Google Scholar] [CrossRef]
- Kirpich, I.A.; Marsano, L.S.; McClain, C.J. Gut-liver axis, nutrition, and non-alcoholic fatty liver disease. Clin. Biochem. 2015, 48, 923–930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anstee, Q.M.; Day, C.P. The genetics of NAFLD. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 645. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Tao, A.; Zhang, S.; Deng, Y.; Chen, G. Association Between Patatin-Like Phospholipase Domain Containing 3 Gene (PNPLA3) Polymorphisms and Nonalcoholic Fatty Liver Disease: A HuGE Review and Meta-Analysis. Sci. Rep. 2015, 5, 9284. [Google Scholar] [CrossRef] [PubMed]
- Bach-Faig, A.; Berry, E.M.; Lairon, D.; Reguant, J.; Trichopoulou, A.; Dernini, S.; Medina, F.X.; Battino, M.; Belahsen, R.; Miranda, G.; et al. Mediterranean diet pyramid today. Science and cultural updates. Public Health Nutr. 2011, 14, 2274–2284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abete, I.; Goyenechea, E.; Zulet, M.A.; Martinez, J.A. Obesity and metabolic syndrome: Potential benefit from specific nutritional components. Nutr. Metab. Cardiovasc. Dis. 2011, 21 (Suppl. 2), 1–15. [Google Scholar] [CrossRef]
- Berge, K.; Musa-Veloso, K.; Harwood, M.; Hoem, N.; Burri, L. Krill oil supplementation lowers serum triglycerides without increasing low-density lipoprotein cholesterol in adults with borderline high or high triglyceride levels. Nutr. Res. 2014, 34, 126–133. [Google Scholar] [CrossRef] [Green Version]
- Burri, L.; Berge, K.; Wibrand, K.; Berge, R.K.; Barger, J.L. Differential effects of krill oil and fish oil on the hepatic transcriptome in mice. Front. Genet. 2011, 2, 45. [Google Scholar] [CrossRef] [Green Version]
- Parnell, J.A.; Raman, M.; Rioux, K.P.; Reimer, R.A. The potential role of prebiotic fibre for treatment and management of non-alcoholic fatty liver disease and associated obesity and insulin resistance. Liver Int. 2012, 32, 701–711. [Google Scholar] [CrossRef]
- Francque, S.M.; van der Graaff, D.; Kwanten, W.J. Non-alcoholic fatty liver disease and cardiovascular risk: Pathophysiological mechanisms and implications. J. Hepatol. 2016, 65, 425–443. [Google Scholar] [CrossRef] [Green Version]
- Bozzetto, L.; Prinster, A.; Annuzzi, G.; Costagliola, L.; Mangione, A.; Vitelli, A.; Mazzarella, R.; Longobardo, M.; Mancini, M.; Vigorito, C.; et al. Liver fat is reduced by an isoenergetic MUFA diet in a controlled randomized study in type 2 diabetic patients. Diabetes Care 2012, 35, 1429–1435. [Google Scholar] [CrossRef] [Green Version]
- Ryan, M.C.; Itsiopoulos, C.; Thodis, T.; Ward, G.; Trost, N.; Hofferberth, S.; O’Dea, K.; Desmond, P.V.; Johnson, N.A.; Wilson, A.M. The Mediterranean diet improves hepatic steatosis and insulin sensitivity in individuals with non-alcoholic fatty liver disease. J. Hepatol. 2013, 59, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Miranda, J.; Perez-Jimenez, F.; Ros, E.; De Caterina, R.; Badimon, L.; Covas, M.I.; Escrich, E.; Ordovas, J.M.; Soriguer, F.; Abia, R.; et al. Olive oil and health: Summary of the II international conference on olive oil and health consensus report, Jaen and Cordoba (Spain) 2008. Nutr. Metab. Cardiovasc. Dis. 2010, 20, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, Y.M.; Bemudez, B.; Lopez, S.; Abia, R.; Villar, J.; Muriana, F.J. Minor compounds of olive oil have postprandial anti-inflammatory effects. Br. J. Nutr. 2007, 98, 260–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zelber-Sagi, S.; Salomone, F.; Mlynarsky, L. The Mediterranean dietary pattern as the diet of choice for non-alcoholic fatty liver disease: Evidence and plausible mechanisms. Liver Int. 2017, 37, 936–949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Covas, M.I.; Nyyssonen, K.; Poulsen, H.E.; Kaikkonen, J.; Zunft, H.J.; Kiesewetter, H.; Gaddi, A.; de la Torre, R.; Mursu, J.; Baumler, H.; et al. The effect of polyphenols in olive oil on heart disease risk factors: A randomized trial. Ann. Intern. Med. 2006, 145, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Spooner, M.H.; Jump, D.B. Omega-3 fatty acids and nonalcoholic fatty liver disease in adults and children: Where do we stand? Curr. Opin. Clin. Nutr. Metab. Care 2019, 22, 103–110. [Google Scholar] [CrossRef]
- Parker, H.M.; Johnson, N.A.; Burdon, C.A.; Cohn, J.S.; O’Connor, H.T.; George, J. Omega-3 supplementation and non-alcoholic fatty liver disease: A systematic review and meta-analysis. J. Hepatol. 2012, 56, 944–951. [Google Scholar] [CrossRef] [Green Version]
- Lu, W.; Li, S.; Li, J.; Wang, J.; Zhang, R.; Zhou, Y.; Yin, Q.; Zheng, Y.; Wang, F.; Xia, Y.; et al. Effects of Omega-3 Fatty Acid in Nonalcoholic Fatty Liver Disease: A Meta-Analysis. Gastroenterol. Res. Pract. 2016, 2016, 1459790. [Google Scholar] [CrossRef] [Green Version]
- Scorletti, E.; Bhatia, L.; McCormick, K.G.; Clough, G.F.; Nash, K.; Hodson, L.; Moyses, H.E.; Calder, P.C.; Byrne, C.D.; Study, W. Effects of purified eicosapentaenoic and docosahexaenoic acids in nonalcoholic fatty liver disease: Results from the Welcome* study. Hepatology 2014, 60, 1211–1221. [Google Scholar] [CrossRef]
- Argo, C.K.; Patrie, J.T.; Lackner, C.; Henry, T.D.; de Lange, E.E.; Weltman, A.L.; Shah, N.L.; Al-Osaimi, A.M.; Pramoonjago, P.; Jayakumar, S.; et al. Effects of n-3 fish oil on metabolic and histological parameters in NASH: A double-blind, randomized, placebo-controlled trial. J. Hepatol. 2015, 62, 190–197. [Google Scholar] [CrossRef]
- Zelber-Sagi, S.; Nitzan-Kaluski, D.; Goldsmith, R.; Webb, M.; Blendis, L.; Halpern, Z.; Oren, R. Long term nutritional intake and the risk for non-alcoholic fatty liver disease (NAFLD): A population based study. J. Hepatol. 2007, 47, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Cortez-Pinto, H.; Jesus, L.; Barros, H.; Lopes, C.; Moura, M.C.; Camilo, M.E. How different is the dietary pattern in non-alcoholic steatohepatitis patients? Clin. Nutr. 2006, 25, 816–823. [Google Scholar] [CrossRef] [PubMed]
- Masterton, G.S.; Plevris, J.N.; Hayes, P.C. Review article: Omega-3 fatty acids–A promising novel therapy for non-alcoholic fatty liver disease. Aliment. Pharm. Ther. 2010, 31, 679–692. [Google Scholar] [CrossRef]
- Sanyal, A.J.; Abdelmalek, M.F.; Suzuki, A.; Cummings, O.W.; Chojkier, M.; Group, E.-A.S. No significant effects of ethyl-eicosapentanoic acid on histologic features of nonalcoholic steatohepatitis in a phase 2 trial. Gastroenterology 2014, 147, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Liccardo, D.; Alisi, A.; Porta, G.; Nobili, V. Is there any link between dietary pattern and development of nonalcoholic fatty liver disease in adolescence? An expert review. Expert Rev. Gastroenterol. Hepatol. 2013, 7, 601–604. [Google Scholar] [CrossRef]
- Oddy, W.H.; Herbison, C.E.; Jacoby, P.; Ambrosini, G.L.; O’Sullivan, T.A.; Ayonrinde, O.T.; Olynyk, J.K.; Black, L.J.; Beilin, L.J.; Mori, T.A.; et al. The Western dietary pattern is prospectively associated with nonalcoholic fatty liver disease in adolescence. Am. J. Gastroenterol. 2013, 108, 778–785. [Google Scholar] [CrossRef] [Green Version]
- Barrera, F.; George, J. The role of diet and nutritional intervention for the management of patients with NAFLD. Clin. Liver Dis. 2014, 18, 91–112. [Google Scholar] [CrossRef]
- Ouyang, X.; Cirillo, P.; Sautin, Y.; McCall, S.; Bruchette, J.L.; Diehl, A.M.; Johnson, R.J.; Abdelmalek, M.F. Fructose consumption as a risk factor for non-alcoholic fatty liver disease. J. Hepatol. 2008, 48, 993–999. [Google Scholar] [CrossRef] [Green Version]
- Kani, A.H.; Alavian, S.M.; Esmaillzadeh, A.; Adibi, P.; Azadbakht, L. Effects of a novel therapeutic diet on liver enzymes and coagulating factors in patients with non-alcoholic fatty liver disease: A parallel randomized trial. Nutrition 2014, 30, 814–821. [Google Scholar] [CrossRef]
- Han, J.M.; Jo, A.N.; Lee, S.M.; Bae, H.S.; Jun, D.W.; Cho, Y.K.; Suk, K.T.; Yoon, J.H.; Ahn, S.B.; Cho, Y.J.; et al. Associations between intakes of individual nutrients or whole food groups and non-alcoholic fatty liver disease among Korean adults. J. Gastroenterol. Hepatol. 2014, 29, 1265–1272. [Google Scholar] [CrossRef]
- Lei, S.; Liu, Z.W.; Li, Y.; Gong, C.; Zhang, H.; Song, L.J.; Huang, C.Y.; Li, M. The prevalence of nonalcoholic fatty liver disease and its association with lifestyle/dietary habits among university faculty and staff in Chengdu. Biomed. Environ. Sci. 2012, 25, 383–391. [Google Scholar] [CrossRef]
- Goran, M.I.; Walker, R.; Allayee, H. Genetic-related and carbohydrate-related factors affecting liver fat accumulation. Curr. Opin. Clin. Nutr. Metab. Care 2012, 15, 392–396. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; Welsh, J.A.; Le, N.A.; Holzberg, J.; Sharma, P.; Martin, D.R.; Vos, M.B. Dietary fructose reduction improves markers of cardiovascular disease risk in Hispanic-American adolescents with NAFLD. Nutrients 2014, 6, 3187–3201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiu, S.; Sievenpiper, J.L.; de Souza, R.J.; Cozma, A.I.; Mirrahimi, A.; Carleton, A.J.; Ha, V.; Di Buono, M.; Jenkins, A.L.; Leiter, L.A.; et al. Effect of fructose on markers of non-alcoholic fatty liver disease (NAFLD): A systematic review and meta-analysis of controlled feeding trials. Eur. J. Clin. Nutr. 2014, 68, 416–423. [Google Scholar] [CrossRef]
- Ye, E.Q.; Chacko, S.A.; Chou, E.L.; Kugizaki, M.; Liu, S. Greater whole-grain intake is associated with lower risk of type 2 diabetes, cardiovascular disease, and weight gain. J. Nutr. 2012, 142, 1304–1313. [Google Scholar] [CrossRef]
- Nothlings, U.; Schulze, M.B.; Weikert, C.; Boeing, H.; van der Schouw, Y.T.; Bamia, C.; Benetou, V.; Lagiou, P.; Krogh, V.; Beulens, J.W.; et al. Intake of vegetables, legumes, and fruit, and risk for all-cause, cardiovascular, and cancer mortality in a European diabetic population. J. Nutr. 2008, 138, 775–781. [Google Scholar] [CrossRef] [Green Version]
- Parnell, J.A.; Reimer, R.A. Effect of prebiotic fibre supplementation on hepatic gene expression and serum lipids: A dose-response study in JCR:LA-cp rats. Br. J. Nutr. 2010, 103, 1577–1584. [Google Scholar] [CrossRef] [Green Version]
- Ross, A.B.; Bruce, S.J.; Blondel-Lubrano, A.; Oguey-Araymon, S.; Beaumont, M.; Bourgeois, A.; Nielsen-Moennoz, C.; Vigo, M.; Fay, L.B.; Kochhar, S.; et al. A whole-grain cereal-rich diet increases plasma betaine, and tends to decrease total and LDL-cholesterol compared with a refined-grain diet in healthy subjects. Br. J. Nutr. 2011, 105, 1492–1502. [Google Scholar] [CrossRef] [Green Version]
- Bruce, S.J.; Guy, P.A.; Rezzi, S.; Ross, A.B. Quantitative measurement of betaine and free choline in plasma, cereals and cereal products by isotope dilution LC-MS/MS. J. Agric. Food Chem. 2010, 58, 2055–2061. [Google Scholar] [CrossRef]
- Singh, S.P.; Singh, A.; Misra, D.; Misra, B.; Pati, G.K.; Panigrahi, M.K.; Kar, S.K.; Bhuyan, P.; Pattnaik, K.; Meher, C.; et al. Risk Factors Associated With Non-Alcoholic Fatty Liver Disease in Indians: A Case-Control Study. J. Clin. Exp. Hepatol. 2015, 5, 295–302. [Google Scholar] [CrossRef] [Green Version]
- Roerecke, M.; Nanau, R.; Rehm, J.; Neuman, M. Ethnicity matters: A Systematic Review and Meta-Analysis of the Non-Linear Relationship Between Alcohol Consumption and Prevalence and Incidence of Hepatic Steatosis. EBioMedicine 2016, 8, 317–330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cotrim, H.P.; Freitas, L.A.; Alves, E.; Almeida, A.; May, D.S.; Caldwell, S. Effects of light-to-moderate alcohol consumption on steatosis and steatohepatitis in severely obese patients. Eur. J. Gastroenterol. Hepatol. 2009, 21, 969–972. [Google Scholar] [CrossRef] [PubMed]
- Hajifathalian, K.; Torabi Sagvand, B.; McCullough, A.J. Effect of Alcohol Consumption on Survival in Nonalcoholic Fatty Liver Disease: A National Prospective Cohort Study. Hepatology 2019, 70, 511–521. [Google Scholar] [CrossRef] [PubMed]
- Yesil, A.; Yilmaz, Y. Review article: Coffee consumption, the metabolic syndrome and non-alcoholic fatty liver disease. Aliment. Pharm. Ther. 2013, 38, 1038–1044. [Google Scholar] [CrossRef] [PubMed]
- Catalano, D.; Martines, G.F.; Tonzuso, A.; Pirri, C.; Trovato, F.M.; Trovato, G.M. Protective role of coffee in non-alcoholic fatty liver disease (NAFLD). Dig. Dis. Sci. 2010, 55, 3200–3206. [Google Scholar] [CrossRef]
- Molloy, J.W.; Calcagno, C.J.; Williams, C.D.; Jones, F.J.; Torres, D.M.; Harrison, S.A. Association of coffee and caffeine consumption with fatty liver disease, nonalcoholic steatohepatitis, and degree of hepatic fibrosis. Hepatology 2012, 55, 429–436. [Google Scholar] [CrossRef]
- Bravi, F.; Bosetti, C.; Tavani, A.; Gallus, S.; La Vecchia, C. Coffee reduces risk for hepatocellular carcinoma: An updated meta-analysis. Clin. Gastroenterol. Hepatol. 2013, 11, 1413–1421. [Google Scholar] [CrossRef]
- Sang, L.X.; Chang, B.; Li, X.H.; Jiang, M. Consumption of coffee associated with reduced risk of liver cancer: A meta-analysis. BMC Gastroenterol. 2013, 13, 34. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Teoh, N.C.; Chitturi, S.; Farrell, G.C. Coffee and non-alcoholic fatty liver disease: Brewing evidence for hepatoprotection? J. Gastroenterol. Hepatol. 2014, 29, 435–441. [Google Scholar] [CrossRef] [Green Version]
- Bravi, F.; Tavani, A.; Bosetti, C.; Boffetta, P.; La Vecchia, C. Coffee and the risk of hepatocellular carcinoma and chronic liver disease: A systematic review and meta-analysis of prospective studies. Eur. J. Cancer Prev. 2017, 26, 368–377. [Google Scholar] [CrossRef]
- Miranda, A.M.; Steluti, J.; Fisberg, R.M.; Marchioni, D.M. Association between Coffee Consumption and Its Polyphenols with Cardiovascular Risk Factors: A Population-Based Study. Nutrients 2017, 9, 276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Keefe, J.H.; Bhatti, S.K.; Patil, H.R.; DiNicolantonio, J.J.; Lucan, S.C.; Lavie, C.J. Effects of Habitual Coffee Consumption on Cardiometabolic Disease, Cardiovascular Health, and All-Cause Mortality. J. Am. Coll. Cardiol. 2013, 62, 1043–1051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hino, A.; Adachi, H.; Enomoto, M.; Furuki, K.; Shigetoh, Y.; Ohtsuka, M.; Kumagae, S.-I.; Hirai, Y.; Jalaldin, A.; Satoh, A.; et al. Habitual coffee but not green tea consumption is inversely associated with metabolic syndrome: An epidemiological study in a general Japanese population. Diabetes Res. Clin. Pract. 2007, 76, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Blond, E.; Disse, E.; Cuerq, C.; Drai, J.; Valette, P.J.; Laville, M.; Thivolet, C.; Simon, C.; Caussy, C. EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease in severely obese people: Do they lead to over-referral? Diabetologia 2017, 60, 1218–1222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez-Gonzalez, M.A.; Martin-Calvo, N. Mediterranean diet and life expectancy; beyond olive oil, fruits, and vegetables. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Perez-Guisado, J.; Munoz-Serrano, A. The effect of the Spanish Ketogenic Mediterranean Diet on nonalcoholic fatty liver disease: A pilot study. J. Med. Food 2011, 14, 677–680. [Google Scholar] [CrossRef]
- Abenavoli, L.; Greco, M.; Milic, N.; Accattato, F.; Foti, D.; Gulletta, E.; Luzza, F. Effect of Mediterranean Diet and Antioxidant Formulation in Non-Alcoholic Fatty Liver Disease: A Randomized Study. Nutrients 2017, 9, 870. [Google Scholar] [CrossRef]
- Trovato, F.M.; Catalano, D.; Martines, G.F.; Pace, P.; Trovato, G.M. Mediterranean diet and non-alcoholic fatty liver disease: The need of extended and comprehensive interventions. Clin. Nutr. 2015, 34, 86–88. [Google Scholar] [CrossRef]
- Gelli, C.; Tarocchi, M.; Abenavoli, L.; Di Renzo, L.; Galli, A.; De Lorenzo, A. Effect of a counseling-supported treatment with the Mediterranean diet and physical activity on the severity of the non-alcoholic fatty liver disease. World J. Gastroenterol. 2017, 23, 3150–3162. [Google Scholar] [CrossRef]
- Katsagoni, C.N.; Georgoulis, M.; Papatheodoridis, G.V.; Panagiotakos, D.B.; Kontogianni, M.D. Effects of lifestyle interventions on clinical characteristics of patients with non-alcoholic fatty liver disease: A meta-analysis. Metabolism 2017, 68, 119–132. [Google Scholar] [CrossRef] [Green Version]
- Misciagna, G.; Del Pilar Diaz, M.; Caramia, D.V.; Bonfiglio, C.; Franco, I.; Noviello, M.R.; Chiloiro, M.; Abbrescia, D.I.; Mirizzi, A.; Tanzi, M.; et al. Effect of a Low Glycemic Index Mediterranean Diet on Non-Alcoholic Fatty Liver Disease. A Randomized Controlled Clinici Trial. J. Nutr. Health Aging 2017, 21, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Katsagoni, C.N.; Papatheodoridis, G.V.; Ioannidou, P.; Deutsch, M.; Alexopoulou, A.; Papadopoulos, N.; Papageorgiou, M.V.; Fragopoulou, E.; Kontogianni, M.D. Improvements in clinical characteristics of patients with non-alcoholic fatty liver disease, after an intervention based on the Mediterranean lifestyle: A randomised controlled clinical trial. Br. J. Nutr. 2018, 120, 164–175. [Google Scholar] [CrossRef] [PubMed]
- Kontogianni, M.D.; Tileli, N.; Margariti, A.; Georgoulis, M.; Deutsch, M.; Tiniakos, D.; Fragopoulou, E.; Zafiropoulou, R.; Manios, Y.; Papatheodoridis, G. Adherence to the Mediterranean diet is associated with the severity of non-alcoholic fatty liver disease. Clin. Nutr. 2014, 33, 678–683. [Google Scholar] [CrossRef] [PubMed]
- Della Corte, C.; Mosca, A.; Vania, A.; Alterio, A.; Iasevoli, S.; Nobili, V. Good adherence to the Mediterranean diet reduces the risk for NASH and diabetes in pediatric patients with obesity: The results of an Italian Study. Nutrition 2017, 39, 8–14. [Google Scholar] [CrossRef]
- Katsagoni, C.N.; Georgoulis, M.; Papatheodoridis, G.V.; Fragopoulou, E.; Ioannidou, P.; Papageorgiou, M.; Alexopoulou, A.; Papadopoulos, N.; Deutsch, M.; Kontogianni, M.D. Associations Between Lifestyle Characteristics and the Presence of Nonalcoholic Fatty Liver Disease: A Case-Control Study. Metab. Syndr. Relat. Disord. 2017, 15, 72–79. [Google Scholar] [CrossRef]
- Graif, M.; Yanuka, M.; Baraz, M.; Blank, A.; Moshkovitz, M.; Kessler, A.; Gilat, T.; Weiss, J.; Walach, E.; Amazeen, P.; et al. Quantitative estimation of attenuation in ultrasound video images: Correlation with histology in diffuse liver disease. Investig. Radiol. 2000, 35, 319–324. [Google Scholar] [CrossRef]
- Hamaguchi, M.; Kojima, T.; Itoh, Y.; Harano, Y.; Fujii, K.; Nakajima, T.; Kato, T.; Takeda, N.; Okuda, J.; Ida, K.; et al. The severity of ultrasonographic findings in nonalcoholic fatty liver disease reflects the metabolic syndrome and visceral fat accumulation. Am. J. Gastroenterol. 2007, 102, 2708–2715. [Google Scholar] [CrossRef]
- Stavropoulos, K.; Imprialos, K.; Pittaras, A.; Faselis, C.; Narayan, P.; Kokkinos, P. Lifestyle Modifications in Non-Alcoholic Fatty Liver Disease and Non- Alcoholic Steatohepatitis. Curr. Vasc. Pharm. 2018, 16, 239–245. [Google Scholar] [CrossRef]
- Abenavoli, L.; Peta, V.; Milic, N. Lifestyle changes associated with a new antioxidant formulation in non-alcoholic fatty liver disease: A case series. Ann. Hepatol. 2015, 14, 121–126. [Google Scholar] [CrossRef]
- Papamiltiadous, E.S.; Roberts, S.K.; Nicoll, A.J.; Ryan, M.C.; Itsiopoulos, C.; Salim, A.; Tierney, A.C. A randomised controlled trial of a Mediterranean Dietary Intervention for Adults with Non Alcoholic Fatty Liver Disease (MEDINA): Study protocol. BMC Gastroenterol. 2016, 16, 14. [Google Scholar] [CrossRef] [Green Version]
- Keating, S.E.; Hackett, D.A.; Parker, H.M.; O’Connor, H.T.; Gerofi, J.A.; Sainsbury, A.; Baker, M.K.; Chuter, V.H.; Caterson, I.D.; George, J.; et al. Effect of aerobic exercise training dose on liver fat and visceral adiposity. J. Hepatol. 2015, 63, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Sung, K.C.; Ryu, S.; Lee, J.Y.; Kim, J.Y.; Wild, S.H.; Byrne, C.D. Effect of exercise on the development of new fatty liver and the resolution of existing fatty liver. J. Hepatol. 2016, 65, 791–797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bacchi, E.; Negri, C.; Targher, G.; Faccioli, N.; Lanza, M.; Zoppini, G.; Zanolin, E.; Schena, F.; Bonora, E.; Moghetti, P. Both resistance training and aerobic training reduce hepatic fat content in type 2 diabetic subjects with nonalcoholic fatty liver disease (the RAED2 randomized trial). Hepatology 2013, 58, 1287–1295. [Google Scholar] [CrossRef] [PubMed]
- Romero-Gomez, M.; Zelber-Sagi, S.; Trenell, M. Treatment of NAFLD with diet, physical activity and exercise. J. Hepatol. 2017, 67, 829–846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keating, S.E.; Adams, L.A. Exercise in NAFLD: Just do it. J. Hepatol. 2016, 65, 671–673. [Google Scholar] [CrossRef] [Green Version]
- Hashida, R.; Kawaguchi, T.; Bekki, M.; Omoto, M.; Matsuse, H.; Nago, T.; Takano, Y.; Ueno, T.; Koga, H.; George, J.; et al. Aerobic vs. resistance exercise in non-alcoholic fatty liver disease: A systematic review. J. Hepatol. 2017, 66, 142–152. [Google Scholar] [CrossRef]
- Takahashi, H.; Kotani, K.; Tanaka, K.; Egucih, Y.; Anzai, K. Therapeutic Approaches to Nonalcoholic Fatty Liver Disease: Exercise Intervention and Related Mechanisms. Front. Endocrinol. 2018, 9, 588. [Google Scholar] [CrossRef]
- Lazarus, J.V.; Ekstedt, M.; Marchesini, G.; Mullen, J.; Novak, K.; Pericas, J.M.; Roel, E.; Romero-Gomez, M.; Ratziu, V.; Tacke, F.; et al. A cross-sectional study of the public health response to non-alcoholic fatty liver disease in Europe. J. Hepatol. 2019. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Loomba, R.; Rinella, M.E.; Bugianesi, E.; Marchesini, G.; Neuschwander-Tetri, B.A.; Serfaty, L.; Negro, F.; Caldwell, S.H.; Ratziu, V.; et al. Current and future therapeutic regimens for nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Hepatology 2018, 68, 361–371. [Google Scholar] [CrossRef]
- Rinella, M.E.; Tacke, F.; Sanyal, A.J.; Anstee, Q.M.; The Participants of the AASLD/EASL Workshop. Report on the AASLD/EASL joint workshop on clinical trial endpoints in NAFLD. J. Hepatol 2019, 71, 823–833. [Google Scholar] [CrossRef] [Green Version]

| Reference | Design | Dietary Composition | Study Aim | N Patients, Age (Median) | NAFLD Diagnosis and Assessment at Follow Up | Baseline Parameters | Main Results |
|---|---|---|---|---|---|---|---|
| Perez-Guisado et al. [76] | Prospective study, 12 weeks of dietary treatment. No physical activity Follow up at 12 weeks | Unlimited calories. High doses of virgin olive oil (min 30 mL/day) and ω-3 fatty acids from fish as main source of fat, fish as the main source of protein, green vegetables and salads as the main source of carbohydrates (max. 30 g/day) | Assessment of Spanish ketogenic MD effects on steatosis degree, measured by US and serologic measures of liver function | 14 41 years | NAFLD was diagnosed by ALT levels >40U/L and steatosis on US. Steatosis degree was assessed by US using a four-point validated scale | BMI 36.5 ± 0.54 kg/m2 ALT 71.9 ± 3.5 U/L AST 47.7 ± 2.8 U/L Not stated whether patients had diabetes | -Complete fatty liver regression (21.4% of the patients) with an overall reduction in 92.86% of patients (p < 0.001) -Significant (p<0.001) BM, AST and ALT reduction (mean BMI 32.4, AST 29 and ALT 37.0) |
| Ryan et al. [31] | Randomised cross-over trial: patients received six weeks of MD and six weeks of low fat/high carbohydrate diet. No physical activity Follow up at end of second diet | Unlimited calories. High in MUFAs from olive oil and ω-3 fatty acids from fish; approximate macronutrient composition 40% fat, 40%carbs, 20% proteins | Assessment of MD on steatosis degree, measured by MRI, and on insulin sensitivity | 12 55 years | Biopsy-proven NAFLD diagnosis Steatosis degree was assessed by MRI | BMI 32.0 ± 4.0 kg/m2 ALT 49.0 ± 23 U/L No patients were diabetic (No significant differences between groups) | −39% relative reduction in steatosis in MD intervention group (p < 0.05) -No significant reduction in BMI/ALT in either diet -Improved insulin sensitivity in MD (p = 0.03) |
| Abenavoli et al. [77] | Randomised clinical trial. Physical activity recommended Low-calorie MD was prescribed to Group A and B patients for 6 months. Group B received also antioxidant complex. Group C received no treatment/advice. Follow up at 6 months | Low calorie MD with carbohydrates (50%–60%), proteins (15%–20%, about 50% of which were vegetable proteins), MUFAs and PUFAs (less than 30%), saturated fat (less than 10%), cholesterol (less than 300 mg/day) and fibers (25–30 g/day. Treatment prescribed for six months. | Evaluate the effects of antioxidant complex associated with MD on liver fat accumulation, BMI, glucose, and lipid metabolism | 50 Group A: 52 years Group B: 46 years Group C: 33 years | NAFLD was diagnosed by US Steatosis degree was assessed by FLI and US using the Hamaguchi score | BMI 31 (29–33) kg/m2 in group A; 29 (28–32) kg/m2 in group B; 29 (27–31) kg/m2 in group C AST and ALT median levels within normal range in all groups Not stated if patients were diabetic | Significant reductions in BMI (p = 0.0001), lipid profile (p < 0.001) and in FLI (p < 0.01) and US-Hamaguchi score (p = 0.0001) were reported in groups A and B but not in the control group. |
| Trovato et al. [78] | Prospective, observational No dietary treatment but behavoural and dietary counseling Follow up at six months | Behavioral and dietary counseling | To evaluate the effectiveness of an intervention focused to increase the Adherence to Mediterranean Diet Score (AMDS) and the level of physical exercise | 90 50 years | NAFLD was diagnosed by US Steatosis degree was assessed by BLS | BMI 31 ± 5 kg/m2 ALT 23.3 U/L BLS 1.96 ± 0.69 No patient had diabetes | Significant reduction in BMI (p < 0.0001) and BLS (p < 0.0001). No significant reductions in ALT Significant reductions in liver stiffness, assed by TE |
| Gelli et al. [79] | Prospective, observational Restricted calories MD for six months Follow up at six months | Calorie restriction: maximum calorie reduction, 500 kcal/day. MD: 55%–60% of carbohydrates of which 80% complex carbohydrates (bread, pasta, rice), 10%–15% of proteins about 60% of animal origin (especially white meat, fish), 25%–30% fat (mostly olive oil) | End-points: (1) reduction of at least 1 unit of steatosis grade; (2) A 7% weight reduction; (3) normalization or improvement of metabolic indexes and (4) normalization or improvement of ALT, AST, GGT. | 46 47 years | NAFLD was diagnosed by US Steatosis degree was assessed by a four-grade US scale | BMI 29.3 kg/m2 ALT was abnormal in 67% patients whereas AST and GGT were abnormal in 26% and 34% of patients, respectively 8% of patients had diabetes | -Steatosis regression 20% -In more than 80% of patients at least one grade reduction of steatosis degree -BMI decreased on average from 29.3 ± 6.11 to 27.5 ± 6.21 (p < 0.01) -Significant reduction of ALT, AST and GGT (p < 0.01) |
| Katsagoni et al. [80] | Metanalysis of RCTs about lifestyle interventions in NAFLD | RCTs of intervention with exercise and/or diet in NAFLD-patients, in English language were included. Aim: evaluate the efficacy of lifestyle interventions on liver, anthropometric and metabolic parameters. | 20 RCT with 1073 patients were included | NAFLD diagnosis by means of US or MRI or biopsy | Combination of exercise and diet decreased ALT levels (p < 0.01) and improved NAFLD activity score (SMD = −0.61, 95% CI: −1.09to −0.13). Moderate-carbohydrate diets yielded similar changes in liver enzymes compared to low/moderate-fat diets | ||
| Misciagna et al. [81] | Double blind RCT LGIMD or INRAN diet for six months Follow up at six months | Energy restriction. Low glycemic Index MD vs. Italian National Research Institute for Foods and Nutrition (INRAN) diet. | To estimate the effect of a LGIMD on steatosis as measured by US | 98 Median age not available | NAFLD diagnosis by US Steatosis degree with an US based scale | 4% of patients had normal BMI values, 26% were overweight and 70% were obese. None was diabetic. All patients had moderate/severe steatosis on US. | FLI and steatosis degree significantly decreased in both diets (p < 0.05) but the decrease was greater in the LGIMD group |
| Katsagoni et al. [82] | Single blind RCT Patients randomized in control group, MD group and Mediterranean lifestyle (ML) group. MD and ML group also received regular counseling during follow up, including training and sleep duration counseling for the latter Follow up at six months | All groups received energy restriction and similar dietetic regimen with 45% carbs, 20% protein and 35% lipids MD and ML groups were also counseled regularly | To estimate the effect of MD or Mediterranean lifestyle in NAFLD patients. vStudy primary outcomes (i.e., ALT<40U/l and 50% reduction of ALT levels) | 63 50 years | NAFLD diagnosis based on the following criteria: elevated alanine aminotransferase (ALT) and/or γ-glutamyl-transpeptidase (GGT) levels, evidence of hepatic steatosis on ultrasound and/or compatible liver histology | BMI 31.8 ± 4.5 kg/m2 Not stated if patients were diabetic | Greater BMI reductions (p = 0·008) in MD and ML compared with control ML showed significant improvements compared with the control (p = 0.009), and a higher tendency compared to MD (OR = 0·27; 95% CI 0·07, 1·03, in ALT reduction. Both MD and ML improved liver stiffness in TE |
| Properzi et al. [12] | Single blind RCT Randomization to MD vs. Low-fat diet (LFD )Follow up at 12 weeks | LFD composition: 50% carbs, 30% fat (with <10% of energy as saturated fat), and 20% protein MD composition: 40% carbs, 35–40% fat (with <10% of energy as saturated fat), and 20% protein | Investigate the effect of two ad libitum isocaloric diets (MD or LFD) on hepatic steatosis | 48 52 years | NAFLD diagnosis was made by MRI | BMI 30.2 kg/m2 in LFD BMI 31.5 kg/m2 in MD ALT 66.8 U/L in LFD ALT 76.5 U/L in MD 28% patients in LFD and 30% in MD had diabetes | Hepatic fat content reduced significantly in both groups (p < 0.01), with no difference between groups (P = 0.32). Liver enzymes improved significantly in both groups. Weight loss: minimal and not different between groups |
| Gepner et al. [11] | Single blind RCT Randomization to M-low carbs (MD/LC) vs. LFD. After six months each diet group was further randomized into added physical activity or no added PA groups for another 12 months Follow up at 18 months | -LF diet: fat maximum 30% of calories, with up to 10% of saturated fat, no more than 300 mg/day of cholesterol, increased dietary fiber.-MD/LC diet: MD with less than 70 g/day of carbs, increased protein and fat intake plus 28 g of walnuts/day | Investigate if hepatic fat loss in response to dietary interventions induces specific beneficial effects independently of visceral abdominal fat (VAT) changes. | 278 48 years | NAFLD diagnosis was made by MRI | BMI 30.8 ± 3.8 kg/m2 Overall, 53% of patients had MRI-proven steatosis. All patients had abdominal obesity. 11% of patients had diabetes | Hepatic fat substantially decreased after 18 months (4.0% absolute units (29% Relatively); p < 0.001 vs. baseline) in entire cohort (also in patients without steatosis, i.e., HF < 5%). Hepatic fat was substantially reduced by diet-induced moderate weight loss and more effectively by the MED/LC diet than the LF diet (p = 0.036), independently of VAT changes. |
| Kontogianni et al. [83] | Retrospective study Adherence to MD and physical activity over the last 12 months were assessed | To investigate the associations between adherence to the MD(estimated with MedDietScore) and histological characteristics of NAFLD | 73 45 years | NAFLD diagnosis based on the following criteria: elevated alanine aminotransferase (ALT) and/or γ-glutamyl-transpeptidase (GGT) levels, evidence of hepatic steatosis on ultrasound and/or compatible liver histology | BMI 29.7 ± 4.6 kg/m2 No patient had diabetes. 58 patients were matched with 58 healthy controls for age, sex and BMI. 63% of patient had NASH | MedDietScore negatively correlated to ALT (p = 0.03) and severity of steatosis (p = 0.006) Patients with NASH exhibited lower adherence to MD (29.3 +/− 3.2 vs. 34.1 +/− 4.4, p = 0.004) compared to those with simple fatty liver and had higher BMI (p = 0.028). No difference in the MedDietScore was observed between patients and controls | |
| Della Corte et al. [84] | Observational study Adherence to MD was assessed at time of inclusion | To analyze the association between adherence to the MD(using the KIDMED Index) and NAFLD, with laboratory and histologic evaluation, in a group of children and adolescents with obesity. | 243 Age: 10–17 years | NAFLD diagnosis was made on an US basis with/without abnormal transaminases | BMI 28.1 ± 6.3 kg/m2 No patient had diabetes 166 patients had steatosis, among them 53 had NASH | Low KIDMED score was significantly higher in patients with NASH (p = 0.001) and correlated with a NAFLD activity score (NAS) >5 (p = 0.001), and grade 2 fibrosis (p = 0.02). Low KIDMED correlates with fibrosis (OR 2.58, 95%CI 1.36–3.9, p = 0.05) and NAS (OR 1.03, 95%CI 0.9–2, p = 0.05) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plaz Torres, M.C.; Aghemo, A.; Lleo, A.; Bodini, G.; Furnari, M.; Marabotto, E.; Miele, L.; Giannini, E.G. Mediterranean Diet and NAFLD: What We Know and Questions That Still Need to Be Answered. Nutrients 2019, 11, 2971. https://doi.org/10.3390/nu11122971
Plaz Torres MC, Aghemo A, Lleo A, Bodini G, Furnari M, Marabotto E, Miele L, Giannini EG. Mediterranean Diet and NAFLD: What We Know and Questions That Still Need to Be Answered. Nutrients. 2019; 11(12):2971. https://doi.org/10.3390/nu11122971
Chicago/Turabian StylePlaz Torres, Maria Corina, Alessio Aghemo, Ana Lleo, Giorgia Bodini, Manuele Furnari, Elisa Marabotto, Luca Miele, and Edoardo G. Giannini. 2019. "Mediterranean Diet and NAFLD: What We Know and Questions That Still Need to Be Answered" Nutrients 11, no. 12: 2971. https://doi.org/10.3390/nu11122971
APA StylePlaz Torres, M. C., Aghemo, A., Lleo, A., Bodini, G., Furnari, M., Marabotto, E., Miele, L., & Giannini, E. G. (2019). Mediterranean Diet and NAFLD: What We Know and Questions That Still Need to Be Answered. Nutrients, 11(12), 2971. https://doi.org/10.3390/nu11122971








