Abstract
A series of 4-methoxybenzoylhydrazones 1–30 was synthesized and the structures of the synthetic derivatives elucidated by spectroscopic methods. The compounds showed a varying degree of antiglycation activity, with IC50 values ranging between 216.52 and 748.71 µM, when compared to a rutin standard (IC50 = 294.46 ± 1.50 µM). Compounds 1 (IC50 = 216.52 ± 4.2 µM), 3 (IC50 = 289.58 ± 2.64 µM), 6 (IC50 = 227.75 ± 0.53 µM), 7 (IC50 = 242.53 ± 6.1) and 11 (IC50 = 287.79 ± 1.59) all showed more activity that the standard, and these compounds have the potential to serve as possible leads for drugs to inhibit protein glycation in diabetic patients. A preliminary SAR study was performed.
1. Introduction
Benzoylhydrazones have many applications in medicinal and analytical chemistry [1,2,3]. Benzoylhydrazones of different heterocyclic compounds were reported to possess antiproliferative [4], anticonvulsant [5], antioxidant [6], cytotoxicity and anti-HIV activities [7,8]. Numerous benzoylhydrazones have shown interesting bioactivities, such as antibacterial, antifungal, antiinflammatory, antimalarial, analgesic, antiplatelet, anticancer, antituberculosis [9,10,11,12,13,14,15,16,17], insecticidal, antiplasmodium, and antimycobacterial effects, as adriamycin immunoconjugates, proteinase inhibitors and activity against the parasite Trypanosoma brucei [18,19,20,21,22]. Their hydrazide derivatives have shown β-glucuronidase inhibition activity [23]. In addition, substituted acylhydrazide Schiff bases are reported to have a wide range of bioactivities, including anticancer [24], antitubercular, and anti-inflammatory activities [25]. Hydrazine derivatives also have several commercial applications [26].
Glycation is a non-enzymatic chemical process in which biomolecules (such as proteins, human DNA, and lipids) are damaged by the attachment of reducing sugars (e.g., glucose), finally leading to the formation of highly reactive so-called advanced glycation end products (AGEs). This process has been associated with deleterious health effects. Protein glycation has been implicated in the development of pathologies associated with diabetes and ageing etc. [27]. Therefore, the discovery of anti-glycation agents is among the most promising approaches for the management of late diabetic complications. At present only a few glycation inhibitors are known and the requirement of novel glycation inhibitors is still unmet [28]. With the epidemic-like spread of type-2 diabetes, the onsets of late diabetic complications, such as cardiopathy, retinopathy, neuropathy, nephropathy, are on rise. This is largely due to the formation of advanced glycation end products (AGEs) [29,30]. Major efforts have recently been focused on the discovery of new, safe and effective glycation inhibitors [31]. Few molecules are reported to cleave cross-links formed by AGEs, and possibly provide the exciting opportunity of reversing the process of late diabetic complications [32]. It has been discovered that aged garlic extract possess excellent antiglycation potential in vitro [33,34]. Aminoguanidine was found to inhibit AGE formation and prevent retinopathy and diabetic vascular complications in diabetic animals, but it showed toxicity problems in phase III clinical trials [35]. Some other molecules (e.g., spermine, spermidine and polyamines) were also reported to have potent anti-glycation potential, similar to those of aminoguanidine and carnosine, but these compounds have to be addressed in future in vivo studies [36]. In the search of new, effective and safe antiglycation agents, we have reported several classes of compounds from natural flora, such as cyclopeptide alkaloids from Ziziphus oxyphylla Edgw, polyphenolic compounds from Parmotrema cooperi, kaempferol-7-β-d-glucopyranoside from Carum petroselinum, flavanones and flavones from Iris tenuifolia and Otostegia persica (Burm.) Boiss, respectively [37,38,39,40]. Along with natural compounds we have also reported different classes of synthetic compounds having antiglycation properties in the recent past, such as acylhydrazide [41], benzophenonehydrazone [42], 2,4,6-trichlorophenylhydrazones [43], oxindole derivatives [44], bis-Schiff bases of isatin [45] and metronidazole esters [46]. The work reported here is in continuation of this same systematic study.
2. Results and Discussion
2.1. Chemistry
4-Methoxybenzoylhydrazones 1–30 were synthesized from 4-methoxybenzoylhydrazide, which were obtained from methyl 4-methoxybenzoate by refluxing with hydrazine hydrate for 2 h. The 4-methoxybenzoylhydrazide obtained was recrystallized from methanol. 4-Methoxy- benzoylhydrazones 1–30 were prepared by refluxing 4-methoxybenzoylhydrazide with different aldehydes in methanol for 3 to 4 h (Scheme 1). The crude products were further recrystallized from methanol and mostly needle-like crystals were obtained in 78%–92% yield. The structures of the 4-methoxybenzoylhydrazones were deduced using various spectroscopic techniques and CHN analyses. The configuration of C=N double bond is E, which can be seen by various crystal structures of similar structures we have published [47,48,49,50,51,52,53,54,55,56].
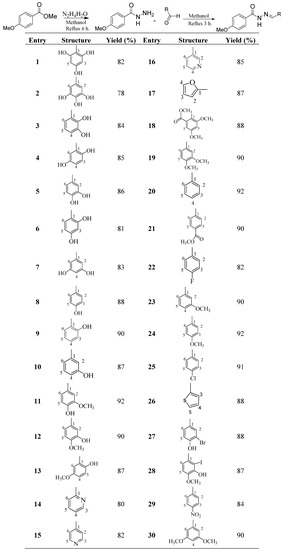
Scheme 1.
Synthesis of 4-Methoxybenzoylhydrazones 1–30.
Scheme 1.
Synthesis of 4-Methoxybenzoylhydrazones 1–30.
2.2. Antiglycation Activity
Structure Activity Relationship
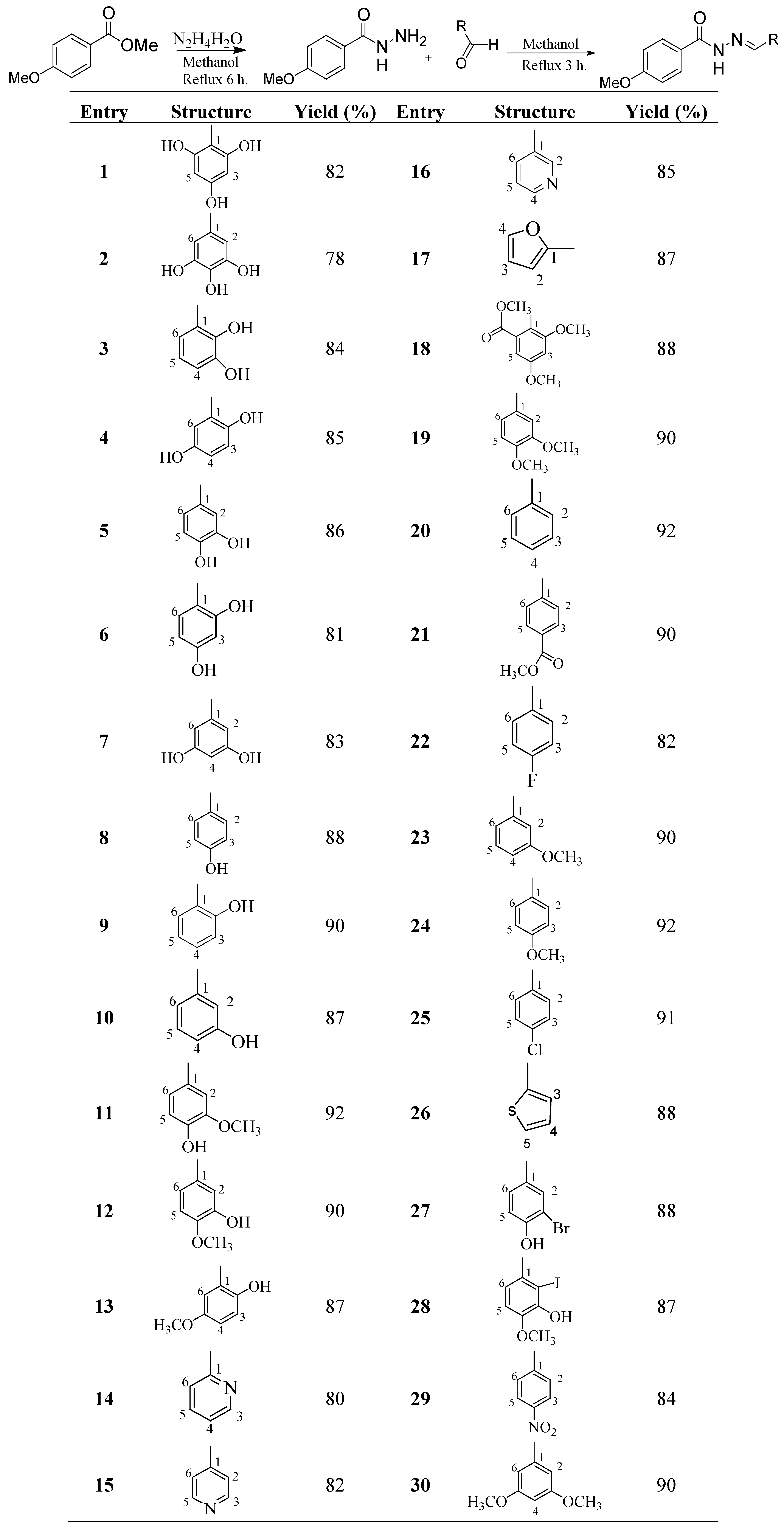
The NH2 groups of aminoguanidine and other nitrogen-containing compounds are well known to form Schiff base adducts with the carbonyl moieties of sugars. This interaction is mainly responsible for inhibiting the formation of advanced glycation end product (AGEs). Additionally, it has been found that compounds with different substituents have varying degree of activity against protein glycation [47,48,49,50,51,52,53,54,55,56]. Based on this, we have prepared a series of 4-methoxybenzoylhydrazones 1–30 and evaluated their antiglycation potential in vitro. For our anti-glycation studies two standards, namely aminoguanidine and rutin, were used. In our protein model system (BSA-MG glycation model), aminoguanidine showed an IC50 value of 1168.24 ± 1.2 µM, while rutin showed an IC50 value of 294.5 ± 1.5 µM. However, as rutin is more active against glycation than aminoguanidine, we therefore decided to use rutin as the standard in this assay. The compounds 1–30 showed potent to moderate antiglycation activities, with IC50 values ranging between 216.52 and 748.71 µM, when compared to the standard compound. Compounds 1, 3, 6, 7, and 11 (IC50 = 216.52 ± 4.2 µM, 289.58 ± 2.64 µM, 227.75 ± 0.53 µM, 242.53 ± 6.1 µM and IC50 = 287.79 ± 1.59 µM, respectively), showed more potent activities than the rutin standard. The compounds 4 (IC50 = 307.1 ± 6.08 µM), 8 (IC50 = 347.62 ± 5.8 µM), 2 (IC50 = 394.76 ± 3.35 µM) and 12 (IC50 = 399.90 ± 7.9 µM) showed good activity. Compounds 5 (IC50 = 420.40 ± 3.3 µM) and 17 (IC50 = 474.97 ± 19.14 µM) showed moderate activities. Compounds 14 (IC50 = 649.18 ± 18.5 µM), 10 (IC50 = 657.75 ± 14.0 µM), 18 (IC50 = 718.96 ± 10.7 µM) and 15 (IC50 = 748.71 ± 7.8 µM) were only weakly active (Table 1).

Table 1.
In vitro protein glycation inhibitory activity of compounds 1–30.
| Compounds | IC50 (µM ± SEM a) | Compounds | IC50 (µM ± SEM a) |
|---|---|---|---|
| 1 | 216.52 ± 4.2 | 16 | NA b |
| 2 | 394.76 ± 3.35 | 17 | 474.97 ± 19.14 |
| 3 | 289.58 ± 2.64 | 18 | 718.96 ± 10.7 |
| 4 | 307.1 ± 6.08 | 19 | NA b |
| 5 | 420.40 ± 3.3 | 20 | NA b |
| 6 | 227.75 ± 0.53 | 21 | NA b |
| 7 | 242.53 ± 6.1 | 22 | NA b |
| 8 | 347.62 ± 5.8 | 23 | NA b |
| 9 | NA b | 24 | NA b |
| 10 | 657.75 ± 14.0 | 25 | NA b |
| 11 | 287.79 ± 1.59 | 26 | NA b |
| 12 | 399.90 ± 7.9 | 27 | NA b |
| 13 | NA b | 28 | NA b |
| 14 | 649.18 ± 18.5 | 29 | NA b |
| 15 | 748.71 ± 7.8 | 30 | NA b |
| Standard Rutin c | 294.5 ± 1.50 | ||
a SEM is the standard error of the mean. b NA Not active. c Rutin: standard inhibitor for antiglycation activity.
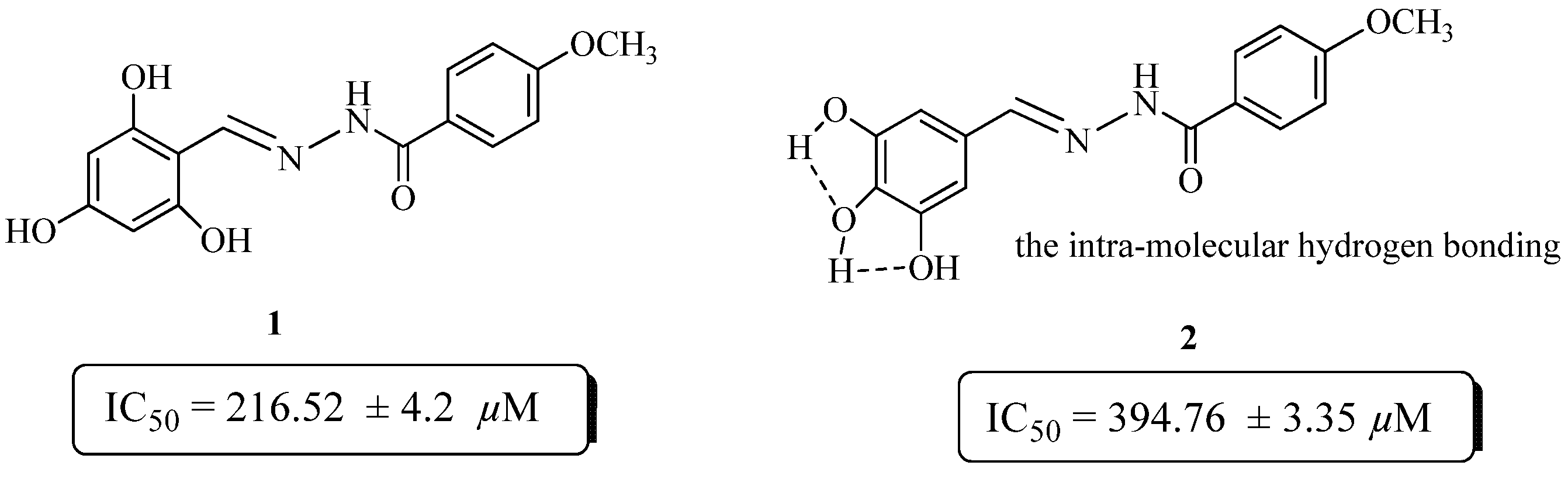
The preliminary structure activity relationship data suggests that the activity mainly depends on the number, as well as the position of hydroxyl substituent’s on the phenyl moiety. Compounds 1 and 2 are both trihydroxy substituted, but compound 1 showed better activity (IC50 = 216.52 ± 4.2 µM) than the standard rutin. The activity of these compounds might be due to their capacity to inhibit glycoxidation. Compound 2 showed very low activity (IC50 = 394.76 ± 3.35 µM), as compared to compound 1. This may be due to the intra-molecular hydrogen bonding in compound 2, which reduce its chances to inhibit glycoxidation as compared to compound 1 (Figure 1).
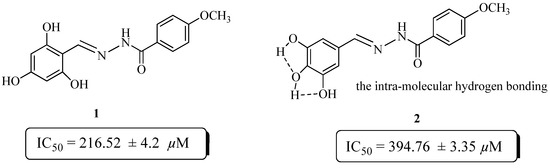
Figure 1.
Comparison of the anti-glycation activity of compounds 1 and 2.
Figure 1.
Comparison of the anti-glycation activity of compounds 1 and 2.
The five compounds having dihydroxy substituents (i.e., 6, 7, 3, 4 and 5) showed excellent to moderate activity, depending upon the position of the hydroxyl groups. Compounds 6 (IC50 = 227.75 ± 0.53 µM), 7 (IC50 = 242.53 ± 6.1 µM) and 3 (IC50 = 289.58 ± 2.64 µM) showed more potent activity than the standard (rutin), whereas compounds 4 (IC50 = 307.1 ± 6.08 µM) showed activity comparable to the standard. Compound 5 showed moderate activity (Table 1). As discussed earlier, the antiglycation activity mainly depends on the position and potential of hydroxy groups to inhibit glycoxidation. In compounds 6, the 2,4-dihydroxy groups, being far apart from each other, have no hydrogen bonding with each other. para-Hydroxy groups easily inhibit glycoxidation and hence a potent anti-glycation activity was observed. In compound 7, both hydroxys are at the meta position and it showed potent anti-glycation activity, with an IC50 value of 242.53 ± 6.1 µM. In compounds 3 and 4, the meta-hydroxy moieties are still free to inhibit glycoxidation, but the activity was decreased with IC50 values of 289.58 ± 2.64 and 307.1 ± 6.08 µM, respectively. In compound 5, the ortho-hydroxyl groups are involved in intramolecular hydrogen bonding therefore a weak activity was observed as compared to its analogs, i.e., compounds 6, 7, 3 and 4 (Table 1).
The monohydroxyl-substituted analogues showed varied activities, mainly depending on the position of the hydroxyl group. Compound 8 (IC50 = 347.62 ± 5.8 µM) is the most active analogue among the monohydroxy derivatives, with a hydroxyl group at the para position. When the hydroxy group is at the meta position, the activity is reduced by half as compared to compound 8, (compound 10; IC50 = 657.75 ± 14.0 µM). Interestingly when the hydroxy is at the ortho position, as in compound 9, the activity was completely lost.
Compounds 11–13 having one hydroxy and one methoxy group showed varied activity, depending upon the position of the hydroxyl substituent. Compound 11 (IC50 = 287.79 ± 1.59 µM) having a para-hydroxy, showed better activity than the standard, whereas its analogue 12 (IC50 = 399.90 ± 7.9 µM) with a meta hydroxy showed a moderate activity against protein glycation. Compound 13 with an ortho hydroxy was found to be inactive.
Compounds 14–16 possess diverse pyridine rings. The most active among the pyridine derivatives was compound 14 (IC50 = 649.18 ± 18.5 µM), with the nitrogen at position-3, near to the hydrazine bridge. The activity decreases sharply when the nitrogen shifts to position-4, as in case of compound 15 (748.71 ± 7.8 µM). Compound 16 with the nitrogen at position-2 was found to be completely inactive (Table 1).
Compounds 17 and 18 showed a weak activity. Compound 17 possess a furfuryl ring and its low activity may be due to the weak interaction of the ring oxygen to inhibit glycoxidation. Furthermore, compound 18 possess an ester moiety, which again interacts weakly with the amino group of the proteins and hence showed a weak activity. Additionally compounds 9, 13 and 18–30 were also found to be inactive.
In conclusion, compounds having hydroxy groups at suitable positions, especially at the para position, can inhibit glycoxidation, and thus exhibit a potent antiglycation activity. However, structural modifications can be optimized to achieve the desired activity in this class of compounds.
3. Experimental
3.1. General Information
NMR experiments were performed on a Bruker Ultra Shield FT NMR 500 MHz (Wissembourg, Switzerland). CHN analysis was performed on a Carlo Erba Strumentazione-Mod-1106 (Milan, Italy). Electron impact mass spectra (EI-MS) were recorded on a Finnigan MAT-311A instrument (Bremen, Germany). Thin layer chromatography (TLC) was performed on pre-coated silica gel aluminum plates (Kieselgel 60, 254, E. Merck, Darmstadt, Germany). Chromatograms were visualized by UV at 254 and 365 nm.
3.2. Experimental Protocol
3.2.1. Synthesis of 4-Methoxybenzohydrazide
Methyl 4-methoxybenzoate (10g) was refluxed with the mixture of hydrazine hydrate (10 mL) and methanol (25 mL) for 6 h. The excess hydrazine and methanol were evaporated to give the crude product which was recrystallized from methanol to yield 92% pure 4-methoxybenzohydrazide.
3.2.2. General Procedure for the Synthesis of 4-Methoxybenzohydrazone Derivatives
The 4-methoxybenzohydrazide derivatives were synthesized by refluxing in methanol a mixture of 2 mmol each of 4-methoxybenzohydrazide with different aldehydes and a catalytic amount of acetic acid for 3 h. After the completion of the reaction, the solvent was evaporated under vacuum to afford the crude products which were further recrystallized from methanol to afford needle-like pure products in most of the cases in good to excellent yields.
N'-(2,4,6-Trihydroxybenzylidiene)-4-methoxybenzohydrazide (1). Solid, M.p.: >250 °C; 1H-NMR (DMSO-d6): δ 11.77 (s, 1H, NH), 11.12 (s, 2H, OH), 9.81 (s, 1H, OH), 8.80 (s, 1H, N=CH-Ar), 7.93 (d, 2H, J2,6/3,5 = 9.0 Hz, H-2, H-6), 7.07 (d, 2H, J3,5/2,6 = 9.0 Hz, H-3, H-5), 5.85 (s, 2H, H-3, H-5), 3.83 (s, 3H, OCH3); Anal. Calcd for C15H14N2O5: C = 59.60, H = 4.67, N = 9.27, O = 26.46, Found C = 59.58, H = 4.65, N = 9.24, O = 26.44; EI MS m/z (% rel. abund.): 302. (M+, 10), 284 (45), 167 (25), 135 (100).
N'-(3,4,5-Trihydroxybenzylidiene)-4-methoxybenzohydrazide (2). Solid, M.p.: >250 °C; 1H-NMR (DMSO-d6): δ 11.46 (s, 1H, NH), 11.32 (s, 2H, OH), 9.61 (s, 1H, OH), 8.16 (s, 1H, N=CH-Ar), 7.89 (d, 2H, J2,6/3,5 = 9.0 Hz, H-2, H-6), 7.07 (d, 2H, J3,5/ 2,6 = 9.0 Hz, H-3, H-5), 6.70 (s, 2H, H-2, H-6), 3.83 (s, 3H, OCH3); Anal. Calcd for C15H14N2O5: C = 59.60, H = 4.67, N = 9.27, O = 26.46, Found C = 59.57, H = 4.64, N = 9.25, O = 26.43; EI MS m/z (% rel. abund.): 302 (M+, 5), 284 (25), 139 (20), 135 (100).
N'-(2,3-Dihydroxybenzylidene)-4-methoxybenzohydrazide (3). Solid, M.p.: 231°C; 1H-NMR (DMSO-d6): δ 12.01 (s, 1H, NH), 11.26 (s, 1H, OH), 9.61 (s, 1H, OH), 8.58 (s, 1H, N=CH-Ar), 7.95 (d, 2H, J2,6/3,5 = 9.0 Hz, H-2 , H-6), 7.10 (d, 2H, J3,5/2,6 = 9.0 Hz, H-3, H-5), 6.96 (dd, 1H, J4,5 = 6.5, J4,6 = 2.0 Hz, H-4), 6.86 (dd, 1H, J6,5 = 6.5, J6,4 = 2.0 Hz, H-6), 6.76 (t, 1H, J5(4,6) = 6.5 Hz, H-5), 3.85 (s, 3H, OCH3); Anal. Calcd for C15H14N2O4: C = 62.93, H = 4.93, N = 9.79, O = 22.35, Found C = 62.91, H = 4.90, N = 9.77, O = 22.32; EI MS m/z (% rel. abund.): 286 (M+, 12), 268 (20), 135 (100), 109 (15).
N'-(2,5-Dihydroxybenzylidene)-4-methoxybenzohydrazide (4). Solid, M.p.: 237 °C; 1H-NMR (DMSO-d6): δ 12.01 (s, 1H, NH), 11.27 (s, 1H, OH), 9.22 (s, 1H, OH), 8.57 (s, 1H, N=CH-Ar), 7.95 (d, 2H, J2,6/ 3,5 = 9.0 Hz, H-2 , H-6), 7.09 (d, 2H, J3,5/2,6 = 9.0 Hz, H-3, H-5), 6.96 (dd, 1H, J4,3 = 8.0, J4,6 = 2.0 Hz, H-3), 6.86 (d, 1H, J6,4 = 2.0 Hz, H-6), 6.75 (d, 1H, J3,4 = 8.0 Hz, H-3), 3.85 (s, 3H, OCH3); Anal. Calcd for C15H14N2O4: C = 62.93, H = 4.93, N = 9.79, O = 22.35, Found C = 62.91, H = 4.90, N = 9.77, O = 22.31; EI MS m/z (% rel. abund.): 286 (M+, 6), 268 (18), 135 (100), 109 (18).
N'-(3,4-Dihydroxybenzylidene)-4-methoxybenzohydrazide (5). Solid, M.p.: 239 °C; 1H-NMR (DMSO-d6): δ 11.47 (s, 1H, NH), 9.41 (s, 2H, OH), δ 8.25 (s, 1H, N=CH-Ar), 7.90 (d, 2H, J2,6/3,5 = 9.0 Hz, H-2, H-6), (s, 1H, H-6), 7.06 (d, 2H, J3,5/2,6 = 9.0 Hz, H-3, H-5), 6.93 (d, 1H, J3,2 = 8.0 Hz, H-4), 6.79 (d, 1H, J2,3 = 8.0 Hz, H-2), 3.84 (s, 3H, OCH3); Anal. Calcd for C15H14N2O4, C = 62.93, H = 4.93, N= 9.79, O = 22.35, Found C = 62.91, H = 4.90, N = 9.77, O = 22.32; EI MS m/z (% rel. abund.): 286 (M+, 17), 268 (22), 135 (100), 109 (9).
N'-(2,4-Dihydroxybenzylidene)-4-methoxybenzohydrazide (6). Solid, M.p.: >250 °C; 1H-NMR (DMSO-d6): δ 11.85 (s, 1H, OH) 11.56 (s, 1H, OH), 9.98 (s, 1H, OH), 8.41 (s, 1H, N=CH-Ar), 7.92 (d, 2H, J2,6/3,5 = 9.0 Hz, H-2, H-6), 7.30(d, 1H, J6,5 = 8.5 Hz, H-6), 7.08 (d, 2H, J3,5/2,6 = 9.0 Hz, H-3, H-5), 6.37 (dd, 1H, J5,6 = 8.5, J5,3 = 2.0 Hz, H-5), 6.32 (d, 1H, J3,5 = 2.0 Hz, H-3), 3.89 (s, 3H, OCH3); Anal. Calcd for C15H14N2O4: C = 62.93, H = 4.93, N = 9.79, O = 22.35, Found C = 62.91, H = 4.90, N = 9.77, O = 22.31; EI MS m/z (% rel. abund.): 286 (M+, 11), 268 (13), 135 (100), 109 (25).
N'-(3,5-Dihydroxybenzylidene)-4-methoxybenzohydrazide (7). Solid, M.p.: >250 °C; 1H-NMR (DMSO-d6): δ 11.60 (s, 1H, OH) 9.49 (s, 2H, OH), 8.23 (s, 1H, N=CH-Ar), 7.92 (d, 2H, J2,6/3,5 = 9.0 Hz, H-2, H-6), 7.06 (d, 2H, J3,5/2,6 = 9.0 Hz, H-3, H-5), 6.60 (s, 2H, H-2, H-6), 6.26 (t, 1H, J4(2,6) = 2.0 Hz, H-4), 3.83 (s, 3H, OCH3); Anal. Calcd for Anal. Calcd for C15H14N2O4: C = 62.93, H = 4.93, N= 9.79, O = 22.35, Found C = 62.91, H = 4.90, N = 9.77, O = 22.31; EI MS m/z (% rel. abund.): 286 (M+, 6), 268 (17), 135 (100), 109 (22).
N'-(4-Hydroxybenzylidene)-4-methoxybenzohydrazide (8). Solid, M.p.: >250 °C; 1H-NMR (DMSO-d6): δ 11.54 (s, 1H, NH), 9.93 (s, 1H, OH), 8.32 (s, 1H, N=CH-Ar), 7.90 (d, 2H, J2,6/3,5 = 9.0 Hz, H-2, H-6), 7.57 (d, 2H, J2,6/3,5 = 8.5 Hz, H-2/H-6), 7.06 (d, 2H, J3,5/2,6 = 9.0 Hz, H-3, H-5) 6.84 (d, 2H J3,5/2,6 = 8.5 Hz, H-3/H-5), 3.83 (s, 3H, OCH3); Anal. Calcd for C15H14N2O3: C = 66.66, H = 5.22, N= 10.36, O = 17.76, Found C = 66.64, H = 5.20, N = 10.33, O = 17.73; EI MS m/z (% rel. abund.): 270 (M+, 30), 268 (15), 135 (100), 93 (45).
N'-(2-Hydroxybenzylidiene)-4-methoxybenzohydrazide (9). Solid, M.p.: 183 °C; 1H-NMR (DMSO-d6): δ 12.02 (s, 1H, NH), 11.40 (s, 1H, OH), 8.62 (s, 1H, N=CH-Ar), 7.95 (d, 2H, J2,6/3,5 = 9.0 Hz, H-2, H-6), 7.53 (d, 1H, J3,4 = 7.5, H-3), 7.32 (t, 1H, J5(4,6) = 8.5 Hz, H-5), 7.09 (d, 2H, J3,5/2,6 = 9.0 Hz, H-3, H-5), 6.95–6.90 (m, 2H, H-4/H-6), 3.84 (s, 3H, OCH3); Anal. Calcd for C15H14N2O3: C = 66.66, H = 5.22, N= 10.36, O = 17.76, Found C = 66.63, H = 5.19, N = 10.32, O = 17.74; EI MS m/z (% rel. abund.): 270 (M+, 70), 268 (14), 135 (100), 93 (15).
N'-(3-Hydroxybenzylidene)-4-methoxybenzohydrazide (10). Solid, M.p.: 219 °C; 1H-NMR (DMSO-d6): δ 11.63 (s, 1H, NH), 9.66 (s, 1H, OH), 8.37 (s, 1H, N=CH-Ar), 7.91 (d, 2H, J2,6/3,5 = 8.5 Hz, H-2, H-6), 7.32 (t, 1H, J5(4,6) = 8.5 Hz, H-5), 7.36 (s, 1H, H-2), 7.62 (d, 1H, J6,5 = 8.0 Hz, H-6), 7.07 (d, 2H, J3,5/2,6 = 8.5 Hz, H-3, H-5), 6.83 (d, 1H, J4,5 = 6.5 Hz, H-4), 3.88 (s, 3H, OCH3); Anal. Calcd for C15H14N2O3: C = 66.66, H = 5.22, N= 10.36, O = 17.76, Found C = 66.63, H = 5.19, N = 10.32, O = 17.74; EI MS m/z (% rel. abund.): 270 (M+, 87), 268 (15), 135 (100), 93 (25).
N'-(4-Hydroxy-3-methoxybenzylidene)-4-methoxybenzohydrazide (11). Solid, M.p.: 181.0 °C; 1H-NMR (DMSO-d6): δ 11.57 (s, 1H, NH), 9.56 (s, 1H, OH), 8.33 (s, 1H, N=CH-Ar), 7.91 (d, 2H, J2,6/3,5 = 8.5 Hz, H-2, H-6), 7.33 (s, 1H, H-2), 7.09 (d, 1H, J6,5 = 8.0 Hz, H-6), 7.06 (d, 2H, J3,5/2,6 = 8.5 Hz, H-3, H-5), 6.83 (d, 1H, J5,6 = 8.0 Hz, H-5), 3.83 (s, 3H, OCH3), 3.68 (s, 3H, OCH3); Anal. Calcd for C16H16N2O4: C = 63.99, H = 5.37, N = 9.33, O = 21.31, Found C = 63.94, H = 5.35, N = 9.31, O = 21.29; EI MS m/z (% rel. abund.): 300 (M+, 90), 135 (100), 122 (25).
N'-(3-Hydroxy-4-methoxybenzylidene)-4-methoxybenzohydrazide (12). Solid, M.p.: 213 °C; 1H-NMR (DMSO-d6): δ 11.56 (s, 1H, NH), 9.33 (s, 1H, OH), 8.29 (s, 1H, N=CH-Ar), 7.90 (d, 2H, J2,6/3,5 = 9.0 Hz, H-2, H-6), 7.27 (s, 1H, H-2), 7.09 (d, 1H, J6,5 = 8.5 Hz, H-6), 7.06 (d, 2H, J3,5/2,6 = 9.0 Hz, H-3, H-5), 6.98 (d, 1H, J5,6 = 8.5 Hz, H-5), 3.84 (s, 3H, OCH3), 3.81 (s, 3H, OCH3); Anal. Calcd for C16H16N2O4: C = 63.99, H = 5.37, N = 9.33, O = 21.31, Found C = 63.94, H = 5.35, N = 9.31, O = 21.29; EI MS m/z (% rel. abund.): 300 (M+, 70), 135 (100), 122 (30).
N'-(2-Hydroxy-5-methoxybenzylidene)-4-methoxybenzohydrazide (13). Solid, M.p.: 202 °C; 1H-NMR (DMSO-d6): 11.99 (s, 1H, NH), 10.77 (s, 1H, OH), δ 8.60 (s, 1H, N=CH-Ar), 7.94 (d, 2H, J2,6/3,5 = 8.5 Hz, H-2, H-6), 7.12 (d, 1H, J3,4 = 8.5 Hz, H-3), 7.09 (d, 2H, J3,5/2,6 = 8.5 Hz, H-3, H-5), 6.95 (dd, 1H, J4,3 = 8.5, J4,6 = 2.0 Hz, H-4), 6.88 (d, 1H, J6,4 = 2.0 Hz, H-6), 3.84 (s, 3H, OCH3), 3.74 (s, 3H, OCH3); Anal. Calcd for C16H16N2O4: C = 63.99, H = 5.37, N = 9.33, O = 21.31, Found C = 63.97, H = 5.34, N = 9.30, O = 21.28; EI MS m/z (% rel. abund.): 300 (M+, 90), 135 (100), 122 (21).
4-Methoxy-N-((pyridine-2-methylene)benzohydrazide (14). Solid, M.p.: 107 °C; 1H-NMR (DMSO-d6): δ 11.96 (s, 1H, NH), 8.62 (d, 1H, J6,5 = 5.0Hz, H-6), 8.46 (s, 1H, N=CH-Ar), 7.99 (d, 1H, J3,4 = 8.0 Hz, H-3), 7.94 (d, 2H, J2,6/3,5 = 8.5 Hz, H-2, H-6), 7.90 (m, 1H, H-4), 7.60 (t, 1H, J5(4,6) = 8.0 Hz, H-6), 7.09 (d, 2H, J3,5/2,6 = 8.5 Hz, H-3, H-5), 3.87 (s, 3H, OCH3); Anal. Calcd for C14H13N3O2: C = 65.87, H = 5.13, N= 16.46, O = 12.54, Found C = 65.84, H = 5.09, N = 16.44, O = 12.52; EI MS m/z (% rel. abund.): 255 (M+, 88), 135 (100), 78 (21).
4-Methoxy-N'-(pyridin-4-methylene)benzohydrazide (15). Solid, M.p.: 180 °C; 1H-NMR (DMSO-d6): δ 12.04 (s, 1H, NH), 8.65 (d, 2H, J2,6/3,5 = 6.0 Hz, H-2, H-6), 8.42 (s, 1H, N=CH-Ar), 7.93 (d, 2H, J2,6/3,5 = 8.5 Hz, H-2, H-6), 7.67 (d, 2H, J3,5/2,6 = 6.0 Hz, H-3, H-5), 7.09 (d, 2H, J3,5/2,6 = 8.5 Hz, H-3, H-5), 3.84 (s, 3H, OCH3); Anal. Calcd for C14H13N3O2: C = 65.87, H = 5.13, N= 16.46, O = 12.54, Found C = 65.83, H = 5.11, N = 16.43, O = 12.52; EI MS m/z (% rel. abund.): 255 (M+, 80), 135 (100), 78 (27).
4-Methoxy-N'-(pyridin-3-methylene)benzohydrazide (16). Solid, M.p.: 222 °C; 1H-NMR (DMSO-d6): δ 11.92 (s, 1H, NH), 11.92 (s, 1H, H-6), 8.61 (d, 1H, J2,4 = 2.0 Hz, H-2), 8.36 (s, 1H, N=CH-Ar), 8.16 (d, 1H, J4,5 = 8.0 Hz, H-4), 7.93 (d, 2H, J2,6/3,5 = 8.5 Hz, H-2, H-6), 7.51 (dd, 1H, J5,4 = 8.0 J5,6 = 5.0 Hz, H-5), 7.08 (d, 2H, J3,5/2,6 = 8.5 Hz, H-3, H-5), 3.88 (s, 3H, OCH3); Anal. Calcd for C14H13N3O2: C = 65.87, H = 5.13, N= 16.46, O = 12.54, Found C = 65.82, H = 5.10, N = 16.42, O = 12.52; EI MS m/z (% rel. abund.): 255 (M+,92), 135 (100), 78 (33).
N'-((Furan-2-yl)methylene)-4-methoxybenzohydrazide (17). Solid, M.p.: 207 °C; 1H-NMR (DMSO-d6): δ 11.69 (s, 1H, NH), 8.32 (s, 1H, N=CH-Ar), 7.89 (d, 2H, J2,6/3,5 = 9.0 Hz, H-2, H-6), 7.84 (s, 1H, H-3), 7.07 (d, 2H, J3,5/2,6 = 9.0 Hz, H-3, H-5), 6.91 (s, 1H, H-3), 6.64 (dd, 1H, J3,4 = 5.0 Hz, J3,5 = 2.0 Hz, H-3), 3.83 (s, 3H, OCH3); Anal. Calcd for C13H12N2O3: C = 63.93, H = 4.95, N= 11.47, O = 19.65, Found C = 63.94, H = 4.97, N = 11.46, O = 19.64; EI MS m/z (% rel. abund.): 244 (M+, 94), 135 (100), 68 (23).
Methyl 2-(4-methoxybenzoylimino)methyl)-3,5-dimethoxybenzoate (18). Solid, M.p.: 162 °C; 1H-NMR (DMSO-d6): δ 11.64 (s, 1H, NH), 8.64 (s, 1H, N=CH-Ar), 7.92 (d, 2H, J2,6/3,5 = 8.5 Hz, H-2, H-6), 7.06 (d, 2H, J3,5/2,6 = 8.5 Hz, H-3, H-5), 6.76 (d, 1H, J4,6 = 2.0 Hz, H-4), 6.61 (d, 1H, J6,4 = 2.0 Hz, H-6), 3.89 (s, 3H, OCH3), 3.85 (s, 3H, OCH3), 3.84 (s, 3H, OCH3); Anal. Calcd for C19H20N2O6: C = 61.28, H = 5.41, N= 7.52, O = 25.78, Found C = 61.26, H = 5.43, N = 7.51, O = 25.79; EI MS m/z (% rel. abund.): 372 (M+, 25), 195 (40), 135 (100).
N'-(3,4-Dimethoxybenzylidene)-4-methoxybenzohydrazide (19). Solid, M.p.: 179 °C; 1H-NMR (DMSO-d6): δ 11.63 (s, 1H, NH), 8.37 (s, 1H, N=CH-Ar), 7.91 (d, 2H, J2,6/3,5 = 8.5 Hz, H-2, H-6), 7.36 (s, 1H, H-2), (d, 1H, J6,5 = 8.5 Hz, H-6), 7.07 (d, 2H, J3,5/2,6 = 8.5 Hz, H-3, H-5), 6.76 (d, 1H, J5,6 = 8.5 Hz, H-4), 3.84 (s, 3H, OCH3), 3.82 (s, 3H, OCH3), 3.81 (s, 3H, OCH3); Anal. Calcd for C17H18N2O4: C = 64.96, H = 5.77, N= 8.91, O = 20.36, Found C = 64.94, H = 5.74, N = 8.88, O = 20.35; EI MS m/z (% rel. abund.): 314 (M+, 90), 137 (40), 135 (100).
N'-Benzylidene-4-methoxybenzohydrazide (20). Solid, M.p.: 202 °C; 1H-NMR (DMSO-d6): δ 11.74 (s, 1H, NH), 8.44 (s, 1H, N=CH-Ar), 7.92 (d, 2H, J2,6/3,5 = 8.5 Hz, H-2, H-6), (d, 2H, J3,5/2,6 = 6.5 Hz, H-5 H-6), 7.48–7.44 (m, 3H, H-3, H-4 ,H-5), 7.08 (d, 2H, J3,5/2,6 = 8.5 Hz, H-3, H-5), 3.84 (s, 3H, OCH3); Anal. Calcd for C15H14N2O2: C = 70.85, H = 5.54, N= 11.02, O = 12.58, Found C = 70.86, H = 5.55, N = 11.01, O = 12.57; EI MS m/z (% rel. abund.): 254 (M+, 70), 135 (100), 77 (30).
Methyl 4-((4-methoxybenzoylimino)methyl)benzoate (21). Solid, M.p.: 206 °C; 1H-NMR (DMSO-d6): δ 11.92 (s, 1H, NH), 8.50 (s, 1H, N=CH-Ar), 8.04 (d, 2H, J2,6/3,5 = 8.0 Hz, H-2/H-6), 7.94 (d, 2H, J2,6/3,5 = 8.5 Hz, H-2, H-6), 7.87 (d, 2H, J3,5/2,6 = 8.0 Hz, H-3/H-5), 7.09 (d, 2H, J3,5/2,6 = 8.5 Hz, H-3, H-5), 3.88 (s, 3H, OCH3), 3.84 (s, 3H, OCH3); Anal. Calcd for C17H16N2O4: C = 65.38, H = 5.16, N= 8.97, O = 20.49, Found C = 65.36, H = 5.15, N = 8.94, O = 20.47; EI MS m/z (% rel. abund.): 312 (M+, 44), 135 (100), 76 (30).
N'-(4-Fluorobenzylidene)-4-methoxybenzohydrazide (22). Solid, M.p.: 186 °C; 1H-NMR (DMSO-d6): δ 11.76 (s, 1H, NH), 8.44 (s, 1H, N=CH-Ar), 7.92 (d, 2H, J2,6/3,5 = 8.5 Hz, H-2, H-6), 7.80 (t, 2H, J2,6/2,6,F = 7.0 Hz, H-2/H-6), 7.32 (t, 2H, J3,5/2,6,F = 7.0 Hz, H-2/H-6), 7.07 (d, 2H, J3,5/2,6 = 8.5 Hz, H-3, H-5), 3.84 (s, 3H, OCH3); Anal. Calcd for C15H13FN2O2: C = 66.17, H = 4.81, F = 6.98, N = 10.29, O = 11.75, Found C = 66.13, H = 4.79, F = 6.95, N = 10.27, O = 11.73; EI MS m/z (% rel. abund.): 272 (M+, 78), 135 (100), 95 (30).
N'-(3-Methoxybenzylidene)-4-methoxybenzohydrazide (23). Solid, M.p.: 121.6 °C; 1H-NMR (DMSO-d6): δ 11.74 (s, 1H, NH), 8.41 (s, 1H, N=CH-Ar), 7.92 (d, 2H, J2,6/3,5 = 9.0 Hz, H-2, H-6), 7.40 (t, 1H, J5(4,6) = 7.5 Hz, H-5), 7.30–725 (m, 1H, H-4), 7.07 (d, 2H, J3,5/2,6 = 9.0 Hz, H-3, H-5), 7.81 (dd, 1H, J6,5 = 7.5 Hz, J6,4 = 2.0 Hz, H-6), 3.84 (s, 3H, OCH3), 3.81 (s, 3H, OCH3); Anal. Calcd for C16H16N2O3: C = 67.59, H = 5.67, N= 9.85, O = 16.88, Found C = 67.57, H = 5.64, N = 9.82, O = 16.85; EI MS m/z (% rel. abund.): 284 (M+, 55), 135 (100), 107 (30).
N'-(4-Methoxybenzylidene)-4-methoxybenzohydrazide (24). Solid, M.p.: 174 °C; 1H-NMR (DMSO-d6): δ 11.62 (s, 1H, NH), 8.37 (s, 1H, N=CH-Ar), 7.91(d, 2H, J2,6/3,5 = 8.5 Hz, H-2, H-6), 7.68 (d, 2H, J2,6/3,5 = 8.0 Hz, H-2, H-6), 7.07 (d, 2H, J3,5/2,6 = 9.0 Hz, H-3, H-5), 7.03(d, 2H, J3,5/2,6 = 8.0 Hz, H-3, H-5), 3.84 (s, 3H, OCH3), 3.81 (s, 3H, OCH3); Anal. Calcd for C16H16N2O3: C = 67.59, H = 5.67, N= 9.85, O = 16.88, Found C = 67.57, H = 5.64, N = 9.82, O = 16.85; EI MS m/z (% rel. abund.): 284 (M+, 85), 135 (100), 95 (40).
N'-(4-Chlorobenzylidene)-4-methoxybenzohydrazide (25). Solid, M.p.: 198 °C; 1H-NMR (DMSO-d6): δ 11.80 (s, 1H, NH), 8.37 (s, 1H, N=CH-Ar), 7.912 (d, 2H, J2,6/3,5 = 9.0 Hz, H-2, H-6), 7.76 (d, 2H, J2,6/3,5 = 8.5 Hz, H-2, H-6), 7.54 (d, 2H, J3,5/2,6 = 8.5 Hz, H-3, H-5), 7.07 (d, 2H, J3,5/ 2,6 = 9.0 Hz, H-3, H-5), 3.84 (s, 3H, OCH3), 3.85 (s, 3H, OCH3); Anal. Calcd for C15H13ClN2O2: C = 62.40, H = 4.54, N= 9.70, O = 11.08, Found C = 62.41, H = 4.53, N = 9.71, O = 11.06; EI MS m/z (% rel. abund.): 290 (M++2, 100), 288 (M+, 32), 135 (100), 113 (15), 111 (50).
4-Methoxy-N'-(thiophen-2-methylene)benzohydrazide (26). Solid, M.p.: 209 °C; 1H-NMR (DMSO-d6): δ 11.67 (s, 1H, NH), 8.65 (s, 1H, N=CH-Ar), 7.90 (d, 2H, J2,6/3,5 = 9.0 Hz, H-2, H-6), 7.65 (d, 1H, J3,4 = 5.0 Hz, H-3), 7.45 (d, 1H, J5,4 = 3.0 Hz, H-5), 7.15 (d, 1H, J4,5 = 5.0, J4,3 = 3.0 Hz, H-4), 7.06 (d, 2H, J3,5/2,6 = 9.0 Hz, H-3, H-5), 3.84 (s, 3H, OCH3); Anal. Calcd for C13H12N2O2S: C = 59.98, H = 4.65, N= 10.76, O = 12.29, S = 12.32, Found C = 59.96, H = 4.63, N = 10.74, O = 12.27, S = 12.30; EI MS m/z (% rel. abund.): 260 (M+, 65), 135 (100), 83 (28).
N'-(3-Bromo-4-hydroxybenzylidiene)-2-methoxybenzohydrazide (27). Solid, M.p.: 209 °C; 1H-NMR (DMSO-d6): δ 11.67 (s, 1H, NH), 10.83 (s, 1H, OH), 8.30 (s, 1H, N=CH-Ar), 7.91 (d, 2H, J2,6/3,5 = 8.5 Hz, H-2, H-6), 7.86 (s, 1H, H-2), 7.56 (d, 1H, J6,5 = 8.0 Hz, H-6), 7.06 (d, 2H, J3,5/2,6 = 9.0 Hz, H-3, H-5), 7.03 (d, 1H, J5,6 = 8.0 Hz, H-5), 3.84 (s, 3H, OCH3); Anal. Calcd for C15H13BrN2O3: C = 51.60, H = 3.75, Br = 22.88, N= 8.02, O = 13.75, Found C = 51.57, H = 3.73, Br = 22.85, N = 7.99, O = 13.73; EI MS m/z (% rel. abund.): 350 (M+2, 56), 348 (M+, 57), 172 (26), 170 (25), 135 (100), 92 (20).
N'-(3-Hydroxy-2-iodo-4-methoxybenzylidene)-4-methoxybenzohydrazide (28). Solid, M.p. = 147 °C; 1H-NMR (DMSO-d6): δ 11.65 (s, 1H, NH), 9.72 (s, 1H, OH), 8.68 (s, 1H, N=CH-Ar), 7.93 (d, 2H, J2,6/3,5 = 8.5 Hz, H-2, H-6), 7.50 (d, 1H, J6,5 = 8.0 Hz, H-6), 7.09 (d, 1H, J5,6 = 8.0 Hz, H-5), 7.06 (d, 2H, J3,5/2,6 = 8.5 Hz, H-3, H-5), 3.87 (s, 3H, OCH3); Anal. Calcd for C16H15IN2O4: C = 45.09, H = 3.55, I = 29.78, N= 6.57, O = 15.02, Found C = 45.07, H = 3.53, I = 29.77, N = 6.55, O = 14.99; EI MS m/z (% rel. abund.): 426 (M+, 15), 299 (36), 248 (20), 135 (100).
N'-(3,5-Dimethoxybenzylidene)-2-methoxybenzohydrazide (29). Solid, M.p. = 184 °C; 1H-NMR (DMSO-d6): δ 11.78 (s, 1H, NH), 8.36 (s, 1H, N=CH-Ar), 9.92 (d, 2H, J2,6/3,5 = 9.0 Hz, H-2, H-6), 7.08 (d, 2H, J3,5/2,6 = 9.0 Hz, H-3, H-5), 6.89 (s, 2H, H-2, H-6), 6.57 (s, 1H, H-4), 3.84 (s, 3H,OCH3), 3.79 (s, 6H, OCH3); Anal. Calcd for C17H18N2O4: C = 64.96, H = 5.77, N = 8.91, O = 20.36, Found C = 64.95, H = 5.77, N = 8.88, O = 20.33; EI MS m/z (% rel. abund.): 314 (M+, 81), 137 (36), 135 (100).
N'-(4-Nitrobenzylidiene)-4-methoxybenzohydrazide (30). Solid, M.p. = 240 °C; 1H-NMR (DMSO-d6): δ 12.08 (s, 1H, NH), 8.53 (s, 1H, N=CH-Ar), 8.31 (d, 2H, J2,6/3,5 = 8.0 Hz, H-2, H-6), 8.00 (d, 2H, J3,5/2,6 = 8.0 Hz, H-3, H-5), 7.94 (d, 2H, J2,6/3,5 = 8.5 Hz, H-2, H-6), 7.09 (d, 2H, J3,5/ 2,6 = 8.5 Hz, H-3, H-5), 3.85 (s, 3H, OCH3); Anal. Calcd for C15H13N3O4: C = 60.20, H = 4.38, N= 14.04, O = 21.38, Found C = 60.17, H = 4.35, N = 14.02, O = 21.37; EI MS m/z (% rel. abund.): 301 (M+, 94), 135 (100). 122 (35), 76 (20).
3.2.3. Protocol for Antiglycation Activity
Bovine Serum Albumin (BSA) was purchased from Merck Marker Pvt. Ltd. (Darmstadt, Germany), rutin and methylglyoxal (MG) (40% aqueous solution) were from Sigma Aldrich (Tokyo, Japan), sodium dihydrogen phosphate (NaH2PO4), disodium hydrogen phosphate (Na2HPO4) and sodium azide (NaN3) were purchased from Scharlau Chemie, S. A. (Barcelona, Spain), while dimethyl sulphoxide (DMSO) was purchased from Fischer Scientific (Loughborough, UK). Bovine Serum Albumin (10 mg/mL), methyl glyoxal (14 mM), various concentrations of the compounds (prepared in DMSO, 10% final concentration), and 0.1 M phosphate buffer (pH 7.4) containing sodium azide (30 mM) was incubated under aseptic conditions at 37 °C for 9 days. After 9 days, each sample was examined for the development of specific fluorescence (excitation, 330 nm; emission, 440 nm) against sample blank [39,57]. Rutin was used as a positive control. All of the experiments were done in a 96-well microplate reader (SpectraMax M2, Molecular Devices, Sunnyvale, CA, USA). The percent inhibition of AGE formation in the test sample versus control was calculated for each inhibitor compound by using the following formula:
% inhibition= (1 − fluorescence of test sample/Fluorescence of the control group) × 100
3.3. Software/Statistical
The obtained results were analysed by SoftMaxPro 4.8 and MS-Excel. Results are presented as means ± SEM from three experiments. IC50 Values were determined by using EZ-FIT, Enzyme kinetics software by Perrella Scientific, Inc., Hillsborough, NH, USA.
4. Conclusions
In conclusion, compounds having hydroxy groups showed good antiglycation activity due to their capacity to inhibit glycoxidation. However, structural modifications can be optimized to achieve the desired activity in this class of compounds.
Supplementary Materials
Supplementary materials can be accessed at: http://www.mdpi.com/1420-3049/19/1/1302/s1.
Acknowledgments
The authors would like to acknowledge Universiti Teknologi MARA for the financial support under the Research Intensive Faculty grant scheme with reference number UiTM 600-RMI/DANA 5/3/RIF (347/2012).
Conflict of Interest
The authors declare no conflict of interest.
References
- Cimerman, Z.; Miljanić, S.; Galic, N. Schiff Bases derived from aminopyridines as spectrofluorimetric analytical reagents. Croat. Chem. Acta 2000, 73, 81–95. [Google Scholar]
- Musharraf, S.G.; Bibi, A.; Shahid, N.; Najam-ul-Haq, M.; Khan, M.; Taha, M.; Mughal, U.R.; Khan, K. Acylhydrazide and isatin Schiff bases as alternate UV-laser desorption ionization (LDI) matrices for low molecular weight (LMW) peptides analysis. Am. J. Anal. Chem. 2012, 3, 779–789. [Google Scholar] [CrossRef]
- Tarafder, M.T.; Kasbollah, A.; Saravan, N.; Crouse, K.A.; Ali, A.M.; Tin, O.K. S-methyldithiocarbazate and its Schiff bases: Evaluation of bondings and biological properties. J. Biochem. Mol. Biol. Biophys. 2002, 6, 85–91. [Google Scholar] [CrossRef]
- Kabak, M.; Elmali, A.; Elerman, Y. Keto-enol tautomerism, conformations and structure of N-(2-hydroxy-5-methylphenyl), 2-hydroxybenzaldehyde-imine. J. Mol. Struct. 1999, 477, 151–158. [Google Scholar] [CrossRef]
- Küçükgüzel, I.; Küçükgüzel, Ş.G.; Rollas, S.; Otuk-Saniş, G.; Özdemir, O.; Bayrak, İ.; Altuğ, T.; Stables, J.P. Synthesis of some 3-(arylalkylthio)-4-alkyl/aryl-5-(4-aminophenyl)-4H-1,2,4-triazole derivatives and their anticonvulsant activity. Il Farmaco 2004, 59, 839–891. [Google Scholar] [CrossRef]
- Taha, M.; Ismail, N.H.; Jamil, W.; Yousuf, S.; Jaafar, F.M.; Ali, M.I.; Kashif, S.M.; Hussain, E. Synthesis, evaluation of antioxidant activity and crystal structure of 2,4-dimethylbenzoylhydrazones. Molecules 2013, 18, 10912–10929. [Google Scholar] [CrossRef]
- Pandeya, S.N.; Sriram, D.; Nath, G.; de Clercq, E. Synthesis, antibacterial, antifungal and anti-HIV evaluation of Schiff and Mannich bases of isatin derivatives with 3-amino-2-methylmercapto quinazolin-4(3H)-one. Pharm. Acta Helv. 1999, 74, 11–17. [Google Scholar] [CrossRef]
- Pandeya, S.N.; Sriram, D.; Nath, G.; DeClercq, E. Synthesis, antibacterial, antifungal and anti-HIV activities of Schiff and Mannich bases derived from isatin derivatives and N-[4-(4'-chlorophenyl)thiazol-2-yl] thiosemicarbazide. Eur. J. Pharm. Sci. 1999, 9, 25–31. [Google Scholar] [CrossRef]
- Loncle, C.; Brunel, J.M.; Vidal, N.; Dherbomez, M.; Letourneux, Y. Synthesis and antifungal activity of cholesterol-hydrazone derivatives. Eur. J. Med. Chem. 2004, 39, 1067–1071. [Google Scholar] [CrossRef]
- Küçükgüzel, S.G.; Mazi, A.; Sahin, F.; Öztürk, S.; Stables, J. Synthesis and biological activities of diflunisal hydrazide-hydrazones. Eur. J. Med. Chem. 2003, 38, 1005–1013. [Google Scholar] [CrossRef]
- Todeschini, A.R.; de Miranda, A.L.; Silva, C.M.; Parrini, S.C.; Barreiro, E.J. Synthesis and evaluation of analgesic, anti-inflammatory and antiplatelet properties of new 2-pyridylarylhydrazone derivatives. Eur. J. Med. Chem. 1998, 33, 189–199. [Google Scholar] [CrossRef]
- Melnyk, P.; Leroux, V.; Sergheraert, C.; Grellier, P. Design, synthesis and in vitro antimalarial activity of an acylhydrazone library. Bioorg. Med. Chem. Lett. 2006, 16, 31–35. [Google Scholar] [CrossRef]
- Leite, L.F.C.C.; Ramos, M.N.; Da Silva, J.B.P.; Miranda, A.L.P.; Fraga, C.A.M.; Barreiro, E.J. Synthesis and analgesic profile of novel N-containing heterocycle derivatives: Arylidene 3-phenyl-1,2,4-oxadiazole-5-carbohydrazide. Il Farmaco 1999, 54, 747–757. [Google Scholar] [CrossRef]
- Lima, P.C.; Lima, L.M.; Da Silva, K.C.; Léda, P.H.; de Miranda, A.L.; Fraga, C.A.; Barreiro, E.J. Synthesis and analgesic activity of novel N-acylarylhydrazones and isosters, derived from natural safrole. Eur. J. Med. Chem. 2000, 35, 187–203. [Google Scholar] [CrossRef]
- Cunha, A.C.; Figueiredo, J.M.; Tributino, J.L.M.; Miranda, A.L.P.; Castro, H.C.; Zingali, R.B.; Fraga, C.A.M.; de Souza, M.C.B.V.; Ferreira, V.F.; Barreiro, E.J. Antiplatelet properties of novel N-substituted-phenyl-1,2,3-triazole-4-acylhydrazone derivatives. Bioorg. Med. Chem. 2003, 11, 2051–2059. [Google Scholar] [CrossRef]
- Kaymakçıoğlu, K.B.; Oruç, E.E.; Unsalan, S.; Kandemirli, F.; Shvets, N.; Rollas, S.; Anatholy, D. Synthesis and characterization of novel hydrazide-hydrazones and the study of their structure antituberculosis activity. Eur. J. Med. Chem. 2006, 41, 1253–1261. [Google Scholar] [CrossRef]
- Terzioglu, N.; Gürsoy, A. Synthesis and anticancer evaluation of some new hydrazone derivatives of 2,6-dimethylimidazo[2,1-b]-[1,3,4]thiadiazole-5-carbohydrazide. Eur. J. Med. Chem. 2003, 38, 781–786. [Google Scholar] [CrossRef]
- Sawada, Y.; Yanai, T.; Nakagawa, H.; Tsukamoto, Y.; Tamagawa, Y.; Yokoi, S.; Yanagi, M.; Toya, T.; Sugizaki, H.; Kato, Y.; et al. Synthesis and insecticidal activity of benzoheterocyclic analogues of N'-benzoyl-N-(tert-butyl)benzohydrazide: Part 1. Design of benzoheterocyclic analogues. Pest Manag. Sci. 2003, 59, 25–35. [Google Scholar] [CrossRef]
- Taha, M.; Baharudin, M.S.; Ismail, N.H.; Khan, K.M.; Jaafar, F.M.; Samreen; Siddiqui, S.; Choudhary, M.I. Synthesis of 2-methoxybenzoylhydrazone and evaluation of their antileishmanial activity. Bioorg. Med. Chem. Lett. 2013, 23, 3463–3466. [Google Scholar] [CrossRef]
- Küçükgüzel, S.G.; Rollas, S. Synthesis, characterization of novel coupling products and 4-arylhydrazono-2-pyrazoline-5-ones as potential antimycobacterial agents. Il Farmaco 2002, 57, 583–587. [Google Scholar] [CrossRef]
- Greenfield, R.S.; Kaneko, T.; Daues, A.; Edson, M.A.; Fitzgerald, K.A.; Olech, L.J.; Grattan, J.A.; Spitalny, G.L.; Braslawsky, G.R. Evaluation in vitro of adriamycin immunoconjugates synthesized using an acid-sensitive hydrazone linker. Cancer Res. 1990, 50, 6600–6007. [Google Scholar]
- Caffery, C.R.; Schanz, M.; Nkemgu-Njinkeng, J.; Brush, M.; Hansell, E.; Cohen, F.E.; Flaherty, T.M.; Mckerrow, J.H.; Steverding, D. Screening of acyl hydrazide proteinase inhibitors for antiparasitic activity against Trypanosoma brucei. Int. J. Anitimicrob. Agents 2002, 19, 227–231. [Google Scholar] [CrossRef]
- Khan, K.M.; Shujaat, S.; Rahat, S.; Hayat, S.; Atta-ur-Rahman; Choudhary, M.I. β-N-Cyanoethyl acyl hydrazide derivatives: A new class of β-glucuronidase inhibitors. Chem. Pharm. Bull. Jpn. 2002, 50, 1443–1446. [Google Scholar] [CrossRef]
- Mansour, A.K.; Eid, M.M.; Khalil, N.S.A.M. Synthesis and reactions of some new heterocyclic carbohydrazides and related compounds as potential anticancer agents. Molecules 2003, 8, 744–755. [Google Scholar] [CrossRef]
- Patole, J.; Sandbhor, U.; Padhye, S.; Deobagkar, D.N.; Anson, C.E.; Powell, A. Structural chemistry and in vitro antitubercular activity of acetylpyridine benzoyl hydrazone and its copper complex against Mycobacterium smegmatis. Bioorg. Med. Chem. Lett. 2003, 13, 51–55. [Google Scholar] [CrossRef]
- Ragnarsson, U. Synthetic methodology for alkyl substituted hydrazines. Chem. Soc. Rev. 2001, 30, 205–213. [Google Scholar] [CrossRef]
- Reddy, V.P.; Beyaz, A. Inhibitors of the Maillard reaction and AGE breakers as therapeutics for multiple diseases. Drug Discov. Today 2006, 11, 646–654. [Google Scholar] [CrossRef]
- Ahmed, N. Advanced glycation end products-role in pathology of diabetic complications. Diabetes Res. Clin. Pract. 2005, 67, 3–21. [Google Scholar] [CrossRef]
- Brownlee, M. Glycation and complications Diabetes. Diabetes 1994, 43, 836–841. [Google Scholar] [CrossRef]
- Peppa, M.; Uribarri, J.; Vlassara, H. Glucose, advanced glycation end products, and diabetes complications: What is new and what works. Clin. Diabetes 2003, 21, 186–187. [Google Scholar] [CrossRef]
- Monnier, V.M. Intervention against the Maillard reaction in vivo. Arch. Biochem. Biophys. 2003, 419, 1–15. [Google Scholar] [CrossRef]
- Vasan, S.; Foiles, P.; Founds, H. Therapeutic potential of breakers of advanced glycation end product–protein crosslinks. Arch. Biochem. Biophys. 2003, 419, 89–96. [Google Scholar] [CrossRef]
- Hunt, J.V.; Bottoms, M.A.; Mitchinson, M.J. Oxidative alterations in the experimental glycation model of diabetes mellitus are due to protein-glucose adduct oxidation. Some fundamental differences in proposed mechanisms of glucose oxidation and oxidant production. Biochem. J. 1993, 291, 529–535. [Google Scholar]
- Ahmed, M.S.; Ahmed, N. Antiglycation properties of aged garlic extract: Possible role in prevention of diabetic complications. Am. Soc. Nutr. 2006, 136, 796–799. [Google Scholar]
- Gugliucci, A. Glycation as the glucose link to diabetic complications. J. Am. Osteopath. Assoc. 2000, 100, 621–634. [Google Scholar]
- Gugliucci, A.; Menini, T. The polyamines spermine and spermidine protect proteins from structural and functional damage by AGE precursors: A new role for old molecules. Life Sci. 2003, 72, 2603–2616. [Google Scholar] [CrossRef]
- Choudhary, M.I.; Adhikari, A.; Rasheed, S.; Marasini, B.P.; Hussain, N.; Kaleem, W.A.; Atta-ur-Rahman. Cyclopeptide alkaloids of Ziziphus oxyphylla Edgw as novel inhibitors of α-glucosidase enzyme and protein glycation. Phytochem. Lett. 2011, 4, 404–406. [Google Scholar] [CrossRef]
- Choudhary, M.I.; Ali, M.; Wahab, A.; Khan, A.; Rasheed, S.; Shyaula, S.L.; Rahman, A.U. New antiglycation and enzyme inhibitors from Parmotrema cooperi. Sci. China Chem. 2011, 54, 1926–1931. [Google Scholar] [CrossRef]
- Atta-ur-Rahman; Choudhary, M.I.; Basha, F.Z.; Abbas, G.; Khan, S.N.; Shah, S.A.A. Science at the interface of chemistry and biology: Discoveries of α-glucosidase inhibitors and antiglycation agents. Pure Appl. Chem. 2007, 79, 2263–2268. [Google Scholar] [CrossRef]
- Ayatollahi, S.A.M.; Kobarfard, F.; Asgarpanah, J.; Choudhary, M.I. Antiglycation activity of Otostegia persica (Burm) Boiss. Afr. J. Biotechnol. 2010, 9, 3645–3648. [Google Scholar]
- Khan, K.M.; Taha, M.; Rahim, F.; Fakhri, M.I.; Jamil, W.; Khan, M.; Rasheed, S.; Karim, A.; Perveen, S.; Choudhary, M.I. Acylhydrazide schiff bases: Synthesis and antiglycation activity. J. Pak. Chem. Soc. 2013, 35, 929–937. [Google Scholar]
- Khan, K.M.; Rahim, F.; Ambreen, N.; Taha, M.; Khan, M.; Jahan, H.; Najeebullah, U.; Shaikh, A.; Iqbal, S.; Perveen, S.; et al. Synthesis of benzophenonehydrazone Schiff bases and their in vitro antiglycating activities. Med. Chem. 2013, 9, 588–595. [Google Scholar] [CrossRef]
- Khan, K.M.; Shah, Z.; Ahmad, V.U.; Khan, M.; Taha, M.; Rahim, F.; Jahun, H.; Perveen, S.; Choudhary, M.I. Synthesis of 2,4,6-trichlorophenyl hydrazones and their inhibitory potential against glycation of protein. Med. Chem. 2011, 7, 572–580. [Google Scholar] [CrossRef]
- Khan, K.M.; Khan, M.; Ambreen, N.; Taha, M.; Rahim, F.; Rasheed, S.; Saied, S.; Shafi, H.; Perveen, S.; Choudhary, M.I. Oxindole derivatives: Synthesis and antiglycation activity. Med. Chem. 2013, 9, 681–688. [Google Scholar] [CrossRef]
- Khan, K.M.; Khan, M.; Ali, M.; Taha, M.; Rasheed, S.; Perveen, S.; Choudhary, M.I. Synthesis of bis-Schiff bases of isatins and their antiglycation activity. Bioorg. Med. Chem. 2009, 17, 7795–7801. [Google Scholar] [CrossRef]
- Zeb, A.; Malik, I.; Rasheed, S.; Choudhary, M.I.; Basha, F.Z. Metronidazole esters: A new class of antiglycation agents. Med. Chem. 2012, 8, 846–852. [Google Scholar] [CrossRef]
- Taha, M.; Ismail, N.H.; Jaafar, F.M.; Aziz, A.N.; Yousuf, S. (E)-N'-(3,4Dihydroxybenzylidene)-2,4-dimethylbenzohydrazide monohydrate. Acta Cryst. 2013, E69, o490. [Google Scholar]
- Baharudin, M.S.; Taha, M.; Ismail, N.H.; Shah, S.A.A. (E)-N'-(4-Chlorobenzylidene)-2-methoxybenzohydrazide. Acta Cryst. 2013, E69, o276. [Google Scholar]
- Taha, M.; Baharudin, M.S.; Ismail, N.H.; Shah, S.A.A.; Yousuf, S. (E)-2-Methoxy-N'-(2,4,6-trihydroxybenzylidene)Benzohydrazide. Acta Cryst. 2013, E69, o277. [Google Scholar]
- Taha, M.; Ismail, N.H.; Jaafar, F.M.; Khan, K.M.; Yousuf, S. (E)-2,4-Dimethyl-N'-(2-methylbenzylidene) benzohydrazide. Acta Cryst. 2013, E69, o400. [Google Scholar]
- Baharudin, M.S.; Taha, M.; Ismail, N.H.; Shah, S.A.A.; Yousuf, S. N-[(E)-2-Hydroxy-5-methoxybenzylidene]-2-methoxybenzohydra-zide. Acta Cryst. 2012, E68, o3255. [Google Scholar]
- Taha, M.; Baharudin, M.S.; Ismail, N.H.; Shah, S.A.A.; Yousuf, S. N'-[(E)-2,3-Dihydroxybenzylidene]-2-methoxybenzohydrazide. Acta Cryst. 2012, E68, o3256. [Google Scholar]
- Taha, M.; Naz, H.; Rahman, A.A.; Ismail, N.H.; Yousuf, S. (E)-4-Methoxy-N'-[(pyridin-4-yl)methylidene] benzohydrazide monohydrate. Acta Cryst. 2012, E68, o2778. [Google Scholar]
- Taha, M.; Naz, H.; Rahman, A.A.; Ismail, N.H.; Yousuf, S. (E)-N-(3,4-Dimethoxybenzylidene)-4-methoxybenzohydrazide. Acta Cryst. 2012, E68, o2780. [Google Scholar]
- Naz, H.; Taha, M.; Rahman, A.A.; Ismail, N.H.; Yousuf, S. Methyl (E)-3,5-dimethoxy-2-{[2-(4-methoxybenzoyl)hydrazin-1-ylidene]- methyl}benzoate. Acta Cryst. 2012, E68, o2671. [Google Scholar]
- Taha, M.; Naz, H.; Rahman, A.A.; Ismail, N.H.; Yousuf, S. (E)-4-Methoxy-N'-(3,4,5-trihydroxybenzylidene)benzohydrazide methanol monosolvate. Acta Cryst. 2012, E68, o2846. [Google Scholar]
- Cho, S.J.; Roman, G.; Yeboah, F.; Konishi, Y. The road to advanced glycation end products: A mechanistic perspective. Curr. Med. Chem. 2007, 14, 1653–1671. [Google Scholar] [CrossRef]
- Sample Availability: Not available.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
