Safety of Short-Term Supplementation with Methylliberine (Dynamine®) Alone and in Combination with TeaCrine® in Young Adults
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Participants
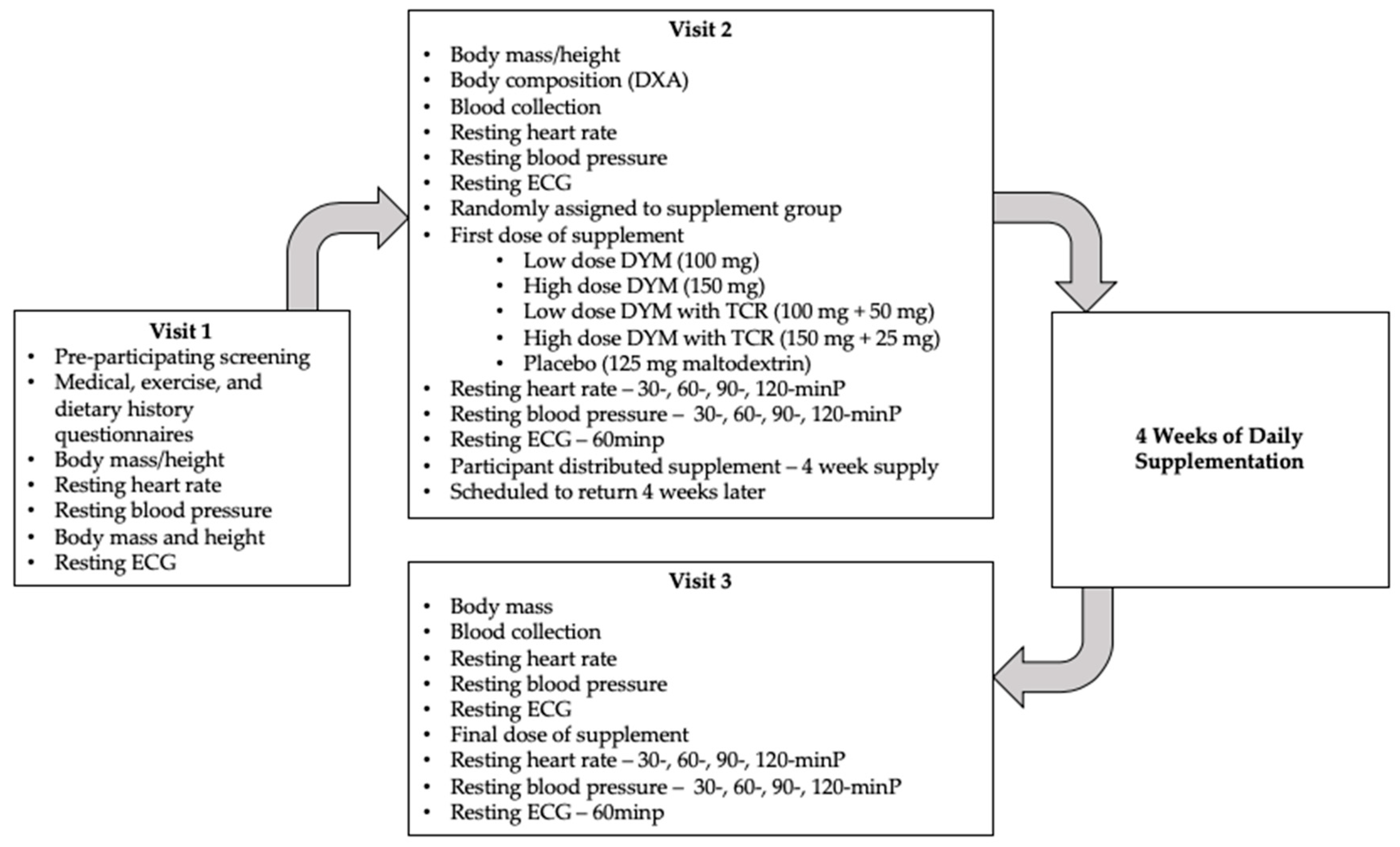
2.3. Visits Overview
2.3.1. Visit 1: Pre-participation screening
2.3.2. Visits 2 and 3
2.4. Laboratory Assessments
2.4.1. Anthropometric Measurements
2.4.2. Blood sampling and analysis
2.4.3. Resting heart rate and blood pressure
2.4.4. Resting Electrocardiogram
2.4.5. Supplementation intervention
2.4.6. Diet Tracking
2.5. Statistical Analysis
3. Results
3.1. Cardiovascular Function
3.2. Blood Biomarkers
3.3. Dietary Tracking
4. Discussion
4.1. Cardiovascular Function
4.2. Blood Biomarkers
5. Conclusions, Limitations, and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Grgic, J.; Grgic, I.; Pickering, C.; Schoenfeld, B.J.; Bishop, D.J.; Pedisic, Z. Wake up and smell the coffee: Caffeine supplementation and exercise performance-an umbrella review of 21 published meta-analyses. Br. J. Sports Med. 2019, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, E.R.; Ziegenfuss, T.; Kalman, D.; Kreider, R.; Campbell, B.; Wilborn, C.; Wildman, R. International society of sports nutrition position stand: Caffeine and performance. J. Int. Soc. Sports Nutr. 2010, 7, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boulenger, J.P.; Patel, J.; Post, R.M.; Parma, A.M.; Marangos, P.J. Chronic caffeine consumption increases the number of brain adenosine receptors. Life Sci. 1983, 32, 1135–1142. [Google Scholar] [CrossRef]
- Hi, D.; Nikodijević, O.; Jacobson, K.A.; Daly, J.W. Chronic caffeine alters the density of adenosine, adrenergic, cholinergic, GABA, and serotonin receptors and calcium channels in mouse brain. Cell. Mol. Neurobiol. 1993, 13, 247–261. [Google Scholar]
- Svenningsson, P.; Nomikos, G.G.; Fredholm, B.B. The stimulatory action and the development of tolerance to caffeine is associated with alterations in gene expression in specific brain regions. J. Neurosci. 1999, 19, 4011–4022. [Google Scholar] [CrossRef] [Green Version]
- Temple, J.L.; Bernard, C.; Lipshultz, S.E.; Czachor, J.D.; Westphal, J.A.; Mestre, M.A. The Safety of Ingested Caffeine: A Comprehensive Review. Front. Psychiatry 2017, 8, 80. [Google Scholar] [CrossRef] [Green Version]
- Petermann, J. A new tetramethyluric acid from Coffea liberica and C. dewevrei. Phytochemistry 1977, 16, 620–621. [Google Scholar] [CrossRef]
- Wanner, H.; Pesakova, M.; Baumann, T.W.; Charubala, R.; Guggisberg, A.; Hesse, M.; Schmid, H. 0(2),1,9-trimethyluric acid and 1,3,7,9-tetramethyluric acid in leaves of different Coffea species. Phytochemistry 1975, 14, 747–750. [Google Scholar] [CrossRef]
- Petermann, J.B.; Baumann, T.W. Metabolic Relations between Methylxanthines and Methyluric Acids in Coffea L. Plant Physiol. 1983, 73, 961–964. [Google Scholar] [CrossRef] [Green Version]
- Baumann, T.W.; Oechslin, M.; Wanner, H. Caffeine and Methylated Uric-Acid-Chemical Patterns during Vegetative Development of Coffea-Liberica. Biochem. Und Physiol. Der Pflanz. 1976, 170, 217–225. [Google Scholar] [CrossRef]
- Daly, J.W.; Butts-Lamb, P.; Padgett, W. Subclasses of adenosine receptors in the central nervous system: Interaction with caffeine and related methylxanthines. Cell. Mol. Neurobiol. 1983, 3, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Ralevic, V.; Burnstock, G. Receptors for purines and pyrimidines. Pharmacol. Rev. 1998, 50, 413–492. [Google Scholar]
- Johnson, T.B. The Discovery and Identification of a New Purine Alkaloid in Tea. Science 1937, 85, 431. [Google Scholar] [CrossRef] [PubMed]
- Ziegenfuss, T.N.; Habowski, S.M.; Sandrock, J.E.; Kedia, A.W.; Kerksick, C.M.; Lopez, H.L. A Two-Part. Approach to Examine the Effects of Theacrine (TeaCrine(R)) Supplementation on Oxygen Consumption, Hemodynamic Responses, and Subjective Measures of Cognitive and Psychometric Parameters. J. Diet Suppl. 2017, 14, 9–24. [Google Scholar] [CrossRef] [PubMed]
- Kuhman, D.J.; Joyner, K.J.; Bloomer, R.J. Cognitive Performance and Mood Following Ingestion of a Theacrine-Containing Dietary Supplement, Caffeine, or Placebo by Young Men and Women. Nutrients 2015, 7, 9618–9632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, H.; Ma, D.; Crone, L.B.; Butawan, M.; Meibohm, B.; Bloomer, R.J.; Yates, C.R. Assessment of the Drug-Drug Interaction Potential Between Theacrine and Caffeine in Humans. J. Caffeine Res. 2017, 7, 95–102. [Google Scholar] [CrossRef]
- Taylor, L.; Mumford, P.; Roberts, M.; Hayward, S.; Mullins, J.; Urbina, S.; Wilborn, C. Safety of TeaCrine(R), a non-habituating, naturally-occurring purine alkaloid over eight weeks of continuous use. J. Int. Soc. Sports Nutr. 2016, 13, 2. [Google Scholar] [CrossRef] [Green Version]
- Bello, M.L.; Walker, A.J.; McFadden, B.A.; Sanders, D.J.; Arent, S.M. The effects of TeaCrine(R) and caffeine on endurance and cognitive performance during a simulated match in high-level soccer players. J. Int. Soc. Sports Nutr. 2019, 16, 20. [Google Scholar] [CrossRef] [Green Version]
- Cesareo, K.R.; Mason, J.R.; Saracino, P.G.; Morrissey, M.C.; Ormsbee, M.J. The effects of a caffeine-like supplement, TeaCrine(R), on muscular strength, endurance and power performance in resistance-trained men. J. Int. Soc. Sports Nutr. 2019, 16, 47. [Google Scholar] [CrossRef] [Green Version]
- Li, W.X.; Li, Y.F.; Zhai, Y.J.; Chen, W.M.; Kurihara, H.; He, R.R.V. Theacrine, a purine alkaloid obtained from Camellia assamica var. kucha, attenuates restraint stress-provoked liver damage in mice. J. Agric. Food Chem. 2013, 61, 6328–6335. [Google Scholar]
- Wang, Y.; Yang, X.; Zheng, X.; Li, J.; Ye, C.; Song, X. Theacrine, a purine alkaloid with anti-inflammatory and analgesic activities. Fitoterapia 2010, 81, 627–631. [Google Scholar] [CrossRef] [PubMed]
- Xie, G.; Wu, M.; Huang, Y.; Cao, Y.; Li, L.; Zhou, H.; Zhu, R.; Liao, Y.; Kurihara, H. Experimental study of theacrine on antidepressant effects. Clin. Pharmacol. Bull. 2009, 9, 13. [Google Scholar]
- Xu, J.K.; Kurihara HI RO SH, I.; Zhao LI AN, G.; Yao, X.S. Theacrine, a special purine alkaloid with sedative and hypnotic properties from Cammelia assamica var. kucha in mice. J. Asian Nat. Prod. Res. 2007, 9, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Feduccia, A.A.; Wang, Y.; Simms, J.A.; Henry, Y.Y.; Li, R.; Bjeldanes, L.; Bartlett, S.E. Locomotor activation by theacrine, a purine alkaloid structurally similar to caffeine: Involvement of adenosine and dopamine receptors. Pharmacol. Biochem. Behav. 2012, 102, 241–248. [Google Scholar] [CrossRef]
- Murbach, T.S.; Glavits, R.; Endres, J.R.; Clewell, A.E.; Hirka, G.; Vertesi, A.; Pasics Szakonyine, P. A Toxicological Evaluation of Methylliberine. J. Toxicol. 2019, 2019, 1–25. [Google Scholar]
- Scicchitano, P.; Cameli, M.; Maiello, M.; Amedeo Modesti, P.; Lorenza Muiesan, M.; Novo, S.; Palmiero, P.; Sergio Saba, P.; Pedrinelli, R.; Matteo Ciccone, M. Nutraceuticals and dyslipidaemia: Beyond the common therapeutics. J. Funct. Foods 2014, 6, 11–32. [Google Scholar] [CrossRef]
- Sachdeva, V.; Roy, A.; Bharadvaja, N. Current prospective of nutraceuticals: A review. Curr. Pharm. Biotechnol. 2020. [Google Scholar] [CrossRef]
- Cesarone, M.R.; Belcaro, G.; Hosoi, M.; Ledda, A.; Feragalli, B.; Maione, C.; Hu, S. Supplementary management with Pycnogenol(R) in Parkinson’s disease to prevent cognitive impairment. J. Neurosurg. Sci. 2020. [Google Scholar]
- Oh, J.H.; Jang, Y.S.; Kang, D.; Chang, D.K.; Min, Y.W. Efficacy and Safety of New Lactobacilli Probiotics for Unconstipated Irritable Bowel Syndrome: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients 2019, 11, 2887. [Google Scholar] [CrossRef] [Green Version]
- Schwarz, N.A.; McKinley-Barnard, S.K.; Blahnik, Z.J. Effect of Bang(R) Pre-Workout Master Blaster(R) combined with four weeks of resistance training on lean body mass, maximal strength, mircoRNA expression, and serum IGF-1 in men: A randomized, double-blind, placebo-controlled trial. J. Int. Soc. Sports Nutr. 2019, 16, 54. [Google Scholar] [CrossRef] [Green Version]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Erlbaum: Hillsdale, MI, USA, 1988. [Google Scholar]
- Clewell, A.; Hirka, G.; Glávits, R.; Palmer, P.A.; Endres, J.R.; Murbach, T.S.; Pasics Szakonyiné, I. A 90-Day Oral Toxicological Evaluation of the Methylurate Purine Alkaloid Theacrine. J. Toxicol. 2016, 2016, 6206859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merri, M.; Benhorin, J.; Alberti, M.; Locati, E.; Moss, A.J. Electrocardiographic quantitation of ventricular repolarization. Circulation 1989, 80, 1301–1308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.B.; Li, Y.F.; Mao, Z.F.; Hu, H.H.; Ouyang, S.H.; Wu, Y.P.; He, R.R. Differing chemical compositions of three teas may explain their different effects on acute blood pressure in spontaneously hypertensive rats. J. Sci. Food Agric. 2015, 95, 1236–1242. [Google Scholar] [CrossRef]
- Cooney, M.T.; Vartiainen, E.; Laakitainen, T.; Juolevi, A.; Dudina, A.; Graham, I.M. Elevated resting heart rate is an independent risk factor for cardiovascular disease in healthy men and women. Am. Heart J. 2010, 159, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey, D.E., Jr.; Collins, K.J.; Himmelfarb, D.C.; MacLaughlin, E.J. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High. Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2018, 71, 1269–1324. [Google Scholar]
- Turnbull, D.; Rodricks, J.V.; Mariano, G.F.; Chowdhury, F. Caffeine and cardiovascular health. Regul. Toxicol. Pharmacol. 2017, 89, 165–185. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration, HHS. International Conference on Harmonisation; guidance on E14 Clinical Evaluation of QT/QTc Interval Prolongation and Proarrhythmic Potential for Non-Antiarrhythmic Drugs; availability. Notice. Fed. Regist. 2005, 70, 61134–61135. [Google Scholar]
- Johnson, J.N.; Grifoni, C.; Bos, J.M.; Saber-Ayad, M.; Ommen, S.R.; Nistri, S.; Ackerman, M.J. Prevalence and clinical correlates of QT prolongation in patients with hypertrophic cardiomyopathy. Eur. Heart J. 2011, 32, 1114–1120. [Google Scholar] [CrossRef] [Green Version]
- Zheng, X.X.; Xu, Y.L.; Li, S.H.; Liu, X.X.; Hui, R.; Huang, X.H. Green tea intake lowers fasting serum total and LDL cholesterol in adults: A meta-analysis of 14 randomized controlled trials. Am. J. Clin. Nutr. 2011, 94, 601–610. [Google Scholar] [CrossRef] [Green Version]

| Sample Size | Sex | Age (year) | Height (cm) | Body Mass (kg) | Body Fat (%) | Resting Heart Rate (bpm) | Resting Systolic Blood Pressure (mmHg) | Resting Diastolic Blood Pressure (mmHg) | |
|---|---|---|---|---|---|---|---|---|---|
| 100 mg DYM | 12 | M | 21.7 ± 1.3 | 174.5 ± 7.8 | 78.2 ± 11.1 | 16.7 ± 6.3 | 63.3 ± 7.5 | 123.0 ± 7.0 | 75.8 ± 8.4 |
| 13 | F | 23.4 ± 2.7 | 162.6 ± 4.4 | 62.7 ± 10.9 | 30.6 ± 6.7 | 66.0 ± 11.3 | 110.2 ± 3.8 | 67.0 ± 7.6 | |
| 100 mg DYM + 50 mg TCR | 12 | M | 23.5 ± 3.0 | 175.1 ± 6.7 | 83.3 ± 10.3 | 24.2 ± 7.0 | 66.9 ± 10.9 | 120.0 ± 6.4 | 74.1 ± 10.2 |
| 13 | F | 24.0 ± 6.5 | 163.3 ± 5.1 | 64.4 ± 14.1 | 31.7 ± 7.5 | 61.3 ± 7.6 | 107.5 ± 4.9 | 64.6 ± 5.2 | |
| 150 mg DYM +25 mg TCR | 12 | M | 24.2 ± 3.3 | 175.7 ± 3.5 | 78.1 ± 5.6 | 19.5 ± 2.9 | 63.6 ± 8.8 | 117.6 ± 5.8 | 71.5 ± 9.1 |
| 13 | F | 23.1 ± 2.6 | 164.4 ± 5.1 | 62.7 ± 8.0 | 30.7 ± 7.1 | 73.8 ± 10.1 | 111.0 ± 6.8 | 71.0 ± 5.4 | |
| 150 mg DYM | 12 | M | 22.8 ± 2.6 | 178.3 ± 7.3 | 80.6 ± 8.2 | 19.8 ± 5.6 | 61.1 ± 10.7 | 116.3 ± 7.8 | 74.4 ± 8.7 |
| 13 | F | 21.8 ± 1.8 | 162.7 ± 8.6 | 63.0 ± 15.0 | 27.7 ± 9.4 | 73.7 ± 11.2 | 111.2 ± 8.0 | 68.1 ± 6.1 | |
| Placebo (maltodextrin) | 12 | M | 22.8 ± 3.3 | 117.2 ± 7.7 | 82.5 ± 14.0 | 23.8 ± 7.8 | 66.9 ± 3.9 | 117.7 ± 7.1 | 76.8 ± 7.0 |
| 13 | F | 22.9 ± 3.8 | 166.5 ± 4.7 | 70.9 ± 11.9 | 31.1 ± 6.9 | 68.8 ± 10.1 | 110.0 ± 5.3 | 67.3 ± 7.6 |
| Visit 1 | Visit 2 | Visit 3 | |||
|---|---|---|---|---|---|
| PRE | PRE | 60 minP | PRE | 60 minP | |
| PR Interval (ms) | 145 ± 17 | 146 ± 17 | 147 ± 17 | 145 ± 16 | 149 ± 34 |
| QRS Duration (ms) | 88.7 ± 9 | 89.7 ± 9 | 89.8 ± 8.8 | 89.6 ± 9.1 | 89.2 ± 8.9 |
| QTC (ms) | 412 ± 23 | 410 ± 23 | 406 ± 24 * | 412 ± 23 | 409 ± 24 |
| P Axis (◦) | 56.4 ± 19.1 | 56.5 ± 19.1 | 51.9 ± 22.3 * | 53.9 ± 20.9 | 51.5 ± 22.2 |
| R Axis (◦) | 75.7 ± 15.3 | 75.5 ± 17.3 | 74.1 ± 17.5 | 75.4 ± 17.4 | 75.1 ± 17.4 |
| T Axis (◦) | 51.8 ± 14 | 53 ± 14.3 | 53.6 ± 17.6 | 52.6 ± 13.6 | 52.4 ± 13.3 |
| P Duration (ms) | 94.5 ± 10.2 | 94.2 ± 10.1 | 93.3 ± 11.3 | 93.4 ± 10 | 94.4 ± 10.2 |
| RR Interval (ms) | 928 ± 158 | 961 ± 157 # | 1030 ± 166 * | 950 ± 185 | 1024 ± 176 * |
| PP Interval (ms) | 935 ± 214 | 959 ± 161 | 1022 ± 173 * | 948 ± 188 | 1010 ± 167 * |
| 100 mg of DYM | 100 mg of DYM + 50 mg of TCR | 150 mg of DYM + 25 mg of TCR | 150 mg of DYM | Placebo | Average | ||
|---|---|---|---|---|---|---|---|
| White Blood Cells (× 103/µL) | Visit 2 | 5.37 ± 1.26 | 6.20 ± 1.62 | 5.78 ± 1.26 | 5.76 ± 1.39 | 5.50 ± 1.58 | 5.71 ± 1.43 |
| Visit 3 | 5.68 ± 1.62 | 5.92 ± 1.97 | 5.71 ± 1.28 | 5.56 ± 1.30 | 5.53 ± 1.29 | 5.68 ± 1.49 | |
| Red Blood Cells (× 106/µL) | Visit 2 | 4.83 ± 0.41 | 4.84 ± 0.41 | 4.64 ± 0.40 | 4.65 ± 0.33 | 4.83 ± 0.57 | 4.76 ± 0.43 |
| Visit 3 | 4.83 ± 0.39 | 4.82 ± 0.47 | 4.61 ± 0.40 | 4.67 ± 0.26 | 4.84 ± 0.53 | 4.76 ± 0.42 | |
| Hemoglobin (g/dL) | Visit 2 | 14.6 ± 1.0 | 14.4 ± 1.0 | 13.6 ± 1.0 | 14.1 ± 0.9 | 14.2 ± 1.2 | 14.1 ± 1.0 |
| Visit 3 | 14.6 ± 1.0 | 14.3 ± 1.1 | 13.7 ± 1.1 | 19.5 ± 25.1 | 14.2 ± 1.2 | 15.2 ± 11.1 | |
| Hematocrit (%) | Visit 2 | 43.4 ± 2.9 | 43.7 ± 11.7 | 40.3 ± 2.2 | 42 ± 3 | 42.3 ± 3.4 | 42.3 ± 5.6 |
| Visit 3 | 44.1 ± 2.9 | 42.7 ± 3.0 | 40.9 ± 2.6 | 41.4 ± 3.4 | 42.7 ± 3.1 | 42.3 ± 3.2 | |
| Mean Corpuscular Volume (fL) | Visit 2 | 89.7 ± 3.6 | 88.5 ± 3.6 | 88.4 ± 4.8 | 88.7 ± 4.7 | 88.0 ± 5.0 | 88.6 ± 4.4 |
| Visit 3 | 91.0 ± 3.6 | 88.9 ± 3.2 | 88.8 ± 4.3 | 89.3 ± 5.5 | 88.6 ± 6.2 | 89.3 ± 4.7* | |
| Mean Corpuscular Hemoglobin (pg) | Visit 2 | 29.9 ± 1.5 | 29.7 ± 1.0 | 29.9 ± 1.8 | 30.1 ± 1.9 | 29.4 ± 1.8 | 29.8 ± 1.6 |
| Visit 3 | 30.2 ± 1.1 | 29.7 ± 1.1 | 30.1 ± 1.6 | 30.5 ± 1.9 | 29.6 ± 1.9 | 30.0 ± 1.6 * | |
| Mean Corpuscular Hemoglobin Concentration (g/dL) | Visit 2 | 33.4 ± 0.8 | 33.6 ± 0.8 | 33.3 ± 1.1 | 33.5 ± 0.8 | 33.6 ± 0.9 | 33.5 ± 0.9 |
| Visit 3 | 33.0 ± 0.8 | 33.5 ± 0.5 | 33.4 ± 1.1 | 33.4 ± 0.9 | 33.7 ± 0.9 | 33.4 ± 0.9 | |
| Red Cell Distribution Width (%) | Visit 2 | 13.4 ± 0.7 | 13.2 ± 0.5 | 13.2 ± 0.9 | 13.2 ± 0.6 | 13.4 ± 0.6 | 13.3 ± 0.6 |
| Visit 3 | 13.3 ± 0.5 | 13.3 ± 0.5 | 13.3 ± 0.7 | 13.3 ± 0.6 | 13.4 ± 0.6 | 13.3 ± 0.6 | |
| Platelets (× 103/µL) | Visit 2 | 236 ± 55 | 265 ± 48 | 254 ± 42 | 240 ± 49 | 255 ± 55 | 250 ± 50 |
| Visit 3 | 220 ± 60 | 246 ± 57 | 256 ± 41 | 247 ± 50 | 266 ± 61 | 247 ± 56 |
| 100 mg of DYM | 100 mg of DYM + 50 mg of TCR | 150 mg of DYM + 25 mg of TCR | 150 mg of DYM | Placebo | Average | ||
|---|---|---|---|---|---|---|---|
| Absolute Neutrophils (× 103/µL) | Visit 2 | 2.68 ± 1.04 | 3.06 ± 1.47 | 2.82 ± 0.85 | 2.77 ± 1.24 | 2.56 ± 1.30 | 2.77 ± 1.18 |
| Visit 3 | 2.78 ± 1.28 | 2.85 ± 1.58 | 2.82 ± 0.85 | 2.58 ± 1.12 | 2.60 ± 0.90 | 2.72 ± 1.15 | |
| Absolute Lymphocytes (× 103/µL) | Visit 2 | 2.09 ± 0.33 | 2.31 ± 0.44 | 2.30 ± 0.50 | 2.13 ± 0.51 | 2.07 ± 0.51 | 2.18 ± 0.47 |
| Visit 3 | 2.25 ± 0.50 | 2.31 ± 0.72 | 2.24 ± 0.51 | 2.20 ± 0.48 | 2.20 ± 0.60 | 2.24 ± 0.56 | |
| Absolute Monocytes (× 103/µL) | Visit 2 | 0.463 ± 0.124 | 0.519 ± 0.172 | 0.432 ± 0.149 | 0.396 ± 0.112 | 0.475 ± 0.165 | 0.455 ± 0.149 |
| Visit 3 | 0.445 ± 0.122 | 0.491 ± 0.216 | 0.470 ± 0.122 | 0.414 ± 0.159 | 0.467 ± 0.134 | 0.458 ± 0.153 | |
| Absolute Eosinophils (× 103/µL) | Visit 2 | 0.183 ± 0.169 | 0.162 ± 0.102 | 0.144 ± 0.112 | 0.138 ± 0.077 | 0.175 ± 0.099 | 0.160 ± 0.116 |
| Visit 3 | 0.186 ± 0.146 | 0.191 ± 0.177 | 0.157 ± 0.112 | 0.181 ± 0.112 | 0.192 ± 0.132 | 0.181 ± 0.136 * | |
| Absolute Basophils (× 103/µL) | Visit 2 | 0.017 ± 0.038 | 0.014 ± 0.036 | 0.012 ± 0.033 | <0.010 | <0.010 | 0.008 ± 0.028 |
| Visit 3 | 0.009 ± 0.029 | 0.009 ± 0.029 | 0.027 ± 0.046 | 0.014 ± 0.036 | 0.008 ± 0.028 | 0.014 ± 0.034 | |
| Immature Granulocytes (× 103/µL) | Visit 2 | 0.208 ± 0.415 | 0.429 ± 0.978 | 0.080 ± 0.277 | 0.261 ± 0.689 | 0.333 ± 0.637 | 0.256 ± 0.632 |
| Visit 3 | 0.318 ± 0.477 | 0.364 ± 0.79 | <0.010 | 0.048 ± 0.218 | 0.208 ± 0.658 | 0.188 ± 0.529 | |
| Absolute Immature Granulocytes (× 103/µL) | Visit 2 | 0.017 ± 0.038 | 0.029 ± 0.072 | 0.004 ± 0.020 | 0.009 ± 0.029 | 0.025 ± 0.044 | 0.016 ± 0.043 |
| Visit 3 | 0.014 ± 0.035 | 0.014 ± 0.035 | <0.010 | 0.010 ± 0.030 | 0.013 ± 0.034 | 0.010 ± 0.030 |
| 100 mg of DYM | 100 mg of DYM + 50 mg of TCR | 150 mg of DYM + 25 mg of TCR | 150 mg of DYM | Placebo | Average | ||
|---|---|---|---|---|---|---|---|
| Glucose (mg/dL) | Visit 2 | 87.5 ± 7.2 | 89.1 ± 7.4 | 90.2 ± 5.2 | 91.2 ± 9.4 | 91.0 ± 8.5 | 89.8 ± 7.7 |
| Visit 3 | 87.2 ± 8.5 | 88.2 ± 9.1 | 88.9 ± 6.4 | 91.9 ± 7.6 | 89.9 ± 8.8 | 89.2 ± 8.2 | |
| Blood Urea Nitrogen (mg/dL) | Visit 2 | 15.2 ± 4.1 | 14.0 ± 4.0 | 14.0 ± 4.6 | 14.2 ± 4.1 | 14.0 ± 3.3 | 14.3 ± 4.0 |
| Visit 3 | 16.5 ± 4.7 | 12.6 ± 3.7# | 14.3 ± 4.9 | 14.0 ± 3.9 | 14.1 ± 2.8 | 14.3 ± 4.2 | |
| Creatinine (mg/dL) | Visit 2 | 0.984 ± 0.220 | 0.955 ± 0.161 | 0.870 ± 0.191 | 0.947 ± 0.246 | 0.908 ± 0.172 | 0.932 ± 0.201 |
| Visit 3 | 1.003 ± 0.210 | 0.999 ± 0.177 | 0.889 ± 0.198 | 0.981 ± 0.230 | 0.914 ± 0.172 | 0.957 ± 0.200 * | |
| Estimated Glomerular Filtration Rate (mL/min/1.73) | Visit 2 | 96.6 ± 17.8 | 94.1 ± 26.3 | 107.8 ± 16.7 | 93.3 ± 29.0 | 104.0 ± 14.9 | 99.3 ± 22.0 |
| Visit 3 | 84.1 ± 31.0 | 92.0 ± 15.4 | 105.7 ± 15.4 | 91.6 ± 26.7 | 103.8 ± 16 | 95.5 ± 22.9 * | |
| Blood Urea Nitrogen–Creatinine Ratio | Visit 2 | 16.1 ± 5.0 | 14.8 ± 4.4 | 16.0 ± 4.2 | 14.9 ± 5.4 | 15.3 ± 3.3 | 15.4 ± 4.5 |
| Visit 3 | 16.7 ± 4.2 | 12.9 ± 4.1 | 16.0 ± 4.0 | 14.7 ± 4.1 | 15.5 ± 3.1 | 15.1 ± 4.1 | |
| Sodium (mmol/L) | Visit 2 | 141 ± 2 | 140 ± 2 | 140 ± 2 | 141 ± 2 | 140 ± 2 | 141 ± 2 |
| Visit 3 | 141 ± 1 | 140 ± 2 | 140 ± 2 | 141 ± 2 | 141 ± 2 | 141 ± 2 | |
| Potassium (mmol/L) | Visit 2 | 4.80 ± 0.42 | 4.62 ± 0.48 | 4.46 ± 0.44 | 4.98 ± 1.91 | 4.35 ± 0.32 | 4.64 ± 0.95 |
| Visit 3 | 4.61 ± 0.52 | 4.63 ± 0.83 | 4.41 ± 0.29 | 4.64 ± 0.70 | 4.60 ± 0.42 | 4.58 ± 0.59 | |
| Chloride (mmol/L) | Visit 2 | 102 ± 3 | 101 ± 4 | 102 ± 2 | 103 ± 2 | 102 ± 2 | 102 ± 3 |
| Visit 3 | 103 ± 2 | 103 ± 2 | 102 ± 2 | 103 ± 3 | 103 ± 2 | 103 ± 2 * | |
| Total Carbon Dioxide (mmol/L) | Visit 2 | 24.3 ± 2.3 | 24.3 ± 2.0 | 23.2 ± 2.5 | 23.4 ± 2.2 | 23.9 ± 2.2 | 23.8 ± 2.2 |
| Visit 3 | 23.2 ± 2.1 | 23.3 ± 1.7 | 22.2 ± 2.3 | 23.2 ± 2.6 | 24.3 ± 2.3 | 23.3 ± 2.3 * | |
| Calcium (mg/dL) | Visit 2 | 9.64 ± 0.27 | 9.53 ± 0.31 | 9.47 ± 0.37 | 9.32 ± 0.45 | 9.53 ± 0.34 | 9.50 ± 0.37 |
| Visit 3 | 16.77 ± 23.90 | 9.40 ± 0.34 | 9.44 ± 0.29 | 9.44 ± 0.33 | 9.50 ± 0.38 | 10.87 ± 10.77 |
| 100 mg of DYM | 100 mg of DYM + 50 mg of TCR | 150 mg of DYM + 25 mg of TCR | 150 mg of DYM | Placebo | Average | ||
|---|---|---|---|---|---|---|---|
| Total Protein (g/dL) | Visit 2 | 7.18 ± 0.34 | 7.26 ± 0.52 | 7.06 ± 0.53 | 6.92 ± 0.72 | 7.18 ± 0.25 | 7.12 ± 0.51 |
| Visit 3 | 7.07 ± 0.31 | 7.17 ± 0.51 | 7.08 ± 0.36 | 7.11 ± 0.76 | 7.18 ± 0.39 | 7.12 ± 0.49 | |
| Albumin (A) (g/dL) | Visit 2 | 4.67 ± 0.26 | 4.61 ± 0.33 | 4.60 ± 0.42 | 4.56 ± 0.30 | 4.66 ± 0.32 | 4.62 ± 0.33 |
| Visit 3 | 4.65 ± 0.26 | 4.53 ± 0.31 | 4.54 ± 0.38 | 4.62 ± 0.42 | 4.64 ± 0.31 | 4.59 ± 0.34 | |
| Total Globulin (G) (g/dL) | Visit 2 | 2.47 ± 0.19 | 2.65 ± 0.40 | 2.58 ± 0.43 | 2.59 ± 0.42 | 2.58 ± 0.35 | 2.57 ± 0.37 |
| Visit 3 | 2.43 ± 0.23 | 2.52 ± 0.42 | 2.67 ± 0.38 | 2.67 ± 0.38 | 2.58 ± 0.36 | 2.57 ± 0.37 | |
| A/G Ratio | Visit 2 | 1.90 ± 0.19 | 1.78 ± 0.31 | 1.83 ± 0.35 | 1.81 ± 0.31 | 1.84 ± 0.32 | 1.83 ± 0.30 |
| Visit 3 | 1.94 ± 0.24 | 1.84 ± 0.3 | 1.73 ± 0.28 | 1.76 ± 0.31 | 1.84 ± 0.29 | 1.82 ± 0.29 | |
| Bilirubin, Total (mg/dL) | Visit 2 | 0.529 ± 0.229 | 0.496 ± 0.272 | 0.504 ± 0.348 | 0.424 ± 0.230 | 0.496 ± 0.321 | 0.489 ± 0.282 |
| Visit 3 | 0.448 ± 0.250 | 0.468 ± 0.281 | 0.483 ± 0.248 | 0.391 ± 0.095 | 0.454 ± 0.213 | 0.449 ± 0.226 * | |
| Alkaline Phosphatase (IU/L) | Visit 2 | 61.8 ± 15.6 | 66.9 ± 23.1 | 66.4 ± 21.3 | 62.5 ± 15.8 | 68.4 ± 18.2 | 65.2 ± 18.9 |
| Visit 3 | 60.3 ± 15.5 | 65.5 ± 20.8 | 67.6 ± 21.2 | 62.8 ± 15.6 | 71.4 ± 24.4 | 65.6 ± 19.9 | |
| Aspartate Aminotransferase (IU/L) | Visit 2 | 30.9 ± 22.8 | 23.4 ± 10.0 | 21.7 ± 7.2 | 23.2 ± 8.2 | 24.6 ± 8.9 | 24.7 ± 12.9 |
| Visit 3 | 28.9 ± 17.7 | 24.7 ± 10.1 | 23.2 ± 8.9 | 24.6 ± 11.6 | 22.0 ± 7.1 | 24.7 ± 11.6 | |
| Alanine Aminotransferase (IU/L) | Visit 2 | 23.8 ± 11.7 | 22.5 ± 22.7 | 19.2 ± 9.7 | 14.8 ± 6.4 | 21.3 ± 10.6 | 20.3 ± 13.6 |
| Visit 3 | 19.6 ± 7.9 | 22.4 ± 18.0 | 19.7 ± 10 | 15.7 ± 6.7 | 18.8 ± 9.6 | 19.3 ± 11.3 * |
| 100 mg of DYM | 100 mg of DYM + 50 mg of TCR | 150 mg of DYM + 25 mg of TCR | 150 mg of DYM | Placebo | Average | ||
|---|---|---|---|---|---|---|---|
| Total Cholesterol (mg/dL) | Visit 2 | 158 ± 26 | 167 ± 30 | 155 ± 34 | 145 ± 22 | 175 ± 22 | 160 ± 28 |
| Visit 3 | 160 ± 35 | 166 ± 24 | 157 ± 34 | 148 ± 23 | 167 ± 34 | 160 ± 31 | |
| Triglycerides (mg/dL) | Visit 2 | 69.8 ± 29.7 | 86.3 ± 39.6 | 84.9 ± 32.2 | 84.3 ± 39.5 | 96.0 ± 60.6 | 84.3 ± 41.8 |
| Visit 3 | 75.3 ± 31.9 | 77.8 ± 35.5 | 91.1 ± 41.3 | 83.4 ± 30.5 | 82.2 ± 44.1 | 81.9 ± 36.8 | |
| High-Density Lipoproteins (mg/dL) | Visit 2 | 63.8 ± 13.9 | 56.7 ± 13.7 | 59.3 ± 12.4 | 54.7 ± 12.3 | 58.2 ± 17.4 | 58.5 ± 14.1 |
| Visit 3 | 63.2 ± 14.0 | 60.0 ± 16.1 | 61.1 ± 15.6 | 59.5 ± 14.2 | 57.4 ± 22.2 | 60.2 ± 16.5 * | |
| Very-Low-Density Lipoproteins (mg/dL) | Visit 2 | 13.9 ± 6.0 | 17.2 ± 7.9 | 15.4 ± 5.5 | 15.8 ± 7.9 | 18.0 ± 11.6 | 16.0 ± 8.0 |
| Visit 3 | 15.0 ± 6.6 | 15.8 ± 7.4 | 16.8 ± 7.8 | 16.3 ± 6.1 | 17.2 ± 9.4 | 16.2 ± 7.4 | |
| Low-Density Lipoproteins (mg/dL) | Visit 2 | 80.8 ± 21.0 | 93.8 ± 23.9 | 80.0 ± 28.5 | 74.9 ± 19.7 | 98.6 ± 22.4 | 85.6 ± 24.6 |
| Visit 3 | 81.6 ± 26.9 | 90.2 ± 24.2 | 79.5 ± 27.3 | 72.3 ± 20.1 | 96.4 ± 24.4 | 84.2 ± 25.7 |
| Sample Size | Sex | Carbohydrates (g) | Fat (g) | Protein (g) | Total Calories | p-Value | ||
|---|---|---|---|---|---|---|---|---|
| 100 mg of DYM | 12 | M | Week 1 | 202 ± 43 | 72 ± 20 | 177 ± 27 | 2166 ± 323 | 0.17 |
| Week 4 | 206 ± 39 | 64 ± 34 | 166 ± 34 | 2060 ± 311 | ||||
| 13 | F | Week 1 | 174 ± 52 | 61 ± 26 | 98 ± 34 | 1637 ± 409 | 0.72 | |
| Week 4 | 188 ± 46 | 63 ± 25 | 90 ± 25 | 1681 ± 356 | ||||
| 100 mg of DYM + 50 mg of TCR | 12 | M | Week 1 | 231 ± 30 | 67 ± 20 | 118 ± 33 | 2002 ± 253 | 0.13 |
| Week 4 | 229 ± 33 | 75 ± 26 | 126 ± 27 | 2091 ± 268 | ||||
| 13 | F | Week 1 | 181 ± 31 | 43 ± 12 | 95 ± 22 | 1488 ± 221 | 0.53 | |
| Week 4 | 176 ± 29 | 52 ± 19 | 89 ± 22 | 1528 ± 174 | ||||
| 150 mg of DYM +25 mg of TCR | 12 | M | Week 1 | 238 ± 57 | 74 ± 34 | 147 ± 51 | 2172 ± 330 | 0.48 |
| Week 4 | 253 ± 52 | 70 ± 34 | 150 ± 62 | 2244 ± 517 | ||||
| 13 | F | Week 1 | 186 ± 79 | 58 ± 13 | 90 ± 28 | 1627 ± 337 | 0.75 | |
| Week 4 | 194 ± 68 | 54 ± 14 | 96 ± 22 | 1652 ± 307 | ||||
| 150 mg of DYM | 12 | M | Week 1 | 198 ± 214 | 72 ± 24 | 161 ± 48 | 2087 ± 345 | 0.66 |
| Week 4 | 214 ± 43 | 65 ± 20 | 149 ± 49 | 2037 ± 388 | ||||
| 13 | F | Week 1 | 166 ± 46 | 56 ± 13 | 83 ± 27 | 1501 ± 232 | 0.12 | |
| Week 4 | 171 ± 58 | 62 ± 16 | 89 ± 21 | 1600 ± 306 | ||||
| Placebo (maltodextrin) | 12 | M | Week 1 | 170 ± 56 | 85 ± 20 | 158 ± 65 | 2078 ± 405 | 0.73 |
| Week 4 | 185 ± 42 | 72 ± 23 | 161 ± 60 | 2028 ± 212 | ||||
| 13 | F | Week 1 | 213 ± 85 | 56 ± 26 | 89 ± 32 | 1711 ± 425 | 0.15 | |
| Week 4 | 186 ± 98 | 57 ± 21 | 84 ± 37 | 1598 ± 434 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
VanDusseldorp, T.A.; Stratton, M.T.; Bailly, A.R.; Holmes, A.J.; Alesi, M.G.; Feito, Y.; Mangine, G.T.; Hester, G.M.; Esmat, T.A.; Barcala, M.; et al. Safety of Short-Term Supplementation with Methylliberine (Dynamine®) Alone and in Combination with TeaCrine® in Young Adults. Nutrients 2020, 12, 654. https://doi.org/10.3390/nu12030654
VanDusseldorp TA, Stratton MT, Bailly AR, Holmes AJ, Alesi MG, Feito Y, Mangine GT, Hester GM, Esmat TA, Barcala M, et al. Safety of Short-Term Supplementation with Methylliberine (Dynamine®) Alone and in Combination with TeaCrine® in Young Adults. Nutrients. 2020; 12(3):654. https://doi.org/10.3390/nu12030654
Chicago/Turabian StyleVanDusseldorp, Trisha A., Matthew T. Stratton, Alyssa R. Bailly, Alyssa J. Holmes, Michaela G. Alesi, Yuri Feito, Gerald T. Mangine, Garrett M. Hester, Tiffany A. Esmat, Megan Barcala, and et al. 2020. "Safety of Short-Term Supplementation with Methylliberine (Dynamine®) Alone and in Combination with TeaCrine® in Young Adults" Nutrients 12, no. 3: 654. https://doi.org/10.3390/nu12030654
APA StyleVanDusseldorp, T. A., Stratton, M. T., Bailly, A. R., Holmes, A. J., Alesi, M. G., Feito, Y., Mangine, G. T., Hester, G. M., Esmat, T. A., Barcala, M., Tuggle, K. R., Snyder, M., & Modjeski, A. S. (2020). Safety of Short-Term Supplementation with Methylliberine (Dynamine®) Alone and in Combination with TeaCrine® in Young Adults. Nutrients, 12(3), 654. https://doi.org/10.3390/nu12030654







