Urinary Metabolic Profiling via LC-MS/MS Reveals Impact of Bovine Lactoferrin on Bone Formation in Growing SD Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Animal Experimental Design
2.3. Measurement for Serum Markers of Both Bone Formation and Resorption by ELISA
2.4. Microcomputed Tomographic (μ-CT) Measures for Bone Analysis
2.5. Hematoxylin and Eosin (H&E) for Bone Staining
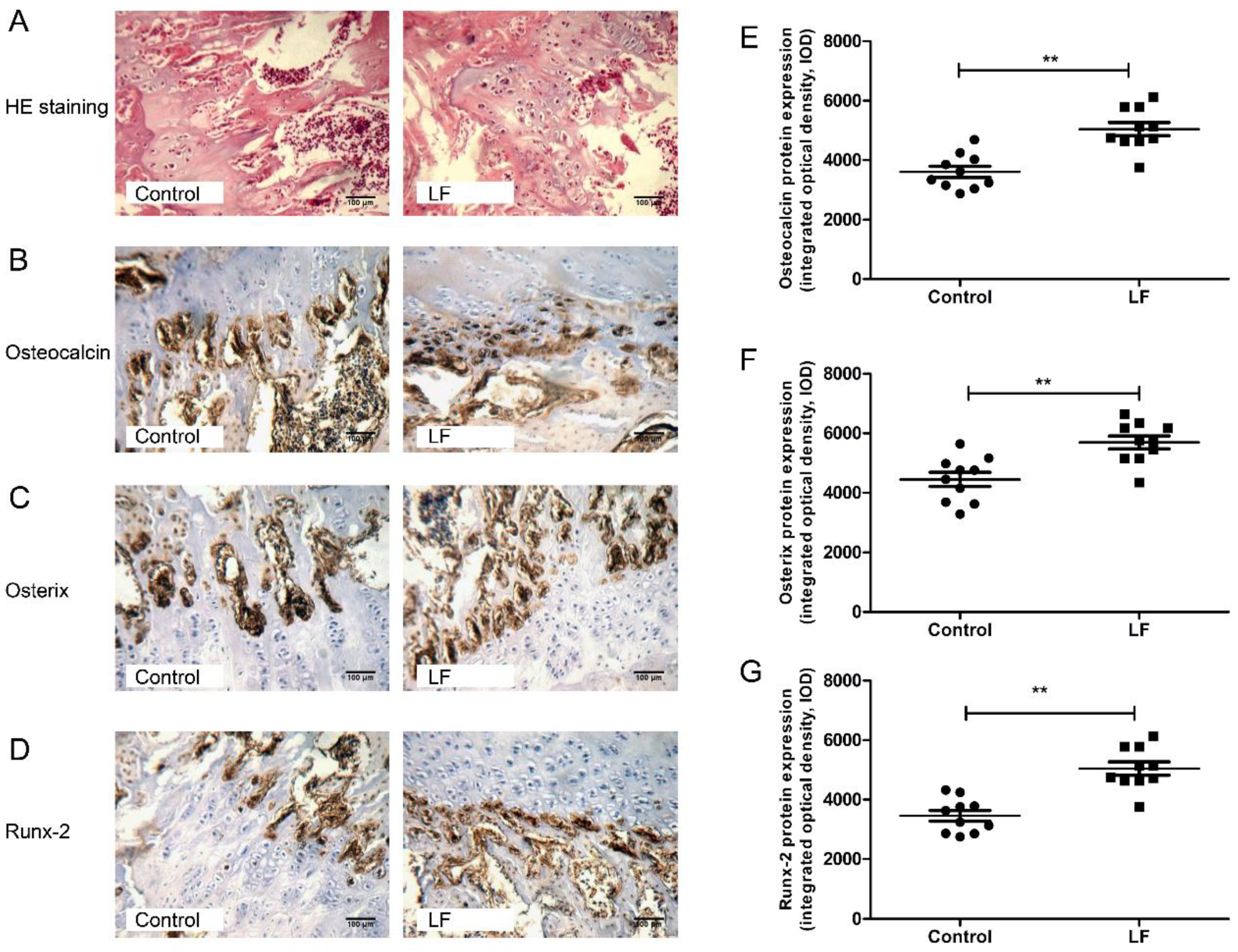
2.6. Immunohistochemical (IHC) Analysis of Osteocalcin, Osterix, and Runx-2 Expression
2.7. Urinary Metabolite Extraction
2.8. Urine Metabolic Profiling Analysis by UPLC-MS/MS
2.9. Urine Metabolomic Data Processing and Annotation
2.10. Statistical Analyses
3. Results
3.1. LF Improved Bone Growth in Growing SD Rat
3.2. LF Impacts on Osteogenesis by Upregulating Osteocalcin, Osterix, and Runx-2
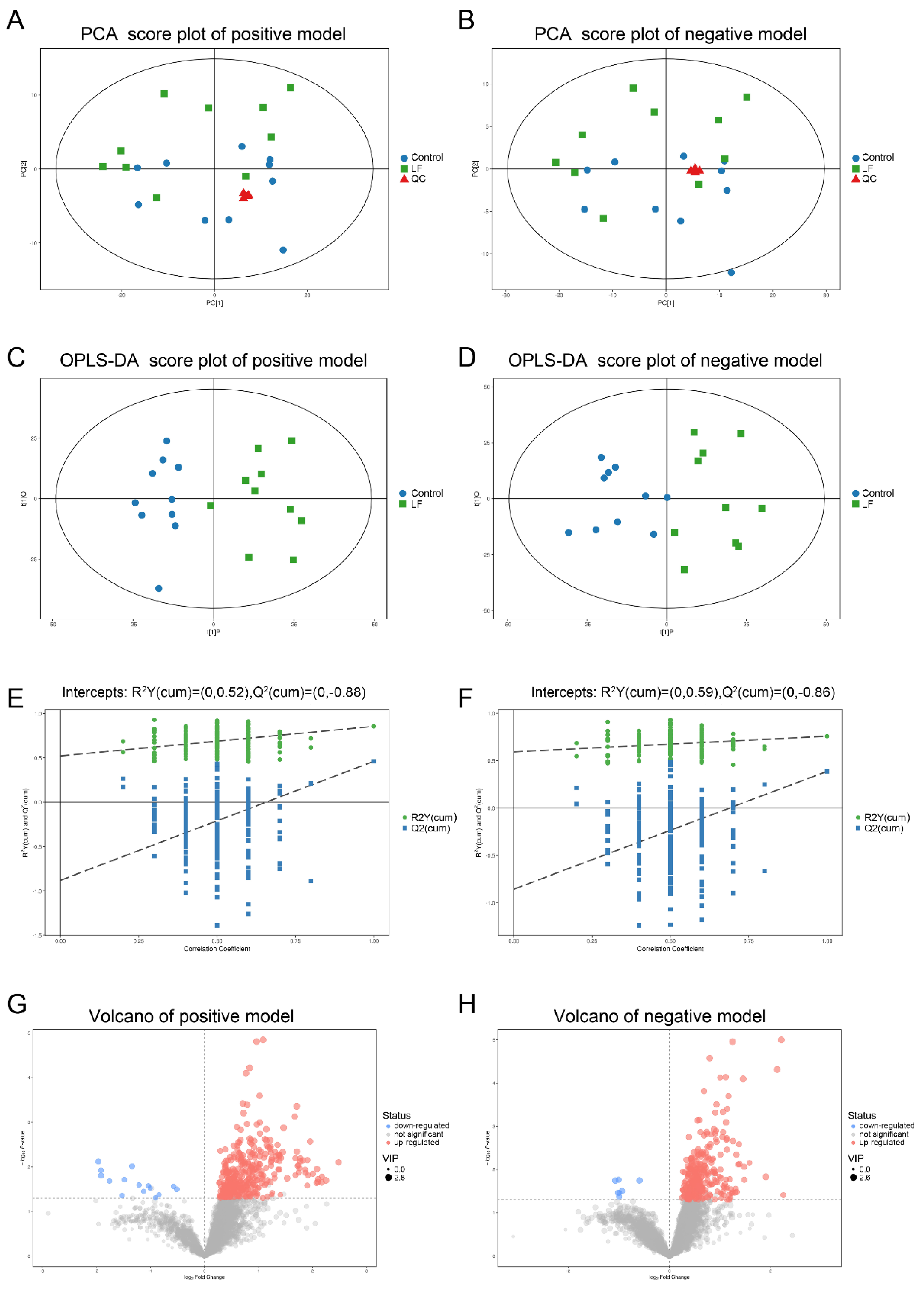
3.3. Quality Assessment and Establishment of the Metabolomic Platform
3.4. Metabolic Profiling Analysis of Urine from Growing SD Rats LF-Supplemented
3.5. Alterations in Metabolic Pathways Analysis
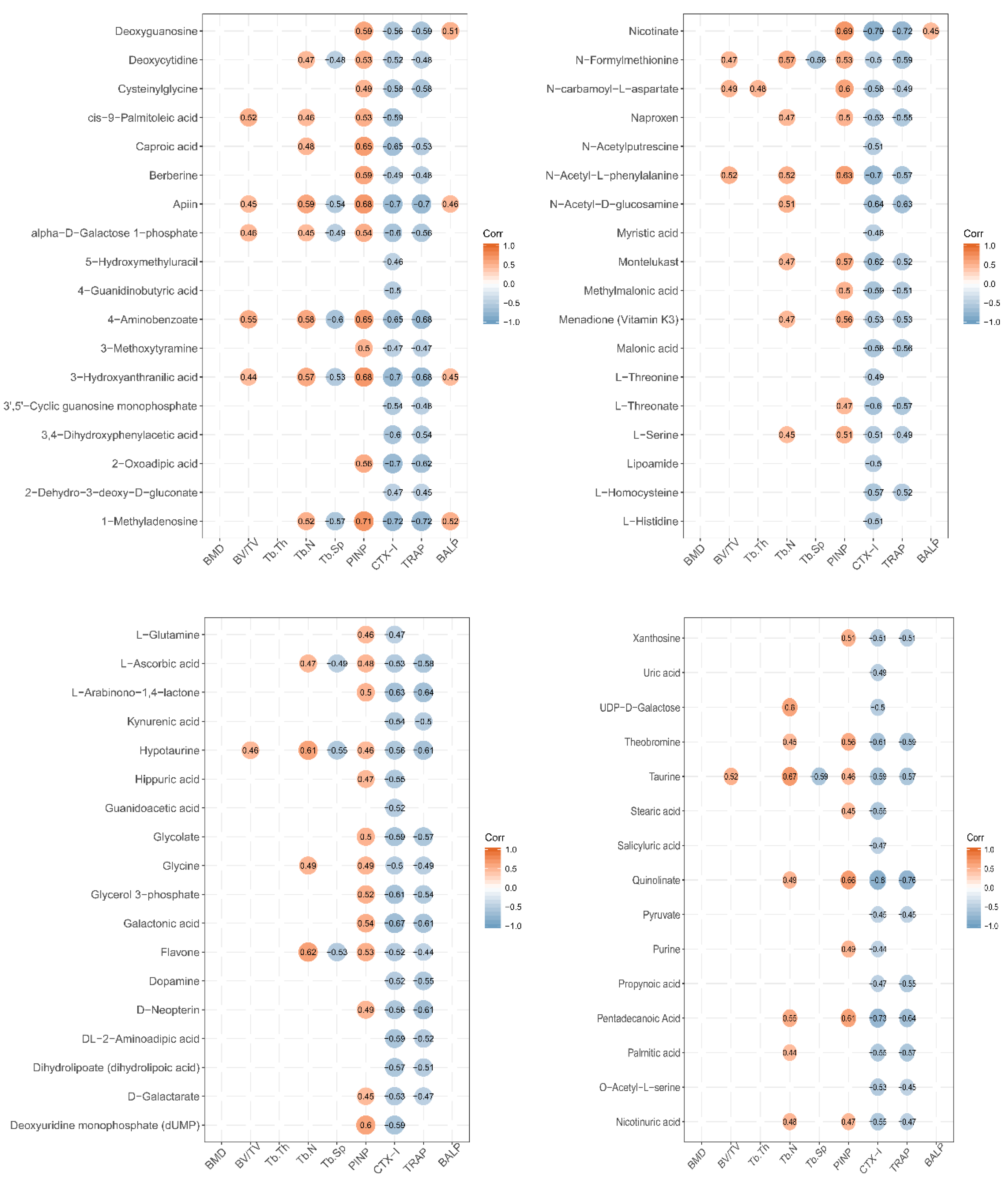
3.6. Correlation between Urinary Metabolites and Bone Growth Induced by LF Supplementation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Weaver, C.M.; Gordon, C.M.; Janz, K.F.; Kalkwarf, H.J.; Lappe, J.M.; Lewis, R.; O’Karma, M.; Wallace, T.C.; Zemel, B.S. The National Osteoporosis Foundation’s position statement on peak bone mass development and lifestyle factors: A systematic review and implementation recommendations. Osteoporos. Int. 2016, 27, 1281–1386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tandon, N.; Fall, C.H.D.; Osmond, C.; Sachdev, H.P.S.; Prabhakaran, D.; Ramakrishnan, L.; Biswas, S.K.D.; Ramji, S.; Khalil, A.; Gera, T.; et al. Growth from birth to adulthood and peak bone mass and density data from the New Delhi Birth Cohort. Osteoporos. Int. 2012, 23, 2447–2459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, F.-P.J.; Montoliu, I.; Kussmann, M. Metabonomics of ageing—Towards understanding metabolism of a long and healthy life. Mech. Ageing Dev. 2017, 165, 171–179. [Google Scholar] [CrossRef]
- Molgaard, C.; Larnkjaer, A.; Mark, A.B.; Michaelsen, K.F. Are early growth and nutrition related to bone health in adolescence? The Copenhagen Cohort Study of infant nutrition and growth. Am. J. Clin. Nutr. 2011, 94, 1865S–1869S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fewtrell, M. Early nutritional predictors of long-term bone health in preterm infants. Curr. Opin. Clin. Nutr. Metab. Care 2011, 14, 297–301. [Google Scholar] [CrossRef]
- Masson, P.L.; Heremans, J.F.; Dive, C.H. An iron-binding protein common to many external secretions. Clin. Chim. Acta 1966, 14, 735–739. [Google Scholar] [CrossRef]
- Gonzalez-Chavez, S.A.; Arevalo-Gallegos, S.; Rascon-Cruz, Q. Lactoferrin: Structure, function and applications. Int. J. Antimicrob. Agents 2009, 33, 301.e301–301.e308. [Google Scholar] [CrossRef]
- Zhang, J.L.; Han, X.; Shan, Y.J.; Zhang, L.W.; Du, M.; Liu, M.; Yi, H.X.; Ma, Y. Effect of bovine lactoferrin and human lactoferrin on the proliferative activity of the osteoblast cell line MC3T3-E1 in vitro. J. Dairy Sci. 2018, 101, 1827–1833. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.X.; Wang, J.X.; Ren, F.Z.; Zhang, W.; Zhang, H.; Zhao, L.; Zhang, M.; Cui, W.; Wang, X.B.; Guo, H.Y. Lactoferrin Promotes Osteogenesis through TGF-ss Receptor II Binding in Osteoblasts and Activation of Canonical TGF-ss Signaling in MC3T3-E1 Cells and C57BL/6J Mice. J. Nutr. 2018, 148, 1285–1292. [Google Scholar] [CrossRef] [Green Version]
- An, J.J.; Xu, Y.; Kong, Z.Q.; Xie, Y.D.; Tabys, D.; Ma, M.; Cao, X.; Ren, H.W.; Liu, N. Effect of lactoferrin and its digests on differentiation activities of bone mesenchymal stem cells. J. Funct. Foods 2019, 57, 202–210. [Google Scholar] [CrossRef]
- Ying, X.Z.; Cheng, S.W.; Wang, W.; Lin, Z.Q.; Chen, Q.Y.; Zhang, W.; Kou, D.Q.; Shen, Y.; Cheng, X.J.; Peng, L.; et al. Effect of lactoferrin on osteogenic differentiation of human adipose stem cells. Int. Orthop. 2012, 36, 647–653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lorget, F.; Clough, J.; Oliveira, M.; Daury, M.C.; Sabokbar, A.; Offord, E. Lactoferrin reduces in vitro osteoclast differentiation and resorbing activity. Biochem. Biophys. Res. Commun. 2002, 296, 261–266. [Google Scholar] [CrossRef]
- Gao, R.; Watson, M.; Callon, K.E.; Tuari, D.; Dray, M.; Naot, D.; Amirapu, S.; Munro, J.T.; Cornish, J.; Musson, D.S. Local application of lactoferrin promotes bone regeneration in a rat critical-sized calvarial defect model as demonstrated by micro-CT and histological analysis. J. Tissue Eng. Regen. Med. 2018, 12, E620–E626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.Y.; Zhu, S.S.; Hu, J. Bone Regeneration Is Promoted by Orally Administered Bovine Lactoferrin in a Rabbit Tibial Distraction Osteogenesis Model. Clin. Orthop. Relat. Res. 2015, 473, 2383–2393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.W.; Li, Y.H.; Zhang, M.J.; Chen, Z.; Ke, D.S.; Xue, Y.; Hou, J.M. Lactoferrin ameliorates aging-suppressed osteogenesis via IGF1 signaling. J. Mol. Endocrinol. 2019, 63, 63–75. [Google Scholar] [CrossRef]
- Bharadwaj, S.; Naidu, A.G.T.; Betageri, G.V.; Prasadarao, N.V.; Naidu, A.S. Milk ribonuclease-enriched lactoferrin induces positive effects on bone turnover markers in postmenopausal women. Osteoporos. Int. 2009, 20, 1603–1611. [Google Scholar] [CrossRef]
- Fan, F.J.; Shi, P.J.; Liu, M.; Chen, H.; Tu, M.L.; Lu, W.H.; Du, M. Lactoferrin preserves bone homeostasis by regulating the RANKL/RANK/OPG pathway of osteoimmunology. Food Funct. 2018, 9, 2653–2660. [Google Scholar] [CrossRef]
- Zhang, W.; Guo, H.Y.; Jing, H.; Li, Y.X.; Wang, X.Y.; Zhang, H.; Jiang, L.; Ren, F.Z. Lactoferrin Stimulates Osteoblast Differentiation Through PKA and p38 Pathways Independent of Lactoferrin’s Receptor LRP1. J. Bone Miner. Res. 2014, 29, 1232–1243. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Fan, F.J.; Shi, P.J.; Tu, M.L.; Yu, C.P.; Yu, C.X.; Du, M. Lactoferrin promotes MC3T3-E1 osteoblast cells proliferation via MAPK signaling pathways. Int. J. Biol. Macromol. 2018, 107, 137–143. [Google Scholar] [CrossRef]
- Zhang, W.; Wu, H.Y.; Li, Y.X.; Ren, F.Z.; Guo, H.Y. Lactoferrin-induced growth factors and cytokines expression profile in pre-osteoblast MC3T3-E1 cell and LRP1 stable knockdown MC3T3-E1 cell. J. Funct. Foods 2017, 37, 147–156. [Google Scholar] [CrossRef]
- Cheung, P.K.; Ma, M.H.; Tse, H.F.; Yeung, K.F.; Tsang, H.F.; Chu, M.K.M.; Kan, C.M.; Cho, W.C.S.; Ng, L.B.W.; Chan, L.W.C.; et al. The applications of metabolomics in the molecular diagnostics of cancer. Expert Rev. Mol. Diagn. 2019, 19, 785–793. [Google Scholar] [CrossRef]
- Sowton, A.P.; Griffin, J.L.; Murray, A.J. Metabolic Profiling of the Diabetic Heart: Toward a Richer Picture. Front. Physiol. 2019, 10, 639. [Google Scholar] [CrossRef]
- Steuer, A.E.; Brockbals, L.; Kraemer, T. Metabolomic Strategies in Biomarker Research-New Approach for Indirect Identification of Drug Consumption and Sample Manipulation in Clinical and Forensic Toxicology? Front. Chem. 2019, 7, 319. [Google Scholar] [CrossRef] [PubMed]
- O’Gorman, A.; Brennan, L. Metabolomic applications in nutritional research: A perspective. J. Sci. Food Agric. 2015, 95, 2567–2570. [Google Scholar] [CrossRef] [PubMed]
- Xie, G.X.; Zhao, A.H.; Zhao, L.J.; Chen, T.L.; Chen, H.Y.; Qi, X.; Zheng, X.J.; Ni, Y.; Cheng, Y.; Lan, K.; et al. Metabolic Fate of Tea Polyphenols in Humans. J. Proteome Res. 2012, 11, 3449–3457. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.Q.; Cao, G.; Chen, H.; Argyopoulos, C.P.; Yu, H.; Su, W.; Chen, L.; Samuels, D.C.; Zhuang, S.G.; Bayliss, G.P.; et al. Identification of serum metabolites associating with chronic kidney disease progression and anti-fibrotic effect of 5-methoxytryptophan. Nat. Commun. 2019, 10, 1476. [Google Scholar] [CrossRef]
- Carvalho, D.V.; Silva, L.M.A.; Alves, E.G.; Santos, F.A.; de Lima, R.P.; Viana, A.; Nunes, P.I.G.; Fonseca, S.G.D.; de Melo, T.S.; Viana, D.D.; et al. Cashew apple fiber prevents high fat diet-induced obesity in mice: An NMR metabolomic evaluation. Food Funct. 2019, 10, 1671–1683. [Google Scholar] [CrossRef]
- Li, Y.; Guo, Y.; Wen, Z.S.; Jiang, X.M.; Ma, X.; Han, X.Y. Weaning Stress Perturbs Gut Microbiome and Its Metabolic Profile in Piglets. Sci. Rep. 2018, 8, 18068. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Yang, X.; Wang, F.; Li, R.; Ning, H.; Na, L.; Huang, Y.; Song, Y.; Liu, L.; Pan, H.; et al. Calcium-deficiency assessment and biomarker identification by an integrated urinary metabonomics analysis. BMC Med. 2013, 11, 86. [Google Scholar] [CrossRef] [Green Version]
- Zhao, H.A.; Cheng, N.; Wang, Q.; Zhou, W.Q.; Liu, C.Y.; Liu, X.Y.; Chen, S.N.; Fan, D.D.; Cao, W. Effects of honey-extracted polyphenols on serum antioxidant capacity and metabolic phenotype in rats. Food Funct. 2019, 10, 2347–2358. [Google Scholar] [CrossRef]
- Cerven, D.; DeGeorge, G.; Bethell, D. 28-Day repeated dose oral toxicity of recombinant human holo-lactoferrin in rats. Regul. Toxicol. Pharmacol. 2008, 52, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Jing, Z.; Wang, C.Y.; Yang, Q.N.; Wei, X.L.; Jin, Y.; Meng, Q.; Liu, Q.; Liu, Z.H.; Ma, X.D.; Liu, K.X.; et al. Luteolin attenuates glucocorticoid-induced osteoporosis by regulatingERK/Lrp-5/GSK-3 signaling pathway in vivo and in vitro. J. Cell. Physiol. 2019, 234, 4472–4490. [Google Scholar] [CrossRef]
- Xia, G.H.; Zhao, Y.L.; Yu, Z.; Tian, Y.Y.; Wang, Y.M.; Wang, S.S.; Wang, J.F.; Xue, C.H. Phosphorylated Peptides from Antarctic Krill (Euphausia superba) Prevent Estrogen Deficiency Induced Osteoporosis by Inhibiting Bone Resorption in Ovariectomized Rats. J. Agric. Food Chem. 2015, 63, 9550–9557. [Google Scholar] [CrossRef]
- Liu, H.X.; Zhu, R.Y.; Liu, C.Y.; Ma, R.F.; Wang, L.L.; Chen, B.B.; Li, L.; Niu, J.Z.; Zhao, D.D.; Mo, F.F.; et al. Evaluation of Decalcification Techniques for Rat Femurs Using HE and Immunohistochemical Staining. Biomed Res. Int. 2017, 9050754. [Google Scholar] [CrossRef]
- Want, E.J.; Wilson, I.D.; Gika, H.; Theodoridis, G.; Plumb, R.S.; Shockcor, J.; Holmes, E.; Nicholson, J.K. Global metabolic profiling procedures for urine using UPLC-MS. Nat. Protoc. 2010, 5, 1005–1018. [Google Scholar] [CrossRef] [PubMed]
- Tautenhahn, R.; Patti, G.J.; Rinehart, D.; Siuzdak, G. XCMS Online: A Web-Based Platform to Process Untargeted Metabolomic Data. Anal. Chem. 2012, 84, 5035–5039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, C.A.; Want, E.J.; O’Maille, G.; Abagyan, R.; Siuzdak, G. XCMS: Processing mass spectrometry data for metabolite profiling using Nonlinear peak alignment, matching, and identification. Anal. Chem. 2006, 78, 779–787. [Google Scholar] [CrossRef] [PubMed]
- Kuhl, C.; Tautenhahn, R.; Bottcher, C.; Larson, T.R.; Neumann, S. CAMERA: An Integrated Strategy for Compound Spectra Extraction and Annotation of Liquid Chromatography/Mass Spectrometry Data Sets. Anal. Chem. 2012, 84, 283–289. [Google Scholar] [CrossRef] [Green Version]
- Chong, J.; Soufan, O.; Li, C.; Caraus, I.; Li, S.Z.; Bourque, G.; Wishart, D.S.; Xia, J.G. MetaboAnalyst 4.0: Towards more transparent and integrative metabolomics analysis. Nucleic Acids Res. 2018, 46, W486–W494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gika, H.G.; Macpherson, E.; Theodoridis, G.A.; Wilson, I.D. Evaluation of the repeatability of ultra-performance liquid chromatography-TOF-MS for global metabolic profiling of human urine samples. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2008, 871, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Malet, A.; Bournaud, E.; Lan, A.; Mikogami, T.; Tome, D.; Blais, A. Bovine lactoferrin improves bone status of ovariectomized mice via immune function modulation. Bone 2011, 48, 1028–1035. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.L.; Zhao, J.; Hu, W.P.; Wang, J.W.; Yu, T.; Dai, Y.P.; Li, N. Effects of Recombinant Human Lactoferrin on Osteoblast Growth and Bone Status in Piglets. Anim. Biotechnol. 2018, 29, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Sun, J.F.; Zhou, Z.Y.; Pan, J.; Zou, S.J.; Chen, J.W. Effects of lactoferrin on bone resorption of midpalatal suture during rapid expansion in rats. Am. J. Orthod. Dentofac. Orthop. 2018, 154, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Tasli, P.N.; Sahin, F. Effect of Lactoferrin on Odontogenic Differentiation of Stem Cells Derived from Human 3rd Molar Tooth Germ. Appl. Biochem. Biotechnol. 2014, 174, 2257–2266. [Google Scholar] [CrossRef]
- Montesi, M.; Panseri, S.; Iafisco, M.; Adamiano, A.; Tampieri, A. Effect of hydroxyapatite nanocrystals functionalized with lactoferrin in osteogenic differentiation of mesenchymal stem cells. J. Biomed. Mater. Res. Part A 2015, 103, 224–234. [Google Scholar] [CrossRef]
- Schroeder, T.M.; Jensen, E.D.; Westendorf, J.J. Runx2: A Master Organizer of Gene Transcription in Developing and Maturing Osteoblasts. Birth Defects Res. C Embryo Today 2005, 75, 213–225. [Google Scholar] [CrossRef]
- Li, Y.X.; Zhang, W.; Ren, F.Z.; Guo, H.Y. Activation of TGF-beta Canonical and Noncanonical Signaling in Bovine Lactoferrin-Induced Osteogenic Activity of C3H10T1/2 Mesenchymal Stem Cells. Int. J. Mol. Sci. 2019, 20, 2880. [Google Scholar] [CrossRef] [Green Version]
- Nakashima, K.; Zhou, X.; Kunkel, G.; Zhang, Z.; Deng, J.M.; Behringer, R.R.; Crombrugghe, B.D. The Novel Zinc Finger-Containing Transcription Factor Osterix Is Required for Osteoblast Differentiation and Bone Formation. Cell 2002, 108, 17–29. [Google Scholar] [CrossRef] [Green Version]
- Montesi, M.; Panseri, S.; Iafisco, M.; Adamiano, A.; Tampieri, A. Coupling Hydroxyapatite Nanocrystals with Lactoferrin as a Promising Strategy to Fine Regulate Bone Homeostasis. PLoS ONE 2015, 10, e0132633. [Google Scholar] [CrossRef]
- Yu, L.S.; Qi, H.H.; An, G.H.; Bao, J.; Ma, B.; Zhu, J.W.; Ouyang, G.; Zhang, P.L.; Fan, H.W.; Zhang, Q. Association between metabolic profiles in urine and bone mineral density of pre- and postmenopausal Chinese women. Menopause J. N. Am. Menopause Soc. 2019, 26, 94–102. [Google Scholar] [CrossRef]
- Kotwal, S.D.; Badole, S.R. Anabolic therapy with Equisetum arvense along with bone mineralising nutrients in ovariectomized rat model of osteoporosis. Indian J. Pharmacol. 2016, 48, 312–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, W.W.; Chen, T.M.; Ding, S.J.; Yang, G.; Xu, Z.W.; Xu, K.M.; Zhang, S.Y.; Ma, T.; Zhang, J. Metabolomic study of the bone trabecula of osteonecrosis femoral head patients based on UPLC-MS/MS. Metabolomics 2016, 12, 48. [Google Scholar] [CrossRef]
- Barlian, A.; Judawisastra, H.; Alfarafisa, N.M.; Wibowo, U.A.; Rosadi, I. Chondrogenic differentiation of adipose-derived mesenchymal stem cells induced by L-ascorbic acid and platelet rich plasma on silk fibroin scaffold. PeerJ 2018, 6, e5809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, H.K.; Kim, G.J.; Yoo, H.S.; Song, D.H.; Chung, K.H.; Lee, K.J.; Koo, Y.T.; An, J.H. Vitamin C Activates Osteoblastogenesis and Inhibits Osteoclastogenesis via Wnt/beta-Catenin/ATF4 Signaling Pathways. Nutrients 2019, 11, 506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyamoto, T.; Hirayama, A.; Sato, Y.; Koboyashi, T.; Katsuyama, E.; Kanagawa, H.; Miyamoto, H.; Mori, T.; Yoshida, S.; Fujie, A.; et al. A serum metabolomics-based profile in low bone mineral density postmenopausal women. Bone 2017, 95, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.L.; Newman, H.; Shen, L.Y.; Sharma, D.; Hu, G.L.; Mirando, A.J.; Zhang, H.Y.; Knudsen, E.; Zhang, G.F.; Hilton, M.J.; et al. Glutamine Metabolism Regulates Proliferation and Lineage Allocation in Skeletal Stem Cells. Cell Metab. 2019, 29, 966–978. [Google Scholar] [CrossRef] [Green Version]
- Karner, C.M.; Esen, E.; Okunade, A.L.; Patterson, B.W.; Long, F. Increased glutamine catabolism mediates bone anabolism in response to WNT signaling. J. Clin. Investig. 2015, 125, 551–562. [Google Scholar] [CrossRef] [Green Version]
- Lee, W.C.; Guntur, A.R.; Long, F.X.; Rosen, C.J. Energy Metabolism of the Osteoblast: Implications for Osteoporosis. Endocr. Rev. 2017, 38, 255–266. [Google Scholar] [CrossRef]
- Berger, F.; Ramirez-Hernandez, M.H.; Ziegler, M. The new life of a centenarian: Signalling functions of NAD(P). Trends Biochem. Sci. 2004, 29, 111–118. [Google Scholar] [CrossRef]
- Kushwaha, P.; Wolfgang, M.J.; Riddle, R.C. Fatty acid metabolism by the osteoblast. Bone 2018, 115, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Comstock, S.S.; Reznikov, E.A.; Contractor, N.; Donovan, S.M. Dietary Bovine Lactoferrin Alters Mucosal and Systemic Immune Cell Responses in Neonatal Piglets. J. Nutr. 2014, 144, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Donovan, S.M. The Role of Lactoferrin in Gastrointestinal and Immune Development and Function: A Preclinical Perspective. J. Pediatrics 2016, 173, S16–S28. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.P.; Zhao, J.; Wang, J.W.; Yu, T.; Wang, J.; Li, N. Transgenic milk containing recombinant human lactoferrin modulates the intestinal flora in piglets. Biochem. Cell Biol. 2012, 90, 485–496. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Ren, F.; Xiong, L.; Zhao, L.; Guo, H. Bovine lactoferrin suppresses high-fat diet induced obesity and modulates gut microbiota in C57BL/6J mice. J. Funct. Foods 2016, 22, 189–200. [Google Scholar] [CrossRef]
- Amini, A.A.; Nair, L.S. Evaluation of the Bioactivity of Recombinant Human Lactoferrins toward Murine Osteoblast-Like Cells for Bone Tissue Engineering. Tissue Eng. Part A 2013, 19, 1047–1055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karner, C.M.; Long, F.X. Glucose metabolism in bone. Bone 2018, 115, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Sortino, O.; Hullsiek, K.H.; Richards, E.; Rupert, A.; Schminke, A.; Tetekpor, N.; Quinones, M.; Prosser, R.; Schacker, T.; Sereti, I.; et al. The Effects of Recombinant Human Lactoferrin on Immune Activation and the Intestinal Microbiome among Persons Living with Human Immunodeficiency Virus and Receiving Antiretroviral Therapy. J. Infect. Dis. 2019, 219, 1963–1968. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Zhao, F.Z.; Zhu, W.Y.; Wang, J. Effects of early-life lactoferrin intervention on growth performance, small intestinal function and gut microbiota in suckling piglets. Food Funct. 2019, 10, 5361–5373. [Google Scholar] [CrossRef]
- Dix, C.; Wright, O. Bioavailability of a Novel Form of Microencapsulated Bovine Lactoferrin and Its Effect on Inflammatory Markers and the Gut Microbiome: A Pilot Study. Nutrients 2018, 10, 1115. [Google Scholar] [CrossRef] [Green Version]


| No. | Metabolites Name | m/z | Exact Mass | RT (min) | Fold Change (LF/Control) | Sub.Class |
|---|---|---|---|---|---|---|
| 1 | 3,4-Dihydroxyphenylacetic acid | 359.067 | 168.042 | 5.429 | 1.315 | Phenols |
| 2 | 3-Hydroxyanthranilic acid | 154.050 | 153.043 | 3.508 | 2.188 | Benzoic acids and derivatives |
| 3 | 3-Methoxytyramine | 234.042 | 167.095 | 2.322 | 1.464 | Phenols |
| 4 | 4-Aminobenzoate | 160.039 | 137.048 | 3.150 | 1.892 | Benzene and substituted derivatives |
| 5 | 4-Guanidinobutyric acid | 146.092 | 145.085 | 5.682 | 1.471 | Carboxylic acids and derivatives |
| 6 | alpha-D-Galactose 1-phosphate | 225.015 | 260.030 | 3.939 | 1.529 | Organooxygen compounds |
| 7 | Apiin | 529.139 | 564.148 | 3.861 | 2.209 | |
| 8 | Berberine | 413.053 | 336.124 | 5.052 | 2.373 | Protoberberine alkaloids and derivatives |
| 9 | Caproic acid | 183.039 | 116.084 | 2.952 | 1.593 | Fatty Acyls |
| 10 | cis-9-Palmitoleic acid | 272.258 | 254.225 | 1.114 | 2.606 | Fatty Acyls |
| 11 | Cysteinylglycine | 223.018 | 178.041 | 5.694 | 1.341 | Carboxylic acids and derivatives |
| 12 | Deoxyguanosine | 267.097 | 267.097 | 4.080 | 1.818 | Purine nucleosides |
| 13 | D-Galactarate | 228.068 | 210.038 | 5.777 | 1.392 | Organooxygen compounds |
| 14 | Dihydrolipoate (dihydrolipoic acid) | 208.063 | 208.059 | 4.757 | 2.017 | Fatty Acyls |
| 15 | DL-2-Aminoadipic acid | 144.064 | 161.069 | 4.751 | 1.674 | Carboxylic acids and derivatives |
| 16 | Dopamine | 136.075 | 153.079 | 4.752 | 1.522 | Benzenediols |
| 17 | Flavone | 223.074 | 222.068 | 4.565 | 1.574 | Flavones |
| 18 | Glycerol 3-phosphate | 345.035 | 172.014 | 5.203 | 1.298 | Glycerophospholipids |
| 19 | Guanidoacetic acid | 118.061 | 117.054 | 5.565 | 1.477 | Amino acids, peptides, and alogues |
| 20 | Hypotaurine | 110.026 | 109.020 | 5.442 | 2.210 | Sulfinic acids and derivatives |
| 21 | Kynurenic acid | 190.052 | 189.043 | 4.751 | 1.890 | Quinolines and derivatives |
| 22 | L-Histidine | 156.076 | 155.069 | 4.959 | 1.465 | Amino acids, peptides, and alogues |
| 23 | L-Threonine | 84.044 | 119.058 | 4.908 | 1.298 | Carboxylic acids and derivatives |
| 24 | Malonic acid | 146.044 | 104.011 | 7.055 | 1.951 | Dicarboxylic acids and derivatives |
| 25 | Montelukast | 603.245 | 585.210 | 6.349 | 1.404 | |
| 26 | Myristic acid | 246.242 | 228.209 | 1.060 | 1.651 | Fatty acids and conjugates |
| 27 | N-Acetyl-D-glucosamine | 222.096 | 221.090 | 5.509 | 1.304 | Organooxygen compounds |
| 28 | N-Acetylputrescine | 131.117 | 130.111 | 5.396 | 1.337 | Carboximidic acids and derivatives |
| 29 | Naproxen | 272.134 | 230.094 | 6.131 | 1.394 | |
| 30 | N-carbamoyl-L-aspartate | 237.074 | 176.043 | 5.530 | 1.324 | Carboxylic acids and derivatives |
| 31 | O-Acetyl-L-serine | 148.060 | 147.053 | 5.509 | 1.272 | Amino acids, peptides, and alogues |
| 32 | Palmitic acid | 274.274 | 256.240 | 0.910 | 1.968 | Fatty acids and conjugates |
| 33 | Pentadecanoic Acid | 260.257 | 242.225 | 0.964 | 2.027 | Fatty Acyls |
| 34 | Propynoic acid | 141.017 | 70.005 | 0.721 | 1.301 | Carboxylic acids and derivatives |
| 35 | Purine | 263.074 | 120.044 | 1.970 | 1.320 | |
| 36 | Stearic acid | 302.304 | 284.272 | 0.876 | 2.451 | Fatty acids and conjugates |
| 37 | UDP-D-Galactose | 567.052 | 566.055 | 2.043 | 1.607 | Pyrimidine nucleosides |
| 38 | 1-Methyladenosine | 262.091 | 281.112 | 3.884 | 2.210 | |
| 39 | 2-Dehydro-3-deoxy-D-gluconate | 177.040 | 178.048 | 4.918 | 1.499 | Keto acids and derivatives |
| 40 | 2-Oxoadipic acid | 159.029 | 160.037 | 5.704 | 1.668 | Keto acids and derivatives |
| 41 | 3′,5′-Cyclic guanosine monophosphate | 344.038 | 345.047 | 5.033 | 1.486 | |
| 42 | 5-Hydroxymethyluracil | 158.053 | 142.038 | 5.955 | 1.275 | Diazines |
| 43 | Deoxycytidine | 226.083 | 227.091 | 3.293 | 1.449 | Pyrimidine nucleotides |
| 44 | Deoxyuridine monophosphate (dUMP) | 307.030 | 308.041 | 0.619 | 1.408 | Pyrimidine nucleosides |
| 45 | D-Neopterin | 312.093 | 253.081 | 5.424 | 1.352 | Pteridines and derivatives |
| 46 | Galactonic acid | 195.051 | 196.058 | 5.715 | 1.608 | Hydroxy acids and derivatives |
| 47 | Glycine | 74.025 | 75.032 | 4.341 | 1.320 | Amino acids, peptides, and alogues |
| 48 | Glycolate | 75.009 | 76.016 | 5.032 | 1.286 | Hydroxy acids and derivatives |
| 49 | Hippuric acid | 178.051 | 179.058 | 3.150 | 1.458 | Benzamides |
| 50 | L-Arabinono-1,4-lactone | 207.125 | 148.114 | 5.082 | 1.516 | |
| 51 | L-Ascorbic acid | 175.025 | 176.032 | 0.716 | 1.218 | Furanones |
| 52 | L-Glutamine | 145.061 | 146.069 | 4.897 | 1.324 | Carboxylic acids and derivatives |
| 53 | L-Homocysteine | 269.069 | 135.035 | 5.363 | 1.387 | Carboxylic acids and derivatives |
| 54 | Lipoamide | 221.083 | 205.060 | 2.891 | 1.531 | Dithiolanes |
| 55 | L-Serine | 104.035 | 105.043 | 4.713 | 1.396 | Amino acids, peptides, and alogues |
| 56 | L-Threonate | 135.030 | 136.037 | 5.029 | 1.349 | Organooxygen compounds |
| 57 | Menadione (Vitamin K3) | 343.102 | 172.052 | 4.616 | 1.476 | phthoquinones |
| 58 | Methylmalonic acid | 117.019 | 118.027 | 1.388 | 1.359 | Dicarboxylic acids and derivatives |
| 59 | N-Acetyl-L-phenylalanine | 206.082 | 207.090 | 2.960 | 1.672 | Carboxylic acids and derivatives |
| 60 | N-Formylmethionine | 176.038 | 177.046 | 3.267 | 1.526 | Carboxylic acids and derivatives |
| 61 | Nicotinate | 122.024 | 123.032 | 6.842 | 1.608 | Pyridinecarboxylic acids and derivatives |
| 62 | Nicotinuric acid | 180.056 | 180.053 | 3.150 | 1.455 | Carboxylic acids and derivatives |
| 63 | Pyruvate | 175.025 | 88.016 | 5.009 | 1.348 | Alpha |
| 64 | Quinolinate | 166.014 | 167.022 | 6.403 | 1.737 | Pyridines and derivatives |
| 65 | Salicyluric acid | 194.047 | 195.053 | 4.692 | 1.354 | Benzene and substituted derivatives |
| 66 | Taurine | 124.008 | 125.015 | 4.668 | 1.549 | Organosulfonic acids and derivatives |
| 67 | Theobromine | 180.066 | 180.065 | 3.501 | 1.661 | Purines and purine derivatives |
| 68 | Uric acid | 167.020 | 168.028 | 3.128 | 1.342 | Purines and purine derivatives |
| 69 | Xanthosine | 283.067 | 284.076 | 4.891 | 1.301 | Purine nucleosides |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Zhao, T.; Ren, H.; Xie, Y.; An, J.; Shang, J.; Tabys, D.; Liu, N. Urinary Metabolic Profiling via LC-MS/MS Reveals Impact of Bovine Lactoferrin on Bone Formation in Growing SD Rats. Nutrients 2020, 12, 1116. https://doi.org/10.3390/nu12041116
Xu Y, Zhao T, Ren H, Xie Y, An J, Shang J, Tabys D, Liu N. Urinary Metabolic Profiling via LC-MS/MS Reveals Impact of Bovine Lactoferrin on Bone Formation in Growing SD Rats. Nutrients. 2020; 12(4):1116. https://doi.org/10.3390/nu12041116
Chicago/Turabian StyleXu, Yan, Tianyu Zhao, Haowei Ren, Yindan Xie, Jingjing An, Jiaqi Shang, Dina Tabys, and Ning Liu. 2020. "Urinary Metabolic Profiling via LC-MS/MS Reveals Impact of Bovine Lactoferrin on Bone Formation in Growing SD Rats" Nutrients 12, no. 4: 1116. https://doi.org/10.3390/nu12041116
APA StyleXu, Y., Zhao, T., Ren, H., Xie, Y., An, J., Shang, J., Tabys, D., & Liu, N. (2020). Urinary Metabolic Profiling via LC-MS/MS Reveals Impact of Bovine Lactoferrin on Bone Formation in Growing SD Rats. Nutrients, 12(4), 1116. https://doi.org/10.3390/nu12041116