Chemical Characterization, Gastrointestinal Motility and Sensory Evaluation of Dark Chocolate: A Nutraceutical Boosting Consumers’ Health
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical Characterization of Chocolate Samples
2.1.1. Chemicals
2.1.2. Chocolate Samples
2.1.3. Proximate Composition
2.2. Total Phenol Content Determination and Antioxidant Activity Evaluation
2.2.1. Sample Preparation
2.2.2. Total Phenol Content Determination
2.3. Polyphenol Investigation by LC/High Resolution Mass Spectrometry
2.3.1. Sample Preparation
2.3.2. LC-MS/MS Analysis
2.4. Polyphenols and Theobromine Quantitation by HPLC-DAD
2.5. Chemical Profiling of Chocolate
2.6. Lipid Analysis (or Analysis of Fatty Acids) by LC-MS/MS
2.6.1. Sample Preparation
2.6.2. LC-MS/MS Analysis
2.7. Protein Characterization by HPLC-MS/MS Analysis
2.7.1. Sample Preparation
2.7.2. LC-MS/MS Analysis and Database Search
3. Clinical Study
3.1. Organoleptic Evaluation
3.2. Gastrointestinal Motility Studies
- -
- Choco-A: chocolate 28.6 g + water 175 mL + Lactulose 15 mL;
- -
- Choco-I: chocolate 27.3 g of + water 176 mL + Lactulose 15 mL;
- -
- Nu: Nutridrink® (Nutricia, Milano, Italy), 200 mL + Lactulose 15 mL.
4. Statistical Analysis
5. Results
5.1. Chemical Analyses
5.2. Organoleptic Evaluation
5.3. Gastrointestinal Motility
5.4. Gallbladder
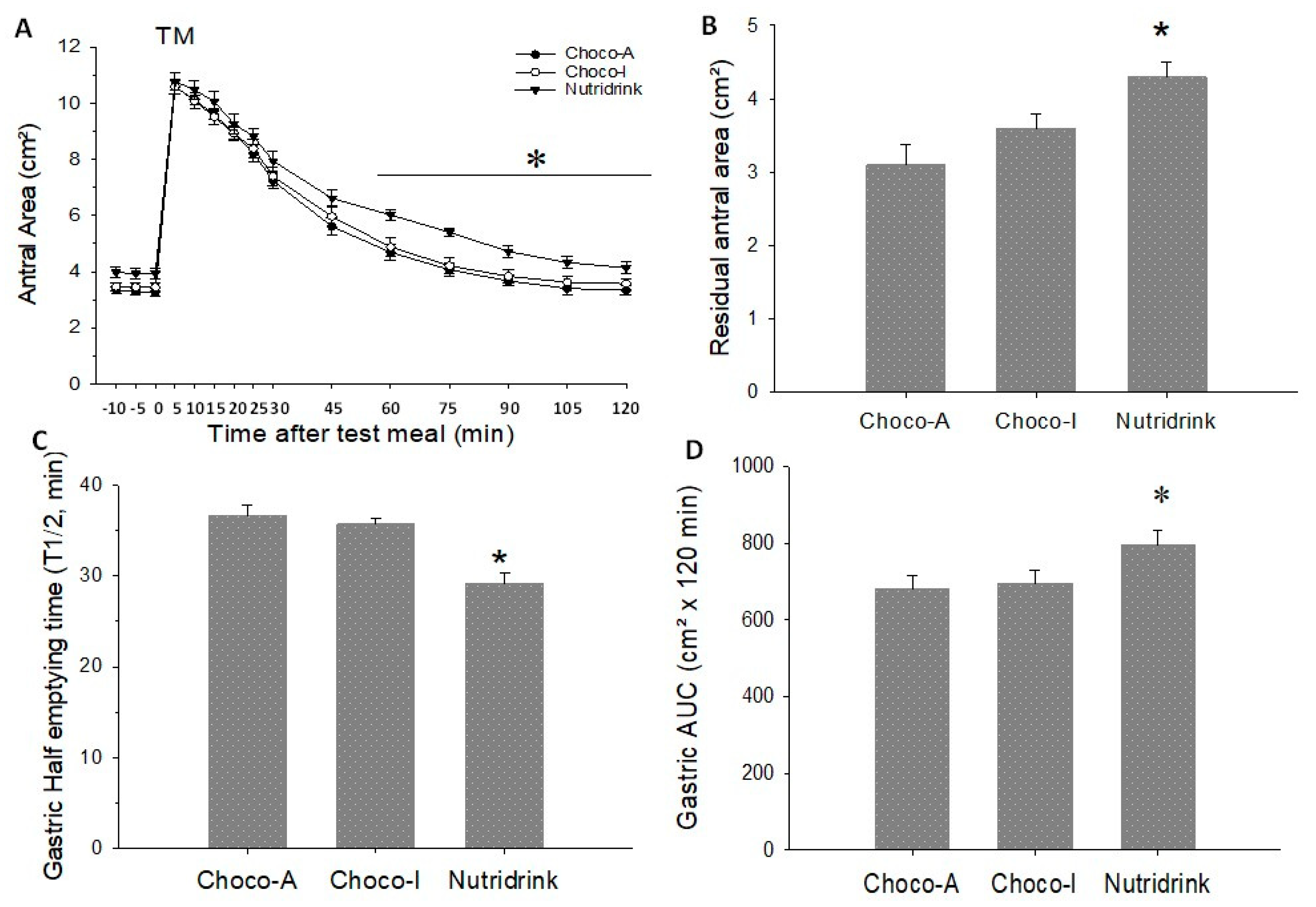
5.5. Stomach
5.6. Small Intestine
6. Discussion
6.1. Perception Study
6.2. Motility Study
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rodriguez-Carrasco, Y.; Gaspari, A.; Graziani, G.; Santini, A.; Ritieni, A. Fast analysis of polyphenols and alkaloids in cocoa-based products by ultra-high performance liquid chromatography and Orbitrap high resolution mass spectrometry (UHPLC-Q-Orbitrap-MS/MS). Food Res. Int. 2018, 111, 229–236. [Google Scholar] [CrossRef]
- Latif, R. Chocolate/cocoa and human health: A review. Netherlands J. Med. 2013, 71, 63–68. [Google Scholar]
- Kris-Etherton, P.M.; Mustad, V.; Derr, J.A. Effects of Dietary Stearic Acid on Plasma Lipids and Thrombosis. Nutr. Today 1993, 28, 30–38. [Google Scholar] [CrossRef]
- Montagna, M.T.; Diella, G.; Triggiano, F.; Caponio, G.R.; Giglio, O.D.; Caggiano, G.; Ciaula, A.D.; Portincasa, P. Chocolate, “Food of the Gods”: History, Science, and Human Health. Int. J. Environ. Res. Public Health 2019, 16, 4960. [Google Scholar] [CrossRef] [PubMed]
- Corti, R.F.A.; Hollenberg, N.K.; Lüscher, T.F. Circulation: Cocoa and cardiovascular health. Circulation 2009, 119, 1433–1441. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Li, X.; Jin, Y.; Lu, J. Chocolate Consumption and Risk of Coronary Heart Disease, Stroke, and Diabetes: A Meta-Analysis of Prospective Studies. Nutrients 2017, 9, 688. [Google Scholar] [CrossRef]
- Cordero-Herrera, I.; Martín, M.Á.; Bravo, L.; Goya, L.; Ramos, S. Cocoa flavonoids improve insulin signalling and modulate glucose production via AKT and AMPK in HepG2 cells. Mol. Nutr. Food Res. 2013, 57, 974–985. [Google Scholar] [CrossRef]
- Tzounis, X.; Rodriguez-Mateos, A.; Vulevic, J.; Gibson, G.R.; Kwik-Uribe, C.; Spencer, J.P. Prebiotic evaluation of cocoa-derived flavanols in healthy humans by using a randomized, controlled, double-blind, crossover intervention study. Am. J. Clin. Nutr. 2011, 93, 62–72. [Google Scholar] [CrossRef]
- Martin, F.P.J.; Rezzi, S.; Peré-Trepat, E.; Kamlage, B.; Collino, S.; Leibold, E.; Kastler, J.; Rein, D.; Laurent, B.F.; Kochhar, S. Metabolic Effects of Dark Chocolate Consumption on Energy, Gut Microbiota, and Stress-Related Metabolism in Free-Living Subjects. J. Proteome Res. 2009, 8, 5568–5579. [Google Scholar] [CrossRef]
- Al Mushref, M.; Srinivasan, S. Effect of high fat-diet and obesity on gastrointestinal motility. Ann. Transl. Med. 2013, 1, 14. [Google Scholar]
- Di Ciaula, A.; Wang, D.Q.; Portincasa, P. Gallbladder and gastric motility in obese newborns, pre-adolescents and adults. J. Gastroenterol. Hepatol. 2012, 27, 1298–1305. [Google Scholar] [CrossRef] [PubMed]
- American Assn. of Cereal Chemists. Approved Methods of the American Association of Cereal Chemists, 10th ed.; AACC: St. Paul, MI, USA, 2000. [Google Scholar]
- AOAC International. AOAC Official Methods of Analysis of AOAC International, 17th ed.; AOAC International: Gaithersburg, MD, USA, 2000. [Google Scholar]
- AOAC International. Official Methods of Analysis, 7th ed.; Association of Analytical Communities: Gaithersburg, MD, USA, 2006. [Google Scholar]
- Gu, L.; House, S.E.; Wu, X.; Ou, B.; Prior, R.L. Procyanidin and Catechin Contents and Antioxidant Capacity of Cocoa and Chocolate Products. J. Agric. Food Chem. 2006, 54, 4057–4061. [Google Scholar] [CrossRef] [PubMed]
- Difonzo, G.; Vollmer, K.; Caponio, F.; Pasqualone, A.; Carle, R.; Steingass, C.B. Characterisation and classification of pineapple (Ananas comosus [L.] Merr.) juice from pulp and peel. Food Control 2019, 96, 260–270. [Google Scholar] [CrossRef]
- Difonzo, G.; Russo, A.; Trani, A.; Paradiso, V.M.; Ranieri, M.; Pasqualone, A.; Summo, C.; Tamma, G.; Silletti, R.; Caponio, F. Green extracts from Coratina olive cultivar leaves: Antioxidant characterization and biological activity. J. Funct. Foods 2017, 31, 63–70. [Google Scholar] [CrossRef]
- Zulueta, A.; Esteve, M.J.; Frigola, A. ORAC and TEAC assays comparison to measure the antioxidant capacity of food products. Food Chem. 2009, 114, 310–316. [Google Scholar] [CrossRef]
- Ortega, N.; Romero, M.P.; Macia, A.; Reguant, J.; Angles, N.; Morello, J.R.; Motilva, M.J. Comparative study of UPLC-MS/MS and HPLC-MS/MS to determine procyanidins and alkaloids in cocoa samples. J. Food Compos. Anal. 2010, 23, 298–305. [Google Scholar] [CrossRef]
- Manirakiza, P.; Covaci, A.; Schepens, P. Comparative Study on Total Lipid Determination using Soxhlet, Roese-Gottlieb, Bligh & Dyer, and Modified Bligh & Dyer Extraction Methods. J. Food Compos. Anal. 2001, 14, 93–100. [Google Scholar]
- Pilolli, R.; De Angelis, E.; Monaci, L. In house validation of a high resolution mass spectrometry Orbitrap-based method for multiple allergen detection in a processed model food. Anal. Bioanal. Chem. 2018, 410, 5653–5662. [Google Scholar] [CrossRef]
- Thamke, I.; Dürrschmid, K.; Rohm, H. Sensory description of dark chocolates by consumers. LWT 2009, 42, 534–539. [Google Scholar] [CrossRef]
- Di Ciaula, A.; Covelli, M.; Berardino, M.; Wang, D.Q.-H.; Lapadula, G.; Palasciano, G.; Portincasa, P. Gastrointestinal symptoms and motility disorders in patients with systemic scleroderma. BMC Gastroenterol. 2008, 8, 7. [Google Scholar] [CrossRef]
- Portincasa, P.; Diciaula, A.; Baldassarre, G.; Palmieri, V.; Gentile, A.; Cimmino, A.; Palasciano, G. Gallbladder Motor Function in Gallstone Patients—Sonographic and in-Vitro Studies on the Role of Gallstones, Smooth-Muscle Function and Gallbladder Wall Inflammation. J. Hepatol. 1994, 21, 430–440. [Google Scholar] [CrossRef]
- Di Ciaula, A.; Grattagliano, I.; Portincasa, P. Chronic alcoholics retain dyspeptic symptoms, pan-enteric dysmotility, and autonomic neuropathy before and after abstinence. J. Dig. Dis. 2016, 17, 735–746. [Google Scholar] [CrossRef] [PubMed]
- Portincasa, P.; Maggipinto, A.; Berardino, M.; Bonfrate, L.; Costin, S.; Todarello, O.; Palasciano, G.; Wang, D.Q.-H.; Dumitrascu, D.L. Assessing gastrointestinal symptoms and perception, quality of life, motility, and autonomic neuropathy in clinical studies. J. Gastrointest. Liver Dis. 2009, 18, 205–211. [Google Scholar]
- Everson, G.T.; Braverman, D.Z.; Johnson, M.L.; Kern, F., Jr. A critical evaluation of real-time ultrasonography for the study of gallbladder volume and contraction. Gastroenterology 1980, 79, 40–46. [Google Scholar] [CrossRef]
- Muresan, C.; Blaga, T.S.; Muresan, L.; Dumitrascu, D.L. Abdominal Ultrasound for the Evaluation of Gastric Emptying Revisited. J. Gastrointest. Liver Dis. 2015, 24, 329–338. [Google Scholar]
- Gasbarrini, A.; Corazza, G.R.; Gasbarrini, G.; Montalto, M.; Di Stefano, M.; Basilisco, G.; Parodi, A.; Usai-Satta, P.; Satta, P.U.; Vernia, P.; et al. Methodology and Indications of H2-Breath Testing in Gastrointestinal Diseases: The Rome Consensus Conference. Aliment. Pharmacol. Ther. 2009, 29, 1–49. [Google Scholar]
- Altomare, D.F.; Portincasa, P.; Rinaldi, M.; Di Ciaula, A.; Martinelli, E.; Amoruso, A.; Palasciano, G.; Memeo, V. Slow-transit constipation: Solitary symptom of a systemic gastrointestinal disease. Dis. Colon Rectum 1999, 42, 231–240. [Google Scholar] [CrossRef]
- Bonfrate, L.; Krawczyk, M.; Lembo, A.; Grattagliano, I.; Lammert, F.; Portincasa, P. Effects of dietary education, followed by a tailored fructose-restricted diet in adults with fructose malabsorption. Eur. J. Gastroenterol. Hepatol. 2015, 27, 785–796. [Google Scholar] [CrossRef]
- Meng, C.C.; Jalil, A.M.M.; Ismail, A. Phenolic and theobromine contents of commercial dark, milk and white chocolates on the Malaysian market. Molecules 2009, 14, 200–209. [Google Scholar] [CrossRef]
- Magrone, T.; Russo, M.A.; Jirillo, E. Cocoa and Dark Chocolate Polyphenols: From Biology to Clinical Applications. Front. Immunol. 2017, 8, 1–13. [Google Scholar] [CrossRef]
- Petyaev, I.M.; Bashmakov, Y.K. Dark Chocolate: Opportunity for an Alliance between Medical Science and the Food Industry. Front. Nutr. 2017, 26, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Apriyanto, M. Analysis of Amino Acids in Cocoa Beans Produced during Fermentation by High Performence Liquid Chromatography (HPLC). Int. J. Food Ferment. Technol. 2017, 7, 25. [Google Scholar] [CrossRef]
- De Melo, C.W.B.; Bandeira, M.D.J.; Maciel, L.F.; Bispo, E.D.S.; De Souza, C.O.; Soares, S.E. Chemical composition and fatty acids profile of chocolates produced with different cocoa (Theobroma cacao L.) cultivars. Food Sci. Technol. 2020, 40, 326–333. [Google Scholar] [CrossRef]
- Perret, D.; Gentili, A.; Marchese, S.; Sergi, M.; Caporossi, L. Determination of free fatty acids in chocolate by liquid chromatography with tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2004, 18, 1989–1994. [Google Scholar] [CrossRef] [PubMed]
- Hunter, J.E.; Zhang, J.; Kris-Etherton, P.M. Cardiovascular disease risk of dietary stearic acid compared with trans, other saturated, and unsaturated fatty acids: A systematic review. Am. J. Clin. Nutr. 2010, 91, 46–63. [Google Scholar] [CrossRef]
- Barceló-Coblijn, G.; Murphy, E.J. Alpha-linolenic acid and its conversion to longer chainn3 fatty acids: Benefitsfor human health and a role in maintaining tissuen3 fatty acid levels. Progr. Lipid Res. 2009, 48, 355–374. [Google Scholar] [CrossRef]
- Jenkins, D.J.A.; Kendall, C.W.C.; Vuksan, V.; Vidgen, E.; Wong, E.; Augustin, L.; Fulgoni, V. Effect of cocoa bran on low-density lipoprotein oxidation and fecal bulking. Arch. Intern. Med. 2000, 160, 2374–2379. [Google Scholar] [CrossRef]
- Weickert, M.O.; Pfeiffer, A.F. Metabolic effects of dietary fiber consumption and prevention of diabetes. J. Nutr. 2008, 138, 439–442. [Google Scholar] [CrossRef]
- Lecumberri, E.; Mateos, R.; Izquierdo-Pulido, M.; Rupérez, P.; Goya, L.; Bravo, L. Dietary fibre composition, antioxidant capacity and physico-chemical properties of a fibre-rich product from cocoa (Theobroma cacao L.). Food Chem. 2007, 104, 948–954. [Google Scholar] [CrossRef]
- Portincasa, P.; Di Ciaula, A.; Wang, H.H.; Palasciano, G.; Van Erpecum, K.J.; Moschetta, A.; Wang, D.Q.-H. Coordinate regulation of gallbladder motor function in the gut-liver axis. Hepatology 2008, 47, 2112–2126. [Google Scholar] [CrossRef]
- Hopman, W.P.; Jansen, J.B.; Rosenbusch, G.; Lamers, C.B. Cephalic Stimulation of Gallbladder Contraction in Humans: Role of Cholecystokinin and the Cholinergic System. Digestion 1987, 38, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Debas, H.T.; Yamagishi, T. Evidence for a pyloro-cholecystic reflex for gallbladder contraction. Ann. Surg. 1979, 190, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.Q.H.; Portincasa, P.; Wang, H.H. Bile Formation and Pathophysiology of Gallstones. In Encyclopedia of Gastroenterology, 2nd ed.; Kujpers, E.J., Ed.; Academic Press: Oxford, UK, 2020; pp. 287–306. [Google Scholar]
- Maljaars, J.; Peters, H.; Mela, D.J.; Masclee, A. Ileal brake: A sensible food target for appetite control. A review. Physiol. Behav. 2008, 95, 271–281. [Google Scholar] [CrossRef] [PubMed]
- van Avesaat, M.; Troost, F.J.; Ripken, D.; Hendriks, H.F.; Masclee, A.A. Ileal brake activation: Macronutrient-specific effects on eating behavior? Int. J. Obes. (Lond.) 2015, 39, 235–243. [Google Scholar] [CrossRef]
- Stolk, M.F.; van Erpecum, K.J.; van Berge Henegouwen, G.P.; Kesselring, O.F.; Hopman, W.P. Gallbladder volume and contraction measured by sum-of-cylinders method compared with ellipsoid and area-length methods. Acta Radiol. 1990, 31, 591–596. [Google Scholar] [CrossRef]
- Diella, G.; Di Ciaula, A.; Lorusso, M.P.; Summo, C.; Caggiano, G.; Caponio, F.; Montagna, M.T.; Portincasa, P. Distinct Effects of two Almond Cultivars on Agreeability and Gastrointestinal Motility in Healthy Subjects: More than mere Nutraceuticals. J. Gastrointestin. Liver Dis. 2018, 27, 31–39. [Google Scholar]
- Portincasa, P.; Altomare, D.F.; Moschetta, A.; Baldassarre, G.; Di Ciaula, A.; Venneman, N.G.; Rinaldi, M.; Vendemiale, G.; Memeo, V.; van Berge-Henegouwen, G.P.; et al. The effect of acute oral erythromycin on gallbladder motility and on upper gastrointestinal symptoms in gastrectomized patients with and without gallstones: A randomized, placebo-controlled ultrasonographic study. Am. J. Gastroenterol. 2000, 95, 3451. [Google Scholar] [CrossRef]






| Organoleptic Evaluation | Gastrointestinal Motility Studies | |
|---|---|---|
| Number | 50 | 16 |
| Males:Females | 25:25 | 8:8 |
| Age, yrs (Range) | 28 ± 0.9 (21–57) | 25 ± 0.6 (21–29) |
| BMI, Kg/m² (Range) | 22.3 ± 0.3 (18.6–31.0) | 21.9 ± 0.4 (18.8–25.1) |
| Choco-A | Choco-I | Nutridrink | |
|---|---|---|---|
| Chocolate (g) | 28.6 | 27.3 | - |
| Water (mL) | 175 | 176 | 200 |
| Lactulose (mL) | 15 | 15 | 15 |
| Final Volume (mL) | 215 | 215 | 215 |
| Fat g (%) | 12 (43) | 12 (45) | 12 (20) |
| Protein g (%) | 2.50 (9) | 2.70 (10) | 12 (20) |
| Carbohydrates g (%) | 10.50 (38) | 9.30 (35) | 36.80 (60) |
| Soluble fiber g (%) | 2.69 (10) | 2.73 (10) | - |
| Salt g (%) | - | 0.07 (0) | - |
| Kcal (kJ) | 165 (684) | 161 (668) | 300 (1260) |
| Density (g/mL) | 1.02 | 1.02 | 1.09 |
| Choco-A | Choco-I | p Value | |
|---|---|---|---|
| Carbohydrates (%) | 45.4 ± 7.78 | 45.1 ± 7.00 | 0.56 |
| Soluble fiber (%) | 5.1 ± 0.78 | 4.9 ± 1.69 | 0.95 |
| Fat (%) | 41.2 ± 0.99 | 41.0 ± 1.41 | 0.63 |
| Protein (%) | 8.3 ± 0.57 | 8.9 ± 0.35 | 0.17 |
| Moisture (%) | 1.4 ± 0.05 | 1.5 ± 0.03 | 0.08 |
| Ash (%) | 1.7 ± 0.04 | 1.8 ± 0.06 | 0.29 |
| Choco-A | Choco-I | p Value | |
|---|---|---|---|
| Total phenol content (TPC) (mg GAE/g) | 20.66 ± 1.28 | 21.53± 2.14 | 0.57 |
| 2,2-Azino-bis(3-ethylbenzothiazoline-6-sulphonic acid (ABTS) (µmol TE/g) | 98.39 ± 1.70 | 88.99 ± 4.20 | 0.02 |
| 2,2-diphenyl-1-picrylhydrazyl (DPPH) (µmol TE/g) | 79.81 ± 5.4 | 68.65 ± 1.39 | 0.02 |
| Theobromine (µg/g) | 1238.68 ± 73.63 | 1436.61 ± 62.64 | 0.02 |
| Catechin (µg/g) | 24.00 ± 4.78 | 21.63 ± 1.98 | 0.47 |
| Procyanidin B2 (µg/g) | 72.95 ± 1.70 | 71.28 ± 4.23 | 0.62 |
| Epicatechin (µg/g) | 112.10 ± 10.83 | 101.44 ± 8.25 | 0.24 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caponio, G.R.; Lorusso, M.P.; Sorrenti, G.T.; Marcotrigiano, V.; Difonzo, G.; De Angelis, E.; Guagnano, R.; Ciaula, A.D.; Diella, G.; Logrieco, A.F.; et al. Chemical Characterization, Gastrointestinal Motility and Sensory Evaluation of Dark Chocolate: A Nutraceutical Boosting Consumers’ Health. Nutrients 2020, 12, 939. https://doi.org/10.3390/nu12040939
Caponio GR, Lorusso MP, Sorrenti GT, Marcotrigiano V, Difonzo G, De Angelis E, Guagnano R, Ciaula AD, Diella G, Logrieco AF, et al. Chemical Characterization, Gastrointestinal Motility and Sensory Evaluation of Dark Chocolate: A Nutraceutical Boosting Consumers’ Health. Nutrients. 2020; 12(4):939. https://doi.org/10.3390/nu12040939
Chicago/Turabian StyleCaponio, Giusy Rita, Michele Pio Lorusso, Giovanni Trifone Sorrenti, Vincenzo Marcotrigiano, Graziana Difonzo, Elisabetta De Angelis, Rocco Guagnano, Agostino Di Ciaula, Giusy Diella, Antonio Francesco Logrieco, and et al. 2020. "Chemical Characterization, Gastrointestinal Motility and Sensory Evaluation of Dark Chocolate: A Nutraceutical Boosting Consumers’ Health" Nutrients 12, no. 4: 939. https://doi.org/10.3390/nu12040939
APA StyleCaponio, G. R., Lorusso, M. P., Sorrenti, G. T., Marcotrigiano, V., Difonzo, G., De Angelis, E., Guagnano, R., Ciaula, A. D., Diella, G., Logrieco, A. F., Montagna, M. T., Monaci, L., De Angelis, M., & Portincasa, P. (2020). Chemical Characterization, Gastrointestinal Motility and Sensory Evaluation of Dark Chocolate: A Nutraceutical Boosting Consumers’ Health. Nutrients, 12(4), 939. https://doi.org/10.3390/nu12040939












