Factors Affecting the Environmentally Induced, Chronic Kidney Disease of Unknown Aetiology in Dry Zonal Regions in Tropical Countries—Novel Findings
Abstract
:1. Introduction
1.1. Demographics Associated with CKDmfo
1.2. Geographical Distribution of CKDmfo
1.3. Diagnosis of CKDmfo
1.4. Urinary Biomarkers for CKDmfo
1.5. Prognosis of CKDmfo
1.6. Summary of Proposed Causes of CKDmfo
1.7. Physiology of Renal Filtration Unit
1.8. CKDmfo: General Pathology
1.9. Toxic Tubular Nephropathy
1.10. Factors that Lead to Prolongation of the Disease
2. Extenuating Factors Affecting CKDmfo
2.1. CKDmfo and Chronic Exposure to Nephrotoxins
2.2. Sources of Water Contamination with Nephrotoxins
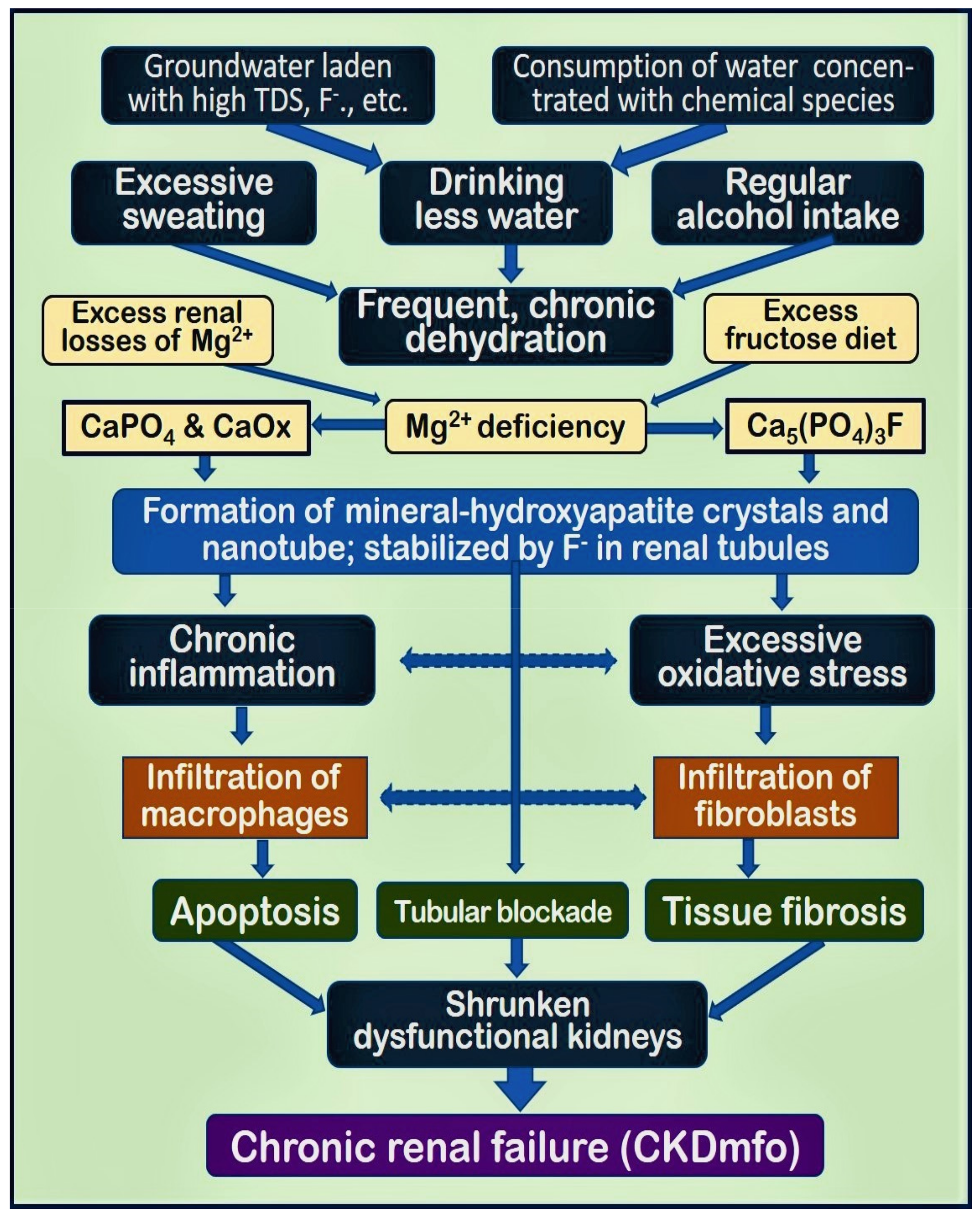
2.3. Mechanisms Initiating Tubular Inflammation and Fibrosis Leading to CKDmfo
2.4. Relationship of Drinking Water and CKDmfo
2.5. Contribution of Agrochemicals and Pollution to CKDmfo
2.6. Fluoride in Drinking Water and CKDmfo
2.7. Excess Fluoride Intake from Water
2.8. Dental and Skeletal Fluorosis and CKDmfo
3. Observations and New Concepts
3.1. Geographical and Other Similarities of CKDmfo-Affected Regions and Countries
3.2. Effects of Diet and Malnutrition
3.3. Intracellular Inclusion Bodies, Tubular Markers, and Chronic Kidney Disease
3.4. Ion Interactions and In Vivo Formation of Apatite
3.5. Nanominerals Causing Pathology
3.6. Other Factors to Consider
3.7. Explanations of Why Wet Zonal Regions are Spared of CKDmfo
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hajat, C.; Stein, E. The global burden of multiple chronic conditions: A narrative review. Prev. Med. Rep. 2018, 12, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.; Chang, Y.; Woo, H.Y.; Lee, K.B.; Kim, S.G.; Kim, D.I.; Kim, W.S.; Suh, B.S.; Jeong, C.; Yoon, K. Time-dependent association between metabolic syndrome and risk of CKD in Korean men without hypertension or diabetes. Am. J. Kidney Dis. 2009, 53, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Glaser, J.; Weiss, I.; La Isla, F. CKDu: Strategies for saving lives now. Med. Rev. 2014, 16, 81–82. [Google Scholar]
- Wimalawansa, S.J. Escalating Chronic Kidney Diseases in Sri Lanka: Causes, Solutions and recommendations. Environ. Health Prev. Med. 2014, 19, 375–394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jayatilake, N.; Mendis, S.; Maheepala, P.; Mehta, F.R. Chronic kidney disease of uncertain aetiology: Prevalence and causative factors in a developing country. BMC Nephrol. 2013, 14, 180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wimalawansa, S.J. The role of ions, heavy metals, fluoride, and agrochemicals: Critical evaluation of potential aetiological factors of chronic kidney disease of multifactorial origin (CKDmfo/CKDu) and recommendations for its eradication. Environ. Geochem. Health 2016, 38, 639–678. [Google Scholar] [CrossRef]
- Dunuweera, R.; Shimomura, R.M.G.; Priyankarage, J.V.; Jayasingha, P.; Wimalawansa, S.J. Chronic kidney disease of multifunctional origin (CKDmfo) prevailing in Sri Lanka: Re-evaluated. World J. Pharm. Res. 2017, 6, 33–66. [Google Scholar]
- Diyabalanage, S.; Abekoon, S.; Watanabe, I.; Watai, C.; Ono, Y.; Wijesekara, S.; Guruge, K.S.; Chandrajith, R. Has irrigated water from Mahaweli River contributed to the kidney disease of uncertain etiology in the dry zone of Sri Lanka? Environ. Geochem. Health 2016, 38, 679–690. [Google Scholar] [CrossRef]
- Wimalawansa, S.J. Chronic Kidney Disease in Rajarata, Worse than Tsunami. Sunday Observer. 26 November 2013. Available online: http://www.sundayobserver.lk/2013/11/24/fea06.asp (accessed on 20 November 2019).
- Wimalawansa, S.J. Molecular and cellular toxicity of fluoride in mystery, tubulointerstitial chronic kidney disease: A systematic review. Rev. Environ. Sci. Biotechnol. 2019, in press. [Google Scholar] [CrossRef]
- Wickremasinghe, A.R.; Peiris-John, R.J.; Wanigasuriya, K.P. Chronic kidney disease of unknown aetiology in the North Central Province of Sri Lanka: Trying to unravel the mystery. Ceylon Med. J. 2011, 56, 143–146. [Google Scholar] [CrossRef] [Green Version]
- Wimalawansa, S.A.; Wimalawansa, S.J. Agrochemical-Related Environmental Pollution: Effects on Human Health. Glob. J. Biol. Agric. Health Sci. 2014, 3, 72–83. [Google Scholar]
- Wimalawansa, S.J.; Wimalawansa, S.A. Chronic kidney disease of multifactorial origin (CKDmfo) in Sri Lanka: Escalating incidence and long-term survival estimates. J. Nephrol. Urol. Res. 2015, 22, 1–17. [Google Scholar]
- Chandrajith, R.; Nanayakkara, S.; Itai, K.; Aturaliya, T.N.; Dissanayake, C.B.; Abeysekera, T.; Harada, K.; Watanabe, T.; Koizumi, A. Chronic kidney diseases of uncertain etiology (CKDue) in Sri Lanka: Geographic distribution and environmental implications. Environ. Geochem. Health 2011, 33, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Bandara, J.M.; Senevirathna, D.M.; Dasanayake, D.M.; Herath, V.; Bandara, J.M.; Abeysekara, T.; Rajapaksha, K.H. Chronic renal failure among farm families in cascade irrigation systems in Sri Lanka associated with elevated dietary cadmium levels in rice and freshwater fish (Tilapia). Environ. Geochem. Health 2008, 30, 465–478. [Google Scholar] [CrossRef] [PubMed]
- Wimalawansa, S.J. Agrochemicals and chronic kidney disease of multifactorial origin: Environmentally induced occupational exposure disease. Int. J. Nephrol. Kidney Fail. 2015, 1, 1–9. [Google Scholar] [CrossRef]
- Wimalawansa, S.J. Effect of water hardness on non-communicable diseases including chronic kidney disease of multifactorial origin (CKDmfo/CKDuo). J. Environ. Health Sci. Eng. 2016, 2, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Faye, M.; Lemrabott, A.T.; Cisse, M.M.; Fall, K.; Keita, Y.; Ngaide, A.A.; Mbaye, A.; Fary Ka, E.H.; Niang, A.; Kane, A.; et al. Prevalence and risk factors of chronic kidney disease in an african semi-urban area: Results from a cross-sectional survey in Gueoul, Senegal. Saudi J. Kidney Dis. Transpl. 2017, 28, 1389–1396. [Google Scholar] [CrossRef]
- Abdulkader, R.; Burdmann, E.A.; Lebrao, M.L.; Duarte, Y.A.O.; Zanetta, D.M.T. Aging and decreased glomerular filtration rate: An elderly population-based study. PLoS ONE 2017, 12, e0189935. [Google Scholar] [CrossRef] [Green Version]
- Noble, A.; Amerasinghe, P.; Manthrithilake, H.; Arasalingam, S. Review of Literature on Chronic Kidney Disease of Unknown Etiology (CKDu) in Sri Lanka; International Water Management Institute (IWMI): Colombo, Sri Lanka, 2014. [Google Scholar]
- Wimalawansa, S.A.; Wimalawansa, S.J. Environmentally induced, occupational diseases with emphasis on chronic kidney disease of multifactorial origin affecting tropical countries. Ann. Occup. Environ. Med. 2016, 28, 33. [Google Scholar] [CrossRef] [Green Version]
- Orantes, C.M. Clinical Characterization and Histopathology of Nephropathy in Salvadorian Agricultural Communities. In Proceedings of the International Conference on Saptial Ecotoxicology, San Salvador, El Salvador, 29 October 2013. [Google Scholar]
- Wijetunge, S.; Ratnatunga, N.V.; Abeysekera, D.T.; Wazil, A.W.; Selvarajah, M.; Ratnatunga, C.N. Retrospective analysis of renal histology in asymptomatic patients with probable chronic kidney disease of unknown aetiology in Sri Lanka. Ceylon Med. J. 2013, 58, 142–147. [Google Scholar] [CrossRef]
- Wyne, K.; Wimalawansa, S.J. Screening and Diagnosis of Chronic Tubular Kidney Disease of Multi-Factorial Origin. In Proceedings of the 5th International Conference on Sustainable Built Environment, Environment Pollution of Prevention of CKD-mfo in Sri Lanka, Kandy, Sri Lanka, 11–13 October 2014; pp. 11–13. [Google Scholar]
- Wimalawansa, S.J. Prevention of CKD of multi-factorial Origin (CKD-mfo): Issues, gravity, & the importance of early diagnosis. In Proceedings of the 5th International Conference on Sustainable Built Environment, Environment Pollution of Prevention of CKD-mfo in Sri Lanka, Kandy, Sri Lanka, 11–13 October 2014; pp. 22–26. [Google Scholar]
- Nanayakkara, S.; Komiya, T.; Ratnatunga, N.; Senevirathna, S.T.; Harada, K.H.; Hitomi, T.; Gobe, G.; Muso, E.; Abeysekera, T.; Koizumi, A. Tubulointerstitial damage as the major pathological lesion in endemic chronic kidney disease among farmers in North Central Province of Sri Lanka. Environ. Health Prev. Med. 2012, 17, 213–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wimalawansa, S.J. Escalating Chronic Kidney Diseases in Sri Lanka: Causes, Solutions and recommendations—Update and responses. Environ. Health Prev. Med. 2015, 20, 152–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Amico, G.; Bazzi, C. Pathophysiology of proteinuria. Kidney Int. 2003, 63, 809–825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yokoyama, T.; Kamijo-Ikemori, A.; Sugaya, T.; Hoshino, S.; Yasuda, T.; Kimura, K. Urinary excretion of liver type fatty acid binding protein accurately reflects the degree of tubulointerstitial damage. Am. J. Pathol. 2009, 174, 2096–2106. [Google Scholar] [CrossRef] [Green Version]
- Kamijo-Ikemori, A.; Ichikawa, D.; Matsui, K.; Yokoyama, T.; Sugaya, T.; Kimura, K. Urinary L-type fatty acid binding protein (L-FABP) as a new urinary biomarker promulgated by the Ministry of Health, Labour and Welfare in Japan. Jpn. J. Clin. Pathol. 2013, 61, 635–640. [Google Scholar]
- Li, Y.; Zhu, M.; Xia, Q.; Wang, S.; Qian, J.; Lu, R.; Che, M.; Dai, H.; Wu, Q.; Ni, Z.; et al. Urinary neutrophil gelatinase-associated lipocalin and L-type fatty acid binding protein as diagnostic markers of early acute kidney injury after liver transplantation. Biomarkers 2012, 17, 336–342. [Google Scholar] [CrossRef]
- Liu, S.; Che, M.; Xue, S.; Xie, B.; Zhu, M.; Lu, R.; Zhang, W.; Qian, J.; Yan, Y. Urinary L-FABP and its combination with urinary NGAL in early diagnosis of acute kidney injury after cardiac surgery in adult patients. Biomarkers 2013, 18, 95–101. [Google Scholar] [CrossRef]
- Doi, K.; Noiri, E.; Sugaya, T. Urinary L-type fatty acid-binding protein as a new renal biomarker in critical care. Curr. Opin. Crit. Care 2010, 16, 545–549. [Google Scholar] [CrossRef]
- Jimenez-Cordova, M.I.; Cardenas-Gonzalez, M.; Aguilar-Madrid, G.; Sanchez-Pena, L.C.; Barrera-Hernandez, A.; Dominguez-Guerrero, I.A.; Gonzalez-Horta, C.; Barbier, O.C.; Del Razo, L.M. Evaluation of kidney injury biomarkers in an adult Mexican population environmentally exposed to fluoride and low arsenic levels. Toxicol. Appl. Pharm. 2018, 352, 97–106. [Google Scholar] [CrossRef]
- Wimalawansa, S.J. Public health interventions for chronic diseases: Cost–benefit modelizations for eradicating chronic kidney disease of multifactorial origin from tropical countries. Heliyon 2019, in press. [Google Scholar] [CrossRef] [Green Version]
- Garcon, G.; Leleu, B.; Marez, T.; Zerimech, F.; Haguenoer, J.M.; Furon, D.; Shirali, P. Biomonitoring of the adverse effects induced by the chronic exposure to lead and cadmium on kidney function: Usefulness of alpha-glutathione S-transferase. Sci. Total Environ. 2007, 377, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Phuc, H.D.; Kido, T.; Oanh, N.T.P.; Manh, H.D.; Anh, L.T.; Oyama, Y.; Okamoto, R.; Ichimori, A.; Nogawa, K.; Suwazono, Y.; et al. Effects of aging on cadmium concentrations and renal dysfunction in inhabitants in cadmium-polluted regions in Japan. J. Appl. Toxicol. 2017, 37, 1046–1052. [Google Scholar] [CrossRef] [PubMed]
- Dharmaratne, R.W. Fluoride in drinking water and diet: The causative factor of chronic kidney diseases in the North Central Province of Sri Lanka. Environ. Health Prev. Med. 2015, 20, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Wimalawansa, S.; Wimalawansa, S.J. Protection of watersheds, and control and responsible use of fertiliser to prevent phosphate eutrophication ofreservoirs. Int. J. Res. Environ. Sci. 2015, 1, 1–18. [Google Scholar]
- Vesey, D.A. Transport pathways for cadmium in the intestine and kidney proximal tubule: Focus on the interaction with essential metals. Toxicol. Lett. 2010, 198, 13–19. [Google Scholar] [CrossRef]
- Wimalawansa, S.J. Vitamin D deficiency: Effects on oxidative stress, epigenetics, gene regulation, and aging. Biology (Basel) 2019, 8, 30. [Google Scholar] [CrossRef] [Green Version]
- Song, C.; Fu, B.; Zhang, J.; Zhao, J.; Yuan, M.; Peng, W.; Zhang, Y.; Wu, H. Sodium fluoride induces nephrotoxicity via oxidative stress-regulated mitochondrial SIRT3 signaling pathway. Sci. Rep. 2017, 7, 672. [Google Scholar] [CrossRef] [Green Version]
- Song, G.H.; Gao, J.P.; Wang, C.F.; Chen, C.Y.; Yan, X.Y.; Guo, M.; Wang, Y.; Huang, F.B. Sodium fluoride induces apoptosis in the kidney of rats through caspase-mediated pathways and DNA damage. J. Physiol. Biochem. 2014, 70, 857–868. [Google Scholar] [CrossRef]
- Herath, S.; Ayala, H.M.; Kawakami, T.; Nagasawa, S.; Serikawa, Y.; Motoyama, A.; Chaminda, G.G.; Weragoda, S.K.; Yatigammana, S.K.; Amarasooriya, A.A.G.D. Arsenic, cadmium, lead, and chromium in well water, rice, and human urine in Sri Lanka in relation to chronic kidney disease of unknown etiology. J. Water Health 2018, 16, 212–222. [Google Scholar] [CrossRef] [Green Version]
- Roncal Jimenez, C.A.; Ishimoto, T.; Lanaspa, M.A.; Rivard, C.J.; Nakagawa, T.; Ejaz, A.A.; Cicerchi, C.; Inaba, S.; Le, M.; Miyazaki, M.; et al. Fructokinase activity mediates dehydration-induced renal injury. Kidney Int. 2014, 86, 294–302. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, N.E. The Role of Calcium in Ameliorating the Oxidative Stress of Fluoride in Rats. Biol. Trace Elem. Res. 2016, 170, 128–144. [Google Scholar] [CrossRef] [PubMed]
- Barnett, L.M.A.; Cummings, B.S. Nephrotoxicity and Renal Pathophysiology: A Contemporary Perspective. Toxicol. Sci. 2018, 164, 379–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nanayakkara, S.; Senevirathna, S.T.; Karunaratne, U.; Chandrajith, R.; Harada, K.H.; Hitomi, T.; Watanabe, T.; Abeysekera, T.; Aturaliya, T.N.; Koizumi, A. Evidence of tubular damage in the very early stage of chronic kidney disease of uncertain etiology in the North Central Province of Sri Lanka: A cross-sectional study. Environ. Health Prev. Med. 2012, 17, 109–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zangeneh, F.; Clarke, B.L.; Hurley, D.L.; Watts, N.B.; Miller, P.D. Chronic Kidney Disease-Mineral and Bone Disorders (CKD-MBDs): What the Endocrinologist Needs to Know. Endocr. Pract. 2014, 20, 500–516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ulinski, T.; Sellier-Leclerc, A.L.; Tudorache, E.; Bensman, A.; Aoun, B. Acute tubulointerstitial nephritis. Pediatr. Nephrol. 2012, 27, 1051–1057. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.G. Chemical-induced nephropathy: A review of the renal tubulointerstitial lesions in humans. Toxicol. Pathol. 2004, 32 (Suppl. 2), 71–84. [Google Scholar] [CrossRef] [Green Version]
- Grollman, A.P.; Jelakovic, B. Role of environmental toxins in endemic (Balkan) nephropathy. October 2006, Zagreb, Croatia. J. Am. Soc. Nephrol. 2007, 18, 2817–2823. [Google Scholar] [CrossRef]
- Futrakul, N.; Futrakul, P. Urgent call for reconsideration of chronic kidney disease. World J. Nephrol. 2012, 1, 155–159. [Google Scholar] [CrossRef] [Green Version]
- Chiang, C.K.; Tanaka, T.; Nangaku, M. Dysregulated oxygen metabolism of the kidney by uremic toxins: Review. J. Ren. Nutr. 2012, 22, 77–80. [Google Scholar] [CrossRef] [Green Version]
- Knochel, J.P.; Dotin, L.N.; Hamburger, R.J. Heat stress, exercise, and muscle injury: Effects on urate metabolism and renal function. Ann. Intern. Med. 1974, 81, 321–328. [Google Scholar] [CrossRef]
- Roncal-Jimenez, C.; Garcia-Trabanino, R.; Barregard, L.; Lanaspa, M.A.; Wesseling, C.; Harra, T.; Aragon, A.; Grases, F.; Jarquin, E.R.; Gonzalez, M.A.; et al. Heat Stress Nephropathy From Exercise-Induced Uric Acid Crystalluria: A Perspective on Mesoamerican Nephropathy. Am. J. Kidney Dis. 2016, 67, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Trabanino, R.; Jarquin, E.; Wesseling, C.; Johnson, R.J.; Gonzalez-Quiroz, M.; Weiss, I.; Glaser, J.; Jose Vindell, J.; Stockfelt, L.; Roncal, C.; et al. Heat stress, dehydration, and kidney function in sugarcane cutters in El Salvador—A cross-shift study of workers at risk of Mesoamerican nephropathy. Environ. Res. 2015, 142, 746–755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coe, F.L.; Evan, A.; Worcester, E. Kidney stone disease. J. Clin. Investig. 2005, 115, 2598–2608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tiselius, H.G. The role of calcium phosphate in the development of Randall’s plaques. Urolithiasis 2013, 41, 369–377. [Google Scholar] [CrossRef]
- Ho, S.P.; Chen, L.; Allen, F.I.; Hsi, R.S.; Shimotake, A.R.; Wiener, S.V.; Stoller, M.L. Architecture-Guided Fluid Flow Directs Renal Biomineralization. Sci Rep. 2018, 8, 14157. [Google Scholar] [CrossRef]
- Verma, R.; Niraimathi, M.; Prasad, P.; Agrawal, V. Dihydroxyadenine crystal-induced nephropathy presenting with rapidly progressive renal failure. Kidney Res. Clin. Pract. 2018, 37, 287–291. [Google Scholar] [CrossRef] [Green Version]
- Larsen, C.P.; Bell, J.M.; Harris, A.A.; Messias, N.C.; Wang, Y.H.; Walker, P.D. The morphologic spectrum and clinical significance of light chain proximal tubulopathy with and without crystal formation. Mod. Pathol. 2011, 24, 1462–1469. [Google Scholar] [CrossRef] [Green Version]
- Sethmann, I.; Wendt-Nordahl, G.; Knoll, T.; Enzmann, F.; Simon, L.; Kleebe, H.J. Microstructures of Randall’s plaques and their interfaces with calcium oxalate monohydrate kidney stones reflect underlying mineral precipitation mechanisms. Urolithiasis 2017, 45, 235–248. [Google Scholar] [CrossRef]
- Wiener, S.V.; Chen, L.; Shimotake, A.R.; Kang, M.; Stoller, M.L.; Ho, S.P. Novel insights into renal mineralization and stone formation through advanced imaging modalities. Connect. Tissue Res. 2018, 59, 102–110. [Google Scholar] [CrossRef] [Green Version]
- Chmielewska, M.; Symonowicz, K.; Pula, B.; Owczarek, T.; Podhorska-Okolow, M.; Ugorski, M.; Dziegiel, P. Expression of metallothioneins I and II in kidney of doxorubicin-treated rats. Exp. Toxicol. Pathol. 2015, 67, 297–303. [Google Scholar] [CrossRef]
- Schanz, M.; Schaaf, L.; Dippon, J.; Biegger, D.; Fritz, P.; Alscher, M.D.; Kimmel, M. Renal effects of metallothionein induction by zinc in vitro and in vivo. BMC Nephrol. 2017, 18, 91. [Google Scholar] [CrossRef] [Green Version]
- Goyer, R.A. Mechanisms of lead and cadmium nephrotoxicity. Toxicol. Lett. 1989, 46, 153–162. [Google Scholar] [CrossRef]
- Rango, T.; Jeuland, M.; Manthrithilake, H.; McCornick, P. Nephrotoxic contaminants in drinking water and urine, and chronic kidney disease in rural Sri Lanka. Sci. Total Environ. 2015, 518, 574–585. [Google Scholar] [CrossRef] [PubMed]
- Dharmawardana, M.W.; Amarasiri, S.L.; Dharmawardene, N.; Panabokke, C.R. Chronic kidney disease of unknown aetiology and ground-water ionicity: Study based on Sri Lanka. Environ. Geochem. Health 2014, 37, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Wimalawansa, S.A.; Wimalawansa, S.J. Clean water, healthy environment, and preservation of watersheds: Correct, enforceable policies are essential. Jacobs J. Hydrol. 2015, 1, 3–15. [Google Scholar] [CrossRef]
- Edirisinghe, E.; Manthrithilake, H.; Pitawala, H.; Dharmagunawardhane, H.A.; Wijayawardane, R.L. Geochemical and isotopic evidences from groundwater and surface water for understanding of natural contamination in chronic kidney disease of unknown etiology (CKDu) endemic zones in Sri Lanka. Isot. Environ. Health Stud. 2018, 54, 244–261. [Google Scholar] [CrossRef]
- Wanigasuriya, K.P.; Peiris-John, R.J.; Wickremasinghe, R. Chronic kidney disease of unknown aetiology in Sri Lanka: Is cadmium a likely cause? BMC Nephrol. 2011, 12, 32. [Google Scholar] [CrossRef] [Green Version]
- Jayathilaka, N.M.P.; Mendis, S.; Mehta, F.R.; Dissanayake, L.J.; Janakan, N. Investigation and Evaluaiton of Chronic Kidney Disease of Uncertain Aetiology in Sri Lanka (Final Report); WHO: Colombo, Sri Lanka, 2013. [Google Scholar]
- WHO. International Expert Consultation on Chronic Kidney Disease of Unknown Etiology (CKDu) in Sri Lanka; WHO Country Office: Colombo, Sri Lanka, 2016; ISBN 978-955-0261-15-4. [Google Scholar]
- Senevirathna, L.; Abeysekera, T.; Nanayakkara, S.; Chandrajith, R.; Ratnatunga, N.; Harada, K.H.; Hitomi, T.; Komiya, T.; Muso, E.; Koizumi, A. Risk factors associated with disease progression and mortality in chronic kidney disease of uncertain etiology: A cohort study in Medawachchiya, Sri Lanka. Environ. Health Prev. Med. 2012, 17, 191–198. [Google Scholar] [CrossRef] [Green Version]
- Nanayakkara, S.; Senevirathna, S.; Harada, K.H.; Chandrajith, R.; Hitomi, T.; Abeysekera, T.; Muso, E.; Watanabe, T.; Koizumi, A. Systematic evaluation of exposure to trace elements and minerals in patients with chronic kidney disease of uncertain etiology (CKDu) in Sri Lanka. J. Trace Elem. Med. Biol. 2019, 54, 206–213. [Google Scholar] [CrossRef]
- WHO. Guidelines for Drinking-Water Quality, Incorporating the 1st Addendum, in Water Sanitation Hygiene, 4th ed.; World Health Organization: Geneva, Switzerland, 2017; ISBN 978-92-4-154995-0. Available online: https://www.who.int/water_sanitation_health/publications/drinking-water-quality-guidelines-4-including-1st-addendum/en/ (accessed on 20 November 2019).
- WHO. Developing Drinking-Water Quality Regulations and Standards; WHO: Geneva, Switzerland, 2018; Available online: https://www.who.int/water_sanitation_health/publications/developing-dwq-regulations/en/ (accessed on 20 November 2019).
- Panabokke, C.R. Nature of occurrence and sustainable use of groundwater resources for agriculture in the North Central, North Western and North Eastern regions in Sri Lanka. Trop. Agric. Res. Ext. 2003, 6, 8–13. [Google Scholar]
- Wimalawansa, S.A.; Wimalawansa, S.J. Impact of changing agricultural practices on human health: Chronic kidney disease of multi-factorial origin in Sri Lanka. Wudpecker J. Agric. Res. 2014, 3, 110–124. [Google Scholar]
- Amerasinghe, F.P.; Munasingha, N.B. A predevelopment mosquito survey in the Mahaweli Development Project area, Sri Lanka: Immatures. J. Med. Entomol. 1988, 25, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Weeraratne, S.; Wimalawansa, S.J. A Major irrigation project (Accelerated Mahaweli Programme) and the chronic kidney disease of multifactorial origin in Sri Lanka. Int. J. Environ. Agric. Res. 2015, 1, 16–27. [Google Scholar]
- Wimalawansa, S.; Ileperuma, O.; Weeraratne, S. Attempts to Change the Globally Accepted Term, CKDu, to KDUCAL, NUCAL, or CINAC Are Inappropriate. Am. J. Kidney Dis. 2018, 71, 914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chandrajith, R.; Dissanayake, C.B.; Tobschall, H.J. The abundances of rarer trace elements in paddy (rice) soils of Sri Lanka. Chemosphere 2005, 58, 1415–1420. [Google Scholar] [CrossRef]
- Dharmagunawardhane, H.A.; Dissanayake, C.B. Fluoride problemsc in Sri Lanka. Environ. Manag. Health 1993, 4, 9–16. [Google Scholar] [CrossRef]
- Dissanayake, C. Water quality in the dry Zone of Sri Lanka—Some interesting health aspects. J. Natl. Sci. Found. Sri Lanka 2005, 33, 161–168. [Google Scholar] [CrossRef] [Green Version]
- Dissanayake, C.B.; Chandrajith, R. Medical geology in tropical countries with special reference to Sri Lanka. Environ. Geochem. Health 2007, 29, 155–162. [Google Scholar] [CrossRef]
- Multiple-References. Estimated “Threshold” Doses for Skeletal Fluorosis. 2012. [cited on 7 July 2019]. Available online: http://fluoridealert.org/studies/skeletal_fluorosis04/ (accessed on 23 December 2019).
- Manji, F.; Kapila, S. Fluorides and fluorosis in Kenya. Part II: The occurrence of dental and skeletal fluorosis. Odontostomatol. Trop. 1986, 9, 71–74. [Google Scholar]
- Arumugam, E.; Harinathbabu, M.; Thillaigovindan, R.; Prabhu, G. Marble Bone Disease: A Rare Bone Disorder. Cureus 2015, 7, e339. [Google Scholar] [CrossRef] [Green Version]
- Askmyr, M.K.; Fasth, A.; Richter, J. Towards a better understanding and new therapeutics of osteopetrosis. Br. J. Haematol. 2008, 140, 597–609. [Google Scholar] [CrossRef] [PubMed]
- Kuno, M. Cooperative electrogenic proton transport pathways in the plasma membrane of the proton-secreting osteoclast. Pflügers Arch. 2018, 470, 851–866. [Google Scholar] [CrossRef] [PubMed]
- Laway, B.A.; Mubarik, I. Renal Tubular Acidosis, Osteopetrosis, and Cerebral Calcification: A Rare Syndrome Caused by Carbonic Anhydrase II Deficiency. Indian J. Nephrol. 2017, 27, 330–331. [Google Scholar] [CrossRef] [PubMed]
- Stefanovic, V.; Cukuranovic, R.; Miljkovic, S.; Marinkovic, D.; Toncheva, D. Fifty years of Balkan endemic nephropathy: Challenges of study using epidemiological method. Ren. Fail. 2009, 31, 409–418. [Google Scholar] [CrossRef] [Green Version]
- Meharg, A.A.; Rahman, M.M. Arsenic contamination of Bangladesh paddy field soils: Implications for rice contribution to arsenic consumption. Environ. Sci. Technol. 2003, 37, 229–234. [Google Scholar] [CrossRef]
- Rahman, M.M.; Chowdhury, U.K.; Mukherjee, S.C.; Mondal, B.K.; Paul, K.; Lodh, D.; Biswas, B.K.; Chanda, C.R.; Basu, G.K.; Saha, K.C.; et al. Chronic arsenic toxicity in Bangladesh and West Bengal, India--a review and commentary. J. Toxicol. Clin. Toxicol. 2001, 39, 683–700. [Google Scholar] [CrossRef]
- Saint-Jacques, N.; Brown, P.; Nauta, L.; Boxall, J.; Parker, L.; Dummer, T.J.B. Estimating the risk of bladder and kidney cancer from exposure to low-levels of arsenic in drinking water, Nova Scotia, Canada. Environ. Int. 2018, 110, 95–104. [Google Scholar] [CrossRef]
- Tao, H.; Xia, S.M.; Chan, Z.Y.; Song, G.; Yanagihara, R. Morphology and morphogenesis of viruses of hemorrhagic fever with renal syndrome. II. Inclusion bodies—Ultrastructural markers of hantavirus-infected cells. Intervirology 1987, 27, 45–52. [Google Scholar] [CrossRef]
- Borges, F.T.; Melo, S.A.; Özdemir, B.C.; Kato, N.; Revuelta, I.; Miller, C.A.; Gattone, V.H.; LeBleu, V.S.; Kalluri, R. TGF-beta1-containing exosomes from injured epithelial cells activate fibroblasts to initiate tissue regenerative responses and fibrosis. J. Am. Soc. Nephrol. 2013, 24, 385–392. [Google Scholar] [CrossRef] [Green Version]
- Okada, H. A new look at tubulointerstitial communication with exosomes. J. Am. Soc. Nephrol. 2013, 24, 330–332. [Google Scholar] [CrossRef] [Green Version]
- Zhou, H.; Cheruvanky, A.; Hu, X.; Matsumoto, T.; Hiramatsu, N.; Cho, M.E.; Berger, A.; Leelahavanichkul, A.; Doi, K.; Chawla, L.S.; et al. Urinary exosomal transcription factors, a new class of biomarkers for renal disease. Kidney Int. 2008, 74, 613–621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, H.; Pisitkun, T.; Aponte, A.; Yuen, P.S.; Hoffert, J.D.; Yasuda, H.; Hu, X.; Chawla, L.; Shen, R.F.; Knepper, M.A.; et al. Exosomal Fetuin-A identified by proteomics: A novel urinary biomarker for detecting acute kidney injury. Kidney Int. 2006, 70, 1847–1857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ranghino, A.; Dimuccio, V.; Papadimitriou, E.; Bussolati, B. Extracellular vesicles in the urine: Markers and mediators of tissue damage and regeneration. Clin. Kidney J. 2015, 8, 23–30. [Google Scholar] [CrossRef]
- Elin, R.J. Assessment of magnesium status. Clin. Chem. 1987, 33, 1965–1970. [Google Scholar] [PubMed]
- Blaine, J.; Chonchol, M.; Levi, M. Renal control of calcium, phosphate, and magnesium homeostasis. Clin. J. Am. Soc. Nephrol. 2015, 10, 1257–1272. [Google Scholar] [CrossRef]
- Wasana, H.M.; Perera, G.D.; De Gunawardena, P.S.; Bandara, J. The impact of aluminum, fluoride, and aluminum-fluoride complexes in drinking water on chronic kidney disease. Environ. Sci. Pollut. Res. Int. 2015, 22, 11001–11009. [Google Scholar] [CrossRef]
- Wasana, H.M.; Perera, G.D.; Gunawardena, P.S.; Fernando, P.S.; Bandara, J. WHO water quality standards Vs Synergic effect(s) of fluoride, heavy metals and hardness in drinking water on kidney tissues. Sci. Rep. 2017, 7, 42516. [Google Scholar] [CrossRef]
- Wasana, H.M.; Aluthpatabendi, D.; Kularatne, W.M.; Wijekoon, P.; Weerasooriya, R.; Bandara, J. Drinking water quality and chronic kidney disease of unknown etiology (CKDu): Synergic effects of fluoride, cadmium and hardness of water. Environ. Geochem. Health 2016, 38, 157–168. [Google Scholar] [CrossRef]
- Gbadebo, A.M. Groundwater fluoride and dental fluorosis in southwestern Nigeria. Environ. Geochem. Health 2012, 34, 597–604. [Google Scholar] [CrossRef]
- Czajka-Jakubowska, A.E.; Liu, J.; Chang, S.R.; Clarkson, B.H. The effect of the surface characteristics of various substrates on fluorapatite crystal growth, alignment, and spatial orientation. Med. Sci. Monit. 2009, 15, MT84–MT88. [Google Scholar]
- Skwarek, E.; Bolbukh, Y.; Janush, W. Hydroxyapatite composites with multiwalled carbon nanotubes. Adsorpt. Sci. Technol. 2017, 35, 534–544. [Google Scholar] [CrossRef]
- Arnich, N.; Lanhers, M.C.; Laurensot, F.; Podor, R.; Montiel, A.; Burnel, D. In vitro and in vivo studies of lead immobilization by synthetic hydroxyapatite. Environ. Pollut. 2003, 124, 139–149. [Google Scholar] [CrossRef]
- Zhu, Y.; Huang, B.; Zhu, Z.; Liu, H.; Huang, Y.; Zhao, X.; Liang, M. Characterization, dissolution and solubility of the hydroxypyromorphite-hydroxyapatite solid solution [(PbxCa1−x)5(PO4)3OH] at 25 degrees C and pH 2-9. Geochem. Trans. 2016, 17, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDowell, H.; Gregory, T.M.; Brown, W.E. Solubility of Ca5(PO4)3OH in the System Ca(OH)2-H3PO4-H2O, at 5, 15, 25, and 37 °C. J. Res. Natl. Bur. Stand. Phys. Chem. 1877, 81, 273–281. [Google Scholar]
- Buzea, C.; Ivan, I.; Pacheco Blandino, I.I.P.; Kevin Robbie, K. Nanomaterials and nanoparticles: Sources and toxicity. Biointerphases 2007, 2, MR17–MR172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aoshima, K. Itai-itai disease: Lessons from the investigations of environmental epidemiology conducted in the 1970’s, with special reference to the studies of the Toyama Institute of Health. Nihon Eiseigaku Zasshi. Jpn. J. Hyg. 2017, 72, 149–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saint-Jacques, N.; Parker, L.; Brown, P.; Dummer, T.J. Arsenic in drinking water and urinary tract cancers: A systematic review of 30 years of epidemiological evidence. Environ. Health 2014, 13, 44. [Google Scholar] [CrossRef] [Green Version]
- Wickramarathna, S.; Balasooriya, S.; Diyabalanage, S.; Chandrajith, R. Tracing environmental aetiological factors of chronic kidney diseases in the dry zone of Sri Lanka-A hydrogeochemical and isotope approach. J. Trace Elem. Med. Biol. 2017, 44, 298–306. [Google Scholar] [CrossRef]
- Reddy, D.V.; Nagabhushanam, P.; Sukhija, B.S.; Reddy, A.G.S.; Smedley, P.L. Fluoride dynamics in the granitic aquifer of the Wailapally watershed, Nalgonda District India. Chem. Geol. 2010, 269, 278–289. [Google Scholar] [CrossRef] [Green Version]
- Smedley, P.L.; Nicolli, H.B.; Macdonald, D.M.J.; Barros, A.J.; Tullio, J.O. Hydrogeochemistry of arsenic and other inorganic constituents in groundwaters from La Pampa, Argentina. Appl. Geochem. 2002, 17, 259–284. [Google Scholar] [CrossRef]
- Battaleb-Looie, S.; Moore, F.; Jacks, G.; Ketabdari, M.R. Geological sources of fluoride and acceptable intake of fluoride in an endemic fluorosis area, southern Iran. Environ. Geochem. Health 2012, 34, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Sayanthooran, S.; Gunerathne, L.; Abeysekera, T.D.J.; Magana-Arachchi, D.N. Transcriptome analysis supports viral infection and fluoride toxicity as contributors to chronic kidney disease of unknown etiology (CKDu) in Sri Lanka. Int. Urol. Nephrol. 2018, 50, 1667–1677. [Google Scholar] [CrossRef] [PubMed]
- Gamage, C.D.; Yoshimatsu, K.; Sarathkumara, Y.D.; Kulendiran, T.; Nanayakkara, N.; Arikawa, J. Serological evidence of hantavirus infection in Girandurukotte, an area endemic for chronic kidney disease of unknown aetiology (CKDu) in Sri Lanka. Int. J. Infect. Dis. 2017, 57, 77–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nanayakkara, S.; Senevirathna, S.T.; Abeysekera, T.; Chandrajith, R.; Ratnatunga, N.; Gunarathne, E.D.; Yan, J.; Hitomi, T.; Muso, E.; Komiya, T.; et al. An integrative study of the genetic, social and environmental determinants of chronic kidney disease characterized by tubulointerstitial damages in the North Central Region of Sri Lanka. J. Occup. Health 2014, 56, 28–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ordak, M.; Maj-Zurawska, M.; Matsumoto, H.; Bujalska-Zadrozny, M.; Kieres-Salomonski, I.; Nasierowski, T.; Muszynska, E.; Wojnar, M. Ionized magnesium in plasma and erythrocytes for the assessment of low magnesium status in alcohol dependent patients. Drug Alcohol. Depend. 2017, 178, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Eberhard, J.; Macdonald, A.; Cundy, T. Severe proton pump inhibitor-induced hypomagnesaemia in a mother and daughter. Intern. Med. J. 2017, 47, 341–342. [Google Scholar] [CrossRef]
- Hess, M.W.; Hoenderop, J.G.; Bindels, R.J.; Drenth, J.P. Systematic review: Hypomagnesaemia induced by proton pump inhibition. Aliment. Pharmacol. Ther. 2012, 36, 405–413. [Google Scholar] [CrossRef]
- Van Angelen, A.A.; Glaudemans, B.; van der Kemp, A.W.; Hoenderop, J.G.; Bindels, R.J. Cisplatin-induced injury of the renal distal convoluted tubule is associated with hypomagnesaemia in mice. Nephrol. Dial. Transpl. 2013, 28, 879–889. [Google Scholar] [CrossRef] [Green Version]
- Lameris, A.L.; Monnens, L.A.; Bindels, R.J.; Hoenderop, J.G. Drug-induced alterations in Mg2+ homoeostasis. Clin. Sci. (Lond.) 2012, 123, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Van de Wal-Visscher, E.R.; Kooman, J.P.; van der Sande, F.M. Magnesium in Chronic Kidney Disease: Should We Care? Blood Purif. 2018, 45, 173–178. [Google Scholar] [CrossRef]





| Factor and/or Condition | Disease Status | |
|---|---|---|
| CKDmfo in Sri Lanka | CKDu in other Countries | |
| Similarities | ||
| First known manifestation of the disease | Mid-1990s | Mid-1970s |
| Duration of exposure required | About 10 to 15 years | Approximately 8 to 10 years |
| Those affected | Disadvantaged populations | Disadvantaged populations |
| Economy | Emerging economy | Developing or emerging |
| Potential source | Contaminated water (food may indirectly contribute) | Contaminated water ± food |
| Type of exposure to toxic agents | Environmental and occupational exposure | Environmental and occupational exposure |
| Potential causes and factors | Multiple factors with synergistic or additive effects | Multiple factors causing chronic renal failure |
| Working conditions | No safety precautions | No safety precautions |
| Chronic dehydration | Highly prevalent: (climate + alcohol) | Highly prevalent (climate and harsh working conditions) |
| Presence of hard water | Common across the affected regions | Common and widespread |
| Drinking water | Insufficient quantities | Insufficient quantities |
| Most-affected gender | Male (approximately 70%) | Male (approximately 80%) |
| Economic status | High prevalence of poverty | Very high prevalence of poverty |
| Access to modern healthcare | Poor (less than optimal) | Very poor (minimal) |
| Access to nutritious food | Low | Very low |
| Nutrition status | Approximately 70% malnourished | Approximately 80% malnourished |
| Micronutrient malnutrition | Uniformly present | Uniformly present |
| Landscape | Flat land with poor drainage | Flat land with poor drainage |
| Closeness to the equator | Located just north of the equator | Both sides of the equator |
| Relation to farming activities | Local farming (significant proportion, mostly daily wage earners); non farmers also affected | Predominately commercial labours (e.g., sugarcane); also affects individual farmer |
| Bioaccumulation | Likely | Possible |
| Evidence of genetic origin | None | None |
| Climatic condition | Prolonged dry spells with short period of torrential rain | Dry spells alternating with flooding |
| Family clusters are affected | Yes | Yes |
| Drinking water source | Mostly shallow wells | Mostly shallow wells |
| Iatrogenic causes | Unlikely | Unlikely |
| Differences | ||
| Agricultural | Rice and vegetables | Cotton and sugarcane, and others |
| Predominant agricultural economic base | Paddy and vegetable | Cotton, sugarcane, rice, and vegetables |
| Type of communities affected | Predominately agricultural communities; lesser number from non-agricultural communities | Disease also present in non-agricultural communities |
| Agrochemical overuse | Mostly fertilizer | Mostly pesticides |
| Group most affected | Individual farm workers | Industrial farm workers |
| Eutrophication of water | With phosphate | Rarely; occasionally nitrates |
| Heavy metal in drinking water | Minimal or none (not consistent) | Present in some locations |
| Presence of fluoride in water | Yes, but not uniformly | Heterogeneously present in most countries |
| Detection of pesticide in drinking water | Virtually none | Common |
| Affected younger population | Children rarely affected | Older children are affected |
| Type of renal tissue affected | Initially, predominantly affecting the tubulointerstitial tissues | Affecting both glomerular and tubulointerstitial tissues |
| Histologic type | Morphology, typical interstitial tubular nephritis | Interstitial nephritis; but may not be typical |
| Proteinuria | Late occurrence | Early occurrence |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wimalawansa, S.J.; Dissanayake, C.B. Factors Affecting the Environmentally Induced, Chronic Kidney Disease of Unknown Aetiology in Dry Zonal Regions in Tropical Countries—Novel Findings. Environments 2020, 7, 2. https://doi.org/10.3390/environments7010002
Wimalawansa SJ, Dissanayake CB. Factors Affecting the Environmentally Induced, Chronic Kidney Disease of Unknown Aetiology in Dry Zonal Regions in Tropical Countries—Novel Findings. Environments. 2020; 7(1):2. https://doi.org/10.3390/environments7010002
Chicago/Turabian StyleWimalawansa, Sunil J., and Chandra B. Dissanayake. 2020. "Factors Affecting the Environmentally Induced, Chronic Kidney Disease of Unknown Aetiology in Dry Zonal Regions in Tropical Countries—Novel Findings" Environments 7, no. 1: 2. https://doi.org/10.3390/environments7010002
APA StyleWimalawansa, S. J., & Dissanayake, C. B. (2020). Factors Affecting the Environmentally Induced, Chronic Kidney Disease of Unknown Aetiology in Dry Zonal Regions in Tropical Countries—Novel Findings. Environments, 7(1), 2. https://doi.org/10.3390/environments7010002






