Synthesis and Electrochemical Properties of Bi2MoO6/Carbon Anode for Lithium-Ion Battery Application
Abstract
:1. Introduction
2. Experimental and Computational Details
2.1. Materials Synthesis
2.2. Materials Characterization
2.3. Electrochemical Measurements
2.4. Computational Details
3. Results and Discussion
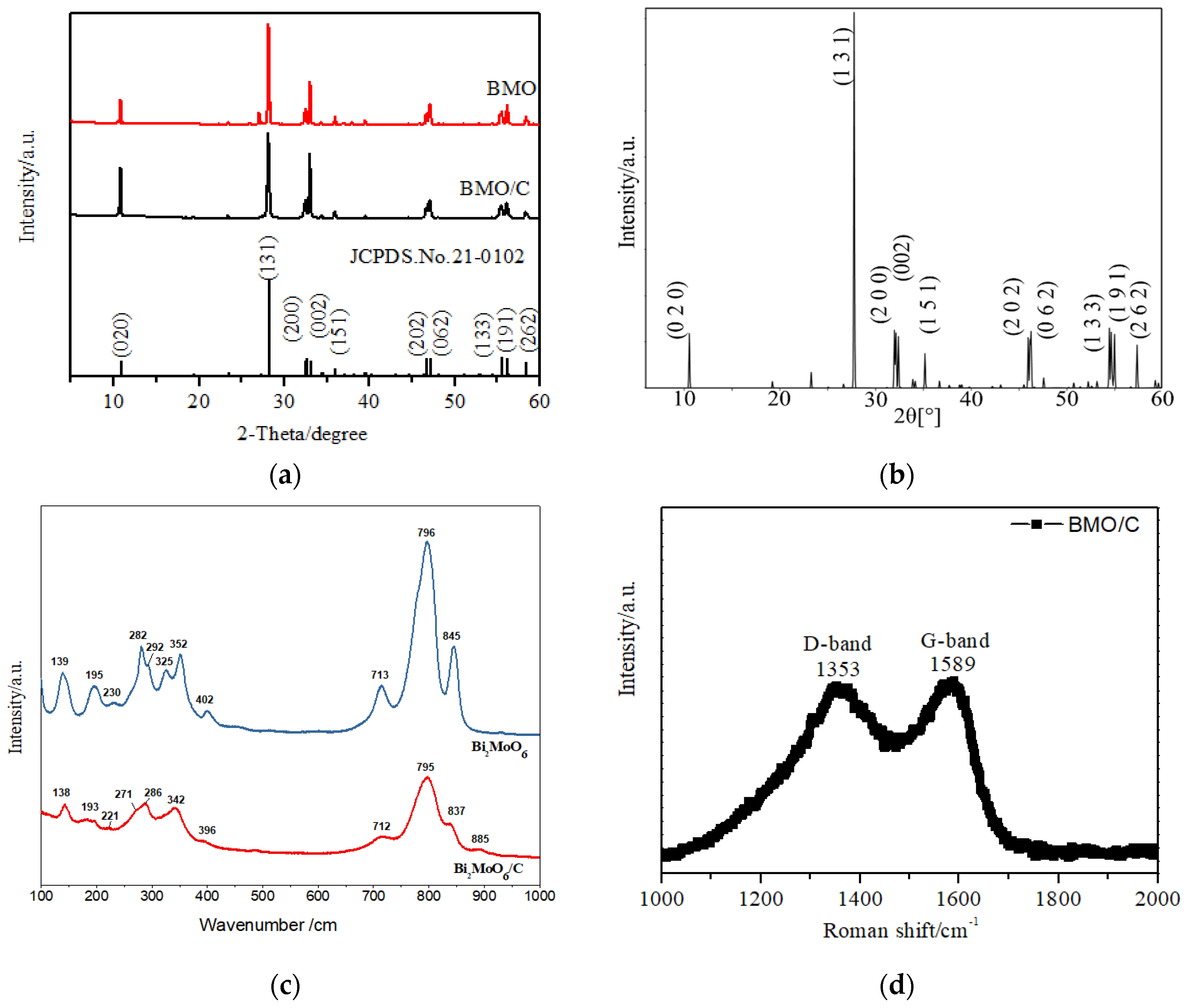
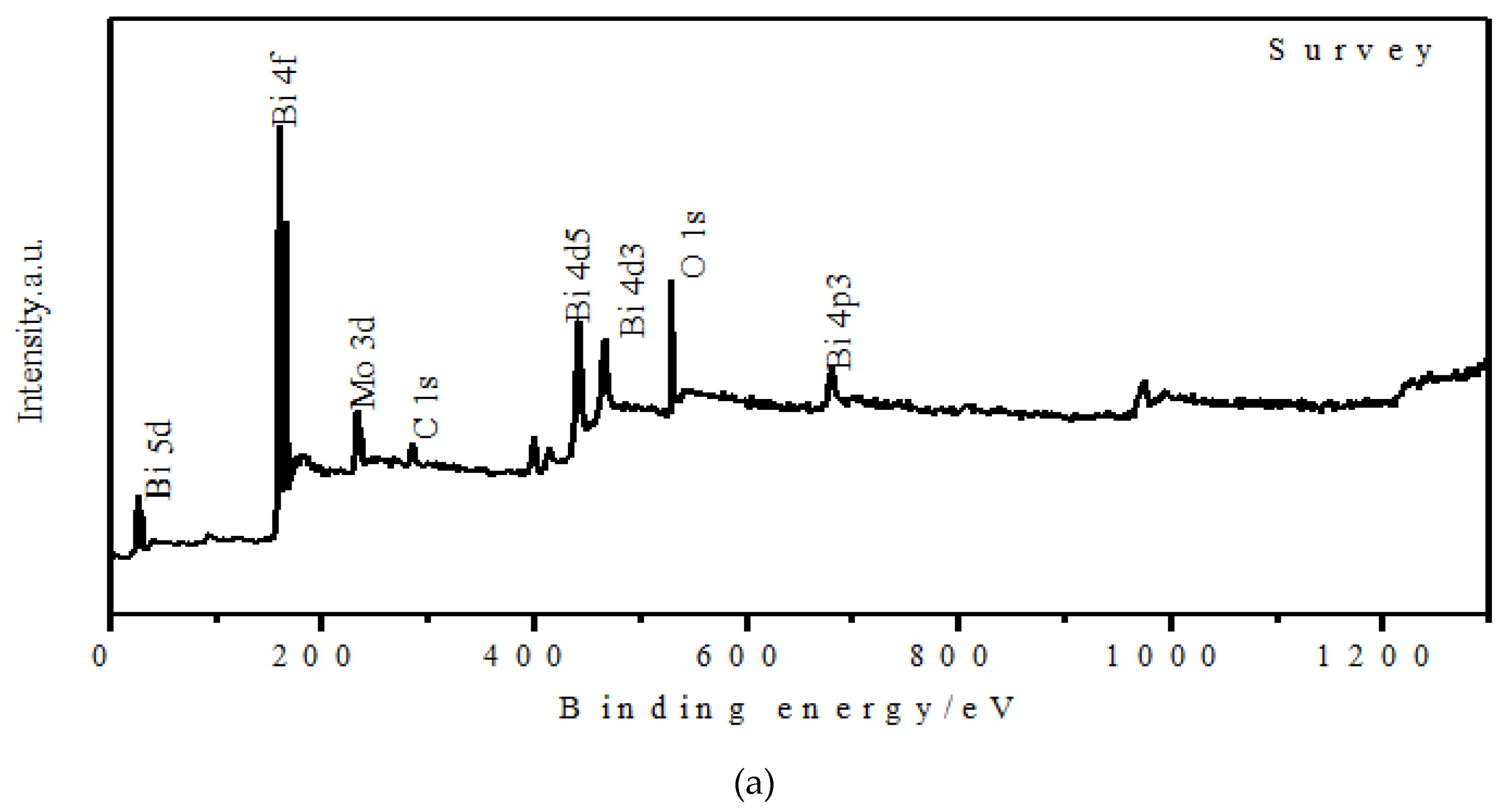
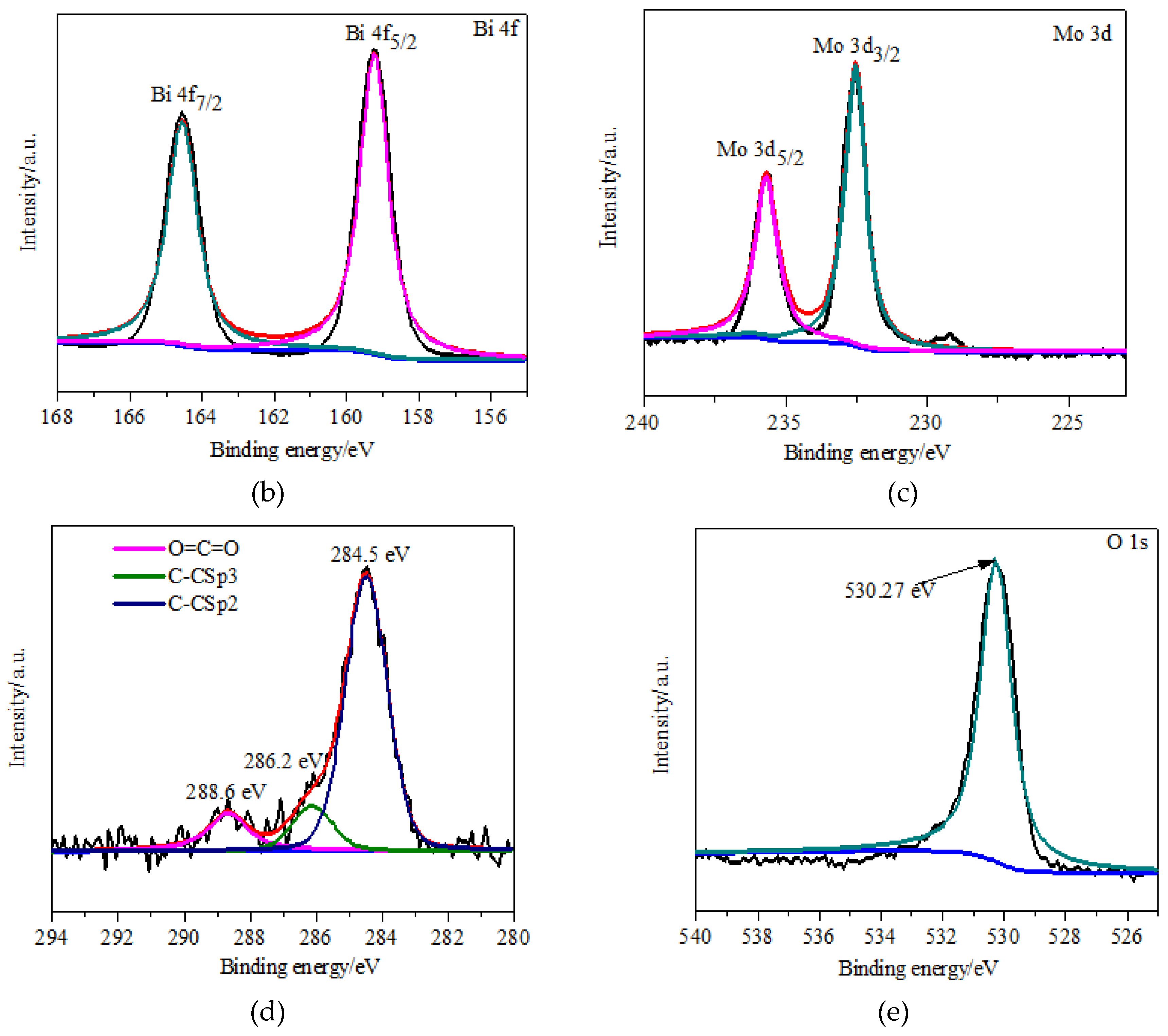
3.1. Materials Characterization
Computational Characterization of Bi2MoO6
3.2. Experimental Characterization
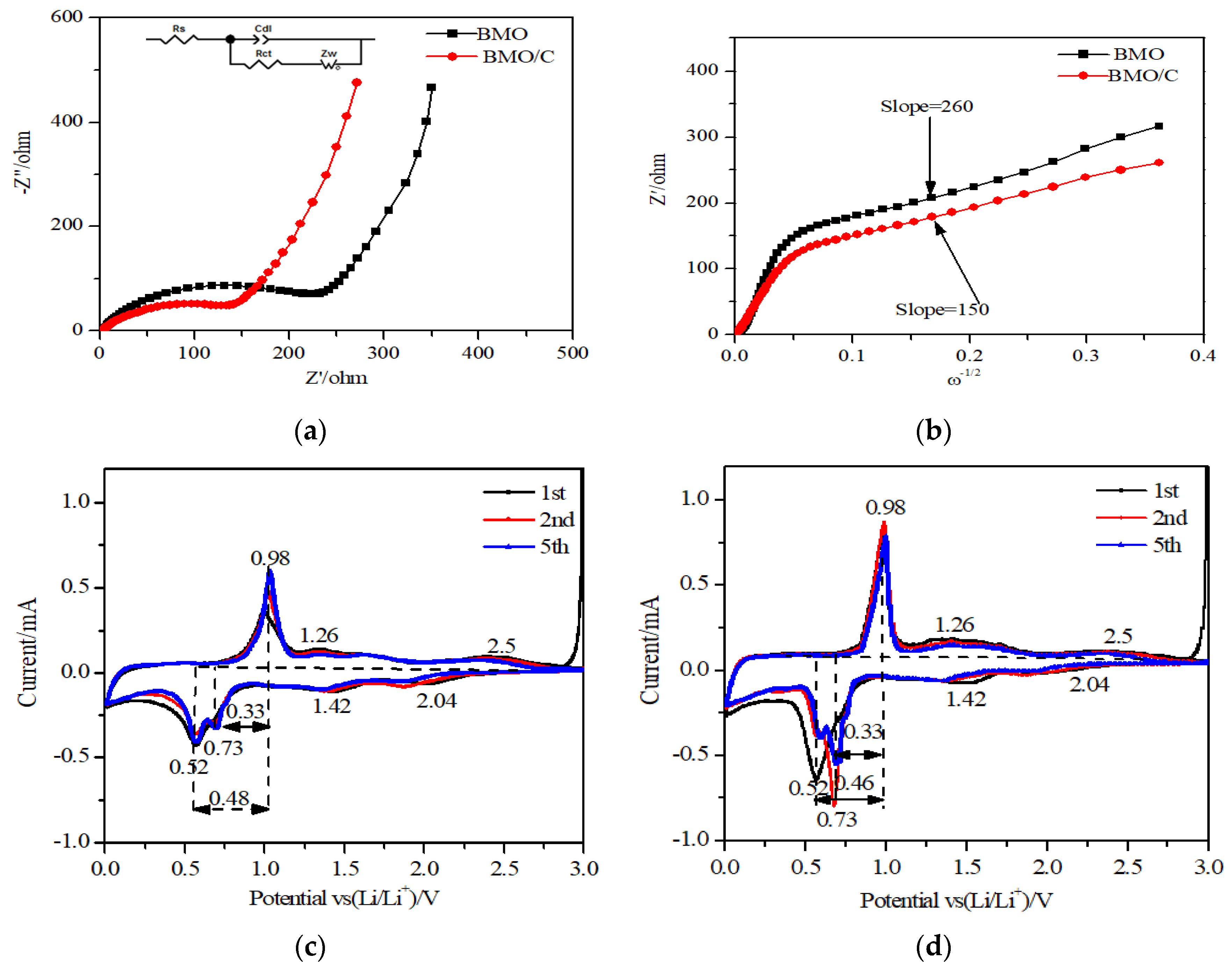
3.3. Electrochemical Properties
3.4. Modelling of Li Insertion in the Bi2MoO6 Lattice
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hawley, W.B.; Li, J. Electrode manufacturing for lithium-ion batteries—Analysis of current and next generation processing. J. Energy Storage 2019, 25, 100862. [Google Scholar] [CrossRef]
- Gauthier, M.; Carney, T.J.; Grimaud, A.; Giordano, L.; Pour, N.; Chang, H.H.; David, P.F.; Simon, F.L.; Odysseas, P.; Christoph, B.; et al. The electrode-electrolyte interface in li-ion batteries: Current understanding and new insights. J. Phys. Chem. Lett. 2015, 6, 4653–4672. [Google Scholar] [CrossRef] [PubMed]
- Nishi, Y. Lithium ion secondary batteries; past 10 years and the future. J. Power Sources 2001, 100, 101–106. [Google Scholar] [CrossRef]
- Giubileo, F.; Bartolomeo, A.D. The role of contact resistance in graphene field-effect devices. Prog. Surf. Sci. 2017, 92, 143–175. [Google Scholar] [CrossRef] [Green Version]
- Huiqiao, L.; Haoshen, Z. Enhancing the performances of Li-ion batteries by carbon-coating:present and future. Chem. Commun. 2012, 48, 1201–1217. [Google Scholar]
- Kang, X. Electrolytes and Interphases in Li-Ion Batteries and Beyond. Chem. Rev. 2014, 114, 11503–11618. [Google Scholar]
- Kang, X. Nonaqueous Liquid Electrolytes for Lithium-Based Rechargeable Batteries. Chem. Rev. 2004, 104, 4303–4417. [Google Scholar]
- Mei, J.; Zhang, Y.; Liao, T.; Sun, Z.; Dou, S.X.; Chemistry, S.O. Strategies for improving the lithium-storage performance of 2D nanomaterials. Natl. Sci. Rev. 2018, 5, 389–416. [Google Scholar] [CrossRef]
- Zahid, A.G.; Zhenhua, S.; Chengguo, S.; Fulai, Q.; Baigang, A.; Feng, L.; Hui, M.C. Key Aspects of Lithium Metal Anodes for Lithium Metal Batteries. Small 2019, 15, 1–26. [Google Scholar]
- Cheng, C.F.; Xue, F.W.; Ying, S.M. Key Issues Hindering a Practical Lithium-Metal Anode. Trend Chem. 2019, 26, 1–7. [Google Scholar]
- Chao, J.N.; Hong, K.L.; Shu, R.C.; Qiu, Y.L.; Jason, D.; Wu, X.; Ji, G.Z.; Whittingham, M.S.; Jie, X.; Jun, L. High-energy lithium metal pouch cells with limited anode swelling and long stable cycles. Nat. Energy 2019, 4, 551–559. [Google Scholar]
- Peng, L.; Guo, Q.Z.; Xiao, B.; Zhen, G.; Xun, X.; Cheng, H.Y.; Wen, P.S.; Shi, X.D. Recent progress on silicon-based anode materials for practical lithium-ion battery applications. Energy Storage Mater. 2018, 15, 422–446. [Google Scholar]
- Poizot, P.; Laruelle, S.; Grugeon, S.; Dupont, L.; Tarascon, J.M. Nano-sized transition-metal oxides as negative-electrode materials for lithium-ion batteries. Nature 2000, 407, 496–499. [Google Scholar] [CrossRef] [PubMed]
- Abbas, S.M.; Hussain, S.T.; Ali, S.; Abbas, M.S. Structure and electrochemical performance of ZnO/CNT composite as anode material for lithium-ion batteries. J. Mater. Sci. 2013, 48, 5429–5436. [Google Scholar] [CrossRef]
- Lu, S.; Wang, H.; Zhou, J.; Wu, X.; Qin, W. Atomic layer deposition of ZnO on carbon black as nanostructured anode materials for high-performance lithium-ion batteries. Nanoscale 2017, 9, 1184–1192. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Kirk, C.; Cai, Q.; Cuadrado- Collados, C.; Silvestre-Albero, J.; Rodríguez- Reinoso, F.; Biggs, M. A High-Volumetric-Capacity Cathode Based on Interconnected Close-Packed N-Doped Porous Carbon Nanospheres for Long-Life Lithium-Sulfur Batteries. Adv. Energy Mater. 2017, 7, 1701082. [Google Scholar] [CrossRef]
- Courtel, F.M.; Duncan, H.; Abu-Lebdeh, Y.; Davidson, I.J. High capacity anode materials for Li-ion batteries based on spinel metal oxides AMn2O4 (A = Co, Ni, and Zn). J. Mater. Chem. 2011, 21, 10206–10218. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Xiong, S.; Li, X.; Qian, Y. Spinel Mn1.5Co1.5O4 core–shell microspheres as Li-ion battery anode materials with a long cycle life and high capacity. J. Mater. Chem. 2012, 22, 23254–23259. [Google Scholar] [CrossRef]
- Hu, L.; Qu, B.; Li, C.; Chen, Y.; Mei, L.; Lei, D. Facile synthesis of uniform mesoporous ZnCo2O4 microspheres as a high-performance anode material for Li-ion batteries. J. Mater. Chem. A 2013, 1, 5596–5602. [Google Scholar] [CrossRef]
- Reddy, M.V.; Rao, G.V.S.; Chowdari, B.V.R. Metal Oxides and Oxysalts as Anode Materials for Li Ion Batteries. Chem. Rev. 2013, 113, 5364–5457. [Google Scholar] [CrossRef]
- Marka, S.K.; Petnikota, S.; Srikanth, V.V.S.S.; Reddy, M.V.; Stefan, A.; Chowdari, B.V.R. Co2Mo3O8/reduced graphene oxide composite: Synthesis, characterization, and its role as a prospective anode material in lithium ion batteries. RSC Adv. 2016, 6, 55167–55175. [Google Scholar] [CrossRef]
- Das, B.; Reddy, M.V.; Krishnamoorthi, C.; Tripathy, S.; Mahendiran, R.; Rao, G.V.S.; Chowdari, B.V.R. Carbothermal synthesis, spectral and magnetic characterization and Li-cyclability of the Mo-cluster compounds, LiYMo3O8 and Mn2Mo3O8. Electrochim. Acta. 2009, 54, 3360–3373. [Google Scholar] [CrossRef]
- Das, B.; Reddy, M.V.; Tripathy, S.; Chowdari, B.V.R. A disc-like Mo-metal cluster compound, Co2Mo3O8, as a high capacity anode for lithium ion batteries. RSC Adv. 2014, 4, 33883. [Google Scholar] [CrossRef]
- Petnikota, S.; Marka, S.K.; Srikanth, V.V.S.S.; Reddy, M.V.; Chowdari, B.V.R. Elucidation of few layered graphene-complex metal oxide (A2Mo3O8, A = Co, Mn and Zn) composites as robust anode materials in Li ion batteries. Electrochim. Acta 2015, 178, 699–708. [Google Scholar] [CrossRef]
- Yuan, S.; Zhao, Y.; Chen, W.; Wu, C.; Wang, X.; Zhang, L.; Wang, Q. Self-assembled 3D hierarchical porous Bi2MoO6 microspheres toward high capacity and ultra-long-life anode material for Li-ion batteries. ACS Appl. Mater. Interfaces 2017, 9, 21781–21790. [Google Scholar] [CrossRef]
- Zhai, X.; Gao, J.; Xue, R.; Xu, X.; Wang, L.; Tian, Q. Facile synthesis of Bi2MoO6/reduced graphene oxide composites as anode materials towards enhanced lithium storage performance. J. Colloid Interface Sci. 2018, 518, 242–251. [Google Scholar] [CrossRef]
- Kudin, T.I.T.; Zainal, N.F.A.; Ali, A.M.M. Electrochemical performance of anode material from palm oils derived carbon nanotubes for lithium ion batteries. Mater. Res. Innov. 2009, 13, 269–271. [Google Scholar] [CrossRef]
- Wang, K.; Zhao, N.; Lei, S. Promising biomass-based activated carbons derived from willow catkins for high performance supercapacitors. Electrochim. Acta. 2015, 166, 1–11. [Google Scholar] [CrossRef]
- Li, Y.; Trujillo, M.A.; Fu, E. Bismuth Oxide: A New Lithium-Ion Battery Anode. J. Mater. Chem. A 2013, 1, 12123–12127. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, T.; Zhao, X.; Zhu, Y. Controllable synthesis of Bi2MoO6 and effect of morphology and variation in local structure on photocatalytic activities. Appl. Catal. B Environ. 2010, 98, 138–146. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhou, T.; Zhao, X.; Pang, W.K.; Gao, H.; Li, S.; Zhou, Z.; Liu, H.; Guo, Z. Atomic interface engineering and electric-field effect in ultrathin Bi2MoO6 nanosheets for superior lithium ion storage. Adv. Mater. 2017, 29, 1700396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 1993, 47, 558–561. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Hafner, J. Ab initio molecular-dynamics simulation of the liquid-metal–amorphous-semiconductor transition in germanium. Phys. Rev. B 1994, 49, 14251–14269. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B. Condens. Matter. 1996, 54, 11169–11186. [Google Scholar] [CrossRef] [PubMed]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Blöchl, P.E.; Jepsen, O.; Andersen, O.K. Improved tetrahedron method for Brillouin-zone integrations. Phys. Rev. B 1994, 49, 16223–16233. [Google Scholar] [CrossRef]
- Perdew, J.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [Green Version]
- Perdew, J.; Burke, K.; Ernzerhof, M. Errata: Generalized gradient approximation made simple. Phys. Rev. Lett. 1997, 78, 1396. [Google Scholar] [CrossRef] [Green Version]
- Bader, R.F.W. Atoms in Molecules: A Quantum Theory; Oxford University Press: Oxford, UK, 1990. [Google Scholar]
- Henkelman, G.; Arnaldsson, A.; Jónsson, H. A fast and robust algorithm for Bader decomposition of charge density. Comput. Mater. Sci. 2006, 36, 354–360. [Google Scholar] [CrossRef]
- Geng, T.; Han, Z.; Zhuang, S. Effective Coulomb interaction in LaMnO3. Phys. B Condens. Matter. 2010, 405, 3714–3716. [Google Scholar] [CrossRef]
- Ravindran, P.; Kjekshus, A.; Fjellvåg, H.; Delin, A.; Eriksson, O. Ground-state and excited-state properties of LaMnO3 from full-potential calculations. Phys. Rev. B. 2002, 65, 064445. [Google Scholar] [CrossRef] [Green Version]
- Ravindran, P.; Vidya, R.; Fjellvåg, H.; Kjekshus, A. Electronic structure and excited-state properties of perovskite-like oxides. J. Cryst. Growth. 2004, 268, 554–559. [Google Scholar] [CrossRef]
- Getsoian, A.; Shapovalov, V.; Bell, A.T. DFT+U investigation of propene oxidation over bismuth molybdate: Active sites, reaction intermediates, and the role of bismuth. J. Phys. Chem. C 2013, 117, 7123–7137. [Google Scholar] [CrossRef]
- Dudarev, S.; Botton, G.; Savrasov, S. Electron-energy-loss spectra and the structural stability of nickel oxide: An LSDA+ U study. Phys. Rev. B 1998, 57, 1505–1509. [Google Scholar] [CrossRef]
- Fan, X.; Zheng, W.T.; Kuo, J.L.; Singh, D.J. Adsorption of single Li and the formation of small Li clusters on graphene for the anode of lithium-ion batteries. ACS Appl. Mater. Interfaces. 2013, 5, 7793–7797. [Google Scholar] [CrossRef]
- Amft, M.; Lebègue, S.; Eriksson, O.; Skorodumova, N.V. Adsorption of Cu, Ag, and Au atoms on graphene including van der Waals interactions. J. Phys. Condens. Matter. 2011, 23, 395001. [Google Scholar] [CrossRef]
- Lee, S.; Lee, M.; Choi, H.; Yoo, D.S.; Chung, Y.C. Effect of nitrogen induced defects in Li dispersed graphene on hydrogen storage. Int. J. Hydrogen Energy. 2013, 38, 4611–4617. [Google Scholar] [CrossRef]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef]
- Yanovskii, V.K.; Voronkova, V.I. Polymorphism and properties of Bi2WO6 and Bi2MoO6. Phys. Status Solidi. 1986, 93, 57–66. [Google Scholar] [CrossRef]
- Bi, J.; Wu, L.; Li, J.; Li, Z.; Wang, X.; Fu, X. Simple solvothermal routes to synthesize nanocrystalline Bi2MoO6 photocatalysts with different morphologies. Acta Mater. 2007, 55, 4699–4705. [Google Scholar] [CrossRef]
- Yin, W.; Wang, W.; Sun, S. Photocatalytic degradation of phenol over cage-like Bi2MoO6 hollow spheres under visible-light irradiation. Catal. Commun. 2010, 11, 647–650. [Google Scholar] [CrossRef]
- Huang, Y.; Li, K.; Li, S.; Lin, Y.; Liu, H.; Tong, Y. Ultrathin Bi2MoO6 nanosheets for photocatalysis: Performance enhancement by atomic interfacial engineering. Chem. Select. 2018, 3, 7423–7428. [Google Scholar]
- Zhang, X.B.; Zhang, L.; Hu, J.S.; Huang, X.H. Facile hydrothermal synthesis and improved photocatalytic activities of Zn2+ doped Bi2MoO6 nanosheets. RSC Adv. 2016, 6, 32349–32357. [Google Scholar] [CrossRef]
- Yang, Z.; Shen, M.; Dai, K.; Zhang, X.; Chen, H. Controllable synthesis of Bi2MoO6 nanosheets and their facet-dependent visible-light-driven photocatalytic activity. Appl. Surf. Sci. 2018, 430, 505–514. [Google Scholar] [CrossRef]
- Murugan, R. Investigation on ionic conductivity and raman spectra of γ-Bi2MoO6. Phys. B Condens. Matter. 2004, 352, 227–232. [Google Scholar] [CrossRef]
- Wu, J.-B.; Lin, M.-L.; Cong, X.; Liu, H.-N.; Tan, P.-H. Raman spectroscopy of graphene-based materials and its applications in related devices. Chem. Soc. Rev. 2018, 47, 1822–1873. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Z.; Jiang, Z.J.; Maiyalagan, T.; Manthiram, A. Cobalt oxide-coated N- and B-doped graphene hollow spheres as bifunctional electrocatalysts for oxygen reduction and oxygen evolution reactions. J. Mater. Chem. A 2016, 4, 5877–5889. [Google Scholar] [CrossRef]
- Lan, L.; Shi, Z.; Zhang, Q.; Li, Y.; Yang, Y.; Wu, S.; Zhao, X. Defects lead to a massive enhancement in the UV-Vis-IR driven thermocatalytic activity of Co3O4 mesoporous nanorods. J. Mater. Chem. A 2018, 6, 7194–7205. [Google Scholar] [CrossRef]
- Fang, J.; Yuan, Y.F.; Wang, L.K.; Ni, H.L.; Zhu, H.L.; Yang, J.L. Synthesis and electrochemical performances of ZnO/MnO2 sea urchin-like sleeve array as anode materials for lithium-ion batteries. Electrochim. Acta 2009, 112, 364–370. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, L.; Preuß, K.; Qiao, M.; Titirici, M.-M.; Varcoe, J.; Cai, Q. Halloysite-derived nitrogen doped carbon electrocatalysts for anion exchange membrane fuel cells. J. Power Sources 2017, 372, 82–90. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.H.; Zhao, T.S.; Tan, P.; Wu, M.C.; Jiang, H.R. Ruthenium dioxide-decorated carbonized tubular polypyrrole as a bifunctional catalyst for non-aqueous lithium-oxygen batteries. Electrochim. Acta 2017, 257, 281–289. [Google Scholar] [CrossRef]
- Kwon, K.C.; Song, M.J.; Kwon, K.H.; Jeoung, H.V.; Kim, D.W.; Lee, G.S.; Hong, J.P.; Park, J.G. Nanoscale CuO solid-electrolyte-based conductive-bridging-random-access-memory cell operating multi-level-cell and 1selector1resistor. J. Mater. Chem. C 2015, 3, 9540–9550. [Google Scholar] [CrossRef]
- Wippermann, K.; Giffin, J.; Kuhri, S.; Lehnert, W.; Korte, C. The influence of water content in a proton-conducting ionic liquid on the double layer properties of the Pt/PIL interface. Phys. Chem. Chem. Phys. 2017, 19, 24706–24723. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Yu, H.; Huang, T.; Wang, H.; Zhu, M. A biomimetic nanofiber-based triboelectric nanogenerator with an ultrahigh transfer charge density. Nano Energy 2018, 48, 464–470. [Google Scholar] [CrossRef]
- Boukamp, B.A. Derivation of a Distribution Function of relaxation times for the (fractal) finite length Warburg. Electrochim. Acta 2017, 252, 154–163. [Google Scholar] [CrossRef]
- Hayamizu, K. Direct relations between ion diffusion constants and ionic conductivity for lithium electrolyte solutions. Electrochim. Acta. 2017, 254, 101–111. [Google Scholar] [CrossRef]
- Billaud, J.; Eames, C.; Tapia-Ruiz, N.; Roberts, M.R.; Naylor, A.J.; Armstrong, A.R.; Islam, M.S.; Bruce, P.G. Evidence of enhanced ion transport in Li-rich silicate intercalation materials. Adv. Energy Mater. 2017, 7, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Liu, H.; Yao, Z.; Cheng, J.; Li, T.; Li, Y.; Wolverton, C.; Wu, J.; Dravid, V.P. Electrochemistry of selenium with sodium and lithium: Kinetics and reaction mechanism. ACS Nano 2016, 10, 8788–8795. [Google Scholar] [CrossRef]
- Islam, M.S.; Fisher, C.A.J. Lithium and sodium battery cathode materials: Computational insights into voltage, diffusion and nanostructural properties. Chem. Soc. Rev. 2014, 43, 185–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.; Northrup, J. Chemical potential dependence of defect formation energies in GaAs: Application to Ga self-diffusion. Phys. Rev. Lett. 1991, 67, 2339–2342. [Google Scholar] [CrossRef]
- Grau-Crespo, R.; Hamad, S.; Catlow, C.R.A.; De Leeuw, N.H. Symmetry-adapted configurational modelling of fractional site occupancy in solids. J. Phys. Condens. Matter. 2007, 19, 256201. [Google Scholar] [CrossRef]
- Olsson, E.; Chai, G.; Dove, M.; Cai, Q. Adsorption and migration of alkali metals (Li, Na, and K) on pristine and defective graphene surfaces. Nanoscale. 2019, 11, 5274–5284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heath, J.; Chen, H.; Islam, M.S. MgFeSiO4 as a potential cathode material for magnesium batteries: Ion diffusion rates and voltage trends. J. Mater. Chem. A. 2017, 5, 13161–13167. [Google Scholar] [CrossRef] [Green Version]
- Eames, C.; Armstrong, A.R.; Bruce, P.G.; Islam, M.S. Insights into changes in voltage and structure of Li2FeSiO4 polymorphs for lithium-ion batterie. Chem. Mater. 2012, 24, 2155–2161. [Google Scholar]
- Aparicio, P.A.; Dawson, J.A.; Islam, M.S.; De Leeuw, N.H. Computational study of NaVOPO4 polymorphs as cathode materials for Na-ion batteries: Diffusion, electronic properties, and cation-doping behavior. J. Phys. Chem. C 2018, 122, 25829–25836. [Google Scholar]
- Aydinol, M.; Kohan, A.; Ceder, G.; Cho, K.; Joannopoulos, J. Ab initio study of lithium intercalation in metal oxides and metal dichalcogenides. Phys. Rev. B Condens Matter Mater. Phys. 1997, 56, 1354–1365. [Google Scholar] [CrossRef] [Green Version]
- Arroyo-de Dompablo, M.E.; Armand, M.; Tarascon, J.M.; Amador, U. On-demand design of polyoxianionic cathode materials based on electronegativity correlations: An exploration of the Li2MSiO4 system (M = Fe, Mn, Co, Ni). Electrochem. Commun. 2006, 8, 1292–1298. [Google Scholar] [CrossRef]
- Armstrong, A.R.; Lyness, C.; Panchmatia, P.M.; Islam, M.S.; Bruce, P.G. The lithium intercalation process in the low-voltage lithium battery anode Li1+xV1-xO2. Nat. Mater. 2011, 10, 223–229. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Rong, Z.; Malik, R.; Canepa, P.; Jain, A.; Ceder, G.; Persson, K.A. Spinel compounds as multivalent battery cathodes: A systematic evaluation based on ab initio calculations. Energy Environ. Sci. 2015, 8, 964–974. [Google Scholar] [CrossRef] [Green Version]







| a (Å) | b (Å) | c (Å) | α = β=γ (°) | Eg (eV) | |
|---|---|---|---|---|---|
| Experimental | 5.45 | 16.47 | 5.47 | 90 | 2.53 [53], 2.56 [54] |
| GGA | 5.658 | 16.491 | 5.676 | 90 | 2.23 |
| GGA + U | 5.448 | 16.506 | 5.467 | 90 | 2.45 |
| Site | Ef | qBi | qBi,NN | qMo | qMo,NN | qO | qO,NN | qLi |
|---|---|---|---|---|---|---|---|---|
| BiO-layer | ||||||||
| 1 | −1.79 | 1.85 ± 0.03 | 1.83, 1.85 | 2.68 ± 0.08 | 2.55, 2.67 | −1.08 ± 0.05 | −1.05, −1.11, −1.10 | 0.51 |
| 2 | −1.44 | 1.83 ± 0.02 | 1.79 | 2.64 ± 0.03 | 2.60, 2.67 | −1.07 ± 0.05 | −1.03, −1.08, −1.15 | 0.52 |
| 3 | −1.79 | 1.85±0.03 | 1.80, 1.82 | 2.70±0.08 | 2.57, 2.73 | −1.09 ± 0.04 | −1.12, −1.07, −1.11 | 0.62 |
| MoO6-layer | ||||||||
| 4 | −2.34 | 1.87 ± 0.02 | 1.89, 1.85 | 2.75 ± 0.06 | 2.66, 2.82 | −1.11 ± 0.04 | −1.08, −1.15 | 0.63 |
| 5 | −2.45 | 1.88 ± 0.02 | 1.87 | 2.72 ± 0.07 | 2.62, 2.80 | −1.10 ± 0.04 | −1.04, −1.09 | 0.61 |
| In-between layers | ||||||||
| 6 | −1.45 | 1.84 ± 0.02 | 1.81, 1.82 | 2.61 ± 0.09 | 2.70, 2.46 | −1.07 ± 0.05 | −1.09, −1.10 | 0.54 |
| nLi | Eincorp (eV/Li) | |
|---|---|---|
| 1 | −2.45 | −2.45 |
| 2 | −4.38 | −2.19 |
| 3 | −6.58 | −2.19 |
| 4 | −7.79 | −1.95 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, T.; Olsson, E.; Choolaei, M.; Stolojan, V.; Feng, C.; Wu, H.; Wang, S.; Cai, Q. Synthesis and Electrochemical Properties of Bi2MoO6/Carbon Anode for Lithium-Ion Battery Application. Materials 2020, 13, 1132. https://doi.org/10.3390/ma13051132
Zhang T, Olsson E, Choolaei M, Stolojan V, Feng C, Wu H, Wang S, Cai Q. Synthesis and Electrochemical Properties of Bi2MoO6/Carbon Anode for Lithium-Ion Battery Application. Materials. 2020; 13(5):1132. https://doi.org/10.3390/ma13051132
Chicago/Turabian StyleZhang, Tingting, Emilia Olsson, Mohammadmehdi Choolaei, Vlad Stolojan, Chuanqi Feng, Huimin Wu, Shiquan Wang, and Qiong Cai. 2020. "Synthesis and Electrochemical Properties of Bi2MoO6/Carbon Anode for Lithium-Ion Battery Application" Materials 13, no. 5: 1132. https://doi.org/10.3390/ma13051132
APA StyleZhang, T., Olsson, E., Choolaei, M., Stolojan, V., Feng, C., Wu, H., Wang, S., & Cai, Q. (2020). Synthesis and Electrochemical Properties of Bi2MoO6/Carbon Anode for Lithium-Ion Battery Application. Materials, 13(5), 1132. https://doi.org/10.3390/ma13051132








