Characterization of the Lipid Oxidation Process of Robusta Green Coffee Beans and Shelf Life Prediction during Accelerated Storage
Abstract
1. Introduction
2. Results and Discussion
2.1. Changes in MC during Accelerated Storage
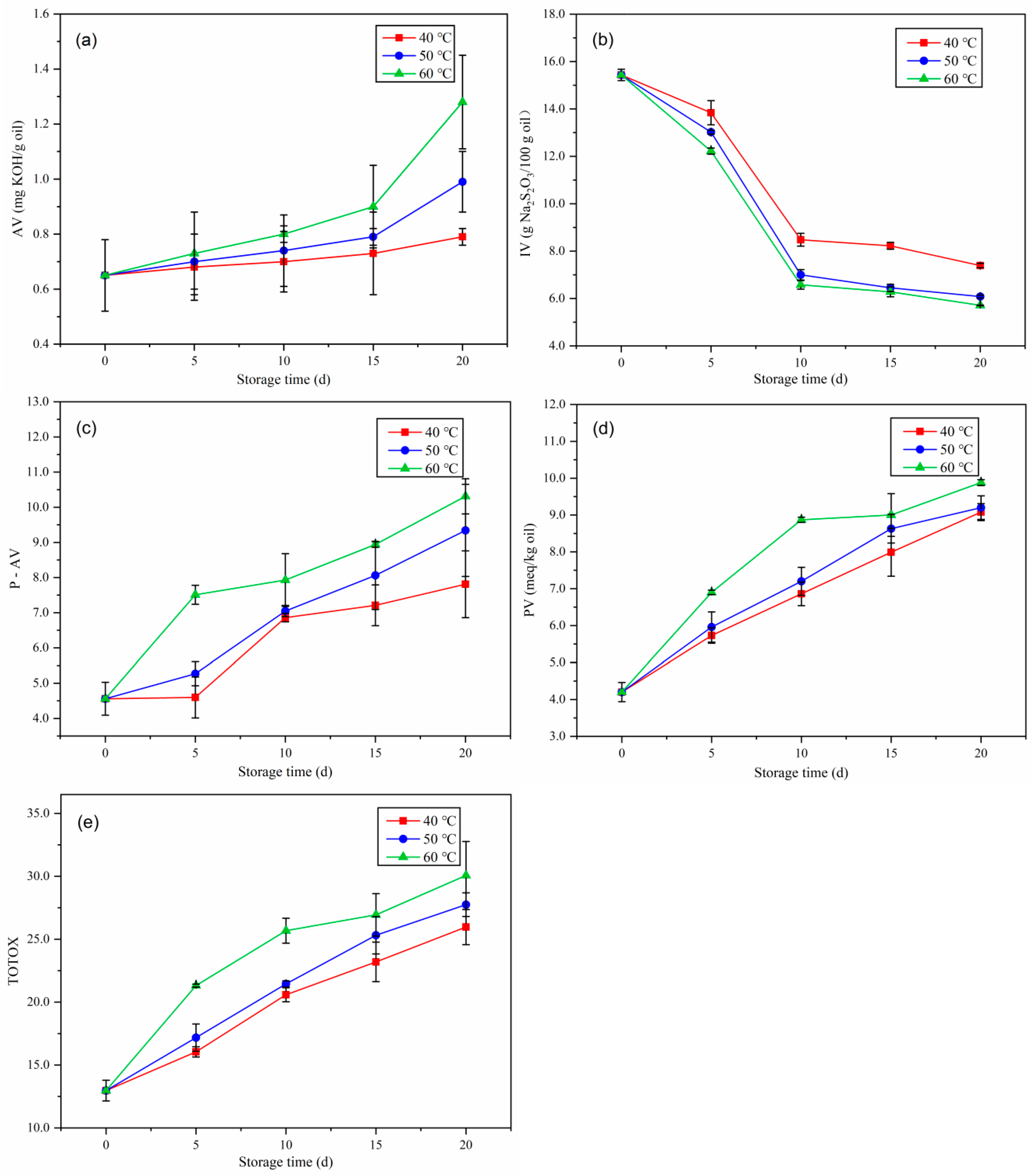
2.2. Changes in Oxidative Indexes during Accelerated Storage
2.2.1. Acid Value
2.2.2. Iodine Value
2.2.3. p-Anisidine Value
2.2.4. Peroxide Value
2.2.5. Total Oxidation
2.3. Changes in Thiobarbituric Acid and FFA Contents during Accelerated Storage
2.3.1. Thiobarbituric Acid (TBARS) Analysis
2.3.2. Free Fatty Acid Analysis
2.4. Changes in K232 nm and K268 nm during Accelerated Storage
2.5. The Effects of Accelerated Storage on the Fatty Acid Content
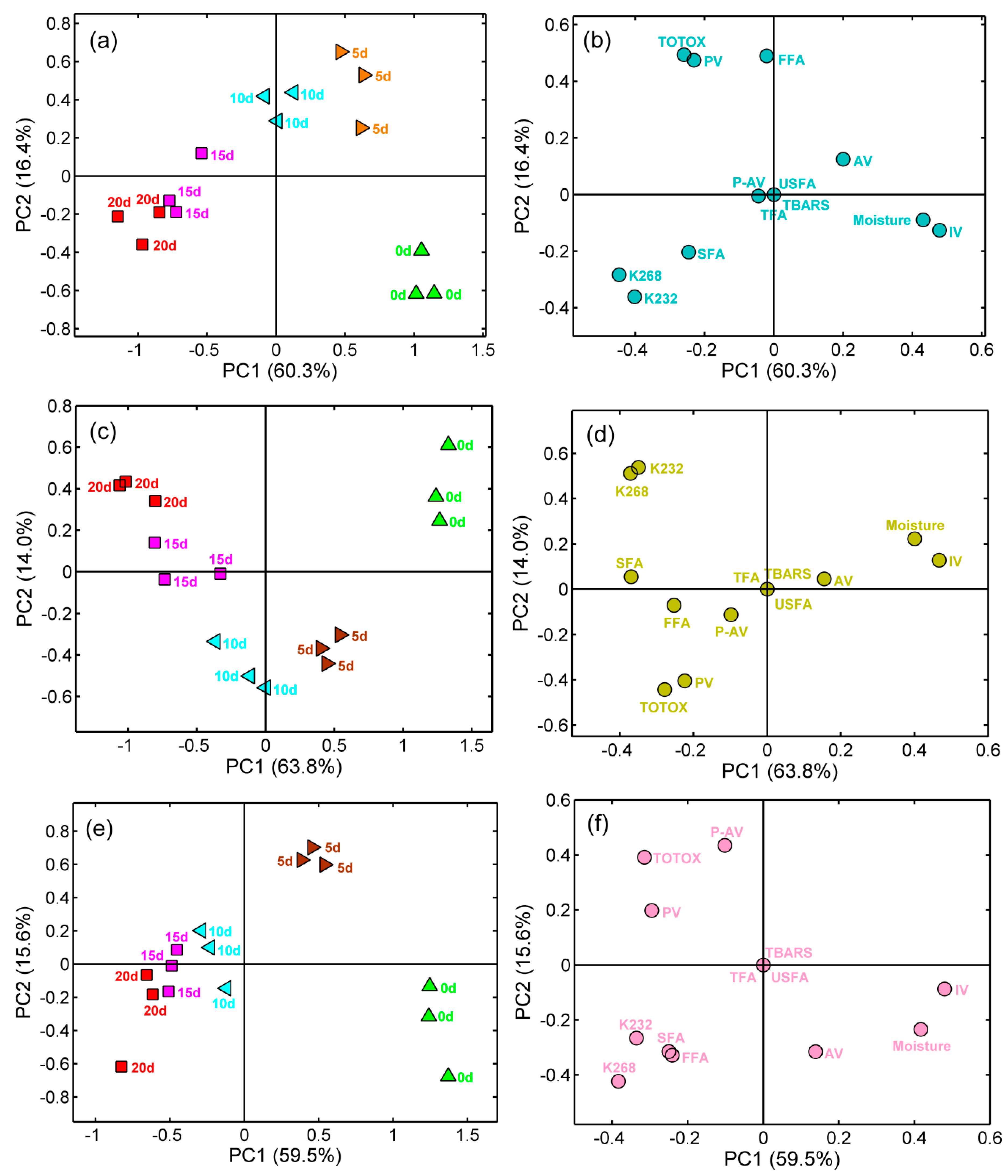
2.6. Principal Component Analysis
2.7. Hierarchical Clustering Analysis
2.8. Shelf Life Prediction
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Samples Preparation and Accelerated Storage Treatment
3.3. Determination of Moisture Content (MC)
3.4. Coffee Oil Extraction
3.5. Determination of Oxidative Indexes
3.5.1. Acid Value (AV)
3.5.2. Iodine Value (IV)
3.5.3. p-Anisidine Value (P-AV)
3.5.4. Peroxide Value (PV)
3.5.5. Total Oxidation Value (TOTOX)
3.6. Measurement of Thiobarbituric Acid Reactive Substances (TBARS)
3.7. Measurement of Free Fatty Acids (FFA)
3.8. Measuring Absorbance at 232 (K232 nm) and 268 nm (K268 nm)
3.9. Fatty Acids Analysis by GC-MS
3.10. Oxidation Kinetics and Shelf Life Prediction
3.11. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- International Coffee Organization (ICO). Historical Data on the Global Coffee Trade. Available online: http://www.ico.org/historical/1990%20onwards/PDF/1a-total-production.pdf (accessed on 14 December 2017).
- Novaes, F.J.M.; Da Silva Junior, A.I.; Kulsing, C.; Nolvachai, Y.; Bizzo, H.R.; De Aquino Neto, F.R.; Rezende, C.M.; Marriott, P.J. New approaches to monitor semi-volatile organic compounds released during coffee roasting using flow-through/active sampling and comprehensive two-dimensional gas chromatography. Food Res. Int. 2019, 119, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Blank, I.; Sen, A.; Grosch, W. Aroma impact compounds of Arabica and Robusta coffee. Qualitative and quantitative investigations. In Proceedings of the 14th International Scientific Colloquium on Coffee, San Francisco, CA, USA, 14–19 July 1991; ASIC: Paris, France, 1991; pp. 117–129. [Google Scholar]
- Yisak, H.; Redi-Abshiro, M.; Chandravanshi, B.S. Selective determination of caffeine and trigonelline in aqueous extract of green coffee beans by FT-MIR-ATR spectroscopy. Vib. Spectrosc. 2018, 97, 33–38. [Google Scholar] [CrossRef]
- Dong, W.J.; Tan, L.H.; Zhao, J.P.; Hu, R.S.; Lu, M.Q. Characterization of fatty acid, amino acid and volatile compound compositions and bioactive components of seven coffee (Coffea robusta) cultivars grown in Hainan province, China. Molecules 2015, 20, 16687–16708. [Google Scholar] [CrossRef] [PubMed]
- Anwar, J.; Spanevello, R.M.; Pimentel, V.C.; Gutierres, J.; Thomé, G.; Cardoso, A.; Zaninia, D.; Martinsa, C.; Palmaa, H.E.; Bagatinic, M.D.; et al. Caffeic acid treatment alters the extracellular adenine nucleotide hydrolysis in platelets and lymphocytes of adult rats. Food Chem. Toxicol. 2013, 56, 459–466. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mirmiran, P.; Carlström, M.; Bahadoran, Z.; Azizi, F. Long-term effects of coffee and caffeine intake on the risk of pre-diabetes and type 2 diabetes: Findings from a population with low coffee consumption. Nutr. Metab. Cardiovas. 2018, 28, 1261–1266. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.K.; Zhou, D.Y.; Hu, X.P.; Liu, Z.Y.; Song, L.; Zhu, B.W. Changes in lipid profiles of dried clams (Mactra chinensis Philippi and Ruditapes philippinarum) during accelerated storage and prediction of shelf life. J. Agric. Food Chem. 2018, 66, 7764–7774. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Xiao, L.E.; Jiang, L.P.; Li, B.; Qian, P. Evaluation of accelerated test factors through the development of predictive models in vacuum-packaged compressed biscuits. Food Anal. Method. 2015, 8, 1618–1628. [Google Scholar] [CrossRef]
- Wang, D.Y.; Fan, W.C.; Guan, Y.F.; Huang, H.N.; Yi, T.; Jin, J.M. Oxidative stability of sunflower oil flavored by essential oil from Coriandrum sativum L. during accelerated storage. LWT Food Sci. Technol. 2018, 98, 268–275. [Google Scholar] [CrossRef]
- Rendón, M.Y.; de Jesus Garcia Salva, T.; Bragagnolo, N. Impact of chemical changes on the sensory characteristics of coffee beans during storage. Food Chem. 2014, 147, 279–286. [Google Scholar] [CrossRef]
- Ribeiro, F.C.; Borém, F.M.; Giomo, G.S.; De Lima, R.R.; Malta, M.R.; Figueiredo, L.P. Storage of green coffee in hermetic packaging injected with CO2. J. Stored Prod. Res. 2011, 47, 341–348. [Google Scholar] [CrossRef]
- Ferreira, L.F.; De Abreu, G.F.; Lago, A.M.T.; Figueiredo, L.P.; Borém, F.M.; Martins, M.A.; Borges, S.V.; Dias, M.V. Development and application of biopolymer coatings to specialty green coffee beans: Influence on water content, color and sensory quality. LWT Food Sci. Technol. 2018, 96, 274–280. [Google Scholar] [CrossRef]
- Caporaso, N.; Whitworth, M.B.; Grebby, S.; Fisk, I.D. Rapid prediction of single green coffee bean moisture and lipid content by hyperspectral imaging. J. Food Eng. 2018, 227, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Prescha, A.; Grajzer, M.; Dedyk, M.; Grajeta, H. The antioxidant activity an oxidative stability of cold-pressed oils. J. Am. Oil Chem. Soc. 2014, 91, 1291–1301. [Google Scholar] [CrossRef] [PubMed]
- Kanner, J.; Gorelik, S.; Roman, S.; Kohen, R. Protection by polyphenols of postprandial human plasma and low-density lipoprotein modification: The stomach as a bioreactor. J. Agric. Food Chem. 2012, 60, 8790–8796. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Gao, S.; Sun, Y.W.; Gao, Y.F.; Wang, X.R.; Zhang, Z.S. Antioxidant efficacy of rosemary ethanol extract in palm oil during frying and accelerated storage. Ind. Crop. Prod. 2016, 94, 82–88. [Google Scholar] [CrossRef]
- Jahurul, M.H.A.; Jing, Y.W.; Foong, C.Y.; Shaarani, S.M.; Zaidul, I.S.M.; Jinap, S.; Hasmadi, M.; Ali, M.E.; Nyam, K. Effect of accelerated storage on chemical compositions and mango seed fat and palm oil mid-fraction blends as cocoa butter replacers. LWT Food Sci. Technol. 2017, 84, 551–554. [Google Scholar] [CrossRef]
- Iqbal, S.; Bhanger, M.I. Stabilization of sunflower oil by garlic extract during accelerated storage. Food Chem. 2007, 100, 246–254. [Google Scholar] [CrossRef]
- Chong, Y.M.; Chang, S.K.; Sia, W.C.M.; Yim, H.S. Antioxidant efficacy of mangosteen (Garcinia mangostana Linn.) peel extracts in sunflower oil during accelerated storage. Food Biosci. 2015, 12, 18–25. [Google Scholar] [CrossRef]
- O’Keefe, S.F.; Pike, O.A. Fat characterization. In Food Analysis, 4th ed.; Nielsen, S.S., Ed.; Springer Science & Business Media: New York, NY, USA, 2010. [Google Scholar]
- Roselló-Soto, E.; Barba, F.J.; Lorenzo, J.M.; Dominguez, R.; Pateiro, M.; Mañes, J.; Moltó, J.C. Evaluating the impact of supercritical-CO2 pressure on the recovery and quality of oil from “horchata” by-products: Fatty acid profile, α-tocopherol, phenolic compounds, and lipid oxidation parameters. Food Res. Int. 2019, 120, 888–894. [Google Scholar] [CrossRef]
- Sobhani, A.; Mohammed, A.S.; Ghobakhlou, F.; Ghazali, H.M. Determining the oxidative stability and quality of tiger nut (Cyperus esculentus) oil and its antioxidant activity during microwave heating. Rev. Esp. De Nutr. Hum. Y Diet. 2018, 22, 52–63. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, L.; Zu, Y.G.; Chen, X.Q.; Wang, F.J.; Liu, F. Oxidative stability of sunflower oil supplemented with carnosic acid with synthetic antioxidants during accelerated storage. Food Chem. 2010, 118, 656–662. [Google Scholar] [CrossRef]
- Peng, Y.L.; Adhiputra, K.; Padayachee, A.; Channon, H.; Ha, M.; Warner, R.D. High oxygen modified atmosphere packaging negative influences consumer acceptability traits of pork. Foods 2019, 8, 567. [Google Scholar] [CrossRef]
- Bouyanfif, A.; Liyanage, S.; Hequet, E.; Moustaid-Moussa, N.; Abidi, N. FTIR microspectroscopy reveals fatty acid-induced biochemical changes in C. elegans. Vib. Spectrosc. 2019, 102, 8–15. [Google Scholar] [CrossRef]
- Shahidi, F.; John, J.A. Oxidative rancidity in nuts. In Improving the Safety and Quality of Nuts; Harris, L.J., Ed.; Woodhead Publishing Limited: Philadelphia, PA, USA, 2013; pp. 198–229. [Google Scholar]
- Keramat, M.; Golmakani, M.T.; Aminlari, M.; Shekarforoush, S. Oxidative stability of virgin olive oil supplemented with Zataria multiflora Boiss. And Rosmarinus officinalis L. essential oils during accelerated storage. J. Food Process. Pres. 2017, 41, e12591. [Google Scholar] [CrossRef]
- Franklin, L.M.; King, E.S.; Chapman, D.; Byrnes, N.; Huang, G.W.; Mitchell, A.E. Flavor and acceptance of roasted California almonds during accelerated storage. J. Agric. Food Chem. 2018, 66, 1222–1232. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.J.; Hu, R.S.; Chu, Z.; Zhao, J.P.; Tan, L.H. Effect of different drying techniques on bioactive components, fatty acid composition, and volatile profile of robusta coffee beans. Food Chem. 2017, 234, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Hua, Q.H.; Wua, Y.Y.; Pei, F.; Kimatu, B.M.; Su, A.X.; Yang, W. Storage time assessment and shelf-life prediction models for postharvest Agaricus bisporus. LWT Food Sci. Technol. 2019, 101, 360–365. [Google Scholar] [CrossRef]
- GB 5009.3-2016. Method for Determination of Moisture in Foods; National Standard of the People’s Republic of China: Beijing, China, 2016. [Google Scholar]
- GB 5009229-2016. Method for Determination of Acid Value in Foods; National Standard of the People’s Republic of China: Beijing, China, 2016. [Google Scholar]
- GB/T 5532-2008. Method for Determination of Iodine Value in Animal and Vegetable Oil; National Standard of the People’s Republic of China: Beijing, China, 2008. [Google Scholar]
- GB/T 24304-2009. Method for Determination of Anisidine Value in Animal and Vegetable Oil; National Standard of the People’s Republic of China: Beijing, China, 2009. [Google Scholar]
- GB/T 5009.227-2016. Method for Determination of Peroxide Value in Food; National Standard of the People’s Republic of China: Beijing, China, 2016. [Google Scholar]
- Chew, S.C.; Tan, C.P.; Long, K.; Nyam, K.L. Effect of chemical refining on the quality of kenaf (Hibiscus cannabinus) seed oil. Ind. Crop. Prod. 2016, 89, 59–65. [Google Scholar] [CrossRef]
- GN/T 5009.181-2016. Method for Determination of Thiobarbituric Acid Reactive Substances in Food; National Standard of the People’s Republic of China: Beijing, China, 2016. [Google Scholar]
- Nhouchi, Z.; Botosoa, E.P.; Chene, C.; Karoui, R. Potentiality of front-face fluorescence and mid-infrared spectroscopies coupled with partial least square regression to predict lipid oxidation in pound cakes during storage. Food Chem. 2019, 275, 322–332. [Google Scholar] [CrossRef]
- GB/T 22500-2008. Method for Determination of UV Absorbance Value in Animal and Vegetable Oil; National Standard of the People’s Republic of China: Beijing, China, 2008. [Google Scholar]
- GB/T 5009.168-2016. Method for Determination of Fatty Acids in Food; National Standard of the People’s Republic of China: Beijing, China, 2016. [Google Scholar]
Sample Availability: Samples of the compounds are available from the authors. |


| Fatty Acids (mg/g oil) | Days | 40 °C | 50 °C | 60 °C |
|---|---|---|---|---|
| C15:0 | 0 | 0.25 ± 0.01b | 0.25 ± 0.01a | 0.25 ± 0.01c |
| 5 | 0.26 ± 0.35b | 0.23 ± 0.03a | 0.37 ± 0.15c | |
| 10 | 0.30 ± 0.09b | 0.26 ± 0.10a | 1.57 ± 0.16b | |
| 15 | 1.16 ± 0.49a | 0.34 ± 0.02a | 1.66 ± 0.01b | |
| 20 | 1.06 ± 0.54a | 0.26 ± 0.06a | 4.89 ± 1.83a | |
| C16:0 | 0 | 109.75 ± 0.01a | 109.75 ± 0.01a | 109.75 ± 0.01a |
| 5 | 73.53 ± 1.99b | 79.96 ± 0.01b | 55.85 ±0.01b | |
| 10 | 70.80 ± 0.40b | 75.22 ± 6.00bc | 51.55 ± 1.86b | |
| 15 | 60.59 ± 2.10c | 67.96 ± 3.55cd | 42.21 ± 0.01c | |
| 20 | 38.46 ± 2.56d | 61.48 ± 4.97d | 45.48 ± 4.28c | |
| C17:0 | 0 | 0.13 ± 0.03b | 0.13 ± 0.03c | 0.13 ± 0.03b |
| 5 | 0.17 ± 0.01b | 0.27 ± 0.04c | 0.12 ± 0.03b | |
| 10 | 0.19 ± 0.06b | 0.29 ± 0.07c | 0.20 ± 0.12b | |
| 15 | 0.35 ± 0.21b | 1.13 ± 0.45b | 0.39 ± 0.06b | |
| 20 | 1.15 ± 0.44a | 1.96 ± 0.38a | 0.88 ± 0.35a | |
| C18:0 | 0 | 109.94 ± 1.47a | 109.94 ± 1.47a | 109.94 ± 1.47a |
| 5 | 65.97 ± 0.14b | 73.13 ± 5.37b | 42.51 ± 2.39b | |
| 10 | 32.60 ± 0.01c | 68.62 ± 5.61bc | 42.27 ± 0.11b | |
| 15 | 32.06 ± 0.22c | 60.97 ± 1.06c | 31.30 ± 1.53c | |
| 20 | 25.16 ± 2.58d | 45.84 ± 2.55d | 21.12 ± 5.95d | |
| C18:1 | 0 | 2.26 ± 0.01a | 2.26 ± 0.01a | 2.26 ± 0.01a |
| 5 | 1.12 ± 0.24b | 0.51 ± 0.03b | 0.28 ± 0.04b | |
| 10 | 1.02 ± 0.18b | 0.28 ± 0.01c | 0.25 ± 0.17b | |
| 15 | 0.38 ± 0.10c | 0.25 ± 0.08c | 0.13 ± 0.02b | |
| 20 | 0.14 ± 0.03c | 0.23 ± 0.02c | 0.12 ± 0.01b | |
| C18:2 | 0 | 354.45 ± 1.77a | 354.45 ± 1.77a | 354.45 ± 1.77a |
| 5 | 69.21 ± 9.72b | 57.75 ± 1.51b | 50.35 ± 9.99bc | |
| 10 | 64.00 ± 0.10b | 45.84 ± 2.55c | 46.64 ± 1.10c | |
| 15 | 50.18 ± 1.10c | 50.32 ± 1.64bc | 62.78 ± 1.00b | |
| 20 | 35.53 ± 1.00d | 25.64 ± 7.00d | 28.19 ± 6.30d | |
| C18:3 | 0 | 2.00 ± 0.60a | 2.00 ± 0.60ab | 2.00 ± 0.60ab |
| 5 | 2.40 ± 0.02a | 2.50 ± 0.36a | 1.27 ± 0.33b | |
| 10 | 2.17 ± 0.02a | 0.83 ± 0.07c | 2.68 ± 0.40a | |
| 15 | 0.49 ± 0.13b | 1.65 ± 0.09b | 1.44 ± 0.70b | |
| 20 | 0.26 ± 0.08b | 1.54 ± 0.17b | 1.41 ± 0.11b | |
| C20:0 | 0 | 0.31 ± 0.03c | 0.31 ± 0.03c | 0.31 ± 0.03c |
| 5 | 0.25 ± 0.03c | 0.54 ± 0.01c | 0.69 ± 0.17c | |
| 10 | 0.45 ± 0.01bc | 1.70 ± 0.02b | 0.70 ± 0.12c | |
| 15 | 0.69 ± 0.01ab | 1.96 ± 0.84b | 2.62 ± 0.18b | |
| 20 | 0.72 ± 0.17a | 4.79 ± 0.71a | 7.24 ± 0.40a | |
| C20:1 | 0 | 2.36 ± 0.84a | 2.36 ± 0.84a | 2.36 ± 0.84a |
| 5 | 1.16 ± 0.07b | 1.10 ± 0.16b | 0.47 ± 0.01c | |
| 10 | 0.95 ± 0.01c | 1.17 ± 1.02b | 0.07 ± 0.01d | |
| 15 | 0.56 ± 0.03d | 0.56 ± 0.25c | 0.43 ± 0.01c | |
| 20 | 0.10 ± 0.02e | 0.75 ± 0.02c | 0.83 ± 0.01b | |
| C20:2 | 0 | 0.48 ± 0.01a | 0.48 ± 0.01a | 0.48 ± 0.01b |
| 5 | 0.10 ± 0.03b | 0.47 ± 0.11a | 0.14 ± 0.00d | |
| 10 | 0.45 ± 0.22a | 0.33 ± 0.26b | 0.79 ± 0.46a | |
| 15 | 0.10 ± 0.09b | 0.32 ± 0.30b | 0.31 ± 0.01c | |
| 20 | 0.40 ± 0.12a | 0.16 ± 0.01c | 0.12 ± 0.01d | |
| C21:0 | 0 | 0.25 ± 0.01a | 0.25 ± 0.01c | 0.25 ± 0.01b |
| 5 | 0.28 ± 0.03a | 0.23 ± 0.05c | 0.20 ± 0.02b | |
| 10 | 0.25 ± 0.01a | 0.24 ± 0.01c | 0.26 ± 0.17b | |
| 15 | 0.30 ± 0.01a | 0.91 ± 0.47a | 1.00 ± 0.56a | |
| 20 | 0.33 ± 0.11a | 0.75 ± 0.02b | 1.20 ± 0.56a | |
| SFA | 0 | 0.94 ± 0.13cd | 0.94 ± 0.13e | 0.94 ± 0.13d |
| 5 | 0.82 ± 0.41d | 1.27 ± 0.12d | 1.38 ± 0.37c | |
| 10 | 1.19 ± 0.75c | 4.98 ± 0.23b | 4.65 ± 0.63b | |
| 15 | 2.50 ± 0.71b | 4.34 ± 1.78c | 4.52 ± 0.79b | |
| 20 | 3.26 ± 1.26a | 7.76 ± 1.17a | 14.21 ± 3.14a | |
| USFA | 0 | 654.01 ± 17.00a | 654.01 ± 17.00a | 654.01 ± 17.00a |
| 5 | 213.39 ± 12.49b | 215.42 ± 8.68b | 150.87 ± 12.45b | |
| 10 | 172.09 ± 3.60c | 192.26 ± 14.53c | 144.25 ± 3.86c | |
| 15 | 144.36 ± 0.63d | 182.06 ± 13.31d | 138.59 ± 7.48d | |
| 20 | 100.05 ± 3.73e | 135.64 ± 14.13e | 97.28 ± 13.50e | |
| TFA | 0 | 654.95 ± 17.13a | 654.95 ± 17.13a | 654.95 ± 17.13a |
| 5 | 214.21 ± 13.10b | 216.69 ± 8.80b | 152.25 ± 12.82b | |
| 10 | 173.28 ± 3.77c | 197.24 ± 14.76c | 148.90 ± 4.49b | |
| 15 | 146.86 ± 1.34d | 186.40 ± 15.09d | 143.11 ± 8.27c | |
| 20 | 103.31 ± 4.99e | 143.40 ± 15.30e | 111.49 ± 16.64d |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cong, S.; Dong, W.; Zhao, J.; Hu, R.; Long, Y.; Chi, X. Characterization of the Lipid Oxidation Process of Robusta Green Coffee Beans and Shelf Life Prediction during Accelerated Storage. Molecules 2020, 25, 1157. https://doi.org/10.3390/molecules25051157
Cong S, Dong W, Zhao J, Hu R, Long Y, Chi X. Characterization of the Lipid Oxidation Process of Robusta Green Coffee Beans and Shelf Life Prediction during Accelerated Storage. Molecules. 2020; 25(5):1157. https://doi.org/10.3390/molecules25051157
Chicago/Turabian StyleCong, Sha, Wenjiang Dong, Jianping Zhao, Rongsuo Hu, Yuzhou Long, and Xiaoxing Chi. 2020. "Characterization of the Lipid Oxidation Process of Robusta Green Coffee Beans and Shelf Life Prediction during Accelerated Storage" Molecules 25, no. 5: 1157. https://doi.org/10.3390/molecules25051157
APA StyleCong, S., Dong, W., Zhao, J., Hu, R., Long, Y., & Chi, X. (2020). Characterization of the Lipid Oxidation Process of Robusta Green Coffee Beans and Shelf Life Prediction during Accelerated Storage. Molecules, 25(5), 1157. https://doi.org/10.3390/molecules25051157





