Methylglyoxal-Dependent Glycative Stress and Deregulation of SIRT1 Functional Network in the Ovary of PCOS Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Estrous Cycle Determination
2.3. Superovulation Induction and Oocyte Collection
2.4. H&E Staining and Ovarian Follicle Classification and Counting
2.5. Heidenhain’s AZAN Trichrome Staining
2.6. Ovarian Immunofluorescence Analysis
2.7. Ovarian Immunohistochemical Analysis
2.8. Western Blot Analysis
2.9. Analysis of DNA Distribution and Spindle Configuration of In Vivo Matured MII Oocytes
2.10. Statistical Analysis
3. Results
3.1. Analysis of PCOS Phenotype in DHEA Mice
3.2. DHEA Induced Ovulatory Dysfunction during Superovulation and Negatively Influences Oocyte Quality
3.3. Glycative Stress is Observed in the Ovary of DHEA Mice
3.4. SIRT1 Functional Network Regulating Mitochondrial Physiology Is Disrupted in DHEA Ovaries
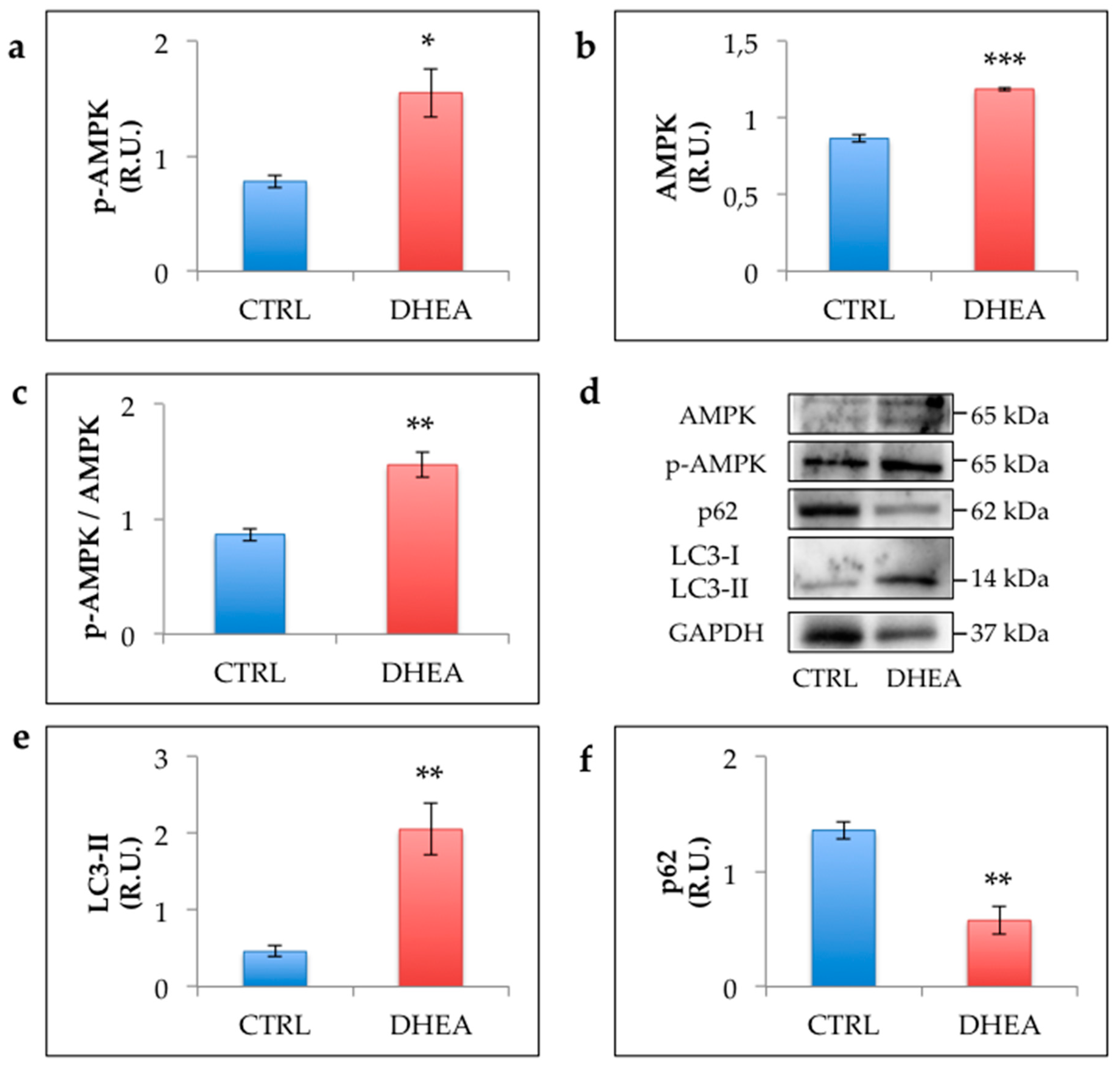
3.5. AMPK and Autophagy Increase in the Ovary of DHEA Mice
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Trikudanathan, S. Polycystic ovarian syndrome. Med. Clin. N. Am. 2015, 99, 221–235. [Google Scholar] [CrossRef] [PubMed]
- Azziz, R.; Carmina, E.; Chen, Z.; Dunaif, A.; Laven, J.S.; Legro, R.S.; Lizneva, D.; Natterson-Horowtiz, B.; Teede, H.J.; Yildiz, B.O. Polycystic ovary syndrome. Nat. Rev. Dis. Primers 2016, 2, 16057. [Google Scholar] [CrossRef] [PubMed]
- Sirmans, S.M.; Pate, K.A. Epidemiology, diagnosis, and management of polycystic ovary syndrome. Clin. Epidemiol. 2013, 6, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papalou, O.; Victor, V.M.; Diamanti-Kandarakis, E. Oxidative Stress in Polycystic Ovary Syndrome. Curr. Pharm. Des. 2016, 22, 2709–2722. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, M. Oxidative Stress and Polycystic Ovary Syndrome: A Brief Review. Int. J. Prev. Med. 2019, 10, 86. [Google Scholar] [CrossRef] [PubMed]
- Peppa, M.; Uribarri, J.; Vlassara, H. Aging and glycoxidant stress. Hormones 2008, 7, 123–132. [Google Scholar] [CrossRef]
- Mano, J. Reactive carbonyl species: Their production from lipid peroxides, action in environmental stress, and the detoxification mechanism. Plant Physiol. Biochem. 2012, 59, 90–97. [Google Scholar] [CrossRef]
- Shipanova, I.N.; Glomb, M.A.; Nagaraj, R.H. Protein modification by methylglyoxal: Chemical nature and synthetic mechanism of a major fluorescent adduct, Arch. Biochem. Biophys. 1997, 344, 29–36. [Google Scholar] [CrossRef]
- Gomes, R.; Sousa Silva, M.; Quintas, A.; Cordeiro, C.; Freire, A.; Pereira, P.; Martins, A.; Monteiro, E.; Barroso, E.; Ponces Freire, A. Argpyrimidine, a methylglyoxal-derived advanced glycation end-product in familial amyloidotic polyneuropathy. Biochem. J. 2005, 385, 339–345. [Google Scholar] [CrossRef] [Green Version]
- Rabbani, N.; Thornalley, P.J. Dicarbonyl stress in cell and tissue dysfunction contributing to ageing and disease. Biochem. Biophys. Res. Commun. 2015, 458, 221–226. [Google Scholar] [CrossRef] [Green Version]
- Diamanti-Kandarakis, E.; Piperi, C.; Patsouris, E.; Korkolopoulou, P.; Panidis, D.; Pawelczyk, L.; Papavassiliou, A.G.; Duleba, A.J. Immunohistochemical localization of advanced glycation end-products (AGEs) and their receptor (RAGE) in polycystic and normal ovaries. Histochem. Cell Biol. 2007, 127, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Diamanti-Kandarakis, E.; Katsikis, I.; Piperi, C.; Alexandraki, K.; Panidis, D. Effect of long-term orlistat treatment on serum levels of advanced glycation end-products in women with polycystic ovary syndrome. Clin. Endocrinol. 2007, 66, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Diamanti-Kandarakis, E.; Alexandraki, K.; Piperi, C.; Aessopos, A.; Paterakis, T.; Katsikis, I.; Panidis, D. Effect of metformin administration on plasma advanced glycation end product levels in women with polycystic ovary syndrome. Metabolism 2007, 56, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Jinno, M.; Takeuchi, M.; Watanabe, A.; Teruya, K.; Hirohama, J.; Eguchi, N.; Miyazaki, A. Advanced glycation end-products accumulation compromises embryonic development and achievement of pregnancy by assisted reproductive technology. Hum. Reprod. 2011, 26, 604–610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garg, D.; Grazi, R.; Lambert-Messerlian, G.M.; Merhi, Z. Correlation between follicular fluid levels of sRAGE and vitamin D in women with PCOS. J. Assist. Reprod. Genet. 2017, 34, 1507–1513. [Google Scholar] [CrossRef] [PubMed]
- Kandaraki, E.; Chatzigeorgiou, A.; Piperi, C.; Palioura, E.; Palimeri, S.; Korkolopoulou, P.; Koutsilieris, M.; Papavassiliou, A.G. Reduced ovarian glyoxalase-I activity by dietary glycotoxins and androgen excess: A causative link to polycystic ovarian syndrome. Mol. Med. 2012, 18, 1183–1189. [Google Scholar] [CrossRef] [PubMed]
- Di Emidio, G.; Santini, S.J.; D’Alessandro, A.M.; Vetuschi, A.; Sferra, R.; Artini, P.G.; Carta, G.; Falone, S.; Amicarelli, F.; Tatone, C. SIRT1 participates in the response to methylglyoxal-dependent glycative stress in mouse oocytes and ovary. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 1389–1401. [Google Scholar] [CrossRef]
- Tatone, C.; Di Emidio, G.; Vitti, M.; Di Carlo, M.; Santini, S., Jr.; D’Alessandro, A.M.; Falone, S.; Amicarelli, F. Sirtuin functions in female fertility: Possible role in oxidative stress and aging. Oxidative Med. Cell Longev. 2015. [Google Scholar] [CrossRef] [Green Version]
- Tatone, C.; Di Emidio, G.; Barbonetti, A.; Carta, G.; Luciano, A.M.; Falone, S.; Amicarelli, F. Sirtuins in gamete biology and reproductive physiology: Emerging roles and therapeutic potential in female and male infertility. Hum. Reprod. Update 2018, 24, 267–289. [Google Scholar] [CrossRef]
- Li, S.Y.; Song, Z.; Song, M.J.; Qin, J.W.; Zhao, M.L.; Yang, Z.M. Impaired receptivity and decidualization in DHEA-induced PCOS mice. Sci. Rep. 2016, 6, 38134. [Google Scholar] [CrossRef]
- Motta, A.B. Dehydroepiandrosterone to induce murine models for the study of polycystic ovary syndrome. J. Steroid Biochem. Mol. Biol. 2010, 119, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Tatone, C.; Carbone, M.C.; Campanella, G.; Festuccia, C.; Artini, P.G.; Talesa, V.; Focarelli, R.; Amicarelli, F. Female reproductive dysfunction during ageing: Role of methylglyoxal in the formation of advanced glycation endproducts in ovaries of reproductively-aged mice. J. Biol. Regul. Homeost. Agents 2010, 24, 63–72. [Google Scholar] [PubMed]
- Di Emidio, G.; D’Alfonso, A.; Leocata, P.; Parisse, V.; Di Fonso, A.; Artini, P.G.; Patacchiola, F.; Tatone, C.; Carta, G. Increased levels of oxidative and carbonyl stress markers in normal ovarian cortex surrounding endometriotic cysts. Gynecol. Endocrinol. 2014, 30, 808–812. [Google Scholar] [CrossRef] [PubMed]
- Falone, S.; Santini, S.J.; Cordone, V.; Grannonico, M.; Cacchio, M.; Di Emidio, G.; Tatone, C.; Amicarelli, F. Regular and Moderate Exercise Counteracts the Decline of Antioxidant Protection but Not Methylglyoxal-Dependent Glycative Burden in the Ovary of Reproductively Aging Mice. Oxid. Med. Cell Longev. 2016. [Google Scholar] [CrossRef]
- Tang, B.L. Sirt1 and the Mitochondria. Mol. Cells 2016, 39, 87–95. [Google Scholar] [CrossRef] [Green Version]
- Campbell, C.T.; Kolesar, J.E.; Kaufman, B.A. Mitochondrial transcription factor A regulates mitochondrial transcription initiation, DNA packaging, and genome copy number. Biochim. Biophys. Acta 2012, 1819, 921–929. [Google Scholar] [CrossRef]
- Fulco, M.; Sartorelli, V. Comparing and contrasting the roles of AMPK and SIRT1 in metabolic tissues. Cell Cycle 2008, 7, 3669–3679. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Qi, J.; Zhu, Q.; He, Y.; Wang, Y.; Lu, Y.; Wu, H.; Sun, Y. The role of androgen in autophagy of granulosa cells from PCOS. Gynecol. Endocrinol. 2019, 35, 669–672. [Google Scholar] [CrossRef]
- Li, D.; You, Y.; Bi, F.F.; Zhang, T.N.; Jiao, J.; Wang, T.R.; Zhou, Y.M.; Shen, Z.Q.; Wang, X.X.; Yang, Q. Autophagy is activated in the ovarian tissue of polycystic ovary syndrome. Reproduction 2018, 155, 85–92. [Google Scholar] [CrossRef] [Green Version]
- Caligioni, C.S. Assessing reproductive status/stages in mice. Curr. Protoc. Neurosci. 2009. [Google Scholar] [CrossRef] [Green Version]
- Gougeon, A. Regulation of ovarian follicular development in primates: Facts and hypotheses. Endocr. Rev. 1996, 17, 121–155. [Google Scholar] [CrossRef] [PubMed]
- Jensen, E.C. Quantitative analysis of histological staining and fluorescence using ImageJ. Anat. Rec. 2013, 296, 378–381. [Google Scholar] [CrossRef] [PubMed]
- Varghese, F.; Bukhari, A.B.; Malhotra, R.; De, A. IHC Profiler: An open source plugin for the quantitative evaluation and automated scoring of immunohistochemistry images of human tissue samples. PLoS ONE 2014, 9, e96801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liou, G.Y.; Storz, P. Detecting reactive oxygen species by immunohistochemistry. Methods Mol. Biol. 2015, 1292, 97–104. [Google Scholar] [PubMed] [Green Version]
- Liu, L.; Keefe, D.L. Ageing-associated aberration in meiosis of oocytes from senescence-accelerated mice. Hum. Reprod. 2002, 17, 2678–2685. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Mizushima, N. LC3- and p62-based biochemical methods for the analysis of autophagy progression in mammalian cells. Methods 2015, 75, 13–18. [Google Scholar] [CrossRef]
- Yoshii, S.R.; Mizushima, N. Monitoring and Measuring Autophagy. Int. J. Mol. Sci. 2017, 18, 1865. [Google Scholar] [CrossRef]
- Tatone, C.; Eichenlaub-Ritter, U.; Amicarelli, F. Dicarbonyl stress and glyoxalases in ovarian function. Biochem. Soc. Trans. 2014, 42, 433–438. [Google Scholar] [CrossRef]
- Tatone, C.; Amicarelli, F. The aging ovary—The poor granulosa cells. Fertil. Steril. 2013, 99, 12–17. [Google Scholar] [CrossRef]
- Lin, P.H.; Chang, C.C.; Wu, K.H.; Shih, C.K.; Chiang, W.; Chen, H.Y.; Shih, Y.H.; Wang, K.L.; Hong, Y.H.; Shieh, T.M.; et al. Dietary Glycotoxins, Advanced Glycation End Products, Inhibit Cell Proliferation and Progesterone Secretion in Ovarian Granulosa Cells and Mimic PCOS-like Symptoms. Biomolecules 2019, 9, 327. [Google Scholar] [CrossRef] [Green Version]
- Walters, K.A.; Allan, C.M.; Handelsman, D.J. Rodent models for human polycystic ovary syndrome. Biol. Reprod. 2012, 86, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Luchetti, C.G.; Solano, M.E.; Sander, V.; Arcos, M.L.; Gonzalez, C.; Di Girolamo, G.; Chiocchio, S.; Cremaschi, G.; Motta, A.B. Effects of dehydroepiandrosterone on ovarian cystogenesis and immune function. J. Reprod. Immunol. 2004, 64, 59–74. [Google Scholar] [CrossRef]
- Caldwell, A.S.; Middleton, L.J.; Jimenez, M.; Desai, R.; McMahon, A.C.; Allan, C.M.; Handelsman, D.J.; Walters, K.A. Characterization of reproductive, metabolic, and endocrine features of polycystic ovary syndrome in female hyperandrogenic mouse models. Endocrinology 2014, 155, 3146–3159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.; Yu, Y.; Gao, J.; Li, R.; Zhang, C.; Zhao, H.; Zhao, Y.; Qiao, J. Impaired oocyte quality induced by dehydroepiandrosterone is partially rescued by metformin treatment. PLoS ONE 2015, 10, e0122370. [Google Scholar] [CrossRef] [PubMed]
- Palomba, S.; Daolio, J.; La Sala, G.B. Oocyte Competence in Women with Polycystic Ovary Syndrome. Trends Endocrinol. Metab. 2017, 28, 186–198. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, N.; Harada, M.; Hirota, Y.; Nose, E.; Azhary, J.M.; Koike, H.; Kunitomi, C.; Yoshino, O.; Izumi, G.; Hirata, T.; et al. Activation of Endoplasmic Reticulum Stress in Granulosa Cells from Patients with Polycystic Ovary Syndrome Contributes to Ovarian Fibrosis. Sci. Rep. 2017, 7, 10824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Pietro, M.; Pascuali, N.; Parborell, F.; Abramovich, D. Ovarian angiogenesis in polycystic ovary syndrome. Reproduction 2018, 155, R199–R209. [Google Scholar] [CrossRef] [Green Version]
- Bousmpoula, A.; Benidis, E.; Demeridou, S.; Kapeta-Kourkouli, R.; Chasiakou, A.; Chasiakou, S.; Kouskouni, E.; Baka, S. Serum and follicular fluid irisin levels in women with polycystic ovaries undergoing ovarian stimulation: Correlation with insulin resistance and lipoprotein lipid profiles. Gynecol. Endocrinol. 2019, 35, 803–806. [Google Scholar] [CrossRef]
- Yang, X.; Wu, L.L.; Chura, L.R.; Liang, X.; Lane, M.; Norman, R.J.; Robker, R.L. Exposure to lipid-rich follicular fluid is associated with endoplasmic reticulum stress and impaired oocyte maturation in cumulus-oocyte complexes. Fertil. Steril. 2012, 97, 1438–1443. [Google Scholar] [CrossRef]
- Tatone, C.; Benedetti, E.; Vitti, M.; Di Emidio, G.; Ciriminna, R.; Vento, M.E.; Cela, V.; Borzì, P.; Carta, G.; Lispi, M.; et al. Italian Society of Embryology, Reproduction and Research (SIERR). Modulating Intrafollicular Hormonal Milieu in Controlled Ovarian Stimulation: Insights From PPAR Expression in Human Granulosa Cells. J. Cell Physiol. 2016, 231, 908–914. [Google Scholar] [CrossRef]
- Nelson, V.L.; Qin, K.N.; Rosenfield, R.L.; Wood, J.R.; Penning, T.M.; Legro, R.S.; Strauss, J.F., 3rd; McAllister, J.M. The biochemical basis for increased testosterone production in theca cells propagated from patients with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2001, 86, 5925–5933. [Google Scholar] [CrossRef] [PubMed]
- Belani, M.; Deo, A.; Shah, P.; Banker, M.; Singal, P.; Gupta, S. Differential insulin and steroidogenic signaling in insulin resistant and non-insulin resistant human luteinized granulosa cells—A study in PCOS patients. J. Steroid Biochem. Mol. Biol. 2018, 178, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Tatone, C.; Heizenrieder, T.; Di Emidio, G.; Treffon, P.; Amicarelli, F.; Seidel, T.; Eichenlaub-Ritter, U. Evidence that carbonyl stress by methylglyoxal exposure induces DNA damage and spindle aberrations, affects mitochondrial integrity in mammalian oocytes and contributes to oocyte ageing. Hum. Reprod. 2011, 26, 1843–1859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elia, E.; Sander, V.; Luchetti, C.G.; Solano, M.E.; Di Girolamo, G.; Gonzalez, C.; Motta, A.B. The mechanisms involved in the action of metformin in regulating ovarian function in hyperandrogenized mice. Mol. Hum. Reprod. 2006, 12, 475–481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buler, M.; Andersson, U.; Hakkola, J. Who watches the watchmen? Regulation of the expression and activity of sirtuins. FASEB J. 2016, 30, 3942–3960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Endo, T.; Kohda, D. Functions of outer membrane receptors in mitochondrial protein import. Biochim. Biophys. Acta 2002, 1592, 3–14. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.T.; Wu, S.B.; Wei, Y.H. Roles of sirtuins in the regulation of antioxidant defense and bioenergetic function of mitochondria under oxidative stress. Free Radic. Res. 2014, 48, 1070–1084. [Google Scholar] [CrossRef]
- Lin, S.; Xing, H.; Zang, T.; Ruan, X.; Wo, L.; He, M. Sirtuins in mitochondrial stress: Indispensable helpers behind the scenes. Ageing Res. Rev. 2018, 44, 22–32. [Google Scholar] [CrossRef]
- Zhang, J.; Bao, Y.; Zhou, X.; Zheng, L. Polycystic ovary syndrome and mitochondrial dysfunction. Reprod. Biol. Endocrinol. 2019, 17, 67. [Google Scholar] [CrossRef]
- Kitada, M.; Ogura, Y.; Koya, D. The protective role of Sirt1 in vascular tissue: Its relationship to vascular aging and atherosclerosis. Aging 2016, 8, 2290–2307. [Google Scholar] [CrossRef] [Green Version]







| CTRL (Mean ± SEM) | DHEA (Mean ± SEM) | |
|---|---|---|
| No. of oocytes from per mouse | 17.4 ± 1.0 | 10.8 ± 1.0 ** |
| Percentage of MII oocytes | 92.1 ± 1.2% | 86.4 ± 3.9% |
| Percentage of MII oocytes with normal metaphase plate | 77.2 ± 4.0% | 44.0 ± 7.5% ** |
| Percentage of degenerated oocytes | 8.0 ± 1.2% | 13.6 ± 3.9% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Emidio, G.; Placidi, M.; Rea, F.; Rossi, G.; Falone, S.; Cristiano, L.; Nottola, S.; D’Alessandro, A.M.; Amicarelli, F.; Palmerini, M.G.; et al. Methylglyoxal-Dependent Glycative Stress and Deregulation of SIRT1 Functional Network in the Ovary of PCOS Mice. Cells 2020, 9, 209. https://doi.org/10.3390/cells9010209
Di Emidio G, Placidi M, Rea F, Rossi G, Falone S, Cristiano L, Nottola S, D’Alessandro AM, Amicarelli F, Palmerini MG, et al. Methylglyoxal-Dependent Glycative Stress and Deregulation of SIRT1 Functional Network in the Ovary of PCOS Mice. Cells. 2020; 9(1):209. https://doi.org/10.3390/cells9010209
Chicago/Turabian StyleDi Emidio, Giovanna, Martina Placidi, Francesco Rea, Giulia Rossi, Stefano Falone, Loredana Cristiano, Stefania Nottola, Anna Maria D’Alessandro, Fernanda Amicarelli, Maria Grazia Palmerini, and et al. 2020. "Methylglyoxal-Dependent Glycative Stress and Deregulation of SIRT1 Functional Network in the Ovary of PCOS Mice" Cells 9, no. 1: 209. https://doi.org/10.3390/cells9010209
APA StyleDi Emidio, G., Placidi, M., Rea, F., Rossi, G., Falone, S., Cristiano, L., Nottola, S., D’Alessandro, A. M., Amicarelli, F., Palmerini, M. G., & Tatone, C. (2020). Methylglyoxal-Dependent Glycative Stress and Deregulation of SIRT1 Functional Network in the Ovary of PCOS Mice. Cells, 9(1), 209. https://doi.org/10.3390/cells9010209









