HSP70 Promoter-Driven Activation of Gene Expression for Immunotherapy Using Gold Nanorods and Near Infrared Light
Abstract
:1. Introduction

2. Experimental
2.1. Materials
2.2. Cloning of HSP70 Promoter-Driven GFP Reporter
2.3. Verification of in Vitro GFP Expression in Cells and Uptake of AuNRs
2.4. In Vitro Optimization of NIR-Induced Expression
2.5. Optimization of in Vivo NIR-Induced GFP Expression
3. Results and Discussion
3.1. Verification of Transfection and Uptake of AuNPs

3.2. In Vitro Optimization of NIR-Driven Expression

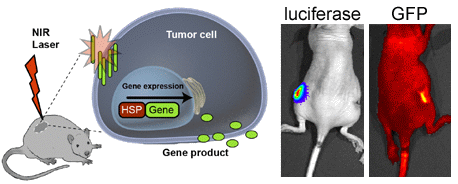
3.3. In Vivo HSP-Promoter-Driven Expression


4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Malyguine, A.M.; Strobl, S.L.; Shurin, M.R. Immunological monitoring of the tumor immunoenvironment for clinical trials. Cancer Immunol. Immunother. 2012, 61, 239–247. [Google Scholar] [CrossRef]
- Jennings, T.; Strouse, G. Past, present, and future of gold nanoparticles. Adv. Exp. Med. Biol. 2007, 620, 34–47. [Google Scholar] [CrossRef]
- Vigderman, L.; Khanal, B.P.; Zubarev, E.R. Functional gold nanorods: Synthesis, self-assembly, and sensing applications. Adv. Mater. 2012, 24, 4811–4841. [Google Scholar] [CrossRef]
- You, J.; Zhang, R.; Xiong, C.; Zhong, M.; Melancon, M.; Gupta, S.; Nick, A.M.; Sood, A.K.; Li, C. Effective photothermal chemotherapy using doxorubicin-loaded gold nanospheres that target ephb4 receptors in tumors. Cancer Res. 2012, 72, 4777–4786. [Google Scholar] [CrossRef]
- Melancon, M.P.; Zhou, M.; Li, C. Cancer theranostics with near-infrared light-activatable multimodal nanoparticles. Acc. Chem. Res. 2011, 44, 947–956. [Google Scholar] [CrossRef]
- Kucharczuk, J.C. Radiofrequency ablation: Emerging therapy for the small pulmonary nodule? Semin. Thorac. Cardiovasc. Surg. 2005, 17, 134–137. [Google Scholar] [CrossRef]
- Maeda, H. The enhanced permeability and retention (epr) effect in tumor vasculature: The key role of tumor-selective macromolecular drug targeting. Adv. Enzym. Regul. 2001, 41, 189–207. [Google Scholar]
- Maeda, H.; Fang, J.; Inutsuka, T.; Kitamoto, Y. Vascular permeability enhancement in solid tumor: Various factors, mechanisms involved and its implications. Int. Immunopharmacol. 2003, 3, 319–328. [Google Scholar] [CrossRef]
- Loo, C.; Lowery, A.; Halas, N.; West, J.; Drezek, R. Immunotargeted nanoshells for integrated cancer imaging and therapy. Nano Lett. 2005, 5, 709–711. [Google Scholar] [CrossRef]
- Lowery, A.R.; Gobin, A.M.; Day, E.S.; Halas, N.J.; West, J.L. Immunonanoshells for targeted photothermal ablation of tumor cells. Int. J. Nanomedicine 2006, 1, 149–154. [Google Scholar] [CrossRef]
- Zhang, X.; Servos, M.R.; Liu, J. Surface science of DNA adsorption onto citrate-capped gold nanoparticles. Langmuir 2012, 28, 3896–3902. [Google Scholar] [CrossRef]
- Yoshida, M.; Takimoto, R.; Murase, K.; Sato, Y.; Hirakawa, M.; Tamura, F.; Sato, T.; Iyama, S.; Osuga, T.; Miyanishi, K.; et al. Targeting anticancer drug delivery to pancreatic cancer cells using a fucose-bound nanoparticle approach. PLoS One 2012, 7, e39545. [Google Scholar] [CrossRef]
- Rana, S.; Bajaj, A.; Mout, R.; Rotello, V.M. Monolayer coated gold nanoparticles for delivery applications. Adv. Drug Deliv. Rev. 2012, 64, 200–216. [Google Scholar] [CrossRef]
- Rippel, R.A.; Seifalian, A.M. Gold revolution—Gold nanoparticles for modern medicine and surgery. J. Nansci. Nanotechnol. 2011, 11, 3740–3748. [Google Scholar] [CrossRef]
- Tiwari, M. Nano cancer therapy strategies. J. Cancer Res. Ther. 2012, 8, 19–22. [Google Scholar] [CrossRef]
- Cherukuri, P.; Glazer, E.S.; Curley, S.A. Targeted hyperthermia using metal nanoparticles. Adv. Drug Deliv. Rev. 2010, 62, 339–345. [Google Scholar] [CrossRef]
- Stieger, K.; Belbellaa, B.; Le Guiner, C.; Moullier, P.; Rolling, F. In vivo gene regulation using tetracycline-regulatable systems. Adv. Drug Deliv. Rev. 2009, 61, 527–541. [Google Scholar] [CrossRef]
- Dorer, D.E.; Nettelbeck, D.M. Targeting cancer by transcriptional control in cancer gene therapy and viral oncolysis. Adv. Drug Deliv. Rev. 2009, 61, 554–571. [Google Scholar] [CrossRef]
- Rome, C.; Couillaud, F.; Moonen, C.T. Spatial and temporal control of expression of therapeutic genes using heat shock protein promoters. Methods 2005, 35, 188–198. [Google Scholar] [CrossRef]
- Akerfelt, M.; Morimoto, R.I.; Sistonen, L. Heat shock factors: Integrators of cell stress, development and lifespan. Nat. Rev. Mol. Cell Biol. 2010, 11, 545–555. [Google Scholar] [CrossRef]
- Halfon, M.S.; Kose, H.; Chiba, A.; Keshishian, H. Targeted gene expression without a tissue-specific promoter: Creating mosaic embryos using laser-induced single-cell heat shock. Proc. Natl. Acad. Sci. USA 1997, 94, 6255–6260. [Google Scholar] [CrossRef]
- Ramos, D.M.; Kamal, F.; Wimmer, E.A.; Cartwright, A.N.; Monteiro, A. Temporal and spatial control of transgene expression using laser induction of the hsp70 promoter. BMC Dev. Biol. 2006, 6, 55. [Google Scholar] [CrossRef]
- Weissleder, R. A clearer vision for in vivo imaging. Nat. Biotechnol. 2001, 19, 316–317. [Google Scholar] [CrossRef]
- Konig, K. Multiphoton microscopy in life sciences. J. Microsc. 2000, 200, 83–104. [Google Scholar] [CrossRef]
- Wei, F.; Wang, H.; Zhang, J.; Chen, X.; Li, C.; Huang, Q. Pharmacokinetics of combined gene therapy expressing constitutive human gm-csf and hyperthermia-regulated human il-12. J. Exp. Clin. Cancer Res. 2013, 32, 5. [Google Scholar] [CrossRef]
- Guilhon, E.; Voisin, P.; de Zwart, J.A.; Quesson, B.; Salomir, R.; Maurange, C.; Bouchaud, V.; Smirnov, P.; de Verneuil, H.; Vekris, A.; et al. Spatial and temporal control of transgene expression in vivo using a heat-sensitive promoter and mri-guided focused ultrasound. J. Gene Med. 2003, 5, 333–342. [Google Scholar] [CrossRef]
- Fiszer-Kierzkowska, A.; Vydra, N.; Wysocka-Wycisk, A.; Kronekova, Z.; Jarzab, M.; Lisowska, K.M.; Krawczyk, Z. Liposome-based DNA carriers may induce cellular stress response and change gene expression pattern in transfected cells. BMC Mol. Biol. 2011, 12, 27. [Google Scholar] [CrossRef]
- Raoof, M.; Zhu, C.; Kaluarachchi, W.D.; Curley, S.A. Luciferase-based protein denaturation assay for quantification of radiofrequency field-induced targeted hyperthermia: Developing an intracellular thermometer. Int. J. Hyperthermia 2012, 28, 202–209. [Google Scholar] [CrossRef]
- Corr, S.J.; Savage, D.J.; Curley, S.A.; Serda, R.E.; Michael, E. DeBakey Department of Surgery, Baylor College of Medicine: Houston, TX, USA, 2014; Unpublished work.
- Raoof, M.; Cisneros, B.T.; Corr, S.J.; Palalon, F.; Curley, S.A.; Koshkina, N.V. Tumor selective hyperthermia induced by short-wave capacitively-coupled rf electric-fields. PLoS One 2013, 8, e68506. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Andersson, H.A.; Kim, Y.-S.; O'Neill, B.E.; Shi, Z.-Z.; Serda, R.E. HSP70 Promoter-Driven Activation of Gene Expression for Immunotherapy Using Gold Nanorods and Near Infrared Light. Vaccines 2014, 2, 216-227. https://doi.org/10.3390/vaccines2020216
Andersson HA, Kim Y-S, O'Neill BE, Shi Z-Z, Serda RE. HSP70 Promoter-Driven Activation of Gene Expression for Immunotherapy Using Gold Nanorods and Near Infrared Light. Vaccines. 2014; 2(2):216-227. https://doi.org/10.3390/vaccines2020216
Chicago/Turabian StyleAndersson, Helen A., Yoo-Shin Kim, Brian E. O'Neill, Zheng-Zheng Shi, and Rita E. Serda. 2014. "HSP70 Promoter-Driven Activation of Gene Expression for Immunotherapy Using Gold Nanorods and Near Infrared Light" Vaccines 2, no. 2: 216-227. https://doi.org/10.3390/vaccines2020216
APA StyleAndersson, H. A., Kim, Y.-S., O'Neill, B. E., Shi, Z.-Z., & Serda, R. E. (2014). HSP70 Promoter-Driven Activation of Gene Expression for Immunotherapy Using Gold Nanorods and Near Infrared Light. Vaccines, 2(2), 216-227. https://doi.org/10.3390/vaccines2020216