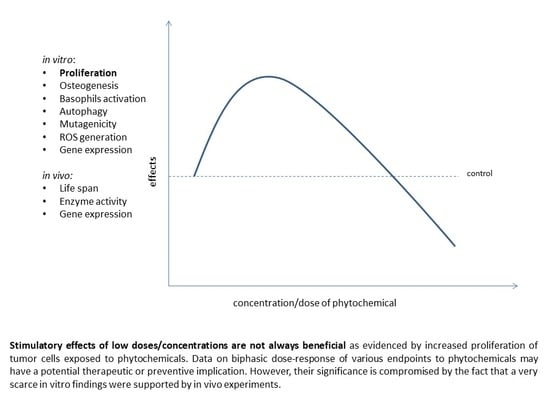
Biphasic Dose-Response Induced by Phytochemicals: Experimental Evidence
Abstract
:1. Introduction
2. Phytoestrogens
3. Resveratrol
4. Other Phytochemicals
5. Comments
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 2-AAF | 2-Acetylaminofluorene |
| 4NQO | 4-nitroquinoline-N-oxide |
| 6-OHDA | 6-Hydroxydopamine |
| AChE | Acetylcholinesterase |
| AFB1 | Aflatoxin B1 |
| AKT | Serine/threonine protein kinase |
| ALP | Alkaline phosphatase |
| apoM | Apolipoprotein M |
| b.w. | Bodyweight |
| B16-F10 | Murine melanoma cell line |
| Bcl-2 | B-cell lymphoma 2 |
| BER | Berberine |
| BMSCs | Bone marrow-derived mesenchymal stem cells |
| C2C12 | Mouse myoblast cell line |
| CA | Caffeic acid |
| CaCo-2 | Human colon cancer cell line |
| CD203c | Basophil-specific ectoenzyme E-NPP3 |
| CD63 | Tetraspan transmembrane protein family |
| CES | Carboxylesterase |
| CIN | Chromosomal instability |
| CLS | Chronologic life span |
| CYN | Cynarin |
| CYP | Cytochrome P450 |
| CYP1A2 | Cytochrome P450 1A2 |
| DAI | Daidzein |
| ER | Estrogen receptor |
| ERK | Extracellular signal-regulated kinase protein-serine/threonine kinase |
| fMLP | Bacterial formyl peptide N-formylmethionine-leucine-phenylalanine |
| FSF-1 | Human skin fibroblasts |
| GEN | Genistein |
| GIR | Genotoxic inhibition rate |
| GPx | Glutathione peroxidase |
| GSH | Reduced glutathione |
| GSSG | Oxidized glutathione |
| GST | Glutathione S-transferase |
| HCT-116 | Colon carcinoma cell lines |
| HepG2 | Human liver cancer cell line |
| HHL-5 | Human normal liver cell line |
| HO-1 | Heme oxygenase-1 |
| Hsp70 | 70 kDa heat shock protein |
| HT-29 | Colon carcinoma cell lines |
| hTERT-MSC | Human normal telomerase-immortalized mesenchymal stem cells |
| HUVEC | Human umbilical vein endothelial cell line |
| ISL | Isoliquiritigenin |
| Jurkat T-CLL | Jurkat T-cell lymphocyte leukemia cells |
| K+ -p-NPPase activity | K+ -p- nitrophenylphosphatase |
| K562 | Immortalized cell line derived from human leukemia |
| KS483 | Murine osteoprogenitor cell line |
| LC3-II | Microtubule-associated protein 2 light chain 3 |
| LNCaP | Androgen-sensitive human prostate adenocarcinoma cell line |
| LoVo | Human colon adenocarcinoma cell line |
| LS-174 | Human colon cancer cell line |
| LVDP | Left ventricular developed pressure |
| MAPK | Mitogen-activated protein kinase |
| MCF-7 | Human breast adenocarcinoma cell line |
| MDA-MB-231, MDA-MB-468, MCF-7MCF-7 | Human breast carcinoma cell lines |
| MeIQ | 2-amino-3,4-dimethylimidazo [4,5-f]quinoline |
| MG-63 | Human osteoblast-like cells |
| MMPs | matrix metalloproteinases |
| MN | Markers micronuclei |
| MSCs | Mesenchymal stem cell line |
| NHDF | Neonatal normal human dermal fibroblasts |
| NHEK | Neonatal normal human epidermal keratinocytes |
| NK | Human natural killer cells |
| NPB | Nucleoplasmic bridge |
| NPCs | Neural progenitor cells; ODC - ornithine decarboxylase |
| OGD | Oxygen-glucose deprivation |
| pBMEC | Primary bovine mammary epithelial cells |
| PC-12 | Phaeochromocytoma cell line |
| PC-3 | Human prostatic carcinoma cell line |
| PCNA | Proliferating cell nuclear antigen |
| PGE2 | Prostaglandin E2 |
| PI3K | Phosphoinositide 3-kinase |
| PKC | Protein kinase C |
| PMA | Phorbol myristate acetate |
| PPARγ | Peroxisome proliferator-activated receptor γ |
| PTS | Panaxatriol saponins |
| PVC | Pericytes |
| QER | Quercetin |
| RA | Rosmarinic acid |
| RAW 264.7 | Murine macrophage cell line |
| RES | Resveratrol |
| ROS | Reactive oxygen species |
| RWPE-1 | Nontumorigenic human prostate epithelial cells |
| SCC-25 | Oral squamous carcinoma cell line |
| SFN | Sulforaphane |
| SKN-1 | Transcription factor skinhead-1 |
| SOX2 | Transcription factor (sex determining region Y-box 2 |
| T24 | Bladder cancer cell line |
| T-47D, T | Human breast cancer cell lines |
| TIMP-2 | Tissue inhibitor of metalloproteinase-2 |
| TRAMP-FVB | Transgenic adenocarcinoma of mouse prostate model |
| UtLM | Human uterine leiomyoma |
| UtSMCs | Uterine smooth muscle cells |
References
- Pal, S.; Konkimalla, V.B. Hormetic potential of sulforaphane (SFN) in switching cells’ fate towards survival or death. Mini Rev. Med. Chem. 2016, 16, 980–995. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.J.; Mattson, M.P.; Calabrese, V. Resveratrol commonly displays hormesis: Occurrence and biomedical significance. Hum. Exp. Toxicol. 2010, 29, 980–1015. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Dudley, J.I.; Das, D.K. Dose-dependency of resveratrol in providing health benefits. Dose Response 2010, 8, 478–500. [Google Scholar] [CrossRef] [PubMed]
- Moghaddam, N.S.A.; Oskouie, M.N.; Butler, A.E.; Petit, P.X.; Barreto, G.E.; Sahebkar, A. Hormetic effects of curcumin: What is the evidence? J. Cell Physiol. 2019, 234, 10060–10071. [Google Scholar] [CrossRef]
- Hayes, D.P. Nutritional hormesis. Eur. J. Clin. Nutr. 2007, 61, 147–159. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Baldwin, L.A. Defining hormesis. Hum. Exp. Toxicol. 2002, 21, 91–97. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Baldwin, L.A. A quantitatively-based methodology for the evaluation of chemical hormesis. Hum. Ecol. Risk Assess. 1997, 3, 545–554. [Google Scholar] [CrossRef]
- Kendig, E.L.; Le, H.H.; Belcher, S.M. Defining hormesis: Evaluation of a complex concentration response phenomenon. Int. J. Toxicol. 2010, 29, 235–246. [Google Scholar] [CrossRef]
- Rietjens, I.M.C.M.; Louisse, J.; Beekmann, K. The potential health effects of dietary phytoestrogens. Br. J. Pharm. 2017, 174, 1263–1280. [Google Scholar] [CrossRef] [Green Version]
- Zava, D.T.; Duwe, G. Estrogenic and antiproliferative properties of genistein and other flavonoids in human breast cancer cells in vitro. Nutr. Cancer 1997, 27, 31–40. [Google Scholar] [CrossRef]
- Hsieh, C.Y.; Santell, R.C.; Haslam, S.Z.; Helferich, W.G. Estrogenic effects of genistein on the growth of estrogen receptor-positive human breast cancer (MCF-7) cells in vitro and in vivo. Cancer Res. 1998, 58, 3833–3838. [Google Scholar] [PubMed]
- Fioravanti, L.; Cappelletti, V.; Miodini, P.; Ronchi, E.; Brivio, M.; Di Fronzo, G. Genistein in the control of breast cancer cell growth: Insights into the mechanism of action in vitro. Cancer Lett. 1998, 130, 143–152. [Google Scholar] [CrossRef]
- Le Bail, J.C.; Champavier, Y.; Chulia, A.J.; Habrioux, G. Effects of phytoestrogens on aromatase, 3beta and 17beta-hydroxysteroid dehydrogenase activities and human breast cancer cells. Life Sci. 2000, 66, 1281–1291. [Google Scholar] [CrossRef]
- Liu, B.; Edgerton, S.; Yang, X.; Kim, A.; Ordonez-Ercan, D.; Mason, T.; Alvarez, K.; McKimmey, C.; Liu, N.; Thor, A. Low-dose dietary phytoestrogen abrogates tamoxifen-associated mammary tumor prevention. Cancer Res. 2005, 65, 879–886. [Google Scholar]
- Limer, J.L.; Parkes, A.T.; Speirs, V. Differential response to phytoestrogens in endocrine sensitive and resistant breast cancer cells in vitro. Int. J. Cancer 2006, 119, 515–521. [Google Scholar] [CrossRef]
- Wang, C.; Kurzer, M.S. Effects of phytoestrogens on DNA synthesis in MCF-7 cells in the presence of estradiol or growth factors. Nutr. Cancer 1998, 31, 90–100. [Google Scholar] [CrossRef]
- Maggiolini, M.; Bonofiglio, D.; Marsico, S.; Panno, M.L.; Cenni, B.; Picard, D.; Andò, S. Estrogen receptor α mediates the proliferative but not the cytotoxic dose-dependent effects of two major phytoestrogens on human breast cancer cells. Mol. Pharmacol. 2001, 60, 595–602. [Google Scholar]
- Miodini, P.; Fioravanti, L.; Fronzo, G.D.; Cappelletti, V. The two phyto-oestrogens genistein and quercetin exert different effects on oestrogen receptor function. Br. J. Cancer 1999, 80, 1150–1155. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.T.; Sathyamoorthy, N.; Phang, J.M. Molecular effects of genistein on estrogen receptor mediated pathways. Carcinogenesis 1996, 17, 271–275. [Google Scholar] [CrossRef] [Green Version]
- El Touny, L.H.; Banerjee, P.P. Identification of a biphasic role for genistein in the regulation of prostate cancer growth and metastasis. Cancer Res. 2009, 69, 3695–3703. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Clubbs, E.A.; Bomser, J.A. Genistein modulates prostate epithelial cell proliferation via estrogen- and extracellular signal-regulated kinase-dependent pathways. J. Nutr. Biochem. 2006, 17, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Moore, A.; Castro, L.; Yu, L.; Zheng, X.; Di, X.; Sifre, M.; Kissling, G.; Newbold, R.; Bortner, C.; Dixon, D. Stimulatory and inhibitory effects of genistein on human uterine leiomyoma cell proliferation are influenced by the concentration. Hum. Reprod. 2007, 22, 2623–2631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dang, Z.C.; Audinot, V.; Papapoulos, S.E.; Boutin, J.A.; Lowik, C.W. Peroxisome proliferator-activated receptor gamma (PPARgamma) as a molecular target for the soy phytoestrogen genistein. J. Biol. Chem. 2003, 278, 962–967. [Google Scholar] [CrossRef] [Green Version]
- Ying, C.; Hsu, J.T.; Hung, H.C.; Lin, D.H.; Chen, L.F.; Wang, L.K. Growth and cell cycle regulation by isoflavones in human breast carcinoma cells. Reprod. Nutr. Dev. 2002, 42, 55–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, J.T.; Jean, T.C.; Chan, M.A.; Ying, C. Differential display screening for specific gene expression induced by dietary nonsteroidal estrogen. Mol. Reprod. Dev. 1999, 52, 141–148. [Google Scholar] [CrossRef]
- Guo, J.M.; Xiao, B.X.; Liu, D.H.; Grant, M.; Zhang, S.; Lai, Y.F.; Guo, Y.B.; Liu, Q. Biphasic effect of daidzein on cell growth of human colon cancer cells. Food Chem. Toxicol. 2004, 42, 1641–1646. [Google Scholar] [CrossRef]
- Dang, Z.; Lowik, C.W. The balance between concurrent activation of ERs and PPARs determines daidzein-induced osteogenesis and adipogenesis. J. Bone Min. Res. 2004, 19, 853–861. [Google Scholar] [CrossRef]
- van der Woude, H.; Gliszczynska-Swiglo, A.; Struijs, K.; Smeets, A.; Alink, G.M.; Rietjens, I.M. Biphasic modulation of cell proliferation by quercetin at concentrations physiologically relevant in humans. Cancer Lett. 2003, 200, 41–47. [Google Scholar] [CrossRef]
- Elattar, T.M.; Virji, A.S. The inhibitory effect of curcumin, genistein, quercetin and cisplatin on the growth of oral cancer cells in vitro. Anticancer Res. 2000, 20, 1733–1738. [Google Scholar]
- Bai, H.W.; Zhu, B.T. Strong activation of cyclooxygenase I and II catalytic activity by dietary bioflavonoids. J. Lipid Res. 2008, 49, 2557–2570. [Google Scholar] [CrossRef] [Green Version]
- Bai, H.W.; Zhu, B.T. Myricetin and quercetin are naturally occurring co-substrates of cyclooxygenases in vivo. Prostaglandins Leukot. Essent. Fat. Acids 2010, 82, 45–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chirumbolo, S.; Conforti, A.; Ortolani, R.; Vella, A.; Marzotto, M.; Bellavite, P. Stimulus-specific regulation of CD63 and CD203c membrane expression in human basophils by the flavonoid quercetin. Int. Immunopharmacol. 2010, 10, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Chirumbolo, S.; Marzotto, M.; Conforti, A.; Vella, A.; Ortolani, R.; Bellavite, P. Bimodal action of the flavonoid quercetin on basophil function: An investigation of the putative biochemical targets. Clin. Mol. Allergy 2010, 8, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pietsch, K.; Saul, N.; Chakrabarti, S.; Sturzenbaum, S.R.; Menzel, R.; Steinberg, C.E. Hormetins, antioxidants and prooxidants: Defining quercetin-, caffeic acid- and rosmarinic acid-mediated life extension in C. elegans. Biogerontology 2011, 12, 329–347. [Google Scholar] [CrossRef]
- Snijman, P.W.; Swanevelder, S.; Joubert, E.; Green, I.R.; Gelderblom, W.C. The antimutagenic activity of the major flavonoids of rooibos (Aspalathus linearis): Some dose-response effects on mutagen activation-flavonoid interactions. Mutat. Res. 2007, 631, 111–123. [Google Scholar] [CrossRef]
- Kang, I.H.; Kim, H.J.; Oh, H.; Park, Y.I.; Dong, M.S. Biphasic effects of the flavonoids quercetin and naringenin on the metabolic activation of 2-amino-3,5-dimethylimidazo[4,5-f]quinoline by Salmonella typhimurium TA1538 co-expressing human cytochrome P450 1A2, NADPH-cytochrome P450 reductase, and cytochrome b5. Mutat. Res. 2004, 545, 37–47. [Google Scholar]
- Hsu, J.T.; Hung, H.C.; Chen, C.J.; Hsu, W.L.; Ying, C. Effects of the dietary phytoestrogen biochanin A on cell growth in the mammary carcinoma cell line MCF-7. J. Nutr. Biochem. 1999, 10, 510–517. [Google Scholar] [CrossRef]
- Pedro, M.; Lourenco, C.F.; Cidade, H.; Kijjoa, A.; Pinto, M.; Nascimento, M.S. Effects of natural prenylated flavones in the phenotypical ER (+) MCF-7 and ER (-) MDA-MB-231 human breast cancer cells. Toxicol. Lett. 2006, 164, 24–36. [Google Scholar] [CrossRef]
- Pedro, M.; Ferreira, M.M.; Cidade, H.; Kijjoa, A.; Bronze-da-Rocha, E.; Nascimento, M.S. Artelastin is a cytotoxic prenylated flavone that disturbs microtubules and interferes with DNA replication in MCF-7 human breast cancer cells. Life Sci. 2005, 77, 293–311. [Google Scholar] [CrossRef]
- Yap, S.P.; Shen, P.; Butler, M.S.; Gong, Y.; Loy, C.J.; Yong, E.L. New estrogenic prenylflavone from Epimedium brevicornum inhibits the growth of breast cancer cells. Planta Med. 2005, 71, 114–119. [Google Scholar] [CrossRef]
- Tamir, S.; Eizenberg, M.; Somjen, D.; Stern, N.; Shelach, R.; Kaye, A.; Vaya, J. Estrogenic and antiproliferative properties of glabridin from licorice in human breast cancer cells. Cancer Res. 2000, 60, 5704–5709. [Google Scholar] [PubMed]
- Tamir, S.; Eizenberg, M.; Somjen, D.; Izrael, S.; Vaya, J. Estrogen-like activity of glabrene and other constituents isolated from licorice root. J. Steroid Biochem. Mol. Biol. 2001, 78, 291–298. [Google Scholar] [CrossRef]
- Feng, J.; Shi, Z.; Ye, Z. Effects of metabolites of the lignans enterolactone and enterodiol on osteoblastic differentiation of MG-63 cells. Biol. Pharm. Bull. 2008, 31, 1067–1070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maggiolini, M.; Statti, G.; Vivacqua, A.; Gabriele, S.; Rago, V.; Loizzo, M.; Menichini, F.; Amdo, S. Estrogenic and antiproliferative activities of isoliquiritigenin in MCF7 breast cancer cells. J. Steroid Biochem. Mol. Biol. 2002, 82, 315–322. [Google Scholar] [CrossRef]
- Kang, S.W.; Choi, J.S.; Choi, Y.J.; Bae, J.Y.; Li, J.; Kim, D.S.; Kim, J.L.; Shin, S.Y.; Lee, Y.J.; Kwun, I.S.; et al. Licorice isoliquiritigenin dampens angiogenic activity via inhibition of MAPK-responsive signaling pathways leading to induction of matrix metalloproteinases. J. Nutr. Biochem. 2010, 21, 55–65. [Google Scholar] [CrossRef]
- Oh, S.M.; Kim, Y.P.; Chung, K.H. Biphasic effects of kaempferol on the estrogenicity in human breast cancer cells. Arch. Pharm. Res. 2006, 29, 354–362. [Google Scholar] [CrossRef]
- Setchell, K.D. Phytoestrogens: The biochemistry, physiology, and implications for human health of soy isoflavones. Am. J. Clin. Nutr. 1998, 68, 1333S–1346S. [Google Scholar] [CrossRef]
- Plauth, A.; Geikowski, A.; Cichon, S.; Wowro, S.J.; Liedgens, L.; Rousseau, M.; Weidner, C.; Fuhr, L.; Kliem, M.; Jenkins, G.; et al. Hormetic shifting of redox environment by pro-oxidative resveratrol protects cells against stress. Free Radic. Biol. Med. 2016, 99, 608–622. [Google Scholar] [CrossRef] [Green Version]
- Kumar, V.; Pandey, A.; Jahan, S.; Shukla, R.K.; Kumar, D.; Srivastava, A.; Singh, S.; Rajpurohit, C.S.; Yadav, S.; Khanna, V.K.; et al. Differential responses of Trans-Resveratrol on proliferation of neural progenitor cells and aged rat hippocampal neurogenesis. Sci. Rep. 2016, 6, 28142. [Google Scholar] [CrossRef] [Green Version]
- San Hipólito-Luengo, Á.; Alcaide, A.; Ramos-González, M.; Cercas, E.; Vallejo, S.; Romero, A.; Talero, E.; Sánchez-Ferrer, C.F.; Motilva, V.; Peiró, C. Dual effects of resveratrol on cell death and proliferation of colon cancer cells. Nutr. Cancer 2017, 69, 1019–1027. [Google Scholar] [CrossRef]
- Tvrdá, E.; Lukac, N.; Lukáčová, J.; Hashim, F.; Massányi, P. In vitro supplementation of resveratrol to bovine spermatozoa: Effects on motility, viability and superoxide production. J. Microbiol. Biotechnol. Food Sci. 2019, 4, 336–341. [Google Scholar] [CrossRef] [Green Version]
- Kuwajerwala, N.; Cifuentes, E.; Gautam, S.; Menon, M.; Barrack, E.R.; Reddy, G.P. Resveratrol induces prostate cancer cell entry into s phase and inhibits DNA synthesis. Cancer Res. 2002, 62, 2488–2492. [Google Scholar] [PubMed]
- Ortega, I.; Wong, D.H.; Villanueva, J.A.; Cress, A.B.; Sokalska, A.; Stanley, S.D.; Duleba, A.J. Effects of resveratrol on growth and function of rat ovarian granulosa cells. Fertil. Steril. 2012, 98, 1563–1573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, X.; Ni, J.; Dai, X.; Zhou, T.; Yang, G.; Xue, J.; Wang, X. Biphasic regulation of spindle assembly checkpoint by low and high concentrations of resveratrol leads to the opposite effect on chromosomal instability. Mutat. Res. Genet. Toxicol. Env. Mutagen. 2018, 825, 19–30. [Google Scholar] [CrossRef]
- Bosutti, A.; Degens, H. The impact of resveratrol and hydrogen peroxide on muscle cell plasticity shows a dose-dependent interaction. Sci. Rep. 2015, 5, 8093. [Google Scholar] [CrossRef] [Green Version]
- Kurano, M.; Hara, M.; Nojiri, T.; Ikeda, H.; Tsukamoto, K.; Yatomi, Y. Resveratrol exerts a biphasic effect on apolipoprotein M. Br. J. Pharm. 2016, 173, 222–233. [Google Scholar] [CrossRef] [Green Version]
- Peltz, L.; Gomez, J.; Marquez, M.; Alencastro, F.; Atashpanjeh, N.; Quang, T.; Bach, T.; Zhao, Y. Resveratrol exerts dosage and duration dependent effect on human mesenchymal stem cell development. PLoS ONE 2012, 7, e37162. [Google Scholar] [CrossRef]
- Lombardi, G.; Vannini, S.; Blasi, F.; Marcotullio, M.C.; Dominici, L.; Villarini, M.; Cossignani, L.; Moretti, M. In vitro safety/protection assessment of resveratrol and pterostilbene in a human hepatoma cell line (HepG2). Nat. Prod. Commun. 2015, 10, 1403–1408. [Google Scholar]
- Li, Q.; Huyan, T.; Ye, L.J.; Li, J.; Shi, J.L.; Huang, Q.S. Concentration-dependent biphasic effects of resveratrol on human natural killer cells in vitro. J. Agric. Food Chem. 2014, 62, 10928–10935. [Google Scholar] [CrossRef]
- Posadino, A.M.; Giordo, R.; Cossu, A.; Nasrallah, G.K.; Shaito, A.; Abou-Saleh, H.; Eid, A.H.; Pintus, G. Flavin oxidase-induced ROS generation modulates PKC biphasic effect of resveratrol on endothelial cell survival. Biomolecules 2019, 9. [Google Scholar] [CrossRef] [Green Version]
- Juhasz, B.; Mukherjee, S.; Das, D.K. Hormetic response of resveratrol against cardioprotection. Exp. Clin. Cardiol. 2010, 15, e134–e138. [Google Scholar] [PubMed]
- Sita, G.; Hrelia, P.; Graziosi, A.; Morroni, F. Sulforaphane from cruciferous vegetables: Recent advances to improve glioblastoma treatment. Nutrients 2018, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bao, Y.; Wang, W.; Zhou, Z.; Sun, C. Benefits and risks of the hormetic effects of dietary isothiocyanates on cancer prevention. PLoS ONE 2014, 9, e114764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calabrese, V.; Cornelius, C.; Dinkova-Kostova, A.T.; Calabrese, E.J.; Mattson, M.P. Cellular stress responses, the hormesis paradigm, and vitagenes: Novel targets for therapeutic intervention in neurodegenerative disorders. Antioxid. Redox Signal. 2010, 13, 1763–1811. [Google Scholar] [CrossRef]
- Mattson, M.P.; Cheng, A. Neurohormetic phytochemicals: Low-dose toxins that induce adaptive neuronal stress responses. Trends Neurosci. 2006, 29, 632–639. [Google Scholar] [CrossRef]
- Zanichelli, F.; Capasso, S.; Cipollaro, M.; Pagnotta, E.; Carteni, M.; Casale, F.; Iori, R.; Galderisi, U. Dose-dependent effects of R-sulforaphane isothiocyanate on the biology of human mesenchymal stem cells, at dietary amounts, it promotes cell proliferation and reduces senescence and apoptosis, while at anti-cancer drug doses, it has a cytotoxic effect. Age (Dordr) 2012, 34, 281–293. [Google Scholar] [CrossRef] [Green Version]
- Jackson, S.J.; Singletary, K.W. Sulforaphane inhibits human MCF-7 mammary cancer cell mitotic progression and tubulin polymerization. J. Nutr. 2004, 134, 2229–2236. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Wang, W.; Shan, Y.; Barrera, L.N.; Howie, A.F.; Beckett, G.J.; Wu, K.; Bao, Y. Synergy between sulforaphane and selenium in the up-regulation of thioredoxin reductase and protection against hydrogen peroxide-induced cell death in human hepatocytes. Food Chem. 2012, 133, 300–307. [Google Scholar] [CrossRef]
- Misiewicz, I.; Skupinska, K.; Kowalska, E.; Lubinski, J.; Kasprzycka-Guttman, T. Sulforaphane-mediated induction of a phase 2 detoxifying enzyme NAD(P)H:quinone reductase and apoptosis in human lymphoblastoid cells. Acta Biochim. Pol. 2004, 51, 711–721. [Google Scholar] [CrossRef] [Green Version]
- Bao, J.; Huang, B.; Zou, L.; Chen, S.; Zhang, C.; Zhang, Y.; Chen, M.; Wan, J.B.; Su, H.; Wang, Y.; et al. Hormetic Effect of Berberine Attenuates the Anticancer Activity of Chemotherapeutic Agents. PLoS ONE 2015, 10, e0139298. [Google Scholar] [CrossRef] [Green Version]
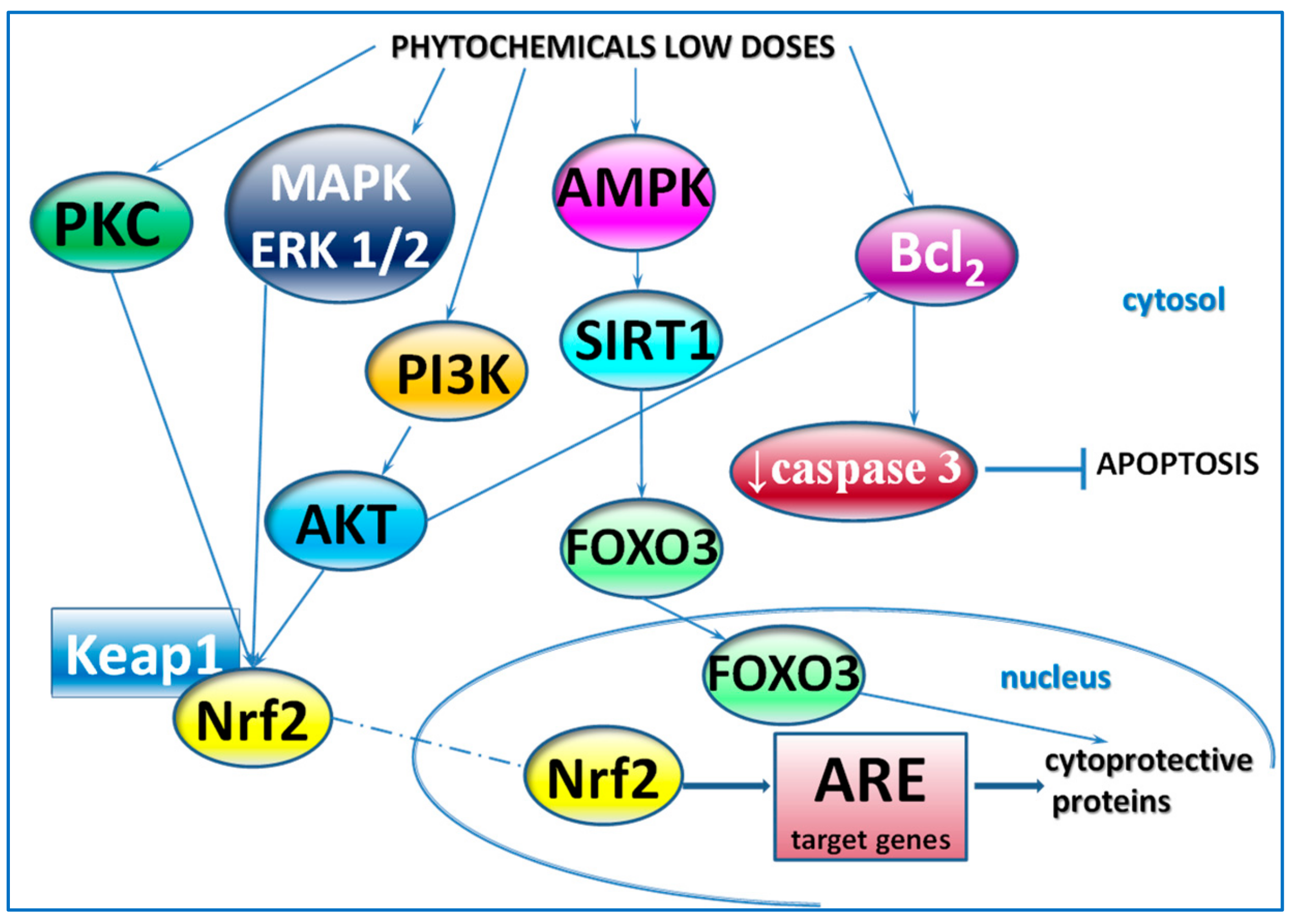
- Zhang, C.; Li, C.; Chen, S.; Li, Z.; Jia, X.; Wang, K.; Bao, J.; Liang, Y.; Wang, X.; Chen, M.; et al. Berberine protects against 6-OHDA-induced neurotoxicity in PC12 cells and zebrafish through hormetic mechanisms involving PI3K/AKT/Bcl-2 and Nrf2/HO-1 pathways. Redox Biol. 2017, 11, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, J.F.; Christensen, L.P.; Theil, P.K.; Oksbjerg, N. The polyacetylenes falcarinol and falcarindiol affect stress responses in myotube cultures in a biphasic manner. Dose Response 2008, 6, 239–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hansen, S.L.; Purup, S.; Christensen, L.P. Bioactivity of falcarinol and the influenceof processing and storage on its content in carrots (Daucus carota L). J. Sci. Food Agric. 2003, 83, 1010–1017. [Google Scholar] [CrossRef]
- Young, J.F.; Duthie, S.J.; Milne, L.; Christensen, L.P.; Duthie, G.G.; Bestwick, C.S. Biphasic effect of falcarinol on caco-2 cell proliferation, DNA damage, and apoptosis. J Agric. Food Chem. 2007, 55, 618–623. [Google Scholar] [CrossRef]
- Chattopadhyay, D.; Sen, S.; Chatterjee, R.; Roy, D.; James, J.; Thirumurugan, K. Context- and dose-dependent modulatory effects of naringenin on survival and development of Drosophila melanogaster. Biogerontology 2016, 17, 383–393. [Google Scholar] [CrossRef]
- Chattopadhyay, D.; Chitnis, A.; Talekar, A.; Mulay, P.; Makkar, M.; James, J.; Thirumurugan, K. Hormetic efficacy of rutin to promote longevity in Drosophila melanogaster. Biogerontology 2017, 18, 397–411. [Google Scholar] [CrossRef]
- Sato, Y.; Sasaki, N.; Saito, M.; Endo, N.; Kugawa, F.; Ueno, A. Luteolin attenuates doxorubicin-induced cytotoxicity to MCF-7 human breast cancer cells. Biol. Pharm. Bull. 2015, 38, 703–709. [Google Scholar] [CrossRef] [Green Version]
- Lascala, A.; Martino, C.; Parafati, M.; Salerno, R.; Oliverio, M.; Pellegrino, D.; Mollace, V.; Janda, E. Analysis of proautophagic activities of Citrus flavonoids in liver cells reveals the superiority of a natural polyphenol mixture over pure flavones. J. Nutr. Biochem. 2018, 58, 119–130. [Google Scholar] [CrossRef]
- Qi, H.; Han, Y.; Rong, J. Potential roles of PI3K/Akt and Nrf2-Keap1 pathways in regulating hormesis of Z-ligustilide in PC12 cells against oxygen and glucose deprivation. Neuropharmacology 2012, 62, 1659–1670. [Google Scholar] [CrossRef]
- Yi, Y.; Dou, G.; Yu, Z.; He, H.; Wang, C.; Li, L.; Zhou, J.; Liu, D.; Shi, J.; Li, G.; et al. Z-ligustilide exerted hormetic effect on growth and detoxification enzymes of spodoptera litura larvae. Evid. Based Complement Altern. Med. 2018, 2018, 7104513. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.R.; Qu, S.X.; Maitz, M.F.; Tan, R.; Weng, J. The effect of the major components of Salvia Miltiorrhiza Bunge on bone marrow cells. J. Ethnopharmacol. 2007, 111, 573–583. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wu, Z.; Feng, S.; Yang, X.; Huang, D. Hormesis of glyceollin I, an induced phytoalexin from soybean, on budding yeast chronological lifespan extension. Molecules 2014, 19, 568–580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gholami, O. Umbelliprenin mediates its apoptotic effect by hormesis: A commentary. Dose Response 2017, 15, 1559325817710035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribeiro, R.A.; Rodriguez de Lores Arnaiz, G. In vitro dose dependent inverse effect of nantenine on synaptosomal membrane K+-p-NPPase activity. Phytomedicine 2001, 8, 107–111. [Google Scholar] [CrossRef]
- Kafi, Z.; Cheshomi, H.; Gholami, O. 7-Isopenthenyloxycoumarin, arctigenin, and hesperidin modify myeloid cell leukemia type-1 (Mcl-1) gene expression by hormesis in K562 cell line. Dose Response 2018, 16, 1559325818796014. [Google Scholar] [CrossRef] [Green Version]
- Hunt, P.R.; Son, T.G.; Wilson, M.A.; Yu, Q.S.; Wood, W.H.; Zhang, Y.; Becker, K.G.; Greig, N.H.; Mattson, M.P.; Camandola, S.; et al. Extension of lifespan in C. elegans by naphthoquinones that act through stress hormesis mechanisms. PLoS ONE 2011, 6, e21922. [Google Scholar] [CrossRef] [Green Version]
- Xiong, L.G.; Chen, Y.J.; Tong, J.W.; Gong, Y.S.; Huang, J.A.; Liu, Z.H. Epigallocatechin-3-gallate promotes healthy lifespan through mitohormesis during early-to-mid adulthood in caenorhabditis elegans. Redox Biol. 2018, 14, 305–315. [Google Scholar] [CrossRef]
- Zhang, C.; Li, C.; Chen, S.; Li, Z.; Ma, L.; Jia, X.; Wang, K.; Bao, J.; Liang, Y.; Chen, M.; et al. Hormetic effect of panaxatriol saponins confers neuroprotection in PC12 cells and zebrafish through PI3K/AKT/mTOR and AMPK/SIRT1/FOXO3 pathways. Sci. Rep. 2017, 7, 41082. [Google Scholar] [CrossRef]
- Gezer, C.; Yücecan, S.; Rattan, S.I.S. Artichoke compound cynarin differentially affects the survival, growth, and stress response of normal, immortalized, and cancerous human cells. Turk. J. Biol. 2019, 39, 299–305. [Google Scholar] [CrossRef]
- Lutz, U.; Lugli, S.; Bitsch, A.; Schlatter, J.; Lutz, W.K. Dose response for the stimulation of cell division by caffeic acid in forestomach and kidney of the male F344 rat. Fundam. Appl. Toxicol. 1997, 39, 131–137. [Google Scholar] [CrossRef] [Green Version]
- Speciale, A.; Chirafisi, J.; Saija, A.; Cimino, F. Nutritional antioxidants and adaptive cell responses: An update. Curr. Mol. Med. 2011, 11, 770–789. [Google Scholar] [CrossRef] [PubMed]
- Birringer, M. Hormetics: Dietary triggers of an adaptive stress response. Pharm. Res. 2011, 28, 2680–2694. [Google Scholar] [CrossRef] [PubMed]
- Murugaiyah, V.; Mattson, M.P. Neurohormetic phytochemicals: An evolutionary-bioenergetic perspective. Neurochem. Int. 2015, 89, 271–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martel, J.; Ojcius, D.M.; Ko, Y.F.; Ke, P.Y.; Wu, C.Y.; Peng, H.H.; Young, J.D. Hormetic effects of phytochemicals on health and longevity. Trends Endocrinol. Metab. 2019, 30, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Thayer, K.A.; Melnick, R.; Burns, K.; Davis, D.; Huff, J. Fundamental flaws of hormesis for public health decisions. Env. Health Perspect 2005, 113, 1271–1276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marques, F.Z.; Morris, B.J. Commentary on resveratrol and hormesis: Resveratrol—A hormetic marvel in waiting? Hum. Exp. Toxicol. 2010, 29, 1026–1028. [Google Scholar] [CrossRef]
- Agathokleous, E.; Calabrese, E.J. A global environmental health perspective and optimisation of stress. Sci. Total Env. 2020, 704, 135263. [Google Scholar] [CrossRef]

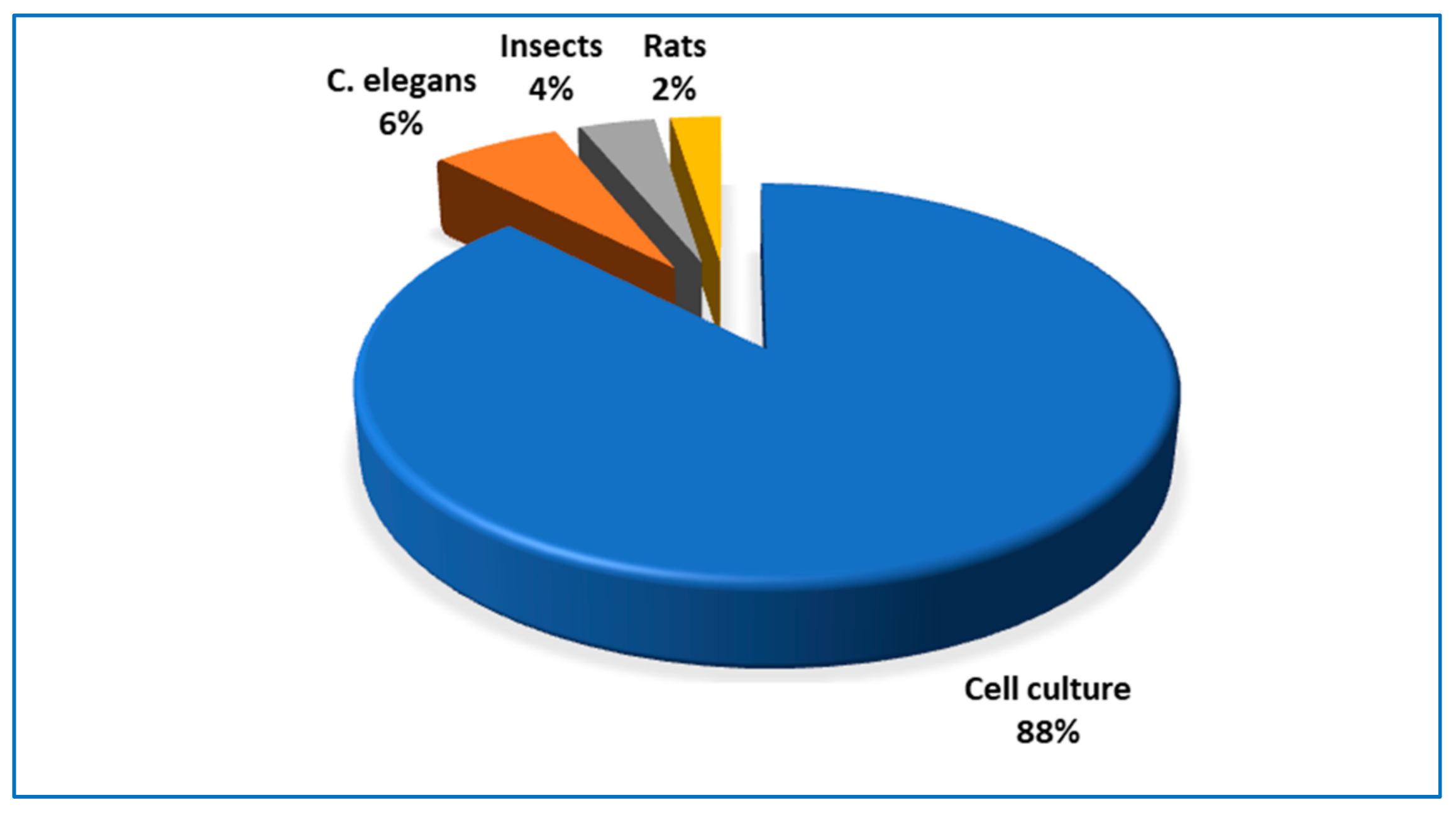
| Compound * | Model | Concentration | Effects | Mechanism | Refs |
|---|---|---|---|---|---|
| Effects Linked to Estrogenic Activity | |||||
| Artelastin Artelastocarpin Artelastochromen Carpelastofuran isolated from Artocarpus elasticus | MCF-7 | 0.02–2.90 μM | ↑proliferation, DNA synthesis | [38,39] | |
| >3.12 μM | ↓proliferation | ||||
| 25 μM | ↓DNA synthesis | ||||
| Biochanin A | MCF-7 | ~4–35 μM | ↑proliferation | [37] | |
| ~106–352 μM | ↓proliferation | ||||
| ~18 μM | ↑DNA synthesis | ||||
| ~70 μM | ↓DNA synthesis | ||||
| T-47D | ~4 μM | ↑proliferation | ↓p53 | [24] | |
| ~70 μM | ↓proliferation | ↑p53 | |||
| Breviflavone B isolated from Epimedium brevicornum | MCF-7 | 450 nM | ↑proliferation | [40] | |
| 2.2–6.6 μM | ↓proliferation | ↓ERα | |||
| Daidzein | T-47D | ~1–79 μM | ↑proliferation | ↓p53 | [24] |
| ~157 μM | ↓proliferation | ↑p53 | |||
| MCF-7 | ~1 μM | ↑proliferation | [25] | ||
| >10 μM | ↓proliferation | ||||
| LoVo | 0.1, 1.0 μM | ↑proliferation | [26] | ||
| 10–100 μM | ↓proliferation | G0/G1 arrest | |||
| ↑caspase-3 | |||||
| KS483, mouse bone marrow cells | <20 μM | ↑osteogenesis ↓adipogenesis | PPARs transactivation | [27] | |
| >30 μM | ↓osteogenesis ↑adipogenesis | ||||
| Enterodiol Enterolactone | MG-63 | ~33 μM | ↑viability | ↑osteonectin ↑collagen I | [43] |
| ~33–333 μM | ↑ALP activity | ||||
| >333 μM | ↓viability | ↓osteonectin ↓collagen I | |||
| ~3–33 mM | ↓ALP activity | ||||
| Genistein | MCF-7 | <1 μM | ↑proliferation | ↑ER transcription | [11,12,13,14,15,17,18,19] |
| >10 μM | ↓proliferation | ||||
| PC-3 | 500–1000 nM | ↑proliferation, | ↑MMP-9 activity | [20] | |
| ↑osteopontin | |||||
| 50,000 nM | ↓proliferation | ↓MMP-9 activity | |||
| RWPE-1 | 1.5–12.5 μM | ↑proliferation | ↑ERK1/2 activity | [21] | |
| 50 and 100 μM | ↓proliferation | ||||
| UtLM | ~4 μM | ↑proliferation | [22] | ||
| ↑PCNA, ↑cells in S phase | |||||
| >37 μM | ↓proliferation ↑apoptosis | ||||
| KS483, mouse bone marrow cells | 0.1–10.0 μM | ↑osteogenesis ↑ALP activity | [23] | ||
| ↑nodule formation and calcium deposition | |||||
| >25 μM | ↓osteogenesis ↓ALP activity | ||||
| ↓nodule formation and calcium deposition | |||||
| KS483, mouse bone marrow cells | 0.1–1.0 μM | ↓adipocytes number | [23] | ||
| 10–50 μM | ↓adipocytes number | ||||
| Glabrene isolated from Glycyrrhiza glabra | T47-D, MCF-7 | 100 nM–10 μM | ↑proliferation | [42] | |
| >15 μM | ↓proliferation | ||||
| Glabridin isolated from Glycyrrhiza glabra | T-46D | 0.1–10 μM | ↑proliferation | [41] | |
| >15 μM | ↓proliferation | ||||
| Isoliquiritigenin synthesized by authors | MCF-7 | <1 μM | ↑proliferation | [44] | |
| 10 μM | ↓proliferation | ||||
| Kaempherol | MCF-7 | <1 μM | ↑proliferation | [46] | |
| >1 μM | ↓proliferation | ||||
| Quercetin | MCF-7 | <1 μM | ↑proliferation | [17] | |
| >10 μM | ↓proliferation | ||||
| HCT-116 | 1–30 μM | ↑proliferation | [28] | ||
| 40–100 μM | ↓proliferation | ||||
| HT-29 | 1–67 μM | ↑proliferation | |||
| 80–100 μM | ↓proliferation | ||||
| SCC-25 | 1–10 μM | ↑proliferation | [29] | ||
| >100 μM | ↓proliferation | ||||
| Activity not Linked to Estrogenic Properties | |||||
| Isoliquiritigenin | HUVEC/PMA | <10 μM | ↑TIMP-2 | ↓JNK, p38 MAPK pathway | [45] |
| 25 μM | ↓TIMP-2 | ||||
| Quercetin | RAW 264.7 | 10–100 nM | ↑PGE2 | [30] | |
| 10–100 μM | ↓PGE2 | ||||
| basophils/fMLP | ~0.03–0.33 μM | ↑CD63, CD203c | [32] | ||
| ~3–33 μM | ↓CD63, CD203c | ||||
| basophils/fMLP | 0.03–0.3 μM | ↑histamine | PI3K involvement | [33] | |
| 33 μM | ↓histamine | ||||
| Caenorhabditis elegans | 100–200 μM | ↑lifespan | ↑hsp | [34] | |
| 250 μM | ↓lifespan | ||||
| Salmonella typhimurium/AFB1 | 0.006–0.01 mM | ↓mutagenicity | [35] | ||
| 0.06–0.12 mM | ↑mutagenicity | ||||
| Salmonella typhimurium/MeIQ | 0.1, 1 μM | ↑mutagenicity, CYP1A2 activity | [36] | ||
| 50, 100 μM | ↓mutagenicity, CYP1A2 activity | ||||
| Model | Concentration | Effects | Mechanism | Refs |
|---|---|---|---|---|
| NHEK | <50 μM | ↑viability | ↑CAT, Nrf2, KEAP1, NQO1, GCLC, GSR, G6PD, FOXO3, SIRT1, DAPK 1 (5–100 µM) | [48] |
| 500 μM | ↓viability | ↓CAT, Nrf2, KEAP1, NQO1, GCLC, GSR, G6PD, FOXO3, SIRT1, DAPK1 150 µM | ||
| NHDF | 1–300 μM | ↑viability | ||
| 500 μM | ↓viability | |||
| HepG2 | 1–100 μM | ↑viability | ||
| 500 μM | ↓viability | |||
| NPCs | 1, 10, 20 μM | ↑proliferation | ↑ERK1/2, p38, p-CREB, Bcl-2, TrkA, synaptophysin, PSA-NCAM | [49] |
| 50, 100 μM | ↓proliferation | ↓p-ERK1/2, p-p38 MAPK | ||
| ↑caspase-3 | ||||
| HT-29 | 1–10 μM | ↑proliferation | [50] | |
| 50, 100 μM | ↓proliferation | ↑NADPH oxidase activity, ↑ɣH2AX, SIRT6 | ||
| Bovine spermatozoa | 1–50 μM | ↑viability | [51] | |
| ↓superoxide anion production | ||||
| 100, 1000 μM | ↓viability | |||
| 100, 200 μM | ↑superoxide anion production | |||
| LNCaP | 5 μM, 10 μM | ↑DNA synthesis | ↓p21cip1, p27kip1 | [52] |
| ↑Cdk2 activity | ||||
| ↑cyclins A, E | ||||
| >15 μM | ↓DNA synthesis | |||
| Rat ovarian | 10 μM | ↑DNA synthesis | [53] | |
| granulosa cells | 30,50 μM | ↓DNA synthesis | ||
| Normal colon epithelial cells | 0.1–1 μM | ↓chromosomal instability, ↑viability | ↑SAC | [54] |
| 100 μM | ↑chromosomal instability, ↓viability | ↓SAC | ||
| C12C12 | 10 μM | ↑cell motility | [55] | |
| 40–60 μM | ↓cell motility | ↓miosin Tpe1 and total ATPase activity | ||
| HepG2 | 1, 10 μM | ↑apoM, | [56] | |
| 100 μM | ↓apoM | |||
| hMSCs | 0.1 μM | ↓cellular senescence | ↑Sirtuin1 | [57] |
| 5, 10 μM | ↑cellular senescence | ↓Sirtuin1, Sirtuin2, Birc4, Birc5 | ||
| ↑Cdk2 | ||||
| HepG2/4NQO | 10, 25, 50 μM | ↓genotoxicity | [58] | |
| 100, 250 μM | ↑genotoxicity | |||
| NK | 1.56, 3.13 μM | ↑cytotoxicity | ↑NKG2D, NKG2D | [59] |
| ↑IFN-γ, IFN-γ | ||||
| 25, 50 μM | ↓cytotoxicity | |||
| HUVEC | 1 μM | ↓ROS | ↑Bcl-2, c-myc, ODC | [60] |
| ↑viability, DNA synthesis | ↑PKC activity | |||
| 10, 50 μM | ↑ROS | ↓Bcl-2, c-myc, ODC | ||
| ↓viability, DNA synthesis | ↓PKC activity | |||
| Rats | 2.5 mg/kg | ↑aortic flow, LVDP, ↓infarct size | ↓cardiomyocyte apoptosis | [61] |
| 25 mg/kg | ↓aortic flow, LVDP, ↑infarct size | ↑cardiomyocyte apoptosis | ||
| 100 mg/kg | no heart function | ↑cardiomyocyte apoptosis |
| Compound * | Model | Concentration | Effect | Mechanism | Refs |
|---|---|---|---|---|---|
| Arctigenin | K-562 | ~27, 54 μM | ↑Mcl-1mRNA | [85] | |
| ~107 μM | ↓Mcl-1mRNA | ||||
| Berberine | B16-F10, | 1.25–5.00 μM | ↑proliferation | ↑MAPK/ERK1/2 ↑PI3K/AKT | [70] |
| MDA-MB-231, | 10–80 μM | ↓proliferation | |||
| MDA-MB-468, | |||||
| MCF-7, LS-174 | |||||
| PC-12 | 0.1–1.0 μM | ↑viability | ↑PI3K/AKT/Bcl-2 | [71] | |
| 2–64 μM | ↓viability | ||||
| Caffeic acid | male F344 rats | 0.14% | ↓proliferation | ↓epithelial cells, S-phase cells | [90] |
| 0.40, 1.64% | ↑proliferation | ↑epithelial cells, ↓S-phase cells in forestomach | |||
| (+) Catechin, rutin | Salmonella typhimurium/2-AAF | 0.01–0.60 mM | ↓mutagenicity | [35] | |
| 1.2, 0.8 mM | ↑mutagenicity | ||||
| Cynarin | FSF-1, | 1–50 µM | ↑viability | ↑HO-1 activity | [89] |
| 75–500 µM | ↓viability | ||||
| hTERT-MSC | 1–00 µM | ↑viability | ↑HO-1 activity | [89] | |
| 75–500 µM | ↓viability | ||||
| EGCG | Caenorhabditis elegans | 50–300 µM | ↑lifespan | ↑ROS; ↑AMPK/SIRT1/FOXO | [87] |
| 800–1000µM | ↓lifespan | ||||
| Falcarinol, Falcarindiol Isolated from carrot roots | primary myotube culture/H2O2 | 1.6–25.0 μM | ↑ROS production | ↑GPx, ↓Hsp70, HO-1 | [72] |
| 50, 100 μM | ↓ROS production | ↓GPx, ↑Hsp70, HO-1 | |||
| Falcarindiol isolated from carrot roots | primary | 0.61–9.80 nM | ↑viability | [72] | |
| myotube culture | 2.5–5.0 μM | ↓viability | |||
| pBMEC | ~0.04–0.20 μM | ↑proliferation | [73] | ||
| ~4–41 μM | ↓proliferation | ||||
| CaCo-2 | 1–10 μM | ↑proliferation | ↓caspase-3, DNA breakage | [74] | |
| ↓apoptosis | |||||
| >20 μM | ↓proliferation | ↑caspase-3, DNA breakage | |||
| ↑apoptosis | |||||
| Glyceollin I isolated from soybean | Saccharomyces cerevisiae | 10–100 nM | ↑CLS | [82] | |
| >1 μM | ↓CLS | ||||
| Luteolin | MCF-7 | 1–10 μM | ↑viability | [77] | |
| 30–1000 μM | ↓viability | ||||
| HepG2 | <35 μM | ↑LC3-II | [78] | ||
| ~105 μM | ↓LC3-II | ||||
| Salmonella typhimurium/2-AAF | 0.006 mM | ↑mutagenicity | [35] | ||
| 1.2 mM | ↓mutagenicity | ||||
| Nanteine isolated from Ocotea macrophilla | synaptosomal membranes | 50 μM, 0.3 mM | ↑K+ -p-NPPase activity | [84] | |
| >0.75 mM | ↓K+ -p-NPPase activity | ||||
| Naringenin | Drosophila melanogaster | 200, 400 μM | ↑lifespan | ↑pupae formation | [75] |
| 600, 800 μM | ↓lifespan | ↓pupae formation | |||
| Naphazarin | Caenorhabditis elegans | 50–500 μM | ↑lifespan | ↑skn-1 | [86] |
| 1000 µM | ↓lifespan | ||||
| Panaxatriol saponins isolated from Panax notoginseng | PC-12 | 0.03–1.00 mg/ml | ↑proliferation | [88] | |
| 4 mg/ml | ↓proliferation | ||||
| PC-12 /6-OHDA | 0.03–2.00 mg/ml | ↑viability | ↑PI3K/AKT/mTOR ↑AMPK/SIRT1/FOXO3 | ||
| 4 mg/ml | ↓viability | ||||
| Plumbagin | Caenorhabditis elegans | 1–45 μM | ↑lifespan | ↑skn-1 | [86] |
| 100 μM | ↓lifespan | ||||
| Rosmarinic acid | Caenorhabditis elegans | 100–300 µM | ↑lifespan | ↑hsp | [34] |
| 600 µM | ↓lifespan | ||||
| Rutin | Drosophila melanogaster | 200, 400 μM | ↑lifespan | ↑longevity associated genes | [76] |
| 600, 800 μM | ↓lifespan | ||||
| Salvianolic acid B | BMSCs | ~4–111 μM | ↑metabolic activity, ALP activity | [81] | |
| ~223 μM | ↓metabolic activity, ALP activity | ||||
| Sulforaphane | T24, HepG2, Caco-2 | 1–5 μM | ↑proliferation | ↑RAS, RAF, MEK, ERK, PI3K, AKT and Nf-kB, FOXO Nrf2 pathways | [63] |
| 10–40 μM | ↓proliferation | ||||
| T24 | 2.50, 3.75 μM | ↑migration | |||
| 5–40 μM | ↓migration | ||||
| HUVEC, PVC | 2.5–5.0 μM | ↑angiogenesis | ↑tube formation | ||
| 10, 20 μM | ↓angiogenesis | ↓tube formation | |||
| Isolated from Brassica oleracea | MSCs | 0.25, 1.00 μM | ↑proliferation | [66] | |
| 20 μM | ↓proliferation | ||||
| <5 μM | ↓apoptotic cells | ||||
| 20 μM | ↑apoptotic cells | ||||
| 0.25, 1.00 μM | ↓senescence cells | ||||
| 5, 20 μM | ↑senescence cells | ||||
| 0.25 μM | ↓ROS production | ||||
| 20 μM | ↑ROS production | ||||
| Commercial source | MCF-7, HHL-5, HepG2, lymphoblastoid cells | <5 μM | ↑proliferation | [67,68,69] | |
| >5 μM | ↓proliferation | ||||
| lymphoblastoid cells | 0.5–5.0 μM | ↑GSH | [69] | ||
| 10 μM | ↓GSH | ||||
| Umbelliprenin isolated from Ferula szowitsiana | Jurkat T-CLL | 10, 25 μM | ↑apoptosis | [83] | |
| 50, 100 μM | ↓apoptosis | ||||
| Z-ligustilide isolated from Ligusticum chuanxiong | PC-12/ OGD | 1–25 μM | ↑viability, ↓apoptosis | ↑HO-1 and Nrf2 translocation | [79] |
| 50 μM | ↓viability, ↑apoptosis | ||||
| Spodoptera litura larvae | 0.1–0.5 mg/g diet | ↑GST, AChE, CYP, CES activities | ↑GSTS1, CYP4S9, CYP4M14 | [80] | |
| 1, 5 mg/g diet | ↓GST, AChE, CYP activity | ↓GSTS1, CYP4S9, CYP4M14 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jodynis-Liebert, J.; Kujawska, M. Biphasic Dose-Response Induced by Phytochemicals: Experimental Evidence. J. Clin. Med. 2020, 9, 718. https://doi.org/10.3390/jcm9030718
Jodynis-Liebert J, Kujawska M. Biphasic Dose-Response Induced by Phytochemicals: Experimental Evidence. Journal of Clinical Medicine. 2020; 9(3):718. https://doi.org/10.3390/jcm9030718
Chicago/Turabian StyleJodynis-Liebert, Jadwiga, and Małgorzata Kujawska. 2020. "Biphasic Dose-Response Induced by Phytochemicals: Experimental Evidence" Journal of Clinical Medicine 9, no. 3: 718. https://doi.org/10.3390/jcm9030718
APA StyleJodynis-Liebert, J., & Kujawska, M. (2020). Biphasic Dose-Response Induced by Phytochemicals: Experimental Evidence. Journal of Clinical Medicine, 9(3), 718. https://doi.org/10.3390/jcm9030718