Absolute Configuration of Mycosporine-Like Amino Acids, Their Wound Healing Properties and In Vitro Anti-Aging Effects
Abstract
:1. Introduction
2. Results
2.1. Determination of the Absolute Configurations of Amino Acids in MAAs by the Advanced Marfey’s Method Using LC-MS
2.2. Determination of Absolute Configuration of Isolated MAAs by Quantum Chemical Calculation Method
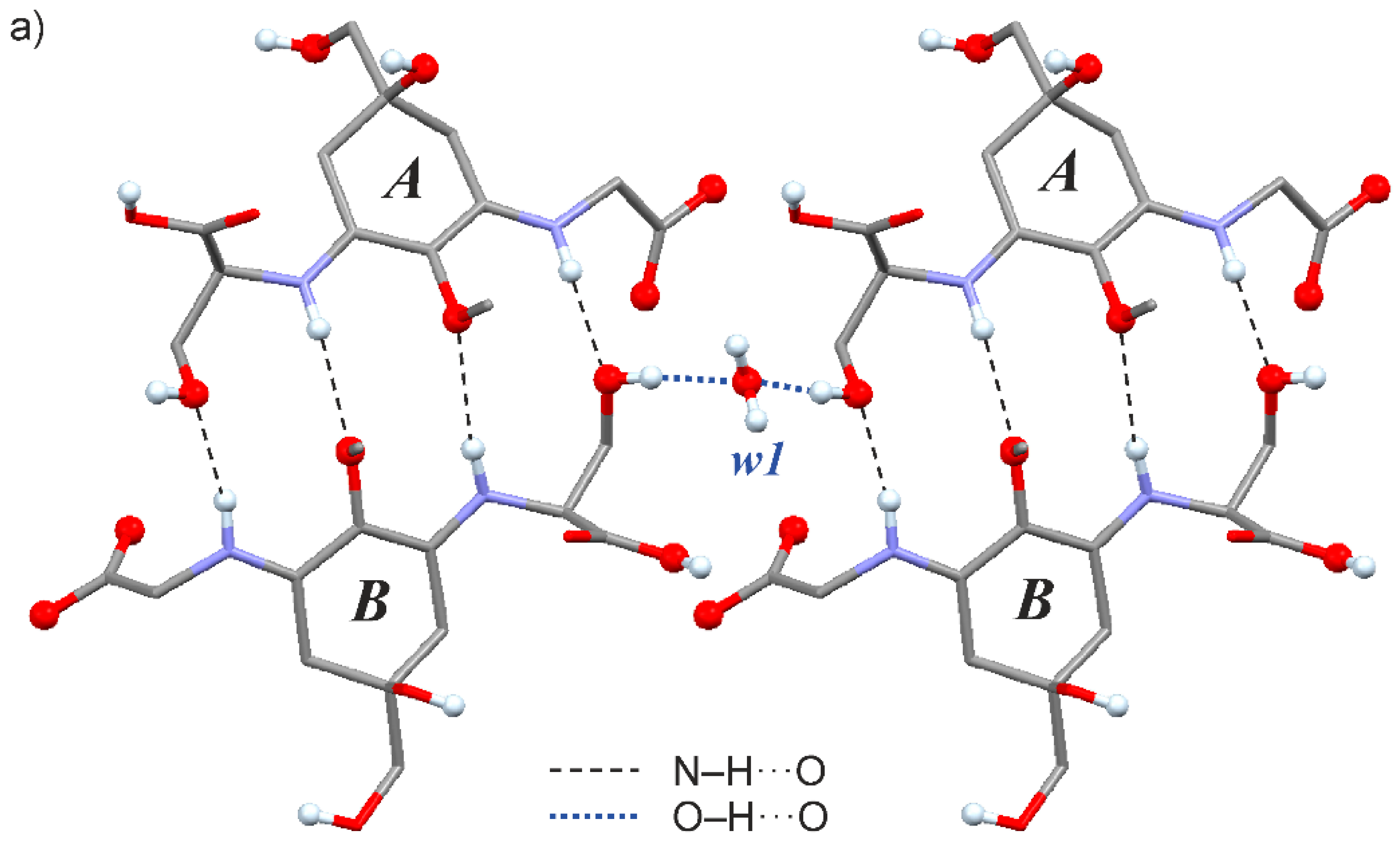
2.3. Crystal Structure of the Shinorine Hydrate 1H
2.4. Collagenase Inhibition Assay
2.5. Advanced Glycation End Products (AGEs) Assay
2.6. Influence of MAAs on Migratrion Behaviour of Human Keratinocytes (Scratch Assay)
3. Discussion and Conclusions
4. Materials and Methods
4.1. Isolated Mycosporine-Like Amino Acids
4.2. Instrumentation
4.3. Chemicals and Reagents
4.4. Determination of the Absolute Configurations of Amino Acids in MAAs by the Advanced Marfey’s Method
4.5. Measurement of ECD Spectra and ECD and Optical Rotation Calculation
4.6. X-ray Crystallography
4.7. Collagenase Inhibition Assay
4.8. Inhibition of Anti-Advanced Glycation End Products (AGEs)
4.9. Wound Scratch Assay
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chrapusta, E.; Kaminski, A.; Duchnik, K.; Bober, B.; Adamski, M.; Bialczyk, J. Mycosporine-Like Amino Acids: Potential Health and Beauty Ingredients. Mar. Drugs 2017, 15, 326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nazifi, E.; Wada, N.; Yamaba, M.; Asano, T.; Nishiuchi, T.; Matsugo, S.; Sakamoto, T. Glycosylated Porphyra-334 and Palythine-Threonine from the Terrestrial Cyanobacterium Nostoc commune. Mar. Drugs 2013, 11, 3124–3154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wada, N.; Sakamoto, T.; Matsugo, S. Mycosporine-Like Amino Acids and Their Derivatives as Natural Antioxidants. Antioxidants 2015, 4, 603–646. [Google Scholar] [CrossRef] [PubMed]
- Favre-Bonvin, J.; Bernillon, J.; Salin, N.; Arpin, N. Biosynthesis of mycosporines: Mycosporine glutaminol in Trichothecium roseum. Phytochemistry 1987, 26, 2509–2514. [Google Scholar] [CrossRef]
- Balskus, E.P.; Walsh, C.T. The Genetic and Molecular Basis for Sunscreen Biosynthesis in Cyanobacteria. Science 2010, 329, 1653–1656. [Google Scholar] [CrossRef] [Green Version]
- Furusaki, A.; Matsumoto, T.; Tsujino, I.; Sekikawa, I. The Crystal and Molecular Structure of Palythine Trihydrate. BCSJ 1980, 53, 319–323. [Google Scholar] [CrossRef] [Green Version]
- Uemura, D.; Katayama, C.; Wada, A.; Hirata, Y. Crystal and molecular structure of palythene possessing a novel 360 nm chromophore. Chem. Lett. 1980, 9, 755–756. [Google Scholar] [CrossRef] [Green Version]
- White, J.D.; Cammack, J.H.; Sakuma, K.; Rewcastle, G.W.; Widener, R.K. Transformations of Quinic Acid. Asymmetric Synthesis and Absolute Configuration of Mycosporin I and Mycosporin-gly. J. Org. Chem. 1995, 60, 3600–3611. [Google Scholar] [CrossRef]
- Klisch, M.; Richter, P.; Puchta, R.; Häder, D.-P.; Bauer, W. The Stereostructure of Porphyra-334: An Experimental and Calculational NMR Investigation. Evidence for an Efficient ‘Proton Sponge’. Helv. Chim. Acta 2007, 90, 488–511. [Google Scholar] [CrossRef]
- Kamio, M.; Kicklighter, C.E.; Nguyen, L.; Germann, M.W.; Derby, C.D. Isolation and Structural Elucidation of Novel Mycosporine-Like Amino Acids as Alarm Cues in the Defensive Ink Secretion of the Sea Hare Aplysia californica. Helv. Chim. Acta 2011, 94, 1012–1018. [Google Scholar] [CrossRef]
- Pope, M.A.; Spence, E.; Seralvo, V.; Gacesa, R.; Heidelberger, S.; Weston, A.J.; Dunlap, W.C.; Shick, J.M.; Long, P.F. O-Methyltransferase Is Shared between the Pentose Phosphate and Shikimate Pathways and Is Essential for Mycosporine-Like Amino Acid Biosynthesis in Anabaena variabilis ATCC 29413. ChemBioChem 2015, 16, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Jurica, E.A.; Wu, X.; Williams, K.N.; Hernandez, A.S.; Nirschl, D.S.; Rampulla, R.A.; Mathur, A.; Zhou, M.; Cao, G.; Xie, C.; et al. Discovery of Pyrrolidine-Containing GPR40 Agonists: Stereochemistry Effects a Change in Binding Mode. J. Med. Chem. 2017, 60, 1417–1431. [Google Scholar] [CrossRef] [PubMed]
- Maetani, M.; Zoller, J.; Melillo, B.; Verho, O.; Kato, N.; Pu, J.; Comer, E.; Schreiber, S.L. Synthesis of a Bicyclic Azetidine with In Vivo Antimalarial Activity Enabled by Stereospecific, Directed C(sp3)–H Arylation. J. Am. Chem. Soc. 2017, 139, 11300–11306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sonawane, Y.A.; Zhu, Y.; Garrison, J.C.; Ezell, E.L.; Zahid, M.; Cheng, X.; Natarajan, A. Structure–Activity Relationship Studies with Tetrahydroquinoline Analogs as EPAC Inhibitors. ACS Med. Chem. Lett. 2017, 8, 1183–1187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oren, A.; Gunde-Cimerman, N. Mycosporines and mycosporine-like amino acids: UV protectants or multipurpose secondary metabolites? FEMS Microbiol. Lett. 2007, 269, 1–10. [Google Scholar] [CrossRef]
- Suh, S.-S.; Hwang, J.; Park, M.; Seo, H.H.; Kim, H.-S.; Lee, J.H.; Moh, S.H.; Lee, T.-K. Anti-inflammation activities of mycosporine-like amino acids (MAAs) in response to UV radiation suggest potential anti-skin aging activity. Mar. Drugs 2014, 12, 5174–5187. [Google Scholar] [CrossRef] [Green Version]
- Tarasuntisuk, S.; Patipong, T.; Hibino, T.; Waditee-Sirisattha, R.; Kageyama, H. Inhibitory effects of mycosporine-2-glycine isolated from a halotolerant cyanobacterium on protein glycation and collagenase activity. Lett. Appl. Microbiol. 2018, 67, 314–320. [Google Scholar] [CrossRef]
- Hartmann, A.; Gostner, J.; Fuchs, J.E.; Chaita, E.; Aligiannis, N.; Skaltsounis, L.; Ganzera, M. Inhibition of Collagenase by Mycosporine-like Amino Acids from Marine Sources. Planta Med. 2015, 81, 813–820. [Google Scholar] [CrossRef] [Green Version]
- Kageyama, H.; Waditee-Sirisattha, R. Antioxidative, Anti-Inflammatory, and Anti-Aging Properties of Mycosporine-Like Amino Acids: Molecular and Cellular Mechanisms in the Protection of Skin-Aging. Mar. Drugs 2019, 17, 222. [Google Scholar] [CrossRef] [Green Version]
- Ryu, J.; Park, S.-J.; Kim, I.-H.; Choi, Y.H.; Nam, T.-J. Protective effect of porphyra-334 on UVA-induced photoaging in human skin fibroblasts. Int. J. Mol. Med. 2014, 34, 796–803. [Google Scholar] [CrossRef] [Green Version]
- Helionori®-Natural Sun Protection Thanks to Marine UVA Filters, Gelyma. Available online: http://www.gelyma.com/helionori.html (accessed on 28 December 2019).
- Schmid, D.; Schürch, C.; Zülli, F. Mycosporine-like Amino Acids from Red Algae Protect against Premature Skin-Aging. Euro Cosmet. 2006, 9, 1–4. [Google Scholar]
- Orfanoudaki, M.; Hartmann, A.; Miladinovic, H.; Nguyen Ngoc, H.; Karsten, U.; Ganzera, M. Bostrychines A–F, Six Novel Mycosporine-Like Amino-Acids and a Novel Betaine from the Red Alga Bostrychia scorpioides. Mar. Drugs 2019, 17, 356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mancinelli, M.; Franzini, R.; Renzetti, C.; Marotta, E.; Villani, C.; Mazzanti, A. Determination of the absolute configuration of conformationally flexible molecules by simulation of chiro-optical spectra: A case study. RSC Adv. 2019, 9, 18165–18175. [Google Scholar] [CrossRef] [Green Version]
- Thring, T.S.; Hili, P.; Naughton, D.P. Anti-collagenase, anti-elastase and anti-oxidant activities of extracts from 21 plants. BMC Complement. Altern. Med. 2009, 9, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Özbilgin, S.; Acıkara, Ö.B.; Akkol, E.K.; Süntar, I.; Keleş, H.; İşcan, G.S. In vivo wound-healing activity of Euphorbia characias subsp. wulfenii: Isolation and quantification of quercetin glycosides as bioactive compounds. J. Ethnopharmacol. 2018, 224, 400–408. [Google Scholar] [CrossRef]
- Angelis, A.; Hubert, J.; Aligiannis, N.; Michalea, R.; Abedini, A.; Nuzillard, J.M.; Gangloff, S.C.; Skaltsounis, L.; Renault, J.H. Bio-Guided Isolation of Methanol-Soluble Metabolites of Common Spruce (Picea abies) Bark by-Products and Investigation of Their Dermo-Cosmetic Properties. Molecules 2016, 21, 1586. [Google Scholar] [CrossRef] [Green Version]
- Séro, L.; Sanguinet, L.; Blanchard, P.; Dang, T.B.; Morel, S.; Richomme, P.; Séraphin, D.; Derbré, S. Tuning a 96-Well Microtiter Plate Fluorescence-Based Assay to Identify AGE Inhibitors in Crude Plant Extracts. Molecules 2013, 18, 14320–14339. [Google Scholar] [CrossRef] [Green Version]
- Boisard, S.; Le Ray, A.-M.; Gatto, J.; Aumond, M.-C.; Blanchard, P.; Derbré, S.; Flurin, C.; Richomme, P. Chemical Composition, Antioxidant and Anti-AGEs Activities of a French Poplar Type Propolis. J. Agric. Food Chem. 2014, 62, 1344–1351. [Google Scholar] [CrossRef] [Green Version]
- Dang, B.-T.; Geny, C.; Blanchard, P.; Rouger, C.; Tonnerre, P.; Charreau, B.; Rakolomalala, G.; Iharinjaka Randriamboavonjy, J.; Loirand, G.; Pacaud, P.; et al. Advanced Glycation Inhibition and Protection against Endothelial Dysfunction induced by Coumarins and Procyanidins from Mammea neurophylla. Fitoterapia 2014, 96, 65–75. [Google Scholar] [CrossRef]
- Rouger, C.; Derbré, S.; Charreau, B.; Pabois, A.; Cauchy, T.; Litaudon, M.; Awang, K.; Richomme, P. Lepidotol A from Mesua lepidota Inhibits Inflammatory and Immune Mediators in Human Endothelial Cells. J. Nat. Prod. 2015, 78, 2187–2197. [Google Scholar] [CrossRef]
- Schinkovitz, A.; Le Pogam, P.; Derbré, S.; Roy-Vessieres, E.; Blanchard, P.; Thirumaran, S.-L.; Breard, D.; Aumond, M.-C.; Zehl, M.; Urban, E.; et al. Secondary metabolites from lichen as potent inhibitors of advanced glycation end products and vasodilative agents. Fitoterapia 2018, 131, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Séro, L.; Calard, F.; Sanguinet, L.; Levillain, E.; Richomme, P.; Séraphin, D.; Derbré, S. Synthesis and evaluation of naphthoic acid derivatives as fluorescent probes to screen advanced glycation end-products breakers. Bioorganic Med. Chem. Lett. 2012, 22, 6716–6720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viña, J.; Borrás, C.; Miquel, J. Theories of ageing. IUBMB Life 2007, 59, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Tümen, İ. Research on the antioxidant, wound healing, and anti-inflammatory activities and the phytochemical composition of maritime pine (Pinus pinaster Ait). J. Ethnopharmacol. 2018, 211, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Volkmann, M.; Gorbushina, A.A.; Kedar, L.; Oren, A. Structure of euhalothece-362, a novel red-shifted mycosporine-like amino acid, from a halophilic cyanobacterium (Euhalothece sp.). FEMS Microbiol. Lett. 2006, 258, 50–54. [Google Scholar] [CrossRef]
- Shaikh-Kader, A.; Houreld, N.N.; Rajendran, N.K.; Abrahamse, H. The link between advanced glycation end products and apoptosis in delayed wound healing. Cell Biochem. Funct. 2019, 37, 432–442. [Google Scholar] [CrossRef]
- Van Putte, L.; De Schrijver, S.; Moortgat, P. The effects of advanced glycation end products (AGEs) on dermal wound healing and scar formation: A systematic review. Scars Burn. Heal. 2016, 2, 1–14. [Google Scholar] [CrossRef]
- Peyroux, J.; Sternberg, M. Advanced glycation endproducts (AGEs): Pharmacological inhibition in diabetes. Pathol. Biol. 2006, 54, 405–419. [Google Scholar] [CrossRef]
- Lawrence, K.P.; Gacesa, R.; Long, P.F.; Young, A.R. Molecular photoprotection of human keratinocytes in vitro by the naturally occurring mycosporine-like amino acid palythine. Br. J. Dermatol. 2018, 178, 1353–1363. [Google Scholar] [CrossRef] [Green Version]
- Choi, Y.-H.; Yang, D.J.; Kulkarni, A.; Moh, S.H.; Kim, K.W. Mycosporine-Like Amino Acids Promote Wound Healing through Focal Adhesion Kinase (FAK) and Mitogen-Activated Protein Kinases (MAP Kinases) Signaling Pathway in Keratinocytes. Mar. Drugs 2015, 13, 7055–7066. [Google Scholar] [CrossRef] [Green Version]
- Oyamada, C.; Kaneniwa, M.; Ebitani, K.; Murata, M.; Ishihara, K. Mycosporine-Like Amino Acids Extracted from Scallop (Patinopecten yessoensis) Ovaries: UV Protection and Growth Stimulation Activities on Human Cells. Mar. Biotechnol. 2008, 10, 141–150. [Google Scholar] [CrossRef]
- Orfanoudaki, M.; Hartmann, A.; Karsten, U.; Ganzera, M. Chemical profiling of mycosporine-like amino acids in twenty-three red algal species. J. Phycol. 2019, 55, 393–403. [Google Scholar] [CrossRef]
- Fujii, K.; Ikai, Y.; Oka, H.; Suzuki, M.; Harada, K. A Nonempirical Method Using LC/MS for Determination of the Absolute Configuration of Constituent Amino Acids in a Peptide: Combination of Marfey’s Method with Mass Spectrometry and Its Practical Application. Anal. Chem. 1997, 69, 5146–5151. [Google Scholar] [CrossRef]
- Rigaku, O.D. CrysAlis PRO; Rigaku Corporation: Tokyo, Japan, 2015. [Google Scholar]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. C 2015, C71, 3–8. [Google Scholar] [CrossRef]
- Parsons, S.; Flack, H.D.; Wagner, T. Use of intensity quotients and differences in absolute structure refinement. Acta Cryst. 2013, B69, 249–259. [Google Scholar] [CrossRef] [Green Version]
- Balekar, N.; Katkam, N.G.; Nakpheng, T.; Jehtae, K.; Srichana, T. Evaluation of the wound healing potential of Wedelia trilobata (L.) leaves. J. Ethnopharmacol. 2012, 141, 817–824. [Google Scholar] [CrossRef]
- Grada, A.; Otero-Vinas, M.; Prieto-Castrillo, F.; Obagi, Z.; Falanga, V. Research Techniques Made Simple: Analysis of Collective Cell Migration Using the Wound Healing Assay. J. Investig. Dermatol. 2017, 137, e11–e16. [Google Scholar] [CrossRef] [Green Version]





| Compound | Collagenase Inhibition | Pentosidine-Like AGEs |
|---|---|---|
| IC50 µM (CI 95 ±) | IC50 µM | |
| Compound 1 + | 104.0 (95.54 to 110.8) | 103 |
| Compound 2 + | 105.9 (94.43 to 117.8) | 90 |
| Compound 3 | 250.5 (237.4 to 264.3) | 150 |
| Compound 4 | 158.1 (153.0 to 163.4) | 75 |
| Compound 5 | 80.71 (73.29 to 88.89) | 400 |
| Compound 6 | 70.91 (65.53 to 76.73) | 125 |
| Compound 7 + | 158.9 (141.4 to 175.7) | 700 |
| Compound 8 | 104.5 (98.51 to 111.0) | - |
| Compound 9 | 58.39 (55.51 to 61.41) | - |
| Compound 10 | 118.0 (108.1 to 128.7) | 85 |
| Compound 11 | 163.0 (150.6 to 176.5) | 150 |
| Compound 12 | 90.03 (82.18 to 98.64) | 200 |
| Compound 13 | 80.52 (74.56 to 86.96) | - |
| Phosphoramidon ** | 1.90 (1.765 to 2.039) | - |
| Epigallocatechingalleate ** | 46.62 (41.30 to 52.63) | - |
| Rutin ** | 100 µM -> 52% Inhibition * | 85 µM |
| Aminoguanidine ** | - | 1.4 mM |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orfanoudaki, M.; Hartmann, A.; Alilou, M.; Gelbrich, T.; Planchenault, P.; Derbré, S.; Schinkovitz, A.; Richomme, P.; Hensel, A.; Ganzera, M. Absolute Configuration of Mycosporine-Like Amino Acids, Their Wound Healing Properties and In Vitro Anti-Aging Effects. Mar. Drugs 2020, 18, 35. https://doi.org/10.3390/md18010035
Orfanoudaki M, Hartmann A, Alilou M, Gelbrich T, Planchenault P, Derbré S, Schinkovitz A, Richomme P, Hensel A, Ganzera M. Absolute Configuration of Mycosporine-Like Amino Acids, Their Wound Healing Properties and In Vitro Anti-Aging Effects. Marine Drugs. 2020; 18(1):35. https://doi.org/10.3390/md18010035
Chicago/Turabian StyleOrfanoudaki, Maria, Anja Hartmann, Mostafa Alilou, Thomas Gelbrich, Patricia Planchenault, Séverine Derbré, Andreas Schinkovitz, Pascal Richomme, Andreas Hensel, and Markus Ganzera. 2020. "Absolute Configuration of Mycosporine-Like Amino Acids, Their Wound Healing Properties and In Vitro Anti-Aging Effects" Marine Drugs 18, no. 1: 35. https://doi.org/10.3390/md18010035
APA StyleOrfanoudaki, M., Hartmann, A., Alilou, M., Gelbrich, T., Planchenault, P., Derbré, S., Schinkovitz, A., Richomme, P., Hensel, A., & Ganzera, M. (2020). Absolute Configuration of Mycosporine-Like Amino Acids, Their Wound Healing Properties and In Vitro Anti-Aging Effects. Marine Drugs, 18(1), 35. https://doi.org/10.3390/md18010035