Stress Hyperglycemia as Predictive Factor of Recurrence in Children with Febrile Seizures
Abstract
:1. Introduction
2. Material and Methods
2.1. Design and Subjects
2.2. Blood Glucose, Lactate and Acid–Base Status Evaluation
2.3. Data Analysis
2.3.1. Clinical and Laboratory Data
2.3.2. Statistical Methods
3. Results
3.1. Study Group Characteristics
3.2. Univariate Analysis on Clinical and Laboratory Characteristics in Stress versus Non- Stress Hyperglycemia Groups
3.3. Multiple Logistic Regression Analysis in Stress Hyperglycemia Group
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
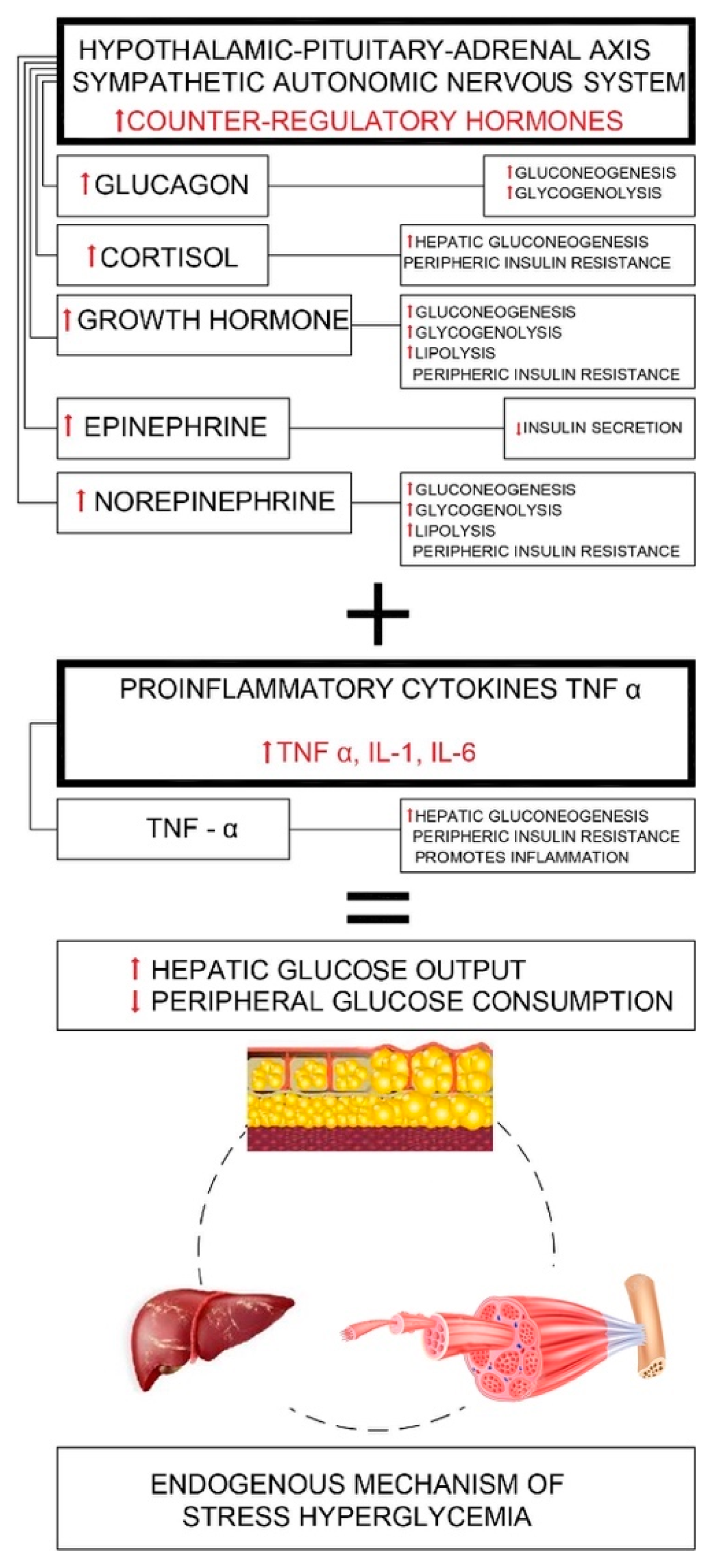
Appendix B

References
- Dungan, K.M.; Braithwaite, S.S.; Preiser, J.C. Stress hyperglycaemia. Lancet 2009, 373, 1798–1807. [Google Scholar] [CrossRef]
- Moghissi, E.S.; Korytkowski, M.T.; DiNardo, M.; Einhorn, D.; Hellman, R.; Hirsch, I.B.; Inzucchi, S.E.; Ismail-Beigi, F.; Kirkman, M.S.; Umpierrez, G.E. American Association of Clinical Endocrinologists and American Diabetes Association Consensus Statement on Inpatient Glycemic Control. Diabetes Care 2009, 32, 1119–1131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, B.; Chen, Z. Generation of Febrile Seizures and Subsequent Epileptogenesis. Neurosci. Bull. 2016, 32, 481–492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marik, P.E. Chapter 76—Endocrinology of the Stress Response During Critical Illness. In Critical Care Nephrology; Elsevier: Amsterdam, The Netherlands, 2016; pp. 446–454. [Google Scholar]
- Marik, P.E.; Bellomo, R. Stress hyperglycemia: An essential survival response! Crit. Care 2013, 17, 305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viana, M.B.; Moraes, R.B.; Rodrigues, A.; Santos, M.F. Assessment and treatment of hyperglycemia in critically ill patients. Rev. Bras. Ter. Intensiva 2014, 26, 71–76. [Google Scholar] [CrossRef]
- Kajbaf, F.; Mojtahedzadeh, M. Mechanism underlying stress-induced hyperglycemia in critical ill children. Therapy 2007, 4, 97. [Google Scholar] [CrossRef]
- Esposito, K.; Nappo, F.; Marfella, R.; Giugliano, G.; Giugliano, F.; Ciotola, M.; Quagliaro, L.; Ceriello, A.; Giugliano, D. Inflammatory Cytokine Concentrations Are Acutely Increased by Hyperglycemia in Humans. Circulation 2002, 106, 2067–2072. [Google Scholar] [CrossRef] [Green Version]
- Bhutia, T.D.; Lodha, R.; Kabra, S.K. Abnormalities in glucose homeostasis in critically ill children. Pediatric Crit. Care Med. 2013, 14, e16–e25. [Google Scholar] [CrossRef]
- Bar-Or, D.; Rael, L.T.; Madayag, R.M.; Banton, K.L.; Tanner, A.; Acuna, D.L.; Lieser, M.J.; Marshall, G.T.; Mains, C.W.; Brody, E. Stress Hyperglycemia in Critically Ill Patients: Insight Into Possible Molecular Pathways. Front. Med. 2019. [Google Scholar] [CrossRef]
- Weiss, S.L.; Alexander, J.; Agus, M.S. Extreme stress hyperglycemia during acute illness in a pediatric emergency department. Pediatrics Emerg. Care 2010, 26, 626–632. [Google Scholar] [CrossRef] [Green Version]
- Marfella, R.; Quagliaro, L.; Nappo, F.; Ceriello, A.; Giugliano, D. Acute hyperglycemia induces an oxidative stress in healthy subjects. J. Clin. Investig. 2001, 108, 635–636. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Shen, X. Oxidative stress and adipokine levels were significantly correlated in diabetic patients with hyperglycemic crises. Diabetol. Metab. Syndr. 2019, 11, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soeters, M.R.; Soeters, P.B. The evolutionary benefit of insulin resistance. Clin. Nutr. 2012, 31, 1002–1007. [Google Scholar] [CrossRef] [PubMed]
- Losser, M.R.; Damoisel, C.; Payen, D. Bench-to-bedside review: Glucose and stress conditions in the intensive care unit. Crit. Care 2010, 14, 231. [Google Scholar] [CrossRef] [Green Version]
- Malfitano, C.; Alba Loureiro, T.C.; Rodrigues, B.; Sirvente, R.; Salemi, V.M.; Rabechi, N.B.; Lacchini, S.; Curi, R.; Irigoyen, M.C. Hyperglycaemia protects the heart after myocardial infarction: Aspects of programmed cell survival and cell death. Eur. J. Heart Fail. 2010, 12, 659–667. [Google Scholar] [CrossRef] [Green Version]
- Lang, C.H.; Dobrescu, C. Gram-negative infection increases noninsulin-mediated glucose disposal. Endocrinology 1991, 128, 645–653. [Google Scholar] [CrossRef]
- Meszaros, K.; Lang, C.H.; Bagby, G.J.; Spitzer, J.J. In vivo glucose utilization by individual tissues during nonlethal hypermetabolic sepsis. FASEB J. 1988, 2, 3083–3086. [Google Scholar] [CrossRef]
- San-Millán, I.; Brooks, G.A. Reexamining cancer metabolism: Lactate production for carcinogenesis could be the purpose and explanation of the Warburg Effect. Carcinogenesis 2017, 38, 119–133. [Google Scholar] [CrossRef]
- Ronco, C.; Bellomo, R.; Kellum, J.; Ricci, Z. Critical Care Nephrology, 3rd ed.; Elsevier Health Sciences: Amsterdam, The Netherlands, 2019; pp. 394–404. [Google Scholar]
- Yang, H.; Wu, J.; Guo, R.; Peng, Y.; Zheng, W.; Liu, D.; Song, Z. Glycolysis in energy metabolism during seizures. Neural Regen. Res. 2013, 8, 1316–1326. [Google Scholar] [CrossRef]
- McDonald, T.; Puchowicz, M.; Borges, K. Impairments in Oxidative Glucose Metabolism in Epilepsy and Metabolic Treatments Thereof. Front. Cell. Neurosci. 2018, 12, 274. [Google Scholar] [CrossRef] [Green Version]
- Guidelines for epidemiologic studies on epilepsy. Commission on Epidemiology and Prognosis, International League against Epilepsy. Epilepsia 1993, 34, 592–596. [Google Scholar] [CrossRef] [PubMed]
- Small, E.; Clements, C.M. Defining fever: Likelihood of infection diagnosis as a function of body temperature in the emergency department. Crit. Care 2014, 18, 42. [Google Scholar] [CrossRef] [Green Version]
- Higgins, C. Lactate and Lactic Acidosis. 2017. Available online: https://acutecaretesting.org/-/media/acutecaretesting/files/pdf/lactate-and-lactic-acidosis.pdf (accessed on 23 October 2019).
- Higgins, C. Central Venous Blood Gas. 2011. Available online: https://acutecaretesting.org/-/media/acutecaretesting/files/pdf/central-venous-blood-gas-analysis.pdf (accessed on 23 October 2019).
- Yildizds, D.; Yapicoglu, H.; Yilmaz, H.; Sertdemir, Y. Correlation of simultaneously obtained capillary, venous and arterial blood gases of patients in a paediatric intensive care unit. Arch. Dis. Child. 2004, 89, 176–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malatesha, G.; Singh, N.; Bharija, A.; Rehani, B.; Goel, A. Comparison of arterial and venous pH, bicarbonate, PCO2 and PO2 in initial emergency department assessment. Emerg. Med. J. 2007, 24, 569–571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koul, P.; Khan, U.; Wani, A. Comparison and agreement between venous and arterial gasanalysis in cardiopulmonary patients in Kashmir valley of the Indian subcontinent. Ann. Thorac. Med. 2011, 6, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Malinoski, D.; Todd, S.; Slone, S.; Mullins, R.J.; Schreiber, M.A. Correlation of central venous and arterial blood gas measurements in mechanically ventilated trauma patients. Arch. Surg. 2005, 140, 1122–1125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Middleton, P.; Kelly, A.-M.; Brown, J.; Robertson, M. Agreement between arterial and central venous values for bicarbonate base excess and lactate. Emerg. Med. J. 2006, 23, 622–624. [Google Scholar] [CrossRef] [Green Version]
- Higgins, C. Lactate Measurement: Arterial Versus Venous Blood Sampling. 2017. Available online: https://acutecaretesting.org/en/articles/lactate-measurement-arterial-versus-venous-blood-sampling (accessed on 23 October 2019).
- Byrne, A.L.; Bennett, M.; Chatterji, R.; Symons, R.; Pace, N.L.; Thomas, P.S. Peripheral venous and arterial blood gas analysis in adults: Are they comparable? A systematic review and meta-analysis. Respirology 2014, 19, 168. [Google Scholar] [CrossRef]
- Theodore, A.C. Venous Blood Gases and Other Alternatives to Arterial Blood Gases. Up to Date. 2019. Available online: https://www.uptodate.com/contents/venous-blood-gases-and-other-alternatives-to-arterial-blood-gases/print (accessed on 23 October 2019).
- Gunnerson, K.J. Lactic Acidosis. Updated: 27 April 2018. Available online: https://emedicine.medscape.com/article/167027-#a4 (accessed on 23 October 2019).
- Nicks, B.A. Acute Lactic Acidosis Overview. Updated: 02 October 2018. Available online: https://emedicine.medscape.com/article/768159-overview (accessed on 23 October 2019).
- Cohen, R.; Woods, H. Clinical and Biochemical Aspects of Lactic Acidosis; Blackwell Scientific Publications: London, UK, 1976. [Google Scholar]
- Mizock, B.A.; Falk, J.L. Lactic acidosis in critical illness. Crit. Care Med. 1992, 20, 80–93. [Google Scholar] [CrossRef]
- WHO Multicentre Growth Reference Study Group. WHO Child Growth Standards: Length/Height-for-Age, Weight-for-Age, Weight-for-Length, Weight-for-Height and Body Mass Index-for-Age: Methods and Development; World Health Organization: Geneva, Switzerland, 2006; p. 312. [Google Scholar]
- Lee, J.Y.; Kim, J.H. Children Experiencing First-Time or Prolonged Febrile Seizure Are Prone to Stress Hyperglycemia. J. Child Neurol. 2016, 31, 439–443. [Google Scholar] [CrossRef]
- Altman, D.G. Practical Statistics for Medical Research; Chapman & Hall: London, UK, 1991; ISBN 0-412-27630-5. [Google Scholar]
- Valerio, G.; Franzese, A.; Carlin, E.; Pecile, P.; Perini, R.; Tenore, A. High prevalence of stress hyperglycemia in children with febrile seizures and traumatic injuries. Acta Paediatr. 2001, 90, 618–622. [Google Scholar] [CrossRef] [PubMed]
- Fattorusso, V.; Nugnes, R.; Casertano, A.; Valerio, G.; Mozzillo, E.; Franzese, A. Non-Diabetic Hyperglycemia in the Pediatric Age: Why, How, and When to Treat? Curr. Diabetes Rep. 2018, 18, 140. [Google Scholar] [CrossRef] [PubMed]
- Chou, H.F.; Shen, E.Y.; Kuo, Y.T. Utility of laboratory tests for children in the emergency department with a first seizure. Pediatric Emerg. Care 2011, 27, 1142–1145. [Google Scholar] [CrossRef]
- Brinkman, J.E.; Sharma, S. Physiology, Respiratory Alkalosis. [Updated 2019 Jun 23]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2019; Available online: https://www.ncbi.nlm.nih.gov/books/NBK482117/ (accessed on 31 January 2020).
- O’Dempsey, T.J.; Laurence, B.E.; McArdle, T.F.; Todd, J.E.; Lamont, A.C.; Greenwood, B.M. The effect of temperature reduction on respiratory rate in febrile illnesses. Arch. Dis. Child. 1993, 68, 492–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazarati, A.M. Respiratory alkalosis: "basic" mechanism of febrile seizures? Epilepsy Curr. 2007, 7, 25–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schuchmann, S.; Schmitz, D.; Rivera, C.; Vanhatalo, S.; Salmen, B.; Mackie, K.; Sipila, S.T.; Voipio, J.; Kaila, K. Experimental febrile seizures are precipitated by hyperthermia-induced respiratory alkalosis. Nat. Med. 2006, 12, 817–823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersen, L.W.; Mackenhauer, J.; Roberts, J.C.; Berg, K.M.; Cocchi, M.N.; Donnino, M.W. Etiology and therapeutic approach to elevated lactate levels. Mayo Clin. Proc. 2013, 88, 1127–1140. [Google Scholar] [CrossRef] [Green Version]
- Lipka, K.; Bulow, H.H. Lactic acidosis following convulsions. Acta Anaesthesiol. Scand. 2003, 47, 616–618. [Google Scholar] [CrossRef]
- Orringer, C.E.; Eustace, J.C.; Wunsch, C.D.; Gardner, L.B. Natural history of lactic acidosis after grand-mal seizures. A model for the study of an anion-gap acidosis not associated with hyperkalemia. N. Engl. J. Med. 1977, 297, 796–799. [Google Scholar] [CrossRef]
- LeBlanc, P.J.; Parolin, M.L.; Jones, N.L.; Heigenhauser, G.J. Effects of respiratory alkalosis on human skeletal muscle metabolism at the onset of submaximal exercise. J. Physiol. 2002, 544, 303–313. [Google Scholar] [CrossRef]
- Lewis, R. Childhood Febrile Seizures Linked to Epilepsy, Psych Disorders—Medscape—8 October 2019. Available online: https://www.medscape.com/viewarticle/919571 (accessed on 23 October 2019).
- Dreier, J.W.; Li, J.; Sun, Y.; Christensen, J. Evaluation of Long-term Risk of Epilepsy, Psychiatric Disorders, and Mortality among Children with Recurrent Febrile Seizures. JAMA Pediatrics 2019. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Choi, S.A.; Kim, S.Y.; Kim, H.; Lim, B.C.; Hwang, H.; Chae, J.H.; Kim, K.J.; Oh, S.; Kim, E.Y.; et al. Association Analysis of Interleukin-1β, Interleukin-6, and HMGB1 Variants with Postictal Serum Cytokine Levels in Children with Febrile Seizure and Generalized Epilepsy with Febrile Seizure Plus. J. Clin. Neurol. 2019, 15, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Aggarwal, A.; Faridi, M.M.A.; Rai, G.; Das, S.; Kotru, M. Serum Interleukin-6 Levels in Children with Febrile Seizures. Indian Pediatrics 2018, 55, 411. [Google Scholar] [CrossRef] [PubMed]
- Mkhize, N.V.P.; Qulu, L.; Mabandla, M.V. The Effect of Quercetin on Pro- and Anti-Inflammatory Cytokines in a Prenatally Stressed Rat Model of Febrile Seizures. J. Exp. Neurosci. 2017, 11, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, A.; Kwak, B.O.; Kim, K.; Ha, J.; Kim, S.J.; Bae, S.H.; Son, J.S.; Kim, S.N.; Lee, R. Cytokine levels in febrile seizure patients: A systematic review and meta-analysis. Seizure 2018, 59, 5–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freund, T.F.; Buzsaki, G.; Prohaska, O.J.; Leon, A.; Somogyi, F. Simultaneous recording of local electrical activity, partial oxygen tension and temperature in the rat hippocampus with a chamber- type microelectrode. Effects of anaesthesia, ischemia and epilepsy. Neuroscience 1989, 28, 539–549. [Google Scholar] [CrossRef]
- Chapman, A.G.; Meldrum, B.S.; Siesjo, B.K. Cerebral metabolic changes during prolonged epileptic seizures in rats. J. Neurochem. 1977, 28, 1025–1035. [Google Scholar] [CrossRef]
- Kloiber, O.; Bockhorst, K.; Hoehn-Berlage, M.; Hossmann, K.A. Effect of hypoxia on bicuculline seizures of rat: NMR spectroscopy and bioluminescence imaging. NMR Biomed. 1993, 6, 333–338. [Google Scholar] [CrossRef]
- Yamada, K.; Ji, J.J.; Yuan, H.; Miki, T.; Sato, S.; Horimoto, N.; Shimizu, T.; Seino, S.; Inagaki, N. Protective role of ATP-sensitive potassium channels in hypoxia-induced generalized seizure. Science 2001, 292, 1543–1546. [Google Scholar] [CrossRef]
- Somjen, G.G.; Tombaugh, G.C. pH modulation of neuronal excitability and central nervous system functions. pH Brain Funct. 1998, 373–393. [Google Scholar]
- Costa Leite, T.; Da Silva, D.; Guimarães Coelho, R.; Zancan, P.; Sola-Penna, M. Lactate favours the dissociation of skeletal muscle 6-phosphofructo-1-kinase tetramers down-regulating the enzyme and muscle glycolysis. Biochem. J. 2007, 408, 123–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zancan, P.; Sola-Penna, M. Regulation of human erythrocyte metabolism by insulin: Cellular distribution of 6-phosphofructo-1-kinase and its implication for red blood cell function. Mol. Genet. Metab. 2005, 86, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Zancan, P.; Rosas, A.O.; Marcondes, M.C.; .Marinho-Carvalho, M.M.; Sola-Penna, M. Clotrimazole inhibits and modulates heterologous association of the key glycolytic enzyme 6-phosphofructo-1-kinase. Biochem. Pharmacol. 2007, 73, 1520–1527. [Google Scholar] [CrossRef] [PubMed]
- Traynelis, S.F.; Cull-Candy, S.G. Proton inhibition of N-methyl-D-aspartate receptors in cerebellar neurons. Nature 1990, 345, 347–350. [Google Scholar] [CrossRef]
- Coryell, M.W.; Ziemann, A.E.; Westmoreland, P.J.; Haenfler, J.M.; Kurjakovic, Z.; Zha, X.M.; Price, M.; Schnizler, M.K.; Wemmie, J.A. Targeting ASIC1a reduces innate fear and alters neuronal activity in the fear circuit. Biol. Psychiatry. 2007, 62, 1140–1148. [Google Scholar] [CrossRef]
- Ziemann, A.E.; Schnizler, M.K.; Albert, G.W.; Severson, M.A.; Howard, M.A., 3rd; Welsh, M.J.; Wemmie, J.A. Seizure termination by acidosis depends on ASIC1a. Nat. Neurosci. 2008, 11, 816–822. [Google Scholar] [CrossRef] [Green Version]
- Kosmidis, I.; Nikolaidis, S.; Chatzis, A.; Christoulas, K.; Metaxas, T.; Mougios, V. Reliability of the Urine Lactate Concentration after Alternating-Intensity Interval Exercise. Proceedings 2019, 25, 1. [Google Scholar] [CrossRef] [Green Version]
- Scheijen, J.L.; Hanssen, N.M.; van de Waarenburg, M.P.; Jonkers, D.M.; Stehouwer, C.D.; Schalkwijk, C.G. L(+) and D(-) lactate are increased in plasma and urine samples of type 2 diabetes as measured by a simultaneous quantification of L(+) and D(-) lactate by reversed-phase liquid chromatography tandem mass spectrometry. Exp. Diabetes Res. 2012, 2012, 234812. [Google Scholar] [CrossRef] [Green Version]
- Mohammadi, M.; Mohebbi, M.M.; Naderi, F. CSF glucose concentrations in infants with febrile convulsions and the possible effect of Acetaminophen. Indian Pediatrics 2003, 40, 1183–1186. [Google Scholar]
- Ehsanipoor, F.; Ardalan, M.; Rafati, A.H. Correlations between Acetaminophen Consumption and CSF Glucose in febrile convulsions. JK Sci. 2011, 13, 176. [Google Scholar] [CrossRef] [Green Version]
| Non-Stress Hyperglycemia Group <140 (n = 138) | Stress-Hyperglycemia Group ≥140 (n = 28) | Sig. | |
|---|---|---|---|
| Age (months) a | 23.38 ± 12.22 | 21.75 ± 11.15 | 0.516 |
| Gender (m/f) | 71 (51.4%)/67 (48.6%) | 22 (78.6%)/6 (21.4%) | 0.008 |
| Body temperature (°C) a | 39.24 ± 0.70 | 39.34 ± 0.88 | 0.489 |
| Body temperature (≥39.5 °C) b | 56 (40.6%) | 15 (53.6%) | 0.205 |
| Seizure semiology (generalized/focal) b | 136 (98.6%)/2 (1.4%) | 26 (92.9%)/2 (7.1%) | 0.073 |
| Type of seizure ( simple/complex) b | 125 (90.6%)/16 (9.4%) | 19 (67.9%)/9 (32.1%) | 0.001 |
| Seizure semiology (motor/non motor) b | 99 (71.7%)/39 (28.3%) | 22 (78.6%)/6 (21.4%) | 0.458 |
| Time interval from fever onset to seizure event (<6 h/≥6 h) b | 76 (55.1%)/62 (44.9%) | 20 (71.4%)/8 (28.6%) | 0.110 |
| Time interval from last fever episode seizure (<15 min/<1 h/1–6 h/6–24 h) b | 91 (65.9%)/21 (15.2%)/ 14 (10.1%)/ 12(8.7%) | 18 (64.3%)/3 (10.7%)/ 3 (10.7%)/ 4(14.3%) | 0.778 |
| Recurrence of FS >1 episode | 33 (23.9%) | 11 (39.3%) | 0.093 |
| Multiple FS within 24 h | 7 (5.1%) | 1 (3.6%) | 0.597 |
| Seizure duration (≤15/>15 min) | 136 (98.6%)/2 (1.4%) | 24 (85.7%)/4 (14.3%) | 0.001 |
| pH a | 7.47 ± 0.07; 7.48 (7.42, 7.53) | 7.44 ± 0.08; 7.46 (7.37, 7.53) | 0.049 |
| Anion-gap a | 3.88 ± 3.29; 4.25 (1.90, 5.70) | 4.64 ± 3.00; 5.05 (1.55, 6.05) | 0.264 |
| Lactate (mg/dl) a | 19.50 (15.00, 27.00) | 30.50 (15.00, 36.00) | 0.000 |
| Glycemia (mg/dl) a | 113.50 (101.00, 128.00) | 167.00 (152.00, 180.50) | 0.000 |
| PCO2 (mmHg) a | 24.35 (21.10, 28.70) | 26.55 (21.25, 32.15) | 0.074 |
| BE (mmol/L) a | −4.62 ± 2.22; −4.60 (−6.00, −2.90) | −5.49 ± 2.31; −5.90 (−6.95, −4.20) | 0.061 |
| HCO3 (mmol/L) a | 21.23 ± 1.71; 21.35 (20.00, 22.40) | 20.36 ± 2.10; 20.15 (20.20, 21.45) | 0.020 |
| Variable | OR (95% CI) | p-Value |
|---|---|---|
| Lactate (>30 mg/dl) | 1.098 (1.047, 1.151) | 0.000 |
| Recurrence of FS > 1 episode | 3.673 (1.285, 10.499) | 0.015 |
| Type of seizure (focal) | 16.993 (1.217, 237.186) | 0.035 |
| Body temperature (≥39.5 °C) | 2.875 (1.025, 8.061) | 0.045 |
| Seizure duration (>15 min) | 20.852 (2.356, 184.568) | 0.006 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costea, R.M.; Maniu, I.; Dobrota, L.; Neamtu, B. Stress Hyperglycemia as Predictive Factor of Recurrence in Children with Febrile Seizures. Brain Sci. 2020, 10, 131. https://doi.org/10.3390/brainsci10030131
Costea RM, Maniu I, Dobrota L, Neamtu B. Stress Hyperglycemia as Predictive Factor of Recurrence in Children with Febrile Seizures. Brain Sciences. 2020; 10(3):131. https://doi.org/10.3390/brainsci10030131
Chicago/Turabian StyleCostea, Raluca Maria, Ionela Maniu, Luminita Dobrota, and Bogdan Neamtu. 2020. "Stress Hyperglycemia as Predictive Factor of Recurrence in Children with Febrile Seizures" Brain Sciences 10, no. 3: 131. https://doi.org/10.3390/brainsci10030131
APA StyleCostea, R. M., Maniu, I., Dobrota, L., & Neamtu, B. (2020). Stress Hyperglycemia as Predictive Factor of Recurrence in Children with Febrile Seizures. Brain Sciences, 10(3), 131. https://doi.org/10.3390/brainsci10030131





