Light and Circadian Signaling Pathway in Pregnancy: Programming of Adult Health and Disease
Abstract
:1. Introduction
2. The Impacts of Light and Circadian Signals in Pregnancy and Fetal Development
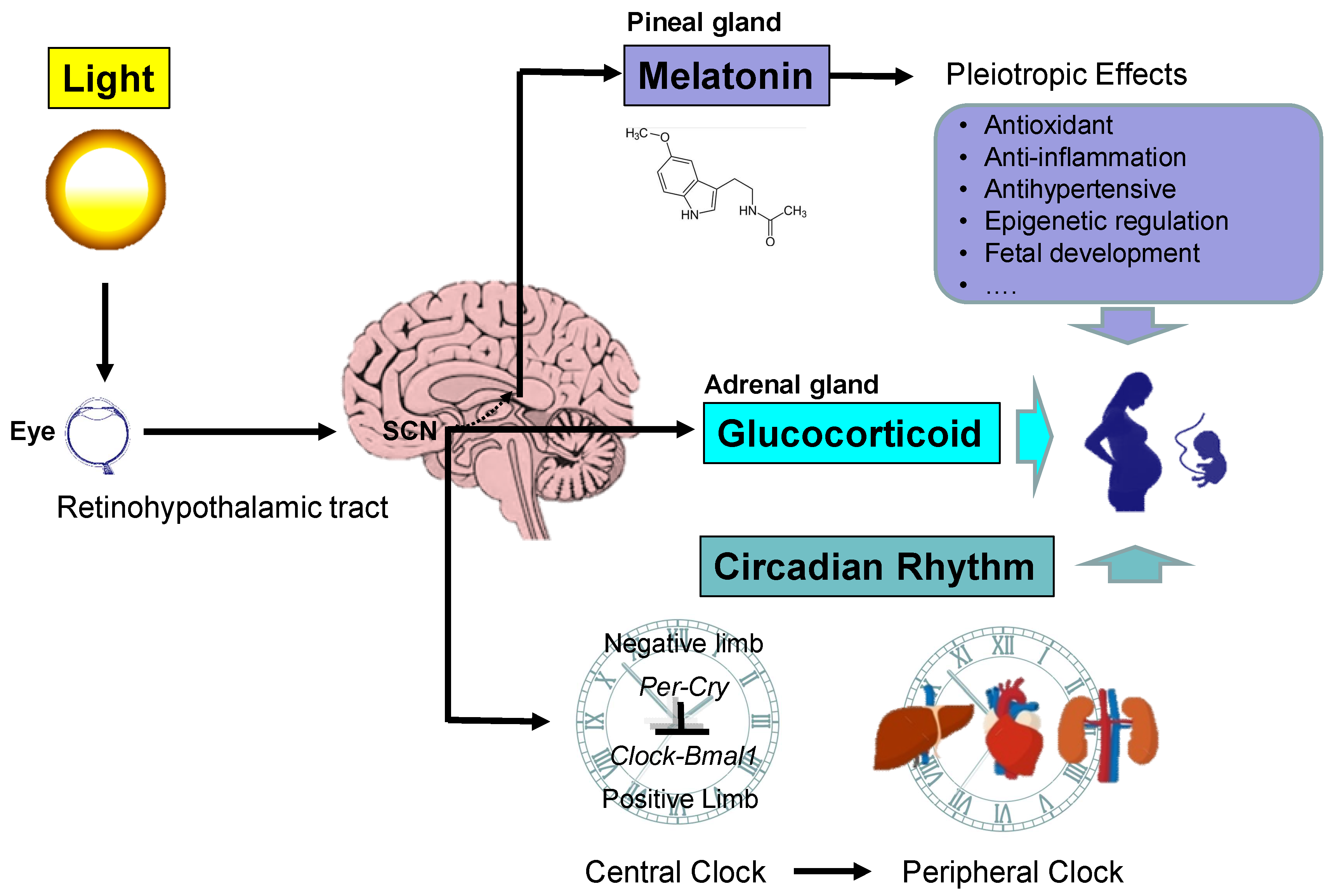
2.1. The Retinohypothalamic Pathway
2.2. Maternal and Fetal Circadian Rhythms
2.3. Ontogenesis of the Circadian Clock
2.4. Maternal Circadian Signals: Melatonin and Glucocorticoid
2.5. Melatonin in Pregnancy and the Fetus
2.6. Placental Melatonin
2.7. Glucocorticoids in Pregnancy and the Fetus
3. Maternal Chronodisruption and Offspring Health
3.1. Human Studies for Programming of Adult Diseases Related to Maternal Chronodisruption
3.2. Animal Models of Maternal Chronodisruption
3.3. Mechanisms Underlying Maternal Chronodisruption-Induced Programmed Diseases
4. Targeting on Light and Circadian Signaling Pathway as a Reprogramming Therapy
4.1. Light and Circadian Signaling-Related Therapy in Human Diseases
4.2. Melatonin as a Reprogramming Therapy in Animal Models
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| ACE | Angiotensin-converting enzyme |
| AT1R | Angiotensin type 1 receptor |
| BMAL1 | Brain and muscle aryl-hydrocarbon receptor nuclear translocator-like 1 |
| CLOCK | Circadian locomotor output cycles kaput |
| CPH | Chronic photoperiod shift |
| DEX | Dexamethasone |
| DNMT | DNA methyltransferases |
| DOHaD | Developmental origins of health and disease |
| GC | Glucocorticoid |
| HDAC | Histone deacetylase |
| HPA | Hypothalamic–pituitary–adrenal |
| L-NAME | NG-nitro-L-arginine-methyl ester |
| MT | Melatonin receptor |
| PBM | Photobiomodulation |
| RAS | Renin-angiotensin system |
| RHT | Retinohypothalamic tract |
| ROS | Reactive oxygen species |
| SCN | Suprachiasmatic nucleus |
References
- Tsao, J.Y.; Han, J.; Haitz, R.H.; Pattison, P.M. The blue LED Nobel prize: Historical context, current scientific understanding, human benefit. Ann. Phys. 2015, 527, A53–A61. [Google Scholar] [CrossRef] [Green Version]
- Hardeland, R.; Pandi-Perumal, S.R.; Cardinali, D.P. Melatonin. Int. J. Biochem. Cell Biol. 2006, 38, 313–316. [Google Scholar] [CrossRef] [PubMed]
- Ishida, A.; Mutoh, T.; Ueyama, T.; Bando, H.; Masubuchi, S.; Nakahara, D.; Tsujimoto, G.; Okamura, H. Light activates the adrenal gland: Timing of gene expression and glucocorticoid release. Cell Metab. 2005, 2, 297–307. [Google Scholar] [CrossRef] [Green Version]
- Scheer, F.A.; Hilton, M.F.; Mantzoros, C.S.; Shea, S.A. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc. Natl. Acad. Sci. USA 2009, 106, 4453–4458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stevens, R.G.; Brainard, G.C.; Blask, D.E.; Lockley, S.W.; Motta, M.E. Breast cancer and circadian disruption from electric lighting in the modern world. CA Cancer J. Clin. 2014, 64, 207–218. [Google Scholar] [CrossRef]
- Leproult, R.; Holmbäck, U.; Van Cauter, E. Circadian misalignment augments markers of insulin resistance and inflammation, independently of sleep loss. Diabetes 2014, 63, 1860–1869. [Google Scholar] [CrossRef] [Green Version]
- Morris, C.J.; Purvis, T.E.; Mistretta, J.; Scheer, F.A. Effects of the internal circadian system and circadian misalignment on glucose tolerance in chronic shift workers. J. Clin. Endocrinol. Metab. 2016, 101, 1066–1074. [Google Scholar] [CrossRef]
- Wirz-Justice, A.; Benedetti, F.; Terman, M.; Basel, S. Chronotherapeutics for affective disorders: A clinician’s manual for light and wake therapy. Ann. Clin. Psychiatry 2010, 22, 67. [Google Scholar]
- Brainard, G.C.; Barger, L.K.; Soler, R.R.; Hanifin, J.P. The development of lighting countermeasures for sleep disruption and circadian misalignment during spaceflight. Curr. Opin. Pulm. Med. 2016, 22, 535–544. [Google Scholar] [CrossRef]
- Hardeland, R.; Tan, D.X.; Reiter, R.J. Kynuramines, metabolites of melatonin and other indoles: The resurrection of an almost forgotten class of biogenic amines. J. Pineal Res. 2009, 47, 109–126. [Google Scholar] [CrossRef]
- Chen, Y.C.; Tain, Y.L.; Sheen, J.M.; Huang, L.T. Melatonin utility in neonates and children. J. Formos. Med. Assoc. 2012, 111, 57–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reiter, R.J.; Mayo, J.C.; Tan, D.X.; Sainz, R.M.; Alatorre-Jimenez, M.; Qin, L. Melatonin as an antioxidant: Under promises but over delivers. J. Pineal Res. 2016, 61, 253–278. [Google Scholar] [CrossRef] [PubMed]
- Barker, D.J. The origins of the developmental origins theory. J. Intern. Med. 2007, 261, 412–417. [Google Scholar] [CrossRef]
- Haugen, A.C.; Schug, T.T.; Collman, G.; Heindel, J.J. Evolution of DOHaD: The impact of environmental health sciences. J. Dev. Orig. Health Dis. 2015, 6, 55–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, J.S. Transcriptional architecture of the mammalian circadian clock. Nat. Rev. Genet. 2017, 18, 164–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berson, D.M.; Dunn, F.A.; Takao, M. Phototransduction by retinal ganglion cells that set the circadian clock. Science 2002, 295, 1070–1073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewy, A.J.; Wehr, T.A.; Goodwin, F.K.; Newsome, D.A.; Markey, S.P. Light suppresses melatonin secretion in humans. Science 1980, 210, 1267–1269. [Google Scholar] [CrossRef]
- Spencer, R.L.; Chun, L.E.; Hartsock, M.J.; Woodruff, E.R. Glucocorticoid hormones are both a major circadian signal and major stress signal: How this shared signal contributes to a dynamic relationship between the circadian and stress systems. Front. Neuroendocrinol. 2018, 49, 52–71. [Google Scholar] [CrossRef]
- Gumz, M.L. Molecular origin of the kidney clock. Kidney Int. 2014, 86, 873–874. [Google Scholar] [CrossRef] [Green Version]
- Bonny, O.; Vinciguerra, M.; Gumz, M.L.; Mazzoccoli, G. Molecular bases of circadian rhythmicity in renal physiology and pathology. Nephrol. Dial. Transpl. 2013, 28, 2421–2431. [Google Scholar] [CrossRef] [Green Version]
- Seron-Ferre, M.; Valenzuela, G.J.; Torres-Farfan, C. Circadian clocks during embryonic and fetal development. Birth Defects Res. C Embryo Today 2007, 81, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Weinert, D. Ontogenetic development of the mammalian circadian system. Chronobiol. Int. 2005, 22, 179–205. [Google Scholar] [CrossRef] [PubMed]
- Sumová, A.; Bendová, Z.; Sládek, M.; El-Hennamy, R.; Laurinová, K.; Jindráková, Z.; Illnerová, H. Setting the biological time in central and peripheral clocks during ontogenesis. FEBS Lett. 2006, 580, 2836–2842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torres-Farfan, C.; Rocco, V.; Monso’, C.; Valenzuela, F.J.; Campino, C.; Germain, A.; Torrealba, F.; Valenzuela, G.J.; Seron-Ferre, M. Maternal melatonin effects on clock gene expression in a nonhuman primate fetus. Endocrinology 2006, 147, 4618–4626. [Google Scholar] [CrossRef]
- Astiz, M.; Oster, H. Perinatal Programming of Circadian Clock-Stress Crosstalk. Neural Plast. 2018, 2018, 5689165. [Google Scholar] [CrossRef] [Green Version]
- Vermes, I.; Dohanics, J.; Tóth, G.; Pongrácz, J. Maturation of the circadian rhythm of the adrenocortical functions in human neonates and infants. Horm. Res. Paediatr. 1980, 12, 237–244. [Google Scholar] [CrossRef]
- Rivkees, S.A.; Hofman, P.L.; Fortman, J. Newborn primate infants are entrained by low intensity lighting. Proc. Natl. Acad. Sci. USA 1997, 94, 292–297. [Google Scholar] [CrossRef] [Green Version]
- Kennaway, D.J.; Stamp, G.E.; Goble, F.C. Development of melatonin production in infants and the impact of prematurity. J. Clin. Endocrinol. Metab. 1992, 75, 367–369. [Google Scholar]
- Ekmekcioglu, C. Melatonin receptors in humans: Biological role and clinical relevance. Biomed. Pharmacother. 2006, 60, 97–108. [Google Scholar] [CrossRef]
- Korkmaz, A.; Reiter, R.J. Epigenetic regulation: A new research area for melatonin? J. Pineal Res. 2008, 44, 41–44. [Google Scholar] [CrossRef]
- Tain, Y.L.; Huang, L.T.; Hsu, C.N. Developmental Programming of Adult Disease: Reprogramming by Melatonin? Int. J. Mol. Sci. 2017, 18, 426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Napso, T.; Yong, H.E.J.; Lopez-Tello, J.; Sferruzzi-Perri, A.N. The Role of Placental Hormones in Mediating Maternal Adaptations to Support Pregnancy and Lactation. Front. Physiol. 2018, 9, 1091. [Google Scholar] [CrossRef] [PubMed]
- Soliman, A.; Lacasse, A.A.; Lanoix, D.; Sagrillo-Fagundes, L.; Boulard, V.; Vaillancourt, C. Placental melatonin system is present throughout pregnancy and regulates villous trophoblast differentiation. J. Pineal Res. 2015, 59, 38–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lanoix, D.; Beghdadi, H.; Lafond, J.; Vaillancourt, C. Human placental trophoblasts synthesize melatonin and express its receptors. J. Pineal Res. 2008, 45, 50–60. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.X.; Korkmaz, A.; Rosales-Corral, S.A. Melatonin and stable circadian rhythms optimize maternal, placental and fetal physiology. Hum. Reprod. Update 2014, 20, 293–307. [Google Scholar] [CrossRef] [Green Version]
- Okatani, Y.; Okamoto, K.; Hayashi, K.; Wakatsuki, A.; Tamura, S.; Sagara, Y. Maternal-fetal transfer of melatonin in pregnant women near term. J. Pineal Res. 1998, 25, 129–134. [Google Scholar] [CrossRef]
- Seron-Ferre, M.; Torres-Farfan, C.; Forcelledo, M.L.; Valenzuela, G.J. The development of circadian rhythms in the fetus and neonate. Semin. Perinatol. 2001, 25, 363–370. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.H.; Zhou, J.N.; Balesar, R.; Unmehopa, U.; Bao, A.; Jockers, R.; Van Heerikhuize, J.; Swaab, D.F. Distribution of MT1 melatonin receptor immunoreactivity in the human hypothalamus and pituitary gland: Colocalization of MT1 with vasopressin, oxytocin, and corticotropin-releasing hormone. J. Comp. Neurol. 2006, 499, 897–910. [Google Scholar] [CrossRef]
- da Silveira Cruz-Machado, S.; Tamura, E.K.; Carvalho-Sousa, C.E.; Rocha, V.A.; Pinato, L.; Fernandes, P.A.C.; Markus, R.P. Daily corticosterone rhythm modulates pineal function through NFκB-related gene transcriptional program. Sci. Rep. 2017, 7, 2091. [Google Scholar] [CrossRef] [Green Version]
- Balsalobre, A.; Brown, S.A.; Marcacci, L.; Tronche, F.; Kellendonk, C.; Reichardt, H.M.; Schütz, G.; Schibler, U. Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science 2000, 289, 2344–2347. [Google Scholar] [CrossRef] [Green Version]
- Moisiadis, V.G.; Matthews, S.G. Glucocorticoids and fetal programming part 1: Outcomes. Nat. Rev. Endocrinol. 2014, 10, 391–402. [Google Scholar] [CrossRef] [PubMed]
- Cole, T.J.; Blendy, J.A.; Monaghan, A.P.; Krieglstein, K.; Schmid, W.; Aguzzi, A.; Fantuzzi, G.; Hummler, E.; Unsicker, K.; Schütz, G. Targeted disruption of the glucocorticoid receptor gene blocks adrenergic chromaffin cell development and severely retards lung maturation. Genes Dev. 1995, 9, 1608–1621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wharfe, M.D.; Mark, P.J.; Waddell, B.J. Circadian variation in placental and hepatic clock genes in rat pregnancy. Endocrinology 2011, 152, 3552–3560. [Google Scholar] [CrossRef] [PubMed]
- Navailles, S.; Zimnisky, R.; Schmauss, C. Expression of glucocorticoid receptor and early growth response gene 1 during postnatal development of two inbred strains of mice exposed to early life stress. Dev. Neurosci. 2010, 32, 139–148. [Google Scholar] [CrossRef] [Green Version]
- Chau, Y.M.; West, S.; Mapedzahama, V. Night work and the reproductive health of women: An integrated literature review. J. Midwifery Womens Health 2014, 59, 113–126. [Google Scholar] [CrossRef]
- Bonzini, M.; Palmer, K.T.; Coggon, D.; Carugno, M.; Cromi, A.; Ferrario, M.M. Shift work and pregnancy outcomes: A systematic review with meta-analysis of currently available epidemiological studies. Int. J. Obstet. Gynaecol. 2011, 118, 1429–1437. [Google Scholar] [CrossRef] [Green Version]
- Strohmaier, S.; Devore, E.E.; Huang, T.; Vetter, C.; Eliassen, A.H.; Rosner, B.; Okereke, O.I.; Austin, S.B.; Schernhammer, E.S. Maternal rotating night shift work before pregnancy and offspring stress markers. Physiol. Behav. 2019, 207, 185–193. [Google Scholar] [CrossRef]
- Begtrup, L.M.; Specht, I.O.; Hammer, P.E.C.; Flachs, E.M.; Garde, A.H.; Hansen, J.; Hansen, Å.M.; Kolstad, H.A.; Larsen, A.D.; Bonde, J.P. Night work and miscarriage: A Danish nationwide register-based cohort study. Occup. Environ. Med. 2019, 76, 302–308. [Google Scholar] [CrossRef]
- Suzumori, N.; Ebara, T.; Matsuki, T.; Yamada, Y.; Kato, S.; Omori, T.; Saitoh, S.; Kamijima, M.; Sugiura-Ogasawara, M. Japan Environment & Children’s Study Group. Effects of long working hours and shift work during pregnancy on obstetric and perinatal outcomes: A large prospective cohort study-Japan Environment and Children’s Study. Birth 2019, 47, 67–79. [Google Scholar] [CrossRef] [Green Version]
- Davari, M.H.; Naghshineh, E.; Mostaghaci, M.; Mirmohammadi, S.J.; Bahaloo, M.; Jafari, A.; Mehrparvar, A.H. Shift Work Effects and Pregnancy Outcome: A Historical Cohort Study. J. Fam. Reprod. Health 2018, 12, 84–88. [Google Scholar]
- Aspholm, R.; Lindbohm, M.L.; Paakkulainen, H.; Taskinen, H.; Nurminen, T.; Tiitinen, A. Spontaneous abortions among Finnish flight attendants. J. Occup. Environ. Med. 1999, 41, 486–491. [Google Scholar] [CrossRef] [PubMed]
- Cone, J.E.; Vaughan, L.M.; Huete, A.; Samuels, S.J. Reproductive health outcomes among female flight attendants: An exploratory study. J. Occup. Environ. Med. 1998, 40, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.; Vandermeer, B.; Khurana, R.; Nerenberg, K.; Featherstone, R.; Sebastianski, M.; Davenport, M.H. The impact of occupational shift work and working hours during pregnancy on health outcomes: A systematic review and meta-analysis. Am. J. Obstet. Gynecol. 2019, 221, 563–576. [Google Scholar] [CrossRef] [PubMed]
- Nehme, P.A.; Amaral, F.G.; Middleton, B.; Lowden, A.; Marqueze, E.; França-Junior, I.; Antunes, J.L.F.; Cipolla-Neto, J.; Skene, D.J.; Moreno, C.R.C. Melatonin profiles during the third trimester of pregnancy and health status in the offspring among day and night workers: A case series. Neurobiol. Sleep Circadian Rhythm. 2019, 6, 70–76. [Google Scholar] [CrossRef]
- Strohmaier, S.; Devore, E.E.; Vetter, C.; Eliassen, A.H.; Rosner, B.; Okereke, O.I.; Schernhammer, E.S. Night shift work before and during pregnancy in relation to depression and anxiety in adolescent and young adult offspring. Eur. J. Epidemiol. 2019, 34, 625–635. [Google Scholar] [CrossRef] [Green Version]
- Tain, Y.L.; Lin, Y.J.; Chan, J.Y.H.; Lee, C.T.; Hsu, C.N. Maternal melatonin or agomelatine therapy prevents programmed hypertension in male offspring of mother exposed to continuous light. Biol. Reprod. 2017, 97, 636–643. [Google Scholar] [CrossRef]
- Voiculescu, S.E.; Le Duc, D.; Roșca, A.E.; Zeca, V.; Chiţimuș, D.M.; Arsene, A.L.; Drăgoi, C.M.; Nicolae, A.C.; Zăgrean, L.; Schöneberg, T.; et al. Behavioral and molecular effects of prenatal continuous light exposure in the adult rat. Brain Res. 2016, 1650, 51–59. [Google Scholar] [CrossRef]
- Vilches, N.; Spichiger, C.; Mendez, N.; Abarzua-Catalan, L.; Galdames, H.A.; Hazlerigg, D.G.; Richter, H.G.; Torres-Farfan, C. Gestational chronodisruption impairs hippocampal expression of NMDA receptor subunits Grin1b/Grin3a and spatial memory in the adult offspring. PLoS ONE 2014, 9, e91313. [Google Scholar] [CrossRef] [Green Version]
- Varcoe, T.J.; Wight, N.; Voultsios, A.; Salkeld, M.D.; Kennaway, D.J. Chronic phase shifts of the photoperiod throughout pregnancy programs glucose intolerance and insulin resistance in the rat. PLoS ONE 2011, 6, e18504. [Google Scholar] [CrossRef] [Green Version]
- Mendez, N.; Halabi, D.; Spichiger, C.; Salazar, E.R.; Vergara, K.; Alonso-Vasquez, P.; Carmona, P.; Sarmiento, J.M.; Richter, H.G.; Seron-Ferre, M.; et al. Gestational chronodisruption impairs circadian physiology in rat male offspring, increasing the risk of chronic disease. Endocrinology 2016, 157, 4654–4668. [Google Scholar] [CrossRef]
- Smarr, B.L.; Grant, A.D.; Perez, L.; Zucker, I.; Kriegsfeld, L.J. Maternal and Early-Life Circadian Disruption Have Long-Lasting Negative Consequences on Offspring Development and Adult Behavior in Mice. Sci. Rep. 2017, 7, 3326. [Google Scholar] [CrossRef] [PubMed]
- Carmona, P.; Pérez, B.; Trujillo, C.; Espinosa, G.; Miranda, F.; Mendez, N.; Torres-Farfan, C.; Richter, H.G.; Vergara, K.; Brebi, P.; et al. Long-Term Effects of Altered Photoperiod During Pregnancy on Liver Gene Expression of the Progeny. Front. Physiol. 2019, 10, 1377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vázquez, N.; Díaz, E.; Fernández, C.; Jiménez, V.; Esquifino, A.; Díaz, B. Seasonal variations of gonadotropins and prolactin in the laboratory rat. Role of maternal pineal gland. Physiol. Res. 2007, 56, 79–88. [Google Scholar] [PubMed]
- Workman, J.L.; Weil, Z.M.; Tuthill, C.R.; Nelson, R.J. Maternal pinealectomy increases depressive-like responses in Siberian hamster offspring. Behav. Brain Res. 2008, 189, 387–391. [Google Scholar] [CrossRef]
- Ferreira, D.S.; Amaral, F.G.; Mesquita, C.C.; Barbosa, A.P.; Lellis-Santos, C.; Turati, A.O.; Santos, L.R.; Sollon, C.S.; Gomes, P.R.; Faria, J.A.; et al. Maternal melatonin programs the daily pattern of energy metabolism in adult offspring. PLoS ONE 2012, 7, e38795. [Google Scholar] [CrossRef] [Green Version]
- Spulber, S.; Conti, M.; DuPont, C.; Raciti, M.; Bose, R.; Onishchenko, N.; Ceccatelli, S. Alterations in circadian entrainment precede the onset of depression-like behavior that does not respond to fluoxetine. Transl. Psychiatry 2015, 5, e603. [Google Scholar] [CrossRef]
- Tsai, C.C.; Tiao, M.M.; Sheen, J.M.; Huang, L.T.; Tain, Y.L.; Lin, I.C.; Lin, Y.J.; Lai, Y.J.; Chen, C.C.; Chang, K.A.; et al. Obesity programmed by prenatal dexamethasone and postnatal high-fat diet leads to distinct alterations in nutrition sensory signals and circadian-clock genes in visceral adipose tissue. Lipids Health Dis. 2019, 18, 19. [Google Scholar] [CrossRef] [Green Version]
- Tain, Y.L.; Sheen, J.M.; Chen, C.C.; Yu, H.R.; Tiao, M.M.; Kuo, H.C.; Huang, L.T. Maternal citrulline supplementation prevents prenatal dexamethasone-induced programmed hypertension. Free Radic. Res. 2014, 48, 580–586. [Google Scholar] [CrossRef]
- Tiao, M.M.; Huang, L.T.; Chen, C.J.; Sheen, J.M.; Tain, Y.L.; Chen, C.C.; Kuo, H.C.; Huang, Y.H.; Tang, K.S.; Chu, E.W.; et al. Melatonin in the regulation of liver steatosis following prenatal glucocorticoid exposure. Biomed. Res. Int. 2014, 2014, 942172. [Google Scholar] [CrossRef] [Green Version]
- Lui, C.C.; Hsu, M.H.; Kuo, H.C.; Chen, C.C.; Sheen, J.M.; Yu, H.R.; Tiao, M.M.; Tain, Y.L.; Chang, K.A.; Huang, L.T. Effects of melatonin on prenatal dexamethasone-induced epigenetic alterations in hippocampal morphology and reelin and glutamic acid decarboxylase 67 levels. Dev. Neurosci. 2015, 37, 105–114. [Google Scholar] [CrossRef]
- Sheen, J.M.; Yu, H.R.; Tiao, M.M.; Chen, C.C.; Huang, L.T.; Chang, H.Y.; Tain, Y.L. Prenatal dexamethasone-induced programmed hypertension and renal programming. Life Sci. 2015, 132, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Kuo, A.H.; Li, J.; Li, C.; Huber, H.F.; Schwab, M.; Nathanielsz, P.W.; Clarke, G.D. Prenatal steroid administration leads to adult pericardial and hepatic steatosis in male baboons. Int. J. Obes. 2017, 41, 1299–1302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, L.; Carey, L.C.; Bi, J.; Valego, N.; Sun, X.; Deibel, P.; Perrott, J.; Figueroa, J.P.; Chappell, M.C.; Rose, J.C. Gender differences in the effects of antenatal betamethasone exposure on renal function in adult sheep. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 296, R309–R317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sloboda, D.M.; Moss, T.J.; Li, S.; Matthews, S.G.; Challis, J.R.; Newnham, J.P. Expression of glucocorticoid receptor, mineralocorticoid receptor, and 11beta-hydroxysteroid dehydrogenase 1 and 2 in the fetal and postnatal ovine hippocampus: Ontogeny and effects of prenatal glucocorticoid exposure. J. Endocrinol. 2008, 197, 213–220. [Google Scholar] [CrossRef] [Green Version]
- Borisenkov, M.F.; Kosova, A.L.; Kasyanova, O.N. Impact of perinatal photoperiod on the chronotype of 11- to 18-year-olds in northern European Russia. Chronobiol. Int. 2012, 29, 305–310. [Google Scholar] [CrossRef]
- Cheng, T.S.; Loy, S.L.; Cheung, Y.B.; Cai, S.; Colega, M.T.; Godfrey, K.M.; Chong, Y.S.; Tan, K.H.; Shek, L.P.; Lee, Y.S.; et al. Plasma Vitamin D Deficiency Is Associated With Poor Sleep Quality and Night-Time Eating at Mid-Pregnancy in Singapore. Nutrients 2017, 9, 340. [Google Scholar] [CrossRef] [Green Version]
- Versteeg, R.I.; Serlie, M.J.; Kalsbeek, A.; la Fleur, S.E. Serotonin, a possible intermediate between disturbed circadian rhythms and metabolic disease. Neuroscience 2015, 301, 155–167. [Google Scholar] [CrossRef]
- Shibata, S.; Moore, R.Y. Development of a fetal circadian rhythm after disruption of the maternal circadian system. Brain Res. 1988, 469, 313–317. [Google Scholar] [CrossRef]
- Hoogerwerf, W.A.; Hellmich, H.L.; Cornélissen, G.; Halberg, F.; Shahinian, V.B.; Bostwick, J.; Savidge, T.C.; Cassone, V.M. Clock gene expression in the murine gastrointestinal tract: Endogenous rhythmicity and effects of a feeding regimen. Gastroenterology 2007, 133, 1250–1260. [Google Scholar] [CrossRef] [Green Version]
- Dolatshad, H.; Campbell, E.A.; O’Hara, L.; Maywood, E.S.; Hastings, M.H.; Johnson, M.H. Developmental and reproductive performance in circadian mutant mice. Hum. Reprod. 2006, 21, 68–79. [Google Scholar] [CrossRef] [Green Version]
- Tain, Y.L.; Hsu, C.N. Interplay between oxidative stress and nutrient sensing signaling in the developmental origins of cardiovascular disease. Int. J. Mol. Sci. 2017, 18, 841. [Google Scholar] [CrossRef] [PubMed]
- Thompson, L.P.; Al-Hasan, Y. Impact of oxidative stress in fetal programming. J. Pregnancy 2012, 2012, 582748. [Google Scholar] [CrossRef] [PubMed]
- Bogdarina, I.; Welham, S.; King, P.J.; Burns, S.P.; Clark, A.J. Epigenetic modification of the renin-angiotensin system in the fetal programming of hypertension. Circ. Res. 2007, 100, 520–526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bianco-Miotto, T.; Craig, J.M.; Gasser, Y.P.; van Dijk, S.J.; Ozanne, S.E. Epigenetics and DOHaD: From basics to birth and beyond. J. Dev. Orig. Health Dis. 2017, 8, 513–519. [Google Scholar] [CrossRef]
- Cottrell, E.C.; Seckl, J.R. Prenatal stress, glucocorticoids and the programming of adult disease. Front. Behav. Neurosci. 2009, 3, 19. [Google Scholar] [CrossRef] [Green Version]
- Colin-Gonzalez, A.L.; Aguilera, G.; Serratos, I.N.; Escribano, B.M.; Santamaria, A.; Tunez, I. On the Relationship between the Light/Dark Cycle, Melatonin and Oxidative Stress. Curr. Pharm. Des. 2015, 21, 3477–3488. [Google Scholar] [CrossRef]
- Cugini, P.; Lucia, P. Circadian rhythm of the renin-angiotensin-aldosterone system: A summary of our research studies. Clin. Ter. 2004, 155, 287–291. [Google Scholar]
- Du, S.; Chen, L.; Ge, L.; Huang, W. A Novel Loop: Mutual Regulation between Epigenetic Modification and the Circadian Clock. Front. Plant Sci. 2019, 10, 22. [Google Scholar] [CrossRef] [Green Version]
- Nicolaides, N.C.; Charmandari, E.; Chrousos, G.P.; Kino, T. Circadian endocrine rhythms: The hypothalamic-pituitary-adrenal axis and its actions. Ann. N. Y. Acad. Sci. 2014, 1318, 71–80. [Google Scholar] [CrossRef]
- Chen, Y.C.; Sheen, J.M.; Tiao, M.M.; Tain, Y.L.; Huang, L.T. Roles of melatonin in fetal programming in compromised pregnancies. Int. J. Mol. Sci. 2013, 14, 5380–5401. [Google Scholar] [CrossRef] [Green Version]
- Reiter, R.J.; Tan, D.X.; Terron, M.P.; Flores, L.J.; Czarnocki, Z. Melatonin and its metabolites: New findings regarding their production and their radical scavenging actions. Acta Biochim. Pol. 2007, 54, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tain, Y.L.; Huang, L.T.; Hsu, C.N.; Lee, C.T. Melatonin therapy prevents programmed hypertension and nitric oxide deficiency in offspring exposed to maternal caloric restriction. Oxidative Med. Cell Longev. 2014, 2014, 283180. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Zhao, Y.C.; Liang, Y.; Lin, X.H.; Tan, Y.J.; Wu, D.D.; Li, X.Z.; Ye, B.Z.; Kong, F.Q.; Sheng, J.Z.; et al. The impaired myocardial ischemic tolerance in adult offspring of diabetic pregnancy is restored by maternal melatonin treatment. J. Pineal Res. 2016, 61, 340–352. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.Y.; Lee, W.C.; Hsu, C.N.; Lee, W.C.; Huang, L.T.; Lee, C.T.; Lin, C.Y. Asymmetric dimethylarginine is associated with developmental programming of adult kidney disease and hypertension in offspring of streptozotocin-treated mothers. PLoS ONE 2013, 8, e55420. [Google Scholar] [CrossRef]
- Shirpoor, A.; Nemati, S.; Ansari, M.H.; Ilkhanizadeh, B. The protective effect of vitamin E against prenatal and early postnatal ethanol treatment-induced heart abnormality in rats: A 3-month follow-up study. Int. Immunopharmacol. 2015, 26, 72–79. [Google Scholar] [CrossRef]
- Wang, X.; Wang, J.; Luo, H.; Chen, C.; Pei, F.; Cai, Y.; Yang, X.; Wang, N.; Fu, J.; Xu, Z.; et al. Prenatal lipopolysaccharide exposure causes mesenteric vascular dysfunction through the nitric oxide and cyclic guanosine monophosphate pathway in offspring. Free Radic. Biol. Med. 2015, 86, 322–330. [Google Scholar] [CrossRef]
- Gwathmey, T.M.; Shaltout, H.A.; Rose, J.C.; Diz, D.I.; Chappell, M.C. Glucocorticoid-induced fetal programming alters the functional complement of angiotensin receptor subtypes within the kidney. Hypertension 2011, 57, 620–626. [Google Scholar] [CrossRef] [Green Version]
- Tain, Y.L.; Lee, C.T.; Chan, J.Y.; Hsu, C.N. Maternal melatonin or N-acetylcysteine therapy regulates hydrogen sulfide-generating pathway and renal transcriptome to prevent prenatal N(G)-Nitro-L-arginine-methyl ester (L-NAME)-induced fetal programming of hypertension in adult male offspring. Am. J. Obstet. Gynecol. 2016, 215, 636. [Google Scholar] [CrossRef]
- Giussani, D.A.; Camm, E.J.; Niu, Y.; Richter, H.G.; Blanco, C.E.; Gottschalk, R.; Blake, E.Z.; Horder, K.A.; Thakor, A.S.; Hansell, J.A.; et al. Developmental programming of cardiovascular dysfunction by prenatal hypoxia and oxidative stress. PLoS ONE 2012, 7, e31017. [Google Scholar] [CrossRef] [Green Version]
- Tain, Y.L.; Chan, J.Y.H.; Lee, C.T.; Hsu, C.N. Maternal Melatonin Therapy Attenuates Methyl-Donor Diet-Induced Programmed Hypertension in Male Adult Rat Offspring. Nutrients 2018, 10, 1407. [Google Scholar] [CrossRef] [Green Version]
- Tain, Y.L.; Leu, S.; Wu, K.L.; Lee, W.C.; Chan, J.Y. Melatonin prevents maternal fructose intake-induced programmed hypertension in the offspring: Roles of nitric oxide and arachidonic acid metabolites. J. Pineal Res. 2014, 57, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Te Riet, L.; van Esch, J.H.; Roks, A.J.; van den Meiracker, A.H.; Danser, A.H. Hypertension: Renin-angiotensin-aldosterone system alterations. Circ. Res. 2015, 116, 960–975. [Google Scholar] [CrossRef] [PubMed]
- Lemmer, B. Signal Transduction and Chronopharmacology of Regulation of Circadian Cardiovascular Rhythms in Animal Models of Human Hypertension. Heart Fail. Clin. 2017, 13, 739–757. [Google Scholar] [CrossRef] [PubMed]
- Simko, F.; Reiter, R.J.; Pechanova, O.; Paulis, L. Experimental models of melatonin-deficient hypertension. Front. Biosci. 2013, 18, 616–625. [Google Scholar] [CrossRef] [Green Version]
- Kett, M.M.; Denton, K.M. Renal programming: Cause for concern? Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 300, R791–R803. [Google Scholar] [CrossRef]
- Tain, Y.L.; Huang, L.T.; Chan, J.Y.; Lee, C.T. Transcriptome analysis in rat kidneys: Importance of genes involved in programmed hypertension. Int. J. Mol. Sci. 2015, 16, 4744–4758. [Google Scholar] [CrossRef] [Green Version]
- Chappell, M.C.; Marshall, A.C.; Alzayadneh, E.M.; Shaltout, H.A.; Diz, D.I. Update on the Angiotensin converting enzyme 2-Angiotensin (1-7)-MAS receptor axis: Fetal programing, sex differences, and intracellular pathways. Front. Endocrinol. 2014, 4, 201. [Google Scholar] [CrossRef] [Green Version]
- Cisternas, C.D.; Compagnucci, M.V.; Conti, N.R.; Ponce, R.H.; Vermouth, N.T. Protective effect of maternal prenatal melatonin administration on rat pups born to mothers submitted to constant light during gestation. Braz. J. Med. Biol. Res. 2010, 43, 874–882. [Google Scholar] [CrossRef] [Green Version]
- Tain, Y.L.; Sheen, J.M.; Yu, H.R.; Chen, C.C.; Tiao, M.M.; Hsu, C.N.; Lin, Y.J.; Kuo, K.C.; Huang, L.T. Maternal Melatonin Therapy Rescues Prenatal Dexamethasone and Postnatal High-Fat Diet Induced Programmed Hypertension in Male Rat Offspring. Front. Physiol. 2015, 6, 377. [Google Scholar] [CrossRef] [Green Version]
- Naruse, Y.; Oh-hashi, K.; Iijima, N.; Naruse, M.; Yoshioka, H.; Tanaka, M. Circadian and light-induced transcription of clock gene Per1 depends on histone acetylation and deacetylation. Mol. Cell. Biol. 2004, 24, 6278–6287. [Google Scholar] [CrossRef] [Green Version]
- Sahar, S.; Sassone-Corsi, P. The epigenetic language of circadian clocks. Handb. Exp. Pharmacol. 2013, 217, 29–44. [Google Scholar]
- Wu, T.H.; Kuo, H.C.; Lin, I.C.; Chien, S.J.; Huang, L.T.; Tain, Y.L. Melatonin prevents neonatal dexamethasone induced programmed hypertension: Histone deacetylase inhibition. J. Steroid Biochem. Mol. Biol. 2014, 144, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Chen, C.C.; Sheen, J.M.; Yu, H.R.; Tiao, M.M.; Kuo, H.C.; Huang, L.T. Melatonin attenuates prenatal dexamethasone-induced blood pressure increase in a rat model. J. Am. Soc. Hypertens. 2014, 8, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Pattison, P.M.; Tsao, J.Y.; Brainard, G.C.; Bugbee, B. LEDs for photons, physiology and food. Nature 2018, 563, 493–500. [Google Scholar] [CrossRef]
- Azeemi, S.T.Y.; Rafiq, H.M.; Ismail, I.; Kazmi, S.R.; Azeemi, A. The mechanistic basis of chromotherapy: Current knowledge and future perspectives. Complement. Ther. Med. 2019, 46, 217–222. [Google Scholar] [CrossRef]
- Chien, A.L.; Qi, J.; Rainer, B.; Sachs, D.L.; Helfrich, Y.R. Treatment of Acne in Pregnancy. J. Am. Board Fam. Med. 2016, 29, 254–262. [Google Scholar] [CrossRef] [Green Version]
- Traverzim, M.A.D.S.; Makabe, S.; Silva, D.F.T.; Pavani, C.; Bussadori, S.K.; Fernandes, K.S.P.; Motta, L.J. Effect of led photobiomodulation on analgesia during labor: Study protocol for a randomized clinical trial. Medicine 2018, 97, e11120. [Google Scholar] [CrossRef]
- Riemann, D.; Baglioni, C.; Bassetti, C.; Bjorvatn, B.; Dolenc Groselj, L.; Ellis, J.G.; Espie, C.A.; Garcia-Borreguero, D.; Gjerstad, M.; Gonçalves, M.; et al. European guideline for the diagnosis and treatment of insomnia. J. Sleep Res. 2017, 26, 675–700. [Google Scholar] [CrossRef]
- Leach, M.J.; Page, A.T. Herbal medicine for insomnia: A systematic review and meta-analysis. Sleep Med. Rev. 2015, 24, 1–12. [Google Scholar] [CrossRef]
- Gitto, E.; Aversa, S.; Reiter, R.J.; Barberi, I.; Pellegrino, S. Update on the use of melatonin in pediatrics. J. Pineal Res. 2011, 50, 21–28. [Google Scholar] [CrossRef]
- Sanchez-Barcelo, E.J.; Rueda, N.; Mediavilla, M.D.; Martinez-Cue, C.; Reiter, R.J. Clinical Uses of Melatonin in Neurological Diseases and Mental and Behavioural Disorders. Curr. Med. Chem. 2017, 24, 3851–3878. [Google Scholar] [CrossRef] [PubMed]
- Carpentieri, A.; Díaz de Barboza, G.; Areco, V.; Peralta López, M.; Tolosa de Talamoni, N. New perspectives in melatonin uses. Pharmacol. Res. 2012, 65, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Foley, H.M.; Steel, A.E. Adverse events associated with oral administration of melatonin: A critical systematic review of clinical evidence. Complement. Ther. Med. 2019, 42, 65–81. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.N.; Huang, L.T.; Tain, Y.L. Perinatal Use of Melatonin for Offspring Health: Focus on Cardiovascular and Neurological Diseases. Int. J. Mol. Sci. 2019, 20, 5681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tain, Y.L.; Joles, J.A. Reprogramming: A preventive strategy in hypertension focusing on the kidney. Int. J. Mol. Sci. 2015, 17, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baydas, G.; Koz, S.T.; Tuzcu, M.; Nedzvetsky, V.S. Melatonin prevents gestational hyperhomocysteinemia-associated alterations in neurobehavioral developments in rats. J. Pineal Res. 2008, 44, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Dubovický, M.; Ujházy, E.; Kovacovský, P.; Navarová, J.; Juráni, M.; Soltés, L. Effect of melatonin on neurobehavioral dysfunctions induced by intrauterine hypoxia in rats. Cent. Eur. J. Public Health 2004, 12 (Suppl.), S23–S25. [Google Scholar]
- Tain, Y.L.; Leu, S.; Lee, W.C.; Wu, K.L.H.; Chan, J.Y.H. Maternal Melatonin Therapy Attenuated Maternal High-Fructose Combined with Post-Weaning High-Salt Diets-Induced Hypertension in Adult Male Rat Offspring. Molecules 2018, 23, 886. [Google Scholar] [CrossRef] [Green Version]
- Tsai, C.C.; Lin, Y.J.; Yu, H.R.; Sheen, J.M.; Lin, I.C.; Lai, Y.J.; Tain, Y.L.; Huang, L.T.; Tiao, M.M. Regulation of Leptin Methylation Not via Apoptosis by Melatonin in the Rescue of Chronic Programming Liver Steatosis. Int. J. Mol. Sci. 2018, 19, 3565. [Google Scholar] [CrossRef] [Green Version]
- Tsai, C.C.; Lin, Y.J.; Yu, H.R.; Sheen, J.M.; Tain, Y.L.; Huang, L.T.; Tiao, M.M. Melatonin alleviates liver steatosis induced by prenatal dexamethasone exposure and postnatal high-fat diet. Exp. Ther. Med. 2018, 16, 917–924. [Google Scholar] [CrossRef]
- Figueiró, P.W.; de S Moreira, D.; Dos Santos, T.M.; Prezzi, C.A.; Rohden, F.; Faccioni-Heuser, M.C.; Manfredini, V.; Netto, C.A.; Wyse, A.T.S. The neuroprotective role of melatonin in a gestational hypermethioninemia model. Int. J. Dev. Neurosci. 2019, 78, 198–209. [Google Scholar] [CrossRef] [PubMed]

| Model | Technique | Impacts on Offspring Health |
|---|---|---|
| Constant light | 24-h constant light exposure during pregnancy | Induced hypertension in 12-week-old rat offspring [56] Induced behavior changes and melatonin signaling dysregulation in 90-day-old rat offspring [57] Impaired cognition function and altered hippocampal clock gene expression in 90-day-old rat offspring [58] |
| Chronic photoperiod shift | Repeated photoperiod shifts during pregnancy | Induced hyperinsulinemia and insulin intolerance in 12-month-old female rat offspring [59] Altered endocrine, cardiovascular, and metabolic function in 90-day-old rat offspring [60] Induced behavior changes with hyperactivity and social avoidance in 60-day-old rat offspring [61] Disrupted daily rhythms in hepatic clock genes in 3-month-old rat offspring [62] |
| Pinealectomy | Surgical removal of pineal gland | Altered seasonal variations of reproductive hormones in 60-day-old rat offspring [63] Increased depressive-like responses in adult swine offspring [64] Induced glucose intolerance in 18-week-old rat offspring [65] |
| Glucocorticoid exposure | Prenatal dexamethasone treatment | Induced depression-like behavior, arrhythmic glucocorticoid secretion, and absent circadian oscillations in hippocampal clock gene expression in 12-month-old mice offspring [66] Altered clock genes in adipose tissue and enhanced obesity, insulin dysregulation, and hypertension in 6-month-old rat offspring [67] Induced hypertension in 16-week-old rat offspring [68] Induced liver steatosis in 7-day-old offspring [69] Altered hippocampal morphology in 16-week-old rat offspring [70] Altered transcriptome in 16-week-old offspring kidney [71] |
| Prenatal betamethasone treatment | Induced obesity and liver steatosis in 10-year-old baboons [72] Induced hypertension and renal dysfunction in 1.5-year-old sheep [73] Altered hippocampal expression of HPA-related genes in 3.5-year-old sheep [74] |
| Animal Models | Route of Administration | Reprogramming Effects |
|---|---|---|
| Maternal caloric restriction | Drinking water | Prevented hypertension in 12-week-old rat offspring [92] |
| Maternal L-NAME exposure | Drinking water | Prevented hypertension in 12-week-old rat offspring [98] |
| Maternal high-fructose diet | Drinking water | Prevented hypertension in 12-week-old rat offspring [101] |
| Maternal hyperhomocysteinemia | Subcutaneous injection | Prevented cognition deficit in 75-day-old rat offspring [126] |
| Maternal phenytoin exposure | Drinking water | Protected neurobehavioral dysfunctions in 12-week-old rat offspring [127] |
| Maternal constant light exposure | Drinking water | Prevented hypertension in 12-week-old rat offspring [56] |
| Drinking water | Protected anxiety-like and sexual behaviors in 16-week-old rat offspring [106] | |
| Maternal high methyl-donor diet | Drinking water | Attenuated hypertension and altered renal transcriptome in 12-week-old rat offspring [100] |
| Maternal high-fructose diet plus post-weaning high-salt diet | Drinking water | Attenuated hypertension in 12-week-old rat offspring [128] |
| Prenatal GC exposure | Drinking water | Protected hippocampal morphology in 16-week-old rat offspring [70] |
| Drinking water | Prevented hypertension and increased nephron number in 16-week-old rat offspring [113] | |
| Drinking water | Protected liver steatosis in 16-week-old rat offspring [129] | |
| Prenatal GC exposure plus post-weaning high-fat diet | Drinking waterDrinking water | Prevented hypertension in 16-week-old rat offspring [109] Protected liver steatosis in 6-month-old rat offspring [130] |
| Maternal hypermethioninemia | Subcutaneous injection | Protected impaired recognition and neurons in 30-day-old rat offspring [131] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, C.-N.; Tain, Y.-L. Light and Circadian Signaling Pathway in Pregnancy: Programming of Adult Health and Disease. Int. J. Mol. Sci. 2020, 21, 2232. https://doi.org/10.3390/ijms21062232
Hsu C-N, Tain Y-L. Light and Circadian Signaling Pathway in Pregnancy: Programming of Adult Health and Disease. International Journal of Molecular Sciences. 2020; 21(6):2232. https://doi.org/10.3390/ijms21062232
Chicago/Turabian StyleHsu, Chien-Ning, and You-Lin Tain. 2020. "Light and Circadian Signaling Pathway in Pregnancy: Programming of Adult Health and Disease" International Journal of Molecular Sciences 21, no. 6: 2232. https://doi.org/10.3390/ijms21062232
APA StyleHsu, C.-N., & Tain, Y.-L. (2020). Light and Circadian Signaling Pathway in Pregnancy: Programming of Adult Health and Disease. International Journal of Molecular Sciences, 21(6), 2232. https://doi.org/10.3390/ijms21062232






