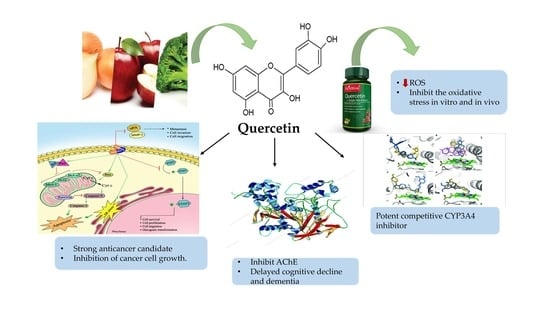
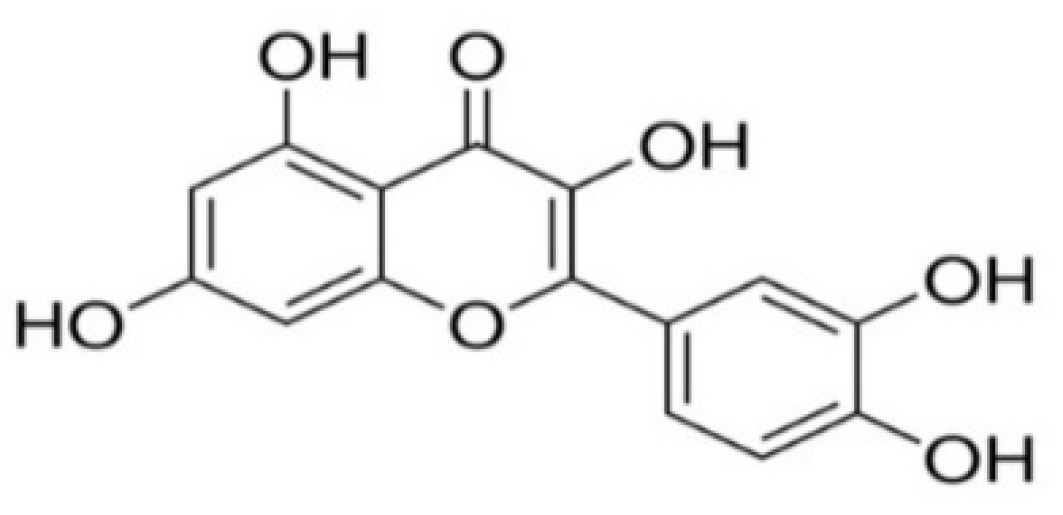
The Pharmacological Activity, Biochemical Properties, and Pharmacokinetics of the Major Natural Polyphenolic Flavonoid: Quercetin
Abstract
1. Introduction
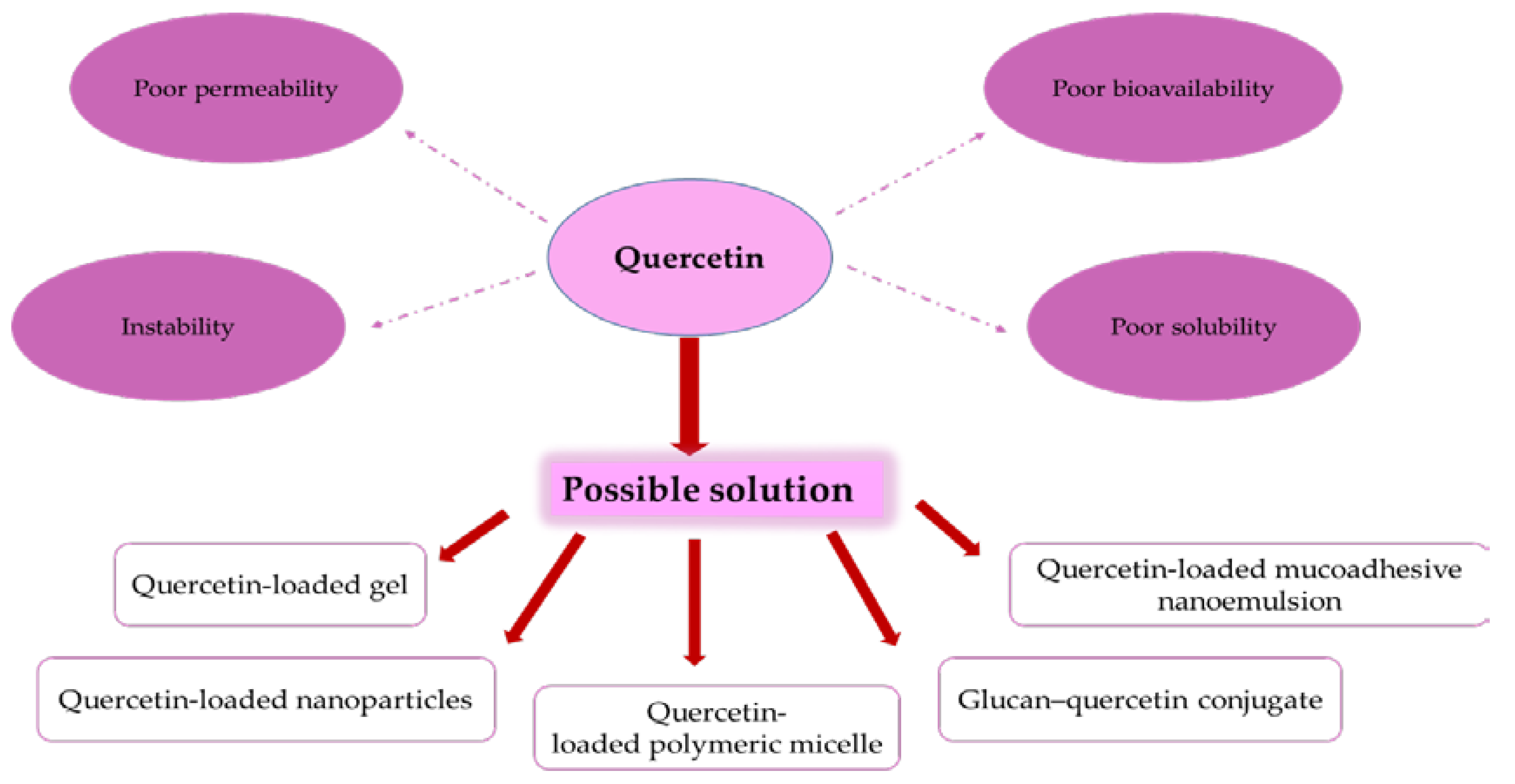
2. Bioavailability and Pharmacokinetics of Quercetin
3. Sources of Quercetin and Its Pharmacological Activity
3.1. General Pharmacological/Biochemical Properties of Quercetin
3.2. Antioxidant Activity
3.3. Antiviral Activity
3.4. Antimicrobial Activity
3.5. Antiprotozoal Activity
3.6. Anti-Inflammatory Effects of Quercetin
3.7. Efficacy in Diseases
3.7.1. Anticancer Activity of Quercetin
3.7.2. Quercitin Hepatoprotective and Antihypertensive Activities
3.7.3. The Important Role of Quercetin in the Treatment of Alzheimer’s Disease
4. Combination Therapy of Quercetin with Other Drugs
5. Dose Use
6. Metabolism and Excretion of Quercetin
7. Toxic Side Effects of Quercetin
8. Quercetin-Drug Interaction
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| IUPAC | International Union of Pure and Applied Chemistry |
| Kf | stability constant value |
| JEV | Japanese encephalitis virus |
| QDP | quercetin 4′,5-diphosphate |
| QPP | quercetin 3′,4′,3,5,7-pentaphosphate |
| QSA | quercetin 5′-sulfonic acid |
| hsp90 | heat shock protein 90 |
| COX | cyclooxygenase |
| LOX | lipoxygenase |
| TLR4 | Toll-like Receptor 4 |
| PKC | Phospho-protein kinase C |
| Th-1 | T helper cell-1 |
| NF-κB | nuclear factor-kappa B |
| AP-1 | activator protein 1 |
| MAPK | mitogen-activated protein kinase |
| NOS | nitric oxide synthase |
| CRP | reactive C-protein |
| IL-1β | interleukin-1β |
| TNF-α | tumor necrosis factor-α |
| LPS | lipopolysaccharide |
| VCAM-1 | vascular cell adhesion molecules |
| ICAM-1 | intracellular cell adhesion molecules |
| CPQN | calcium phosphate–quercetin nanocomplex |
| BBB | blood-brain barrier |
| Nrf2-ARE | NF-E2-related factor 2-antioxidant responsive element |
| SULT 1A1 | phenol sulfotransferase |
| MDR | multi-drug resistant |
| IARC | International Agency for Research on Cancer |
| AChE | acetylcholinesterase |
References
- Batiha, G.-S.; Beshbishy, A.M.; Adeyemi, O.S.; Nadwa, E.H.; Rashwan, E.M.; Alkazmi, L.M.; Elkelish, A.A.; Igarashi, I. Phytochemical screening and antiprotozoal effects of the methanolic Berberis vulgaris and acetonic Rhus coriaria extracts. Molecules 2020, 25, 550. [Google Scholar] [CrossRef]
- Batiha, G.-S.; Alkazmi, L.M.; Wasef, L.G.; Beshbishy, A.M.; Nadwa, E.H.; Rashwan, E.K. Syzygium aromaticum L. (Myrtaceae): Traditional uses, bioactive chemical constituents, pharmacological and toxicological activities. Biomolecules 2020, 10, 202. [Google Scholar] [CrossRef] [PubMed]
- Shakya, A.K. Medicinal plants: Future source of new drugs. Int. J. Herb. Med. IJHM 2016, 59, 59–64. [Google Scholar]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Ríos, J.L.; Recio, M.C. Medicinal plants and antimicrobial activity. J. Ethnopharmacol. 2005, 100, 80–84. [Google Scholar] [CrossRef]
- Lemma, M.T.; Ahmed, A.M.; Elhady, M.T.; Ngo, H.T.; Vu, T.L.-H.; Sang, T.K.; Campos-Alberto, E.; Sayed, A.; Mizukami, S.; Na-Bangchang, K.; et al. Medicinal plants for in vitro antiplasmodial activities: A systematic review of literature. Parasitol. Int. 2017, 66, 713–720. [Google Scholar] [CrossRef]
- Naghibi, F.; Esmaeili, S.; Abdullah, N.R.; Nateghpour, M.; Taghvai, M.; Kamkar, S.; Mosaddegh, M. In vitro and in vivo antimalarial evaluations of myrtle extract, a plant traditionally used for treatment of parasitic disorders. Biomed. Res. Int. 2013, 2013, 1–5. [Google Scholar] [CrossRef]
- Batiha, G.E.S.; Beshbishy, A.M.; Tayebwa, D.S.; Adeyemi, O.S.; Shaheen, H.; Yokoyama, N.; Igarashi, I. The effects of trans-chalcone and chalcone 4 hydrate on the growth of Babesia and Theileria. PLoS Negl. Trop. Dis. 2019, 13, e0007030. [Google Scholar] [CrossRef]
- Beshbishy, A.M.; Batiha, G.E.; Yokoyama, N.; Igarashi, I. Ellagic acid microspheres restrict the growth of Babesia and Theileria in vitro and Babesia microti in vivo. Parasites Vectors 2019, 12, 269. [Google Scholar] [CrossRef]
- Sulaiman, F.A.; Nafiu, M.O.; Yusuf, B.O.; Muritala, H.F.; Adeyemi, S.B.; Omar, S.A.; Dosumu, K.A.; Adeoti, Z.J.; Adegbesan, O.A.; Busari, B.O.; et al. The GC-MS fingerprints of Nicotiana tabacum L. extract and propensity for renal impairment and modulation of serum triglycerides in Wistar rats. J. Pharm. Pharmacogn. Res. 2020, 8, 191–200. [Google Scholar]
- Batiha, G.-S.; Alkazmi, L.M.; Nadwa, E.H.; Rashwan, E.K.; Beshbishy, A.M. Physostigmine: A plant alkaloid isolated from Physostigma venenosum: A review on pharmacokinetics, pharmacological and toxicological activities. J. Drug Deliv. Therap. 2020, 10. [Google Scholar] [CrossRef]
- Anand David, A.V.; Arulmoli, R.; Parasuraman, S. Overviews of Biological Importance of Quercetin: A Bioactive Flavonoid. Pharmacogn. Rev. 2016, 10, 84–89. [Google Scholar] [PubMed]
- Lakhanpal, P.; Rai, D.K. Quercetin: A versatile flavonoid. Int. J. Med. 2007, 2, 22–37. [Google Scholar] [CrossRef]
- Dabeek, W.M.; Marra, M.V. Dietary quercetin and kaempferol: Bioavailability and potential cardiovascular-related bioactivity in humans. Nutrients 2019, 11, 2288. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, L.; Arias, N.; Macarulla, M.T.; Gracia, A.; Portillo, M.P. Beneficial effects of quercetin on obesity and diabetes. Open Nutraceuticals J. 2011, 4, 189–198. [Google Scholar]
- Rich, G.T.; Buchweitz, M.; Winterbone, M.S.; Kroon, P.A.; Wilde, P.J. Towards an understanding of the low bioavailability of quercetin: A study of its interaction with intestinal lipids. Nutrients 2017, 9, 111. [Google Scholar] [CrossRef]
- Walle, T.; Browning, A.M.; Steed, L.L.; Reed, S.G.; Walle, U.K. Flavonoid glucosides are hydrolyzed and thus activated in the oral cavity in humans. J. Nutr. 2005, 135, 48–52. [Google Scholar] [CrossRef]
- Nemeth, K.; Piskula, M.K. Food content, processing, absorption and metabolism of onion flavonoids. Crit. Rev. Food Sci. Nutr. 2007, 47, 397–409. [Google Scholar] [CrossRef]
- Hollman, P.C.; de Vries, J.H.; van Leeuwen, S.D.; Mengelers, M.J.; Katan, M.B. Absorption of dietary quercetin glycosides and quercetin in healthy ileostomy volunteers. Am. J. Clin. Nutr. 1995, 62, 1276–1282. [Google Scholar] [CrossRef]
- Scholz, S.; Williamson, G. Interactions affecting the bioavailability of dietary polyphenols in vivo. Int. J. Vitam. Nutr. Res. 2007, 77, 224–235. [Google Scholar] [CrossRef]
- Ferry, D.R.; Smith, A.; Malkhandi, J.; Fyfe, D.W.; deTakats, P.G.; Anderson, D.; Baker, J.; Kerr, D.J. Phase I clinical trial of the flavonoid quercetin: Pharmacokinetics and evidence for in vivo tyrosine kinase inhibition. Clin. Cancer Res. 1996, 2, 59–68. [Google Scholar]
- Erlund, I.; Kosonen, T.; Alfthan, G.; Mäenpää, J.; Perttunen, K.; Kenraali, J.; Parantainen, J.; Aro, A. Pharmacokinetics of quercetin from quercetin aglycone and rutin in healthy volunteers. Eur. J. Clin. Pharmacol. 2000, 56, 545–553. [Google Scholar] [CrossRef]
- Graefe, E.U.; Wittig, J.; Mueller, S.; Riethling, A.K.; Uehleke, B.; Drewelow, B.; Pforte, H.; Jacobasch, G.; Derendorf, H.; Veit, M. Pharmacokinetics and bioavailability of quercetin glycosides in humans. J. Clin. Pharmacol. 2001, 41, 492–499. [Google Scholar] [CrossRef] [PubMed]
- Salvamani, S.; Gunasekaran, B.; Shaharuddin, N.A.; Ahmad, S.A.; Shukor, M.Y. Antiartherosclerotic effects of plant flavonoids. Biomed. Res. Int. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Lesjak, M.; Beara, I.; Simin, I.; Pintać, D.; Majkić, T.; Bekvalac, K.; Orčić, D.; Mimica-Dukić, N. Antioxidant and anti-inflammatory activities of quercetin and its derivatives. J. Funct. Foods. 2018, 40, 68–75. [Google Scholar] [CrossRef]
- Magar, R.T.; Sohng, J.K. A review on structure, modifications and structure-activity relation of quercetin and its derivatives. J. Microbiol. Biotechnol. 2020, 30, 11–20. [Google Scholar] [CrossRef]
- Boots, A.W.; Haenen, G.R.; Bast, A. Health effects of quercetin: From antioxidant to nutraceutical. Eur. J. Pharmacol. 2008, 585, 325–337. [Google Scholar] [CrossRef]
- Sharmila, G.; Athirai, T.; Kiruthiga, B.; Senthilkumar, K.; Elumalai, P.; Arunkumar, R.; Arunakaran, J. Chemopreventive effect of quercetin in MNU and testosterone-induced prostate cancer of Sprague-Dawley rats. Nutr. Cancer. 2014, 66, 38–46. [Google Scholar] [CrossRef]
- Sharmila, G.; Bhat, F.A.; Arunkumar, R.; Elumalai, P.; Raja Singh, P.; Senthilkumar, K.; Arunakaran, J. Chemopreventive effect of quercetin, a natural dietary flavonoid on prostate cancer in in vivo model. Clin. Nutr. 2014, 33, 718–726. [Google Scholar] [CrossRef]
- Li, B.; Yang, M.; Liu, J.W.; Yin, G.T. Protective mechanism of quercetin on acute myocardial infarction in rats. Genet. Mol. Res. 2016, 15, 15017117. [Google Scholar] [CrossRef]
- Xu, D.; Hu, M.J.; Wang, Y.Q.; Cui, Y.L. Antioxidant activities of quercetin and its complexes for medicinal application. Molecules 2019, 24, 1123. [Google Scholar] [CrossRef]
- Stefek, M.; Karasu, C. Eye lens in aging and diabetes: Effect of quercetin. Rejuvenation Res. 2011, 14, 525–534. [Google Scholar] [CrossRef]
- Coballase-Urrutia, E.; Pedraza-Chaverri, J.; Cardenas Rodriguez, N.; Huerta-Gertrudis, B.; Garcia-Cruz, M.E.; Montesinos-Correa, H.; Sanchez-Gonzalez, D.J.; Camacho-Carranza, R.; Espinosa-Aguirre, J.J. Acetonic and methanolic extracts of Heterotheca inuloides, and quercetin, decrease CCl (4)-oxidative stress in several rat tissues. Evid. Based Complement. Alternat. Med 2013, 2013, 659165. [Google Scholar] [CrossRef]
- Moretti, E.; Mazzi, L.; Terzuoli, G.; Bonechi, C.; Iacoponi, F.; Martini, S.; Rossi, C.; Collodel, G. Effect of quercetin, rutin, naringenin and epicatechin on lipid peroxidation induced in human sperm. Reprod. Toxicol. 2012, 34, 651–657. [Google Scholar] [CrossRef]
- Maciel, R.M.; Costa, M.M.; Martins, D.B.; Franca, R.T.; Schmatz, R.; Graca, D.L.; Duarte, M.M.; Danesi, C.C.; Mazzanti, C.M.; Schetinger, M.R.; et al. Antioxidant and anti-inflammatory effects of quercetin in functional and morphological alterations in streptozotocin-induced diabetic rats. Res. Vet. Sci. 2013, 95, 389–397. [Google Scholar] [CrossRef]
- Tátraaljai, D.; Földes, E.; Pukánszky, B. Efficient melt stabilization of polyethylene with quercetin, a flavonoid type natural antioxidant. Polym. Degrad. Stab. 2014, 102, 41–48. [Google Scholar] [CrossRef]
- Ravichandran, R.; Rajendran, M.; Devapiriam, D. Antioxidant study of quercetin and their metal complex and determination of stability constant by spectrophotometry method. Food Chem. 2014, 146, 472–478. [Google Scholar] [CrossRef]
- Yarahmadi, A.; Zal, F.; Bolouki, A. Protective effects of quercetin on nicotine-induced oxidative stress in ‘HepG2 cells’. Toxicol. Mech. Methods. 2017, 27, 609–614. [Google Scholar] [CrossRef]
- Kalantari, H.; Foruozandeh, H.; Khodayar, M.J.; Siahpoosh, A.; Saki, N.; Kheradmand, P. Antioxidant and hepatoprotective effects of Capparis spinosa L. fractions and quercetin on tert-butyl hydroperoxide- induced acute liver damage in mice. J. Tradit. Complement. Med. 2018, 81, 120–127. [Google Scholar] [CrossRef]
- Coelho-Dos-Reis, J.G.; Gomes, O.A.; Bortolini, D.E.; Martins, M.L.; Almeida, M.R.; Martins, C.S.; Carvalho, L.D.; Souza, J.G.; Vilela, J.M.; Andrade, M.S.; et al. Evaluation of the effects of quercetin and kaempherol on the surface of MT-2 cells visualized by atomic force microscopy. J. Virol. Methods 2011, 174, 47–52. [Google Scholar] [CrossRef][Green Version]
- Johari, J.; Kianmehr, A.; Mustafa, M.; Abubakar, S.; Zandi, K. Antiviral activity of Baicalein and quercetin against the Japanese Encephalitis Virus. Int. J. Mol. Sci. 2012, 13, 16785–16795. [Google Scholar] [CrossRef]
- Zandi, K.; Teoh, B.T.; Sam, S.S.; Wong, P.F.; Mustafa, M.R.; Abubakar, S. Antiviral activity of four types of bioflavonoid against dengue virus type-2. Virol. J. 2011, 8, 560. [Google Scholar] [CrossRef]
- Bachmetov, L.; Gal-Tanamy, M.; Shapira, A.; Vorobeychik, M.; Giterman-Galam, T.; Sathiyamoorthy, P.; Golan Goldhirsh, A.; Benhar, I.; Tur-Kaspa, R.; Zemel, R. Suppression of hepatitis C virus by the flavonoid quercetin is mediated by inhibition of NS3 protease activity. J. Viral. Hepat. 2012, 19, 81–88. [Google Scholar] [CrossRef]
- Ramadan, M.F.; Selim, A.M.M. Antimicrobial and antiviral impact of novel quercetin-enriched lecithin. J. Food Biochem. 2009, 33, 557–571. [Google Scholar] [CrossRef]
- Fan, D.; Zhou, X.; Zhao, C.; Chen, H.; Zhao, Y.; Gong, X. Antiinflammatory, antiviral and quantitative study of quercetin-3-O-beta-D-glucuronide in Polygonum perfoliatum L. Fitoterapia 2011, 82, 805–810. [Google Scholar] [CrossRef]
- Song, J.H.; Shim, J.K.; Choi, H.J. Quercetin 7-rhamnoside reduces porcine epidemic diarrhea virus replication via independent pathway of viral-induced reactive oxygen species. Virol. J. 2011, 8, 460. [Google Scholar] [CrossRef]
- Wang, S.; Yao, J.; Zhou, B.; Yang, J.; Chaudry, M.T.; Wang, M.; Xiao, F.; Li, Y.; Yin, W. Bacteriostatic effect of quercetin as an antibiotic alternative in vivo and its antibacterial mechanism in vitro. J. Food Prot. 2018, 81, 68–78. [Google Scholar] [CrossRef]
- Jaisinghani, R. Antibacterial properties of quercetin. Microb. Res. 2017, 8. [Google Scholar] [CrossRef]
- Osonga, F.J.; Akgul, A.; Miller, R.M.; Eshun, G.B.; Yazgan, I.; Akgul, A.; Sadik, O.A. Antimicrobial activity of a new class of phosphorylated and modified flavonoids. ACS. Omega 2019, 4, 12865–12871. [Google Scholar] [CrossRef]
- Abd-Allah, W.E.; Awad, H.M.; Abdel Mohsen, M.M. HPLC analysis of quercetin and antimicrobial activity of comparative methanol extracts of Shinus molle L. Int. J. Curr. Microbiol. Appl. Sci. 2015, 4, 550–558. [Google Scholar]
- Tasdemir, D.; Kaiser, M.; Brun, R.; Yardley, V.; Schmidt, T.J.; Tosun, F.; Rüedi, P. Antitrypanosomal and antileishmanial activities of flavonoids and their analogues: In vitro, in vivo, structure-activity relationship, and quantitative structure-activity relationship studies. Antimicrob. Agents Chemother. 2006, 50, 1352–1364. [Google Scholar] [CrossRef]
- Weiss, L.M.; Ma, Y.F.; Takvorian, P.M.; Tanowitz, H.B.; Wittner, M. Bradyzoite development in Toxoplasma gondii and the hsp70 stress response. Infect. Immun. 1998, 66, 3295–3302. [Google Scholar] [CrossRef]
- Lehane, A.M.; Saliba, K.J. Common dietary flavonoids inhibit the growth of the intraerythrocytic malaria parasite. BMC Res. Notes 2008, 1, 26. [Google Scholar] [CrossRef]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.T.; Wang, S.; Liu, H.; Yin, Y. Quercetin, inflammation and immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef]
- Bureau, G.; Longpre, F.; Martinoli, M.G. Resveratrol and quercetin, two natural polyphenols, reduce apoptotic neuronal cell death induced by neuroinflammation. J. Neurosci. Res. 2008, 86, 403–410. [Google Scholar] [CrossRef]
- Boots, A.W.; Wilms, L.C.; Swennen, E.L.; Kleinjans, J.C.; Bast, A.; Haenen, G.R. In vitro and ex vivo anti-inflammatory activity of quercetin in healthy volunteers. Nutrition 2008, 24, 703–710. [Google Scholar] [CrossRef]
- Garcia-Mediavilla, V.; Crespo, I.; Collado, P.S.; Esteller, A.; Sanchez-Campos, S.; Tunon, M.J.; Gonzalez-Gallego, J. The anti-inflammatory flavones quercetin and kaempferol cause inhibition of inducible nitric oxide synthase, cyclooxygenase-2 and reactive C-protein, and down-regulation of the nuclear factor kappaB pathway in Chang Liver cells. Eur. J. Pharmacol. 2007, 557, 221–229. [Google Scholar] [CrossRef]
- Lin, C.F.; Leu, Y.L.; Al-Suwayeh, S.A.; Ku, M.C.; Hwang, T.L.; Fang, J.Y. Anti-inflammatory activity and percutaneous absorption of quercetin and its polymethoxylated compound and glycosides: The relationships to chemical structures. Eur. J. Pharm. Sci. 2012, 47, 857–864. [Google Scholar] [CrossRef]
- Ginwala, R.; Bhavsar, R.; Chigbu, D.I.; Jain, P.; Khan, Z.K. Potential role of flavonoids in treating chronic inflammatory diseases with a special focus on the anti-Inflammatory activity of apigenin. Antioxidants 2019, 8, 35. [Google Scholar] [CrossRef]
- Oh, S.J.; Kim, O.; Lee, J.S.; Kim, J.A.; Kim, M.R.; Choi, H.S.; Shim, J.H.; Kang, K.W.; Kim, Y.C. Inhibition of angiogenesis by quercetin in tamoxifen-resistant breast cancer cells. Food Chem. Toxicol. 2010, 48, 3227–3234. [Google Scholar] [CrossRef]
- Dajas, F. Life or death: Neuroprotective and anticancer effects of quercetin. J. Ethno. Pharmacol. 2012, 143, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Baghel, S.S.; Shrivastava, N.; Baghel, R.S.; Agrawal, P.; Rajput, S. A review of quercetin: Antioxidant and anticancer properties. World J. Pharm. Pharm. Sci. 2012, 1, 146–160. [Google Scholar]
- Gibellini, L.; Pinti, M.; Nasi, M.; Montagna, J.P.; De Biasi, S.; Roat, E.; Bertoncelli, L.; Cooper, E.L.; Cossarizza, A. Quercetin and cancer chemoprevention. Evid. Based Complement. Alternat. Med. 2011, 2011, 591356. [Google Scholar] [CrossRef]
- Du, G.; Lin, H.; Yang, Y.; Zhang, S.; Wu, X.; Wang, M.; Ji, L.; Lu, L.; Yu, L.; Han, G. Dietary quercetin combining intratumoral doxorubicin injection synergistically induces rejection of established breast cancer in mice. Int. Immunopharmacol. 2010, 10, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Rauf, A.; Imran, M.; Khan, I.A.; ur-Rehman, M.; Gilani, S.A.; Mehmood, Z.; Mubarak, M.S. Anticancer potential of quercetin: A comprehensive review. Phytother. Res. 2018, 32, 2109–2130. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.F.; He, H.F.; Chen, Q. Quercetin inhibits proliferation and invasion acts by up-regulating miR-146a in human breast cancer cells. Mole. Cell. Biochem. 2015, 402, 93–100. [Google Scholar] [CrossRef]
- Zhang, X.A.; Zhang, S.; Yin, Q.; Zhang, J. Quercetin induces human colon cancer cells apoptosis by inhibiting the nuclear factor-kappa B Pathway. Pharm. Mag. 2015, 11, 404–409. [Google Scholar] [CrossRef]
- Lekic, N.; Canova, N.K.; Horinek, A.; Farghali, H. The involvement of heme oxygenase 1 but not nitric oxide synthase 2 in a hepatoprotective action of quercetin in lipopolysaccharide-induced hepatotoxicity of D-galactosamine sensitized rats. Fitoterapia 2013, 87, 20–26. [Google Scholar] [CrossRef]
- Liu, S.; Hou, W.; Yao, P.; Li, N.; Zhang, B.; Hao, L.; Nussler, A.K.; Liu, L. Heme oxygenase-1 mediates the protective role of quercetin against ethanol-induced rat hepatocytes oxidative damage. Toxicol. In Vitro 2012, 26, 74–80. [Google Scholar] [CrossRef]
- Duarte, J.; Pérez-Palencia, R.; Vargas, F.; Ocete, M.A.; Pérez-Vizcaino, F.; Zarzuelo, A.; Tamargo, J. Antihypertensive effects of the flavonoid quercetin in spontaneously hypertensive rats. Br. J. Pharm. 2001, 133, 117–124. [Google Scholar] [CrossRef]
- Brown, R.C.; Lockwood, A.H.; Sonawane, B.R. Neurodegenerative diseases: An overview of environmental risk factors. Environ. Health Pers. 2005, 113, 1250–1256. [Google Scholar] [CrossRef]
- Choi, G.N.; Kim, J.H.; Kwak, J.H.; Jeong, C.H.; Jeong, H.R.; Lee, U.; Heo, H.J. Effect of quercetin on learning and memory performance in ICR mice under neurotoxic trimethyltin exposure. Food Chem. 2012, 132, 1019–1024. [Google Scholar] [CrossRef]
- Arlt, S. Non-Alzheimer’s disease-related memory impairment and dementia. Dialog. Clinic. Neurosc. 2013, 15, 465–473. [Google Scholar]
- Vauzour, D.; Vafeiadou, K.; Rodriguez-Mateos, A.; Rendeiro, C.; Spencer, J.P. The neuroprotective potential of flavonoids: A multiplicity of effects. Genes Nutr. 2008, 3, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Haleagrahara, N.; Siew, C.J.; Ponnusamy, K. Effect of quercetin and desferrioxamine on 6hydroxydopamine (6-OHDA) induced neurotoxicity in striatum of rats. J. Toxicol. Sci. 2013, 38, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Jazvinscak, J.M.; Cipak, G.A.; Vukovic, L.; Vlainic, J.; Zarkovic, N.; Orsolic, N. Quercetin supplementation: Insight into the potentially harmful outcomes of neurodegenerative prevention. Naunyn-Schmiedeberg Arch. Pharmacol. 2012, 385, 1185–1197. [Google Scholar] [CrossRef]
- Sabogal-Guáqueta, A.M.; Muñoz-Manco, J.I.; Ramírez-Pineda, J.R.; Lamprea-Rodriguez, M.; Osorio, E.; Cardona-Gómez, G.P. The flavonoid quercetin ameliorates Alzheimer’s disease pathology and protects cognitive and emotional function in aged triple transgenic Alzheimer’s disease model mice. Neuropharm 2015, 93, 134–145. [Google Scholar] [CrossRef]
- Denny Joseph, K.M. Enhanced neuroprotective effect of fish oil in combination with quercetin against 3-nitropropionic acid-induced oxidative stress in rat brain. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2013, 40, 83–92. [Google Scholar] [CrossRef]
- Denny Joseph, K.M. Combined oral supplementation of fish oil and quercetin enhance neuroprotection in a chronic rotenone rat model: relevance to Parkinson’s disease. Neurochem. Res. 2015, 40, 894–905. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.; Johnson, J.A. Oxidative damage and the Nrf2-ARE pathway in neurodegenerative diseases. Biochem. Biophys. Acta. 2014, 842, 1208–1218. [Google Scholar] [CrossRef]
- Ferri, P.; Angelino, D.; Gennari, L.; Benedetti, S.; Amrogini, P.; Del Grande, P.; Ninfali, P. Enhancement of flavonoid ability to cross the blood-brain barrier of rats by co-administration with α-tocopherol. Food Funct. 2015, 6, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Lund, K.C.; Pantuso, T. Combination effects of quercetin, resveratrol and curcumin on in vitro intestinal absorption. J. Rest. Med. 2014, 3, 112–120. [Google Scholar] [CrossRef]
- Sahyon, H.A.; Ramadan, E.N.M.; Mashaly, M.M.A. Synergistic effect of quercetin in combination with sulfamethoxazole as new antibacterial agent: In vitro and in vivo study. Pharm. Chem. J. 2019, 53, 803–813. [Google Scholar] [CrossRef]
- Qu, S.; Dai, C.; Shen, Z.; Tang, Q.; Wang, H.; Zhai, B.; Zhyao, L.; Hao, Z. Mechanism of synergy between tetracycline and quercetin against antibiotic resistant Escherichia coli. Front. Microbiol. 2019, 10, 2536. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, V.M.; Carraro, E.; Auler, M.E.; Khalil, N.M. Quercetin and rutin as potential agents antifungal against Cryptococcus spp. Brazil. J. Biol. 2016, 76, 1029–1034. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, C.R.; de Andrade Neto, J.B.; de Sousa Campos, R.; Figueiredo, N.S.; Sampaio, L.S.; Magalhães, H.I.; Cavalcanti, B.C.; Gaspar, D.M.; de Andrade, G.M.; Lima, I.S.; et al. Synergistic effect of the flavonoid catechin, quercetin, or epigallocatechin gallate with fluconazole induces apoptosis in Candida tropicalis resistant to fluconazole. Antimicrob. Agents Chemother. 2014, 58, 1468–1478. [Google Scholar] [CrossRef]
- Harwood, M.; Danielewska-Nikiel, B.; Borzelleca, J.F.; Flamm, G.W.; Williams, G.M.; Lines, T.C. A critical review of the data related to the safety of quercetin and lack of evidence of in vivo toxicity, including lack of genotoxic/carcinogenic properties. Food Chem. Toxicol. 2007, 45, 2179–2205. [Google Scholar] [CrossRef]
- Bischoff, S.C. Quercetin: potentials in the prevention and therapy of disease. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 733–740. [Google Scholar] [CrossRef]
- Russo, M.; Spagnuolo, C.; Tedesco, I.; Bilotto, S.; Russo, G.L. The flavonoid quercetin in disease prevention and therapy: Facts and fancies. Biochem. Pharmacol. 2012, 83, 6–15. [Google Scholar] [CrossRef]
- Lee, J.; Mitchell, A.E. Pharmacokinetics of quercetin absorption from apples and onions in healthy humans. J. Agric. Food Chem. 2012, 60, 3874–3881. [Google Scholar] [CrossRef]
- Edwards, R.L.; Lyon, T.; Litwin, S.E.; Rabovsky, A.; Symons, J.D.; Jalili, T. Quercetin reduces blood pressure in hypertensive subjects. J. Nutr. 2007, 137, 2405–2411. [Google Scholar] [CrossRef] [PubMed]
- Kressler, J.; Millard-Stafford, M.; Warren, G.L. Quercetin and endurance exercise capacity: A systematic review and meta-analysis. Med. Sci. Sports Exerc. 2011, 43, 2396–2404. [Google Scholar] [CrossRef] [PubMed]
- Jin, F.; Nieman, D.C.; Shanely, R.A.; Knab, A.M.; Austin, M.D.; Sha, W. The variable plasma quercetin response to 12-week quercetin supplementation in humans. Eur. J. Clin. Nutr. 2010, 64, 692–697. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.H.; Nakata, R.; Oshima, S.; Inakuma, T.; Terao, J. Accumulation of quercetin conjugates in blood plasma after the short-term ingestion of onion by women. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000, 279, R461–R467. [Google Scholar] [CrossRef]
- Hollman, P.C. Absorption, bioavailability, and metabolism of flavonoids. Pharm. Biol. 2004, 42, 74–83. [Google Scholar] [CrossRef]
- Mullen, W.; Edwards, C.A.; Crozier, A. Absorption, excretion and metabolite profiling of methyl-, glucuronyl-, glucosyl- and sulpho-conjugates of quercetin in human plasma and urine after ingestion of onions. Br. J. Nutr. 2006, 96, 107–116. [Google Scholar] [CrossRef]
- Moon, Y.J.; Wang, L.; DiCenzo, R.; Morris, M.E. Quercetin pharmacokinetics in humans. Biopharm. Drug Dispos. 2008, 29, 205–217. [Google Scholar] [CrossRef]
- Chen, X.; Yin, O.Q.P.; Zuo, Z.; Chow, M.S.S. Pharmacokinetics and modeling of quercetin and metabolites. Pharm. Res. 2005, 22, 892–901. [Google Scholar] [CrossRef]
- Patra, M.; Mukherjee, R.; Banik, M.; Dutta, D.; Begum, N.A.; Basu, T. Calcium phosphate-quercetin nanocomposite (CPQN): A multi-functional nanoparticle having pH indicating, highly fluorescent and anti-oxidant properties. Colloids Surf. B Biointerfaces 2017, 154, 63–73. [Google Scholar] [CrossRef]
- Utesch, D.; Feige, K.; Dasenbrock, J.; Harwood, M.; Danielewska-Nikiel, B.; Lines, T.C. Evaluation of the potential in vivo genotoxicity of quercetin. Mutat. Res. 2008, 654, 38–44. [Google Scholar] [CrossRef]
- Okamoto, T. Safety of quercetin for clinical application (Review). Int. J. Mol. Med. 2005, 16, 275–278. [Google Scholar] [CrossRef]
- Pérez-Pastén, R.; Martínez-Galero, E.; Chamorro-Cevallos, G. Quercetin and naringenin reduce abnormal development of mouse embryos produced by hydroxyurea. J. Pharm. Pharmacol. 2010, 62, 1003–1009. [Google Scholar] [CrossRef] [PubMed]
- Vanhees, K.; de Bock, L.; Godschalk, R.W.; van Schooten, F.J.; van Waalwijk van Doorn-Khosrovani, S.B. Prenatal exposure to flavonoids: Implication for cancer risk. Toxicol. Sci. 2011, 120, 59–67. [Google Scholar] [CrossRef]
- Azuma, K.; Ippoushi, K.; Terao, J. Evaluation of tolerable levels of dietary quercetin for exerting its antioxidative effect in high cholesterol-fed rats. Food Chem. Toxicol. 2010, 48, 1117–1122. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.H.; Chao, P.D.; Hsiu, S.L.; Wen, K.C.; Hou, Y.C. Lethal quercetin-digoxin interaction in pigs. Life Sci. 2004, 74, 1191–1197. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud Hashemi, A.; Solahaye Kahnamouii, S.; Aghajani, H.; Frozannia, K.; Pournasrollah, A.; Sadegh, R.; Esmaeeli, H.; Ghadimi, Y.; Razmpa, E. Quercetin decreases Th17 production by down-regulation of MAPK- TLR4 signaling Pathway on T cells in dental pulpitis. J. Dent. 2018, 19, 259–264. [Google Scholar]
- Choi, J.S.; Li, X. Enhanced diltiazem bioavailability after oral administration of diltiazem with quercetin to rabbits. Int. J. Pharm. 2005, 297, 1–8. [Google Scholar] [CrossRef]
- Beshbishy, A.M.; Batiha, G.E.-S.; Alkazmi, L.; Nadwa, E.; Rashwan, E.; Abdeen, A.; Yokoyama, N.; Igarashi, I. Therapeutic Effects of Atranorin towards the Proliferation of Babesia and Theileria Parasites. Pathogen 2020, 9, 127. [Google Scholar] [CrossRef]
- Batiha, G.S.; Beshbishy, A.M.; El-Mleeh, A.; Abdel-Daim, M.M.; Devkota, H.P. Traditional uses, bioactive chemical constituents, and pharmacological and toxicological activities of Glycyrrhiza glabra L. (Fabaceae). Biomolecules 2020, 10, 352. [Google Scholar] [CrossRef]
- Batiha, G.S.; Beshbishy, A.M.; Guswanto, A.; Nugraha, A.B.; Munkhjargal, T.; Abdel-Daim, M.M.; Mosqueda, J.; Igarashi, I. Phytochemical characterization and chemotherapeutic potential of Cinnamomum verum extracts on the multiplication of protozoan parasites in vitro and in vivo. Molecules 2020, 25, 996. [Google Scholar] [CrossRef]

| Plant Name | Family | Pharmacological Activity |
|---|---|---|
| Apium graveolens | Apiaceae | Lowers blood pressure and glucose, anti-inflammatory, antibacterial |
| Allium fistulosum | Amaryllidaceae | Spring onions as food ingredients |
| Allium cepa (red onions) | Amaryllidaceae | Immunostimulant, cardioprotective, antioxidant |
| Calamus scipionum | Arecaceae | Source of cane |
| Moringa oleifera | Moringaceae | Multipurpose medicinal use anti-inflammatory, antihypertensive, antibacterial |
| Centella asiatica | Apiaceae | Wound healing |
| Hypericum hircinum | Hypericaceae | Antioxidant |
| Hypericum perforatum | Hypericaceae | Major depressive disorders, Neurological effects |
| Brassica oleracea var. sabellica (Kale) | Brassicaceae | Reduce the risk of stroke, reduces blood glucose, neuropathy |
| Brassica oleracea var. italica (broccoli) | Brassicaceae | Edible plant prevents fluid retention and cancer |
| Solanum lycopersicum | Solanaceae | Food supplement and salads |
| Coriandrum Sativum | Apiaceae | Reduce blood pressure, cholesterol, and dyspepsia |
| Morua alba | Moraceae | Diet |
| Nasturtium officinale | Brassicaceae | Reduces the risk of cancers |
| Asparagus officinalis | Asparagaceae | Antineoplastic, antiulcer, antitussive |
| Lactuca sativa | Asteraceae | Iron deficiency anemia, osteoporosis |
| Prunus domestica | Rosaceae | Laxative |
| Malus domestica | Rosaceae | Decrease the risk of cardiovascular disease and cancer |
| Capparis spinosa | Capparaceae | Vermifuges, disinfectants, antiatherosclerotic agent |
| Vaccinium oxycoccus | Ericaceae | Urinary tract infections |
| Prunus avium | Rosaceae | Tonic, astringent, diuretic |
| Camellia sinensis | Theaceae | Antiviral, antispasmodic, analgesic, antidiabetic, bronchodilator |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Batiha, G.E.-S.; Beshbishy, A.M.; Ikram, M.; Mulla, Z.S.; El-Hack, M.E.A.; Taha, A.E.; Algammal, A.M.; Elewa, Y.H.A. The Pharmacological Activity, Biochemical Properties, and Pharmacokinetics of the Major Natural Polyphenolic Flavonoid: Quercetin. Foods 2020, 9, 374. https://doi.org/10.3390/foods9030374
Batiha GE-S, Beshbishy AM, Ikram M, Mulla ZS, El-Hack MEA, Taha AE, Algammal AM, Elewa YHA. The Pharmacological Activity, Biochemical Properties, and Pharmacokinetics of the Major Natural Polyphenolic Flavonoid: Quercetin. Foods. 2020; 9(3):374. https://doi.org/10.3390/foods9030374
Chicago/Turabian StyleBatiha, Gaber El-Saber, Amany Magdy Beshbishy, Muhammad Ikram, Zohair S. Mulla, Mohamed E. Abd El-Hack, Ayman E. Taha, Abdelazeem M. Algammal, and Yaser Hosny Ali Elewa. 2020. "The Pharmacological Activity, Biochemical Properties, and Pharmacokinetics of the Major Natural Polyphenolic Flavonoid: Quercetin" Foods 9, no. 3: 374. https://doi.org/10.3390/foods9030374
APA StyleBatiha, G. E.-S., Beshbishy, A. M., Ikram, M., Mulla, Z. S., El-Hack, M. E. A., Taha, A. E., Algammal, A. M., & Elewa, Y. H. A. (2020). The Pharmacological Activity, Biochemical Properties, and Pharmacokinetics of the Major Natural Polyphenolic Flavonoid: Quercetin. Foods, 9(3), 374. https://doi.org/10.3390/foods9030374