Association between Timing of Energy Intake and Insulin Sensitivity: A Cross-Sectional Study
Abstract
:1. Introduction
2. Methods
2.1. Study Design
2.2. Insulin Sensitivity Assessment
2.3. Assessment of Meal Timing
2.4. Covariates
2.5. Statistical Analysis
3. Results
3.1. Proportions of Energy Intake in Morning Versus Evening
3.2. Clock Times at Which Proportions Are Reached
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lorenzo, C.; Wagenknecht, L.E.; D’Agostino, R.B., Jr.; Rewers, M.J.; Karter, A.J.; Haffner, S.M. Insulin resistance, beta-cell dysfunction, and conversion to type 2 diabetes in a multiethnic population: The Insulin Resistance Atherosclerosis Study. Diabetes Care 2010, 33, 67–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balkau, B.; Mhamdi, L.; Oppert, J.M.; Nolan, J.; Golay, A.; Porcellati, F.; Laakso, M.; Ferrannini, E.; on behalf of the EGIR-RISC Study Group. Physical activity and insulin sensitivity: The RISC study. Diabetes 2008, 57, 2613–2618. [Google Scholar] [CrossRef] [Green Version]
- Preis, S.R.; Massaro, J.M.; Robins, S.J.; Hoffmann, U.; Vasan, R.S.; Irlbeck, T.; Meigs, J.B.; Sutherland, P.; D’Agostino, R.B., Sr.; O’Donnell, C.J.; et al. Abdominal subcutaneous and visceral adipose tissue and insulin resistance in the Framingham heart study. Obesity 2010, 18, 2191–2198. [Google Scholar] [CrossRef] [Green Version]
- Sellahewa, L.; Khan, C.; Lakkunarajah, S.; Idris, I. A Systematic Review of Evidence on the Use of Very Low Calorie Diets in People with Diabetes. Curr. Diabetes Rev. 2017, 13, 35–46. [Google Scholar] [CrossRef]
- Kosinski, C.; Jornayvaz, F.R. Effects of Ketogenic Diets on Cardiovascular Risk Factors: Evidence from Animal and Human Studies. Nutrients 2017, 9, 517. [Google Scholar] [CrossRef]
- Garcia-Prieto, C.F.; Fernandez-Alfonso, M.S. Caloric Restriction as a Strategy to Improve Vascular Dysfunction in Metabolic Disorders. Nutrients 2016, 8, 370. [Google Scholar] [CrossRef] [Green Version]
- Damiola, F.; Le Minh, N.; Preitner, N.; Kornmann, B.; Fleury-Olela, F.; Schibler, U. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev. 2000, 14, 2950–2961. [Google Scholar] [CrossRef] [Green Version]
- Buxton, O.M.; Cain, S.W.; O’Connor, S.P.; Porter, J.H.; Duffy, J.F.; Wang, W.; Czeisler, C.A.; Shea, S.A. Adverse metabolic consequences in humans of prolonged sleep restriction combined with circadian disruption. Sci. Transl. Med. 2012, 4, 129ra143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leproult, R.; Holmback, U.; Van Cauter, E. Circadian misalignment augments markers of insulin resistance and inflammation, independently of sleep loss. Diabetes 2014, 63, 1860–1869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wyse, C.A.; Celis Morales, C.A.; Graham, N.; Fan, Y.; Ward, J.; Curtis, A.M.; Mackay, D.; Smith, D.J.; Bailey, M.E.S.; Biello, S.; et al. Adverse metabolic and mental health outcomes associated with shiftwork in a population-based study of 277,168 workers in UK biobank. Ann. Med. 2017, 49, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Kawada, T.; Otsuka, T. Effect of shift work on the development of metabolic syndrome after 3 years in Japanese male workers. Arch. Environ. Occup. Health 2014, 69, 55–61. [Google Scholar] [CrossRef] [PubMed]
- De Bacquer, D.; Van Risseghem, M.; Clays, E.; Kittel, F.; De Backer, G.; Braeckman, L. Rotating shift work and the metabolic syndrome: A prospective study. Int. J. Epidemiol. 2009, 38, 848–854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Y.C.; Hsiao, T.J.; Chen, P.C. Persistent rotating shift-work exposure accelerates development of metabolic syndrome among middle-aged female employees: A five-year follow-up. Chronobiol. Int. 2009, 26, 740–755. [Google Scholar] [CrossRef] [PubMed]
- McHill, A.W.; Phillips, A.J.; Czeisler, C.A.; Keating, L.; Yee, K.; Barger, L.K.; Garaulet, M.; Scheer, F.A.; Klerman, E.B. Later circadian timing of food intake is associated with increased body fat. Am. J. Clin. Nutr. 2017. [Google Scholar] [CrossRef] [PubMed]
- Breda, E.; Cavaghan, M.K.; Toffolo, G.; Polonsky, K.S.; Cobelli, C. Oral glucose tolerance test minimal model indexes of beta-cell function and insulin sensitivity. Diabetes 2001, 50, 150–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muniyappa, R.; Lee, S.; Chen, H.; Quon, M.J. Current approaches for assessing insulin sensitivity and resistance in vivo: Advantages, limitations, and appropriate usage. Am. J. Physiol. Endocrinol. Metab. 2008, 294, E15–E26. [Google Scholar] [CrossRef] [Green Version]
- Matsuda, M.; DeFronzo, R.A. Insulin sensitivity indices obtained from oral glucose tolerance testing: Comparison with the euglycemic insulin clamp. Diabetes Care 1999, 22, 1462–1470. [Google Scholar] [CrossRef]
- Katz, A.; Nambi, S.S.; Mather, K.; Baron, A.D.; Follmann, D.A.; Sullivan, G.; Quon, M.J. Quantitative insulin sensitivity check index: A simple, accurate method for assessing insulin sensitivity in humans. J. Clin. Endocrinol. Metab. 2000, 85, 2402–2410. [Google Scholar] [CrossRef]
- Abdul-Ghani, M.A.; Matsuda, M.; Balas, B.; DeFronzo, R.A. Muscle and liver insulin resistance indexes derived from the oral glucose tolerance test. Diabetes Care 2007, 30, 89–94. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Sullivan, G.; Quon, M.J. Assessing the predictive accuracy of QUICKI as a surrogate index for insulin sensitivity using a calibration model. Diabetes 2005, 54, 1914–1925. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.J.; Kim, M.K.; Hwang, S.H.; Ahn, Y.; Shim, J.E.; Kim, D.H. Relative validities of 3-day food records and the food frequency questionnaire. Nutr. Res. Pr. 2010, 4, 142–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson, F.E.; Subar, A.F. Dietary Assessment Methodology. In Nutrition in the Prevention and Treatment of Disease, 3rd ed.; Coulston, A.M., Boushey, C.J., Ferruzzi, M.G., Eds.; Elsevier/Academic Press: Waltham, MA, USA, 2013; pp. 5–46. [Google Scholar]
- Van Cauter, E.; Blackman, J.D.; Roland, D.; Spire, J.P.; Refetoff, S.; Polonsky, K.S. Modulation of glucose regulation and insulin secretion by circadian rhythmicity and sleep. J. Clin. Investig. 1991, 88, 934–942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, K.J.; Gall, S.L.; McNaughton, S.A.; Blizzard, L.; Dwyer, T.; Venn, A.J. Skipping breakfast: Longitudinal associations with cardiometabolic risk factors in the Childhood Determinants of Adult Health Study. Am. J. Clin. Nutr. 2010, 92, 1316–1325. [Google Scholar] [CrossRef] [PubMed]
- Farshchi, H.R.; Taylor, M.A.; Macdonald, I.A. Deleterious effects of omitting breakfast on insulin sensitivity and fasting lipid profiles in healthy lean women. Am. J. Clin. Nutr. 2005, 81, 388–396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mekary, R.A.; Giovannucci, E.; Willett, W.C.; van Dam, R.M.; Hu, F.B. Eating patterns and type 2 diabetes risk in men: Breakfast omission, eating frequency, and snacking. Am. J. Clin. Nutr. 2012, 95, 1182–1189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mekary, R.A.; Giovannucci, E.; Cahill, L.; Willett, W.C.; van Dam, R.M.; Hu, F.B. Eating patterns and type 2 diabetes risk in older women: Breakfast consumption and eating frequency. Am. J. Clin. Nutr. 2013, 98, 436–443. [Google Scholar] [CrossRef]
- Kobayashi, F.; Ogata, H.; Omi, N.; Nagasaka, S.; Yamaguchi, S.; Hibi, M.; Tokuyama, K. Effect of breakfast skipping on diurnal variation of energy metabolism and blood glucose. Obes. Res. Clin. Pr. 2014, 8, e201–e298. [Google Scholar] [CrossRef]
- Timlin, M.T.; Pereira, M.A. Breakfast frequency and quality in the etiology of adult obesity and chronic diseases. Nutr. Rev. 2007, 65, 268–281. [Google Scholar] [CrossRef]
- Asao, K.; Marekani, A.S.; VanCleave, J.; Rothberg, A.E. Leptin Level and Skipping Breakfast: The National Health and Nutrition Examination Survey III (NHANES III). Nutrients 2016, 8, 115. [Google Scholar] [CrossRef] [Green Version]
- Thomas, E.A.; Higgins, J.; Bessesen, D.H.; McNair, B.; Cornier, M.A. Usual breakfast eating habits affect response to breakfast skipping in overweight women. Obesity 2015, 23, 750–759. [Google Scholar] [CrossRef]
- Odegaard, A.O.; Jacobs, D.R., Jr.; Steffen, L.M.; Van Horn, L.; Ludwig, D.S.; Pereira, M.A. Breakfast frequency and development of metabolic risk. Diabetes Care 2013, 36, 3100–3106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garaulet, M.; Gomez-Abellan, P.; Alburquerque-Bejar, J.J.; Lee, Y.C.; Ordovas, J.M.; Scheer, F.A. Timing of food intake predicts weight loss effectiveness. Int. J. Obes. (Lond.) 2013, 37, 604–611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morgan, L.M.; Shi, J.W.; Hampton, S.M.; Frost, G. Effect of meal timing and glycaemic index on glucose control and insulin secretion in healthy volunteers. Br. J. Nutr. 2012, 108, 1286–1291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jakubowicz, D.; Barnea, M.; Wainstein, J.; Froy, O. High caloric intake at breakfast vs. dinner differentially influences weight loss of overweight and obese women. Obesity 2013, 21, 2504–2512. [Google Scholar] [CrossRef]
- Nas, A.; Mirza, N.; Hagele, F.; Kahlhofer, J.; Keller, J.; Rising, R.; Kufer, T.A.; Bosy-Westphal, A. Impact of breakfast skipping compared with dinner skipping on regulation of energy balance and metabolic risk. Am. J. Clin. Nutr. 2017, 105, 1351–1361. [Google Scholar] [CrossRef] [Green Version]
- Jakubowicz, D.; Wainstein, J.; Ahren, B.; Bar-Dayan, Y.; Landau, Z.; Rabinovitz, H.R.; Froy, O. High-energy breakfast with low-energy dinner decreases overall daily hyperglycaemia in type 2 diabetic patients: A randomised clinical trial. Diabetologia 2015, 58, 912–919. [Google Scholar] [CrossRef]
- Arble, D.M.; Bass, J.; Laposky, A.D.; Vitaterna, M.H.; Turek, F.W. Circadian timing of food intake contributes to weight gain. Obesity 2009, 17, 2100–2102. [Google Scholar] [CrossRef]
- Minaya, D.M.; Rowland, N.E.; Robertson, K.L. Effect of day-night cycle on distribution of food intake and economic choice among imposed food opportunities in mice. Physiol. Behav. 2016, 164, 395–399. [Google Scholar] [CrossRef]
- Minaya, D.M.; Robertson, K.L.; Rowland, N.E. Circadian and economic factors affect food acquisition in rats restricted to discrete feeding opportunities. Physiol. Behav. 2017, 181, 10–15. [Google Scholar] [CrossRef]
- DeSalvo, K.B.; Olson, R.; Casavale, K.O. Dietary Guidelines for Americans. JAMA 2016, 315, 457–458. [Google Scholar] [CrossRef] [Green Version]

| Mean ± SD or n (%) | |
|---|---|
| Age (years) | 29.8 ± 7.6 |
| Sex | |
| Male | 23 (52%) |
| Female | 21 (48%) |
| Race | |
| African American | 17 (39%) |
| Non-Hispanic White | 27 (61%) |
| BMI (kg/m2) | 27.1 ± 5.1 |
| Glucose metabolism parameters | |
| Fasting glucose (mmol/L) | 4.8 ± 0.4 |
| Fasting insulin (pmol/L) | 50.7 ± 56.9 |
| Matsuda Index—180 min | 20.3 ± 10.5 |
| Matsuda Index—300 min (n = 43) | 25.8 ± 13.9 |
| QUICKI | 0.2 ± 0.03 |
| Sleep timing | |
| Sleep start | 00:14 ± 1:24 |
| Sleep end | 7:34 ± 1:24 |
| Sleep midpoint | 03:40 ± 0:21 |
| Mean ± SD | |
|---|---|
| Dietary Proportion Measures | |
| Total calories per day (Kcal) | 2411 ± 744 |
| Proportion of total calories | |
| In morning meals (% Kcal) | 23.1 ± 12.3 |
| In evening meals (% Kcal) | 40.5 ± 12.9 |
| Proportion of carbohydrates | |
| In morning meals (% Kcal) | 26.2 ± 13.7 |
| In evening meals (% Kcal) | 37.1 ± 11.8 |
| Proportion of protein | |
| In morning meals (% Kcal) | 20.8 ± 11.5 |
| In evening meals (% Kcal) | 42.5 ± 15.1 |
| Proportion of fat | |
| In morning meals (% Kcal) | 22.7 ± 14.6 |
| In evening meals (% Kcal) | 39.5 ± 16.2 |
| Dietary Timing Measures | |
| Timing of total calories | |
| Time reached 25% | 11:59 ± 2:17 |
| Time reached 50% | 15:12 ± 2:26 |
| Time reached 75% | 18:35 ± 1:42 |
| Timing of carbohydrates | |
| Time reached 25% | 11:45 ± 2:15 |
| Time reached 50% | 14:40 ± 2:05 |
| Time reached 75% | 18:17 ± 1:55 |
| Timing of protein | |
| Time reached 25% | 12:25 ± 2:03 |
| Time reached 50% | 15:21 ± 2:20 |
| Time reached 75% | 18:23 ± 1:47 |
| Timing of fat | |
| Time reached 25% | 12:29 ± 2:21 |
| Time reached 50% | 15:17 ± 2:31 |
| Time reached 75% | 17:42 ± 2:21 |
| Dependent Variable | Independent Variable | Unadjusted Model | Adjusted Model * | ||
|---|---|---|---|---|---|
| B-Coefficient (95% CI) | p-Value | B-Coefficient (95% CI) | p-Value | ||
| Proportions | |||||
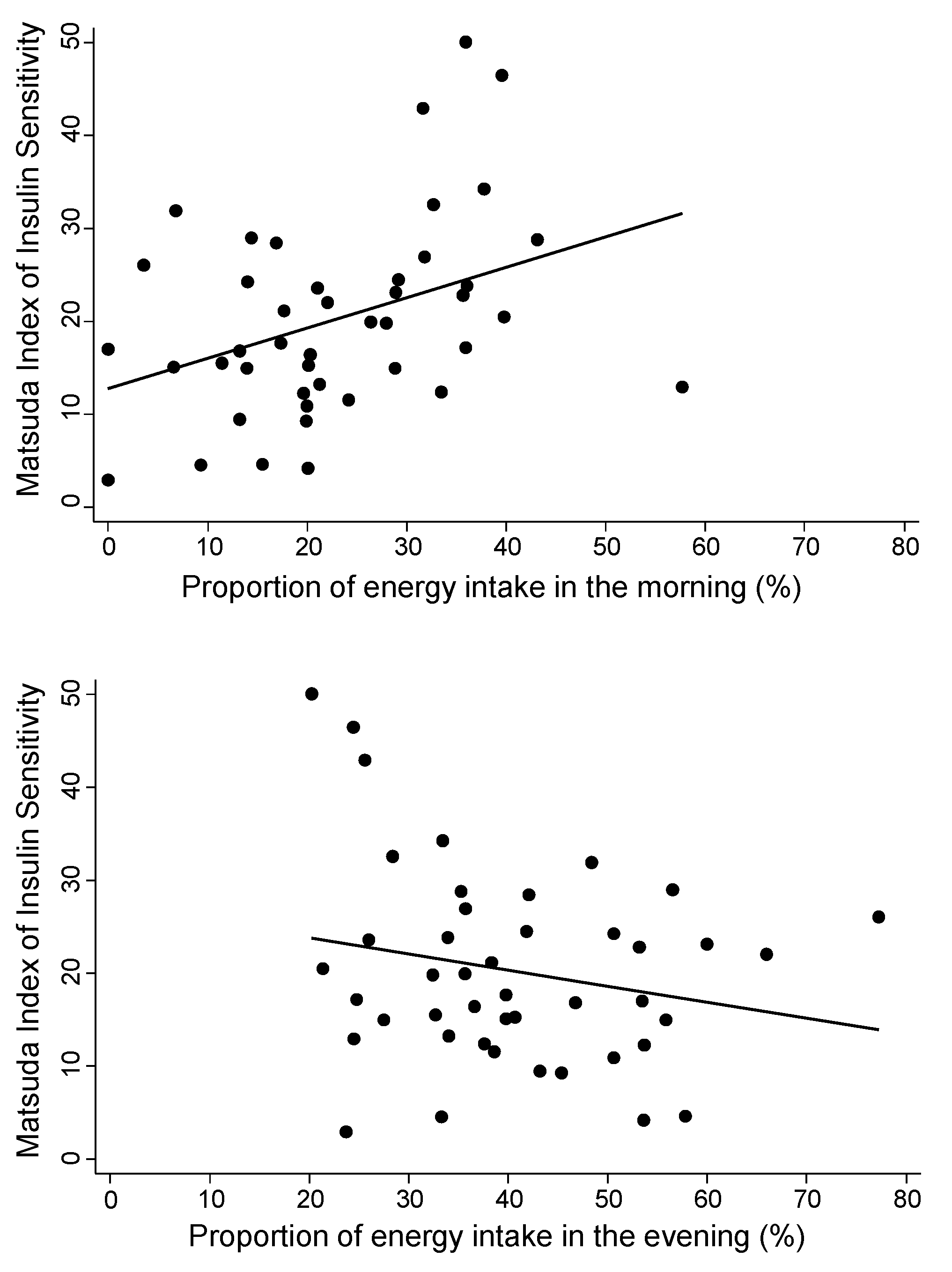
| Matsuda Index | Proportion of morning calories (per 10%) | 3.3 (0.8 to 5.8) | 0.011 | 2.8 (0.3 to 5.2) | 0.03 |
| Proportion of evening calories (per 10%) | −1.7 (−4.2 to 0.8) | 0.17 | −1.9 (−4.4 to 0.6) | 0.13 | |
| QUICKI | Proportion of morning calories (per 10%) | 0.008 (0.001 to 0.013) | 0.018 | 0.006 (−0.0002 to 0.012) | 0.057 |
| Proportion of evening calories (per 10%) | −0.003 (−0.009 to 0.004) | 0.39 | −.003 (−0.009 to 0.003) | 0.28 | |
| Timing | |||||
| Matsuda Index | 25% total calorie intake (per hour) | −1.6 (−3.0 to −0.3) | 0.02 | −1.4 (−2.8 to −0.1) | 0.04 |
| 50% total calorie intake (per hour) | −1.0 (−2.3 to 0.3) | 0.14 | −1.0 (−2.3 to 0.2) | 0.10 | |
| 75% total calorie intake (per hour) | −0.3 (−2.2 to 1.7) | 0.77 | −0.9 (−2.9 to 1.0) | 0.34 | |
| QUICKI | 25% total calorie intake (per hour) | −0.004 (−0.008 to −0.001) | 0.01 | −0.004 (−0.007 to −0.001) | 0.02 |
| 50% total calorie intake (per hour) | −0.002 (−0.006 to 0.001) | 0.14 | −0.002 (−0.006 to 0.001) | 0.11 | |
| 75% total calorie intake (per hour) | −0.001 (−0.006 to 0.004) | 0.70 | −0.002 (−0.007, 0.003) | 0.41 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rangaraj, V.R.; Siddula, A.; Burgess, H.J.; Pannain, S.; Knutson, K.L. Association between Timing of Energy Intake and Insulin Sensitivity: A Cross-Sectional Study. Nutrients 2020, 12, 503. https://doi.org/10.3390/nu12020503
Rangaraj VR, Siddula A, Burgess HJ, Pannain S, Knutson KL. Association between Timing of Energy Intake and Insulin Sensitivity: A Cross-Sectional Study. Nutrients. 2020; 12(2):503. https://doi.org/10.3390/nu12020503
Chicago/Turabian StyleRangaraj, Vittobai Rashika, Alankrita Siddula, Helen J. Burgess, Silvana Pannain, and Kristen L. Knutson. 2020. "Association between Timing of Energy Intake and Insulin Sensitivity: A Cross-Sectional Study" Nutrients 12, no. 2: 503. https://doi.org/10.3390/nu12020503
APA StyleRangaraj, V. R., Siddula, A., Burgess, H. J., Pannain, S., & Knutson, K. L. (2020). Association between Timing of Energy Intake and Insulin Sensitivity: A Cross-Sectional Study. Nutrients, 12(2), 503. https://doi.org/10.3390/nu12020503





