Influence of POR*28 Polymorphisms on CYP3A5*3-Associated Variations in Tacrolimus Blood Levels at an Early Stage after Liver Transplantation
Abstract
:1. Introduction
2. Results
2.1. Patients’ Characteristics
2.2. Demographics of Genetic Polymorphisms
2.3. Influence of the Recipient’s or Donor’s CYP3A5 Polymorphism on the TAC C/D Ratio during the First Month Following Liver Transplantation
2.4. Influence of the Combination of the Recipient’s and Donor’s CYP3A5 Polymorphisms on the TAC C/D Ratio during the First Month Following Liver Transplantation
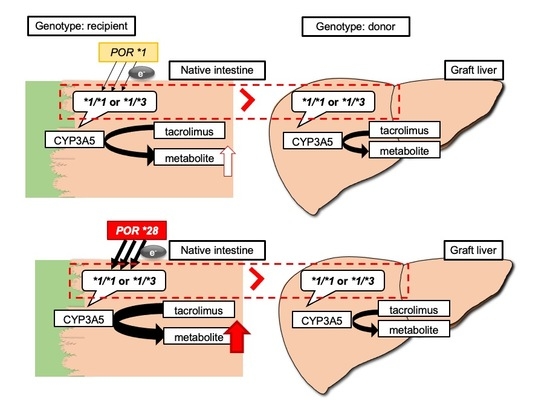
2.5. Impact of Recipient’s or Donor’s POR*28 Genotype on the TAC C/D Ratio Requirement of a CYP3A5 Expressor or Non-Expressor during the First Month Following LIVER transplantation
2.6. Examination of Factors Affecting the TAC C/D Ratio Using Multiple Regression Analysis
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. DNA Extraction and Genotyping of the CYP3A5*3 and POR*28 Polymorphisms
4.3. TAC Trough Concentration Measurement and Immunosuppression Protocol
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kahan, B.D.; Keown, P.; Levy, G.A.; Johnston, A. Therapeutic drug monitoring of immunosuppressant drugs in clinical practice. Clin. Ther. 2002, 24, 330–350. [Google Scholar] [CrossRef]
- Brunet, M.; van Gelder, T.; Asberg, A.; Haufroid, V.; Hesselink, D.A.; Langman, L.; Lemaitre, F.; Marquet, P.; Seger, C.; Shipkova, M.; et al. Therapeutic Drug Monitoring of Tacrolimus-Personalized Therapy: Second Consensus Report. Ther. Drug Monit. 2019, 41, 261–307. [Google Scholar] [CrossRef] [PubMed]
- Staatz, C.E.; Tett, S.E. Clinical pharmacokinetics and pharmacodynamics of tacrolimus in solid organ transplantation. Clin. Pharm. 2004, 43, 623–653. [Google Scholar] [CrossRef] [PubMed]
- Masuda, S.; Inui, K. An up-date review on individualized dosage adjustment of calcineurin inhibitors in organ transplant patients. Pharmacol. Ther. 2006, 112, 184–198. [Google Scholar] [CrossRef]
- Wilkinson, G.R. Drug metabolism and variability among patients in drug response. N. Engl. J. Med. 2005, 352, 2211–2221. [Google Scholar] [CrossRef] [Green Version]
- de Jonge, H.; de Loor, H.; Verbeke, K.; Vanrenterghem, Y.; Kuypers, D.R. In vivo CYP3A4 activity, CYP3A5 genotype, and hematocrit predict tacrolimus dose requirements and clearance in renal transplant patients. Clin. Pharmacol. Ther. 2012, 92, 366–375. [Google Scholar] [CrossRef]
- Hesselink, D.A.; van Schaik, R.H.; van der Heiden, I.P.; van der Werf, M.; Gregoor, P.J.; Lindemans, J.; Weimar, W.; van Gelder, T. Genetic polymorphisms of the CYP3A4, CYP3A5, and MDR-1 genes and pharmacokinetics of the calcineurin inhibitors cyclosporine and tacrolimus. Clin. Pharmacol. Ther. 2003, 74, 245–254. [Google Scholar] [CrossRef]
- Haufroid, V.; Mourad, M.; Van Kerckhove, V.; Wawrzyniak, J.; De Meyer, M.; Eddour, D.C.; Malaise, J.; Lison, D.; Squifflet, J.P.; Wallemacq, P. The effect of CYP3A5 and MDR1 (ABCB1) polymorphisms on cyclosporine and tacrolimus dose requirements and trough blood levels in stable renal transplant patients. Pharmacogenetics 2004, 14, 147–154. [Google Scholar] [CrossRef]
- Press, R.R.; Ploeger, B.A.; den Hartigh, J.; van der Straaten, T.; van Pelt, J.; Danhof, M.; de Fijter, J.W.; Guchelaar, H.J. Explaining variability in tacrolimus pharmacokinetics to optimize early exposure in adult kidney transplant recipients. Ther. Drug Monit. 2009, 31, 187–197. [Google Scholar] [CrossRef]
- Ruiz, J.; Herrero, M.J.; Boso, V.; Megias, J.E.; Hervas, D.; Poveda, J.L.; Escriva, J.; Pastor, A.; Sole, A.; Alino, S.F. Impact of Single Nucleotide Polymorphisms (SNPs) on Immunosuppressive Therapy in Lung Transplantation. Int. J. Mol. Sci. 2015, 16, 20168–20182. [Google Scholar] [CrossRef] [Green Version]
- Yang, T.H.; Chen, Y.K.; Xue, F.; Han, L.Z.; Shen, C.H.; Zhou, T.; Luo, Y.; Zhang, J.J.; Xia, Q. Influence of CYP3A5 genotypes on tacrolimus dose requirement: Age and its pharmacological interaction with ABCB1 genetics in the Chinese paediatric liver transplantation. Int. J. Clin. Pract. 2015, 183, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Picard, N.; Bergan, S.; Marquet, P.; van Gelder, T.; Wallemacq, P.; Hesselink, D.A.; Haufroid, V. Pharmacogenetic Biomarkers Predictive of the Pharmacokinetics and Pharmacodynamics of Immunosuppressive Drugs. Ther. Drug Monit. 2016, 38, S57–S69. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.E.; Schladt, D.P.; Guan, W.; Wu, B.; van Setten, J.; Keating, B.J.; Ikle, D.; Remmel, R.P.; Dorr, C.R.; Mannon, R.B.; et al. Tacrolimus troughs and genetic determinants of metabolism in kidney transplant recipients: A comparison of four ancestry groups. Am. J. Transplant. 2019, 19, 2795–2804. [Google Scholar] [CrossRef] [PubMed]
- Kuehl, P.; Zhang, J.; Lin, Y.; Lamba, J.; Assem, M.; Schuetz, J.; Watkins, P.B.; Daly, A.; Wrighton, S.A.; Hall, S.D.; et al. Sequence diversity in CYP3A promoters and characterization of the genetic basis of polymorphic CYP3A5 expression. Nat. Genet. 2001, 27, 383–391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamba, J.K.; Lin, Y.S.; Schuetz, E.G.; Thummel, K.E. Genetic contribution to variable human CYP3A-mediated metabolism. Adv. Drug Deliv. Rev. 2002, 54, 1271–1294. [Google Scholar] [CrossRef]
- Hart, S.N.; Zhong, X.B. P450 oxidoreductase: Genetic polymorphisms and implications for drug metabolism and toxicity. Expert Opin. Drug Metab. Toxicol. 2008, 4, 439–452. [Google Scholar] [CrossRef]
- Fluck, C.E.; Tajima, T.; Pandey, A.V.; Arlt, W.; Okuhara, K.; Verge, C.F.; Jabs, E.W.; Mendonca, B.B.; Fujieda, K.; Miller, W.L. Mutant P450 oxidoreductase causes disordered steroidogenesis with and without Antley-Bixler syndrome. Nat. Genet. 2004, 36, 228–230. [Google Scholar] [CrossRef] [Green Version]
- Huang, N.; Pandey, A.V.; Agrawal, V.; Reardon, W.; Lapunzina, P.D.; Mowat, D.; Jabs, E.W.; Van Vliet, G.; Sack, J.; Fluck, C.E.; et al. Diversity and function of mutations in p450 oxidoreductase in patients with Antley-Bixler syndrome and disordered steroidogenesis. Am. J. Hum. Genet. 2005, 76, 729–749. [Google Scholar] [CrossRef] [Green Version]
- Huang, N.; Agrawal, V.; Giacomini, K.M.; Miller, W.L. Genetics of P450 oxidoreductase: Sequence variation in 842 individuals of four ethnicities and activities of 15 missense mutations. Proc. Natl. Acad. Sci. USA 2008, 105, 1733–1738. [Google Scholar] [CrossRef] [Green Version]
- Fluck, C.E.; Nicolo, C.; Pandey, A.V. Clinical, structural and functional implications of mutations and polymorphisms in human NADPH P450 oxidoreductase. Fundam. Clin. Pharmacol. 2007, 21, 399–410. [Google Scholar] [CrossRef]
- de Jonge, H.; Metalidis, C.; Naesens, M.; Lambrechts, D.; Kuypers, D.R. The P450 oxidoreductase *28 SNP is associated with low initial tacrolimus exposure and increased dose requirements in CYP3A5-expressing renal recipients. Pharmacogenomics 2011, 12, 1281–1291. [Google Scholar] [CrossRef] [PubMed]
- Elens, L.; Hesselink, D.A.; Bouamar, R.; Budde, K.; de Fijter, J.W.; De Meyer, M.; Mourad, M.; Kuypers, D.R.; Haufroid, V.; van Gelder, T.; et al. Impact of POR*28 on the pharmacokinetics of tacrolimus and cyclosporine A in renal transplant patients. Ther. Drug Monit. 2014, 36, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Lunde, I.; Bremer, S.; Midtvedt, K.; Mohebi, B.; Dahl, M.; Bergan, S.; Asberg, A.; Christensen, H. The influence of CYP3A, PPARA, and POR genetic variants on the pharmacokinetics of tacrolimus and cyclosporine in renal transplant recipients. Eur. J. Clin. Pharmacol. 2014, 70, 685–693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.J.; Liu, S.B.; Xue, L.; Ding, X.L.; Zhang, H.; Miao, L.Y. The genetic polymorphisms of POR*28 and CYP3A5*3 significantly influence the pharmacokinetics of tacrolimus in Chinese renal transplant recipients. Int. J. Clin. Pharmacol. Ther. 2015, 53, 728–736. [Google Scholar] [CrossRef] [PubMed]
- Almeida-Paulo, G.N.; Dapia Garcia, I.; Lubomirov, R.; Borobia, A.M.; Alonso-Sanchez, N.L.; Espinosa, L.; Carcas-Sansuan, A.J. Weight of ABCB1 and POR genes on oral tacrolimus exposure in CYP3A5 nonexpressor pediatric patients with stable kidney transplant. Pharm. J. 2018, 18, 180–186. [Google Scholar] [CrossRef] [PubMed]
- 1000-Genomes-Project-Consortium; Auton, A.; Brooks, L.D.; Durbin, R.M.; Garrison, E.P.; Kang, H.M.; Korbel, J.O.; Marchini, J.L.; McCarthy, S.; McVean, G.A. A global reference for human genetic variation. Nature 2015, 526, 68–74. [Google Scholar]
- Gómez-Bravo, M.A.; Salcedo, M.; Fondevila, C.; Suarez, F.; Castellote, J.; Rufian, S.; Pons, J.A.; Alamo, J.M.; Millán, O.; Brunet, M. Impact of donor and recipient CYP3A5 and ABCB1 genetic polymorphisms on tacrolimus dosage requirements and rejection in Caucasian Spanish liver transplant patients. J. Clin. Pharmacol. 2013, 53, 1146–1154. [Google Scholar] [CrossRef]
- Goto, M.; Masuda, S.; Kiuchi, T.; Ogura, Y.; Oike, F.; Okuda, M.; Tanaka, K.; Inui, K. CYP3A5*1-carrying graft liver reduces the concentration/oral dose ratio of tacrolimus in recipients of living-donor liver transplantation. Pharmacogenetics 2004, 14, 471–478. [Google Scholar] [CrossRef]
- Uesugi, M.; Masuda, S.; Katsura, T.; Oike, F.; Takada, Y.; Inui, K.-I. Effect of intestinal CYP3A5 on postoperative tacrolimus trough levels in living-donor liver transplant recipients. Pharm. Genom. 2006, 16, 119–127. [Google Scholar] [CrossRef]
- Uesugi, M.; Kikuchi, M.; Shinke, H.; Omura, T.; Yonezawa, A.; Matsubara, K.; Fujimoto, Y.; Okamoto, S.; Kaido, T.; Uemoto, S.; et al. Impact of cytochrome P450 3A5 polymorphism in graft livers on the frequency of acute cellular rejection in living-donor liver transplantation. Pharm. Genom. 2014, 24, 356–366. [Google Scholar] [CrossRef]
- Ji, E.; Choi, L.; Suh, K.-S.; Cho, J.-Y.; Han, N.; Oh, J.M. Combinational effect of intestinal and hepatic CYP3A5 genotypes on tacrolimus pharmacokinetics in recipients of living donor liver transplantation. Transplantation 2012, 94, 866–872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolbold, R.; Klein, K.; Burk, O.; Nussler, A.K.; Neuhaus, P.; Eichelbaum, M.; Schwab, M.; Zanger, U.M. Sex is a major determinant of CYP3A4 expression in human liver. Hepatology 2003, 38, 978–988. [Google Scholar] [CrossRef] [PubMed]
- Lamba, V.; Panetta, J.C.; Strom, S.; Schuetz, E.G. Genetic predictors of interindividual variability in hepatic CYP3A4 expression. J. Pharmacol. Exp. Ther. 2010, 332, 1088–1099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.; Zhang, B.; Molony, C.; Chudin, E.; Hao, K.; Zhu, J.; Gaedigk, A.; Suver, C.; Zhong, H.; Leeder, J.S.; et al. Systematic genetic and genomic analysis of cytochrome P450 enzyme activities in human liver. Genome Res. 2010, 20, 1020–1036. [Google Scholar] [CrossRef] [Green Version]
- Paine, M.F.; Ludington, S.S.; Chen, M.L.; Stewart, P.W.; Huang, S.M.; Watkins, P.B. Do men and women differ in proximal small intestinal CYP3A or P-glycoprotein expression? Drug Metab. Dispos. Biol. Fate Chem. 2005, 33, 426–433. [Google Scholar] [CrossRef] [Green Version]
- Cotreau, M.M.; von Moltke, L.L.; Greenblatt, D.J. The influence of age and sex on the clearance of cytochrome P450 3A substrates. Clin. Pharm. 2005, 44, 33–60. [Google Scholar] [CrossRef]
- Herrlinger, C.; Klotz, U. Drug metabolism and drug interactions in the elderly. Best Pract. Res. Clin. Gastroenterol. 2001, 15, 897–918. [Google Scholar] [CrossRef]
- Anderson, B.J.; Holford, N.H. Mechanism-based concepts of size and maturity in pharmacokinetics. Ann. Rev. Pharmacol. Toxicol. 2008, 48, 303–332. [Google Scholar] [CrossRef]
- Sugawara, Y.; Makuuchi, M.; Kaneko, J.; Ohkubo, T.; Imamura, H.; Kawarasaki, H. Correlation between optimal tacrolimus doses and the graft weight in living donor liver transplantation. Clin. Transplant. 2002, 16, 102–106. [Google Scholar] [CrossRef]
- Fukatsu, S.; Yano, I.; Igarashi, T.; Hashida, T.; Takayanagi, K.; Saito, H.; Uemoto, S.; Kiuchi, T.; Tanaka, K.; Inui, K.; et al. Population pharmacokinetics of tacrolimus in adult recipients receiving living-donor liver transplantation. Eur. J. Clin. Pharmacol. 2001, 57, 479–484. [Google Scholar]
- Kuypers, D.R.; de Loor, H.; Naesens, M.; Coopmans, T.; de Jonge, H. Combined effects of CYP3A5*1, POR*28, and CYP3A4*22 single nucleotide polymorphisms on early concentration-controlled tacrolimus exposure in de-novo renal recipients. Pharm. Genom. 2014, 24, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Phupradit, A.; Vadcharavivad, S.; Ingsathit, A.; Kantachuvesiri, S.; Areepium, N.; Sra-Ium, S.; Auamnoy, T.; Sukasem, C.; Sumethkul, V.; Kitiyakara, C. Impact of POR and CYP3A5 Polymorphisms on Trough Concentration to Dose Ratio of Tacrolimus in the Early Post-operative Period Following Kidney Transplantation. Ther. Drug Monit. 2018, 40, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Lesche, D.; Sigurdardottir, V.; Setoud, R.; Oberhansli, M.; Carrel, T.; Fiedler, G.M.; Largiader, C.R.; Mohacsi, P.; Sistonen, J. CYP3A5*3 and POR*28 genetic variants influence the required dose of tacrolimus in heart transplant recipients. Ther. Drug Monit. 2014, 36, 710–715. [Google Scholar] [CrossRef] [Green Version]
- Fu, R.; Tajima, S.; Suetsugu, K.; Watanabe, H.; Egashira, N.; Masuda, S. Biomarkers for individualized dosage adjustments in immunosuppressive therapy using calcineurin inhibitors after organ transplantation. Acta Pharm. Sin. 2019, 40, 151–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cederbaum, A.I. Molecular mechanisms of the microsomal mixed function oxidases and biological and pathological implications. Redox Biol. 2015, 4, 60–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwasaki, K. Metabolism of tacrolimus (FK506) and recent topics in clinical pharmacokinetics. Drug Metab. Pharm. 2007, 22, 328–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomes, A.M.; Winter, S.; Klein, K.; Turpeinen, M.; Schaeffeler, E.; Schwab, M.; Zanger, U.M. Pharmacogenomics of human liver cytochrome P450 oxidoreductase: Multifactorial analysis and impact on microsomal drug oxidation. Pharmacogenomics 2009, 10, 579–599. [Google Scholar] [CrossRef] [Green Version]
- Agrawal, V.; Choi, J.H.; Giacomini, K.M.; Miller, W.L. Substrate-specific modulation of CYP3A4 activity by genetic variants of cytochrome P450 oxidoreductase. Pharm. Genom. 2010, 20, 611–618. [Google Scholar] [CrossRef] [Green Version]
- Oneda, B.; Crettol, S.; Jaquenoud Sirot, E.; Bochud, M.; Ansermot, N.; Eap, C.B. The P450 oxidoreductase genotype is associated with CYP3A activity in vivo as measured by the midazolam phenotyping test. Pharm. Genom. 2009, 19, 877–883. [Google Scholar] [CrossRef]
- Hesselink, D.A.; Ngyuen, H.; Wabbijn, M.; Gregoor, P.J.; Steyerberg, E.W.; van Riemsdijk, I.C.; Weimar, W.; van Gelder, T. Tacrolimus dose requirement in renal transplant recipients is significantly higher when used in combination with corticosteroids. Br. J. Clin. Pharmacol. 2003, 56, 327–330. [Google Scholar] [CrossRef]
- Fukuda, M.; Suetsugu, K.; Tajima, S.; Katsube, Y.; Watanabe, H.; Harada, N.; Yoshizumi, T.; Egashira, N.; Mori, M.; Masuda, S. Neutrophil Gelatinase-Associated Lipocalin Is Not Associated with Tacrolimus-Induced Acute Kidney Injury in Liver Transplant Patients Who Received Mycophenolate Mofetil with Delayed Introduction of Tacrolimus. Int. J. Mol. Sci. 2019, 20, 3103. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Recipients (n = 65) | Donors (n = 65) | |
|---|---|---|
| Preoperative data | ||
| Sex (male/female) | 28/37 (43.1/56.9) | 43/22 (66.2/33.8) |
| Age (years) | 59 (51–65) | 39 (33–48) |
| Body weight (kg) | 61.5 (54.0–67.9) | |
| Scr (mg/dL) | 0.68 (0.52–0.90) | |
| BUN (mg/dL) | 14 (10–19) | |
| eGFR (mL/min/1.73 m2) | 78 (58–97) | |
| AST (U/L) | 47 (31–74) | |
| ALT (U/L) | 24 (17–38) | |
| γ-GTP (U/L) | 36 (25–81) | |
| T-Bil (mg/dL) | 2.5 (1.6–5.0) | |
| Alb (g/dL) | 2.7 (2.4–3.0) | |
| Graft data | ||
| GV (g) | 490 (439–556) | |
| GRWR (%) | 0.82 (0.69–0.91) | |
| Primary disease | ||
| Alcoholic liver disease | 16 (24.6) | |
| Primary biliary cholangitis | 13 (20.0) | |
| Hepatitis C | 10 (15.4) | |
| Hepatitis B | 7 (10.8) | |
| Hepatocellular carcinoma | 6 (9.2) | |
| Non-alcoholic steatohepatitis | 5 (7.7) | |
| Autoimmune hepatitis | 2 (3.1) | |
| Wilson’s disease | 1 (1.5) | |
| Others | 5 (7.7) | |
| ABO blood group match | ||
| Identical | 34 (52.3) | |
| Compatible | 11 (16.9) | |
| Incompatible | 20 (30.8) |
| Category | n (%) | Recipient’s CYP3A5 Genotype | Donor’s CYP3A5 Genotype |
|---|---|---|---|
| R*1/D*1 | 21 (32.3) | *1/*1, *1/*3 | *1/*1, *1/*3 |
| R*1/D*3 | 6 (9.2) | *1/*1, *1/*3 | *3/*3 |
| R*3/D*1 | 9 (13.8) | *3/*3 | *1/*1, *1/*3 |
| R*3/D*3 | 29 (44.6) | *3/*3 | *3/*3 |
| A) Categorization of the recipients by CYP3A5 and POR genotypes | |||
|---|---|---|---|
| Category | n (%) | Recipient’s CYP3A5 Genotype | Recipient’s POR Genotype |
| F/*1 | 8 (12.3) | *1/*1, *1/*3 | *1/*1 |
| F/*28 | 19 (29.2) | *1/*1, *1/*3 | *1/*28, *28/*28 |
| D/*1 | 13 (20.0) | *3/*3 | *1/*1 |
| D/*28 | 25 (38.5) | *3/*3 | *1/*28, *28/*28 |
| B)Categorization of the donors by CYP3A5 and POR genotypes | |||
| Category | n (%) | Donor’s CYP3A5 Genotype | Donor’s POR Genotype |
| F/*1 | 9 (13.8) | *1/*1, *1/*3 | *1/*1 |
| F/*28 | 21 (32.3) | *1/*1, *1/*3 | *1/*28, *28/*28 |
| D/*1 | 10 (15.4) | *3/*3 | *1/*1 |
| D/*28 | 25 (38.5) | *3/*3 | *1/*28, *28/*28 |
| All Patients (65) | Pod 1–7 | Pod 8–14 | Pod 15–21 | Pod 22–28 | ||||
|---|---|---|---|---|---|---|---|---|
| β | p | β | p | Β | p | p | ||
| Recipient sex (male (28) vs. female (37)) | −0.184 | 0.002 | − | − | − | − | − | − |
| Recipient age (years) | 0.156 | 0.002 | 0.095 | 0.018 | 0.100 | 0.043 | − | |
| Recipient BW (kg) | −0.262 | <0.001 | −0.185 | <0.001 | −0.138 | 0.008 | − | − |
| Donor sex (male (43) vs. female (22)) | − | − | − | − | − | − | − | − |
| Donor age (years) | − | − | − | − | − | − | − | − |
| Graft volume (g) | −0.157 | 0.003 | −0.520 | <0.001 | −0.383 | <0.001 | −0.274 | <0.001 |
| Recipient CYP3A5 (f (27) vs. d (38)) | −0.389 | <0.001 | −0.345 | <0.001 | −0.417 | <0.001 | −0.627 | <0.001 |
| Donor CYP3A5 (f (30) vs. d (35)) | − | − | − | − | −0.175 | 0.004 | − | − |
| Recipient POR (*1 (21) vs. *28 (44)) | − | − | − | − | − | − | − | − |
| Donor POR (*1 (19) vs. *28 (46)) | − | − | − | − | − | − | − | − |
| Recipient/ Functional CYP3A5 (27) | Pod 1–7 | Pod 8–14 | Pod 15–21 | Pod 22–28 | ||||
|---|---|---|---|---|---|---|---|---|
| β | p | β | p | Β | p | β | p | |
| Recipient sex (male (11) vs. female (16)) | −0.304 | 0.001 | −0.340 | <0.001 | − | − | −0.440 | 0.001 |
| Recipient age (years) | 0.184 | 0.027 | − | − | 0.641 | <0.001 | 0.941 | <0.001 |
| Recipient BW (kg) | −0.262 | <0.001 | − | − | −0.352 | <0.001 | −0.360 | 0.008 |
| Donor sex (male (19) vs. female (8)) | − | − | − | − | − | − | 0.168 | 0.144 |
| Donor age (years) | − | − | − | − | 0.545 | <0.001 | 1.162 | <0.001 |
| Graft volume (g) | −0.176 | 0.022 | −0.545 | <0.001 | −0.737 | <0.001 | −0.872 | <0.001 |
| Donor CYP3A5 (f (21) vs. d (6)) | − | − | − | − | −0.394 | <0.001 | −0.579 | <0.001 |
| Recipient POR (*1 (8) vs. *28 (19)) | − | − | − | − | 0.390 | <0.001 | − | − |
| Donor POR (*1 (9) vs. *28 (18)) | − | − | − | − | − | − | − | − |
| Donor/ Functional CYP3A5 (30) | Pod 1–7 | Pod 8–14 | Pod 15–21 | Pod 22–28 | ||||
|---|---|---|---|---|---|---|---|---|
| β | p | β | p | β | p | β | p | |
| Recipient sex (male (13) vs. female (17)) | − | − | − | − | − | − | − | − |
| Recipient age (years) | − | − | − | − | 0.312 | <0.001 | 0.187 | 0.074 |
| Recipient BW (kg) | −0.283 | <0.001 | − | − | − | − | − | − |
| Donor sex (male (21) vs. female (9)) | − | − | − | − | − | − | − | − |
| Donor age (years) | 0.310 | <0.001 | − | − | ||||
| Graft volume (g) | −0.178 | 0.032 | −0.617 | <0.001 | −0.730 | <0.001 | −0.301 | 0.006 |
| Recipient CYP3A5 (f (21) vs. d (9)) | − | − | − | − | −0.436 | <0.001 | −0.688 | <0.001 |
| Recipient POR (*1 (11) vs. *28 (19)) | − | − | − | − | − | − | − | − |
| Donor POR (*1 (9) vs. *28 (21)) | − | − | − | − | − | − | − | − |
| Recipient/ Defective CYP3A5 (38) | Pod 1–7 | Pod 8–14 | Pod 15–21 | Pod 22–28 | ||||
|---|---|---|---|---|---|---|---|---|
| β | p | β | p | β | p | β | p | |
| Recipient sex (male (17) vs. female (21)) | −0.198 | 0.009 | −0.205 | <0.001 | − | − | − | − |
| Recipient age (years) | − | − | 0.214 | <0.001 | − | − | − | − |
| Recipient BW (kg) | − | − | −0.332 | <0.001 | −0.221 | 0.004 | −0.288 | 0.011 |
| Donor sex (male (24) vs. female (14)) | − | − | − | − | − | − | − | − |
| Donor age (years) | − | − | − | − | − | − | −0.258 | 0.022 |
| Graft volume (g) | −0.182 | 0.024 | −0.434 | <0.001 | −0.443 | <0.001 | − | − |
| Donor CYP3A5 (f (9) vs. d (29)) | −0.256 | <0.001 | − | − | − | − | − | − |
| Recipient POR (*1 (13) vs. *28 (25)) | − | − | −0.168 | 0.002 | − | − | − | − |
| Donor POR (*1 (10) vs. *28 (28)) | − | − | − | − | − | − | − | − |
| Donor/ Defective CYP3A5 (35) | Pod 1–7 | Pod 8–14 | Pod 15–21 | Pod 22–28 | ||||
|---|---|---|---|---|---|---|---|---|
| β | p | β | p | β | p | β | p | |
| Recipient sex (male (15) vs. female (20)) | −0.322 | <0.001 | − | − | − | − | − | − |
| Recipient age (years) | − | − | 0.305 | <0.001 | 0.138 | <0.001 | − | − |
| Recipient BW (kg) | − | − | −0.664 | <0.001 | −0.564 | <0.001 | −0.495 | <0.001 |
| Donor sex (male (22) vs. female (13)) | − | − | 0.265 | <0.001 | − | − | − | − |
| Donor age (years) | − | − | − | − | − | − | − | − |
| Graft volume (g) | −0.133 | 0.049 | − | − | − | − | − | − |
| Recipient CYP3A5 (f (6) vs. d (29)) | −0.413 | <0.001 | − | − | −0.289 | <0.001 | −0.309 | 0.004 |
| Recipient POR (*1 (10) vs. *28 (25)) | − | − | −0.050 | 0.352 | −0.098 | 0.152 | −0.155 | 0.133 |
| Donor POR (*1 (10) vs. *28 (25)) | − | − | − | − | − | − | − | − |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakamura, T.; Fukuda, M.; Matsukane, R.; Suetsugu, K.; Harada, N.; Yoshizumi, T.; Egashira, N.; Mori, M.; Masuda, S. Influence of POR*28 Polymorphisms on CYP3A5*3-Associated Variations in Tacrolimus Blood Levels at an Early Stage after Liver Transplantation. Int. J. Mol. Sci. 2020, 21, 2287. https://doi.org/10.3390/ijms21072287
Nakamura T, Fukuda M, Matsukane R, Suetsugu K, Harada N, Yoshizumi T, Egashira N, Mori M, Masuda S. Influence of POR*28 Polymorphisms on CYP3A5*3-Associated Variations in Tacrolimus Blood Levels at an Early Stage after Liver Transplantation. International Journal of Molecular Sciences. 2020; 21(7):2287. https://doi.org/10.3390/ijms21072287
Chicago/Turabian StyleNakamura, Takahiro, Mio Fukuda, Ryosuke Matsukane, Kimitaka Suetsugu, Noboru Harada, Tomoharu Yoshizumi, Nobuaki Egashira, Masaki Mori, and Satohiro Masuda. 2020. "Influence of POR*28 Polymorphisms on CYP3A5*3-Associated Variations in Tacrolimus Blood Levels at an Early Stage after Liver Transplantation" International Journal of Molecular Sciences 21, no. 7: 2287. https://doi.org/10.3390/ijms21072287
APA StyleNakamura, T., Fukuda, M., Matsukane, R., Suetsugu, K., Harada, N., Yoshizumi, T., Egashira, N., Mori, M., & Masuda, S. (2020). Influence of POR*28 Polymorphisms on CYP3A5*3-Associated Variations in Tacrolimus Blood Levels at an Early Stage after Liver Transplantation. International Journal of Molecular Sciences, 21(7), 2287. https://doi.org/10.3390/ijms21072287