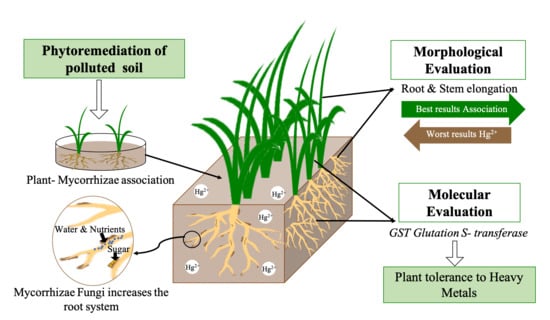
Mercury Phytoremediation with Lolium perenne-Mycorrhizae in Contaminated Soils
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Association Test
2.2.2. KOH Staining
2.2.3. Soil Contamination
2.2.4. Planting in Contaminated Soil
2.2.5. RNA Isolation
2.2.6. Real-Time PCR
2.2.7. Plant Tissue Quantification
2.2.8. Analysis of Data
3. Results
3.1. Association Test
3.2. Morphological Results
3.3. Heavy Metal Analysis
3.4. Molecular Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Slemr, F.; Brunke, E.-G.; Ebinghaus, R.; Temme, C.; Munthe, J.; Wängberg, I.; Schroeder, W.; Steffen, A.; Berg, T. Worldwide trend of atmospheric mercury since 1977. Geophys. Res. Lett. 2003, 30. [Google Scholar] [CrossRef] [Green Version]
- Biester, H.; Bindler, R.; Martinez-Cortizas, A.; Engstrom, D.R. Modeling the Past Atmospheric Deposition of Mercury Using Natural Archives. Environ. Sci. Technol. 2007, 41, 4851–4860. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Cortizas, A.; Pontevedra-Pombal, X.; García-Rodeja, E.; Nóvoa-Muñoz, J.C.; Shotyk, W. Mercury in a Spanish Peat Bog: Archive of Climate Change and Atmospheric Metal Deposition. Science 1999, 284, 939–942. [Google Scholar] [CrossRef] [PubMed]
- Skyllberg, U. Chapter 13—Mercury Biogeochemistry in Soils and Sediments. In Developments in Soil Science; Singh, B., Gräfe, M., Eds.; Synchrotron-Based Techniques in Soils and Sediments; Elsevier: Burlington, VT, USA, 2010; Volume 34, pp. 379–410. [Google Scholar]
- Håkanson, L. A simple model to predict the duration of the mercury problem in Sweden. Ecol. Model. 1996, 93, 251–262. [Google Scholar] [CrossRef]
- Hylander, L.D.; Meili, M. The Rise and Fall of Mercury: Converting a Resource to Refuse after 500 Years of Mining and Pollution. Crit. Rev. Environ. Sci. Technol. 2005, 35, 1–36. [Google Scholar] [CrossRef]
- Glick, B.R. Using soil bacteria to facilitate phytoremediation. Biotechnol. Adv. 2010, 28, 367–374. [Google Scholar] [CrossRef]
- Gupta, P.; Rani, R.; Usmani, Z.; Chandra, A.; Kumar, V. Chapter 5—The Role of Plant-Associated Bacteria in Phytoremediation of Trace Metals in Contaminated Soils. In New and Future Developments in Microbial Biotechnology and Bioengineering; Singh, J.S., Singh, D.P., Eds.; Elsevier: Burlington, VT, USA, 2019; pp. 69–76. ISBN 978-0-444-64191-5. [Google Scholar]
- Girma, G. Microbial Bioremediation of some Heavy Metals in Soils: An updated review. J. Resour. Dev. Manag. 2015, 10, 62–73. [Google Scholar] [CrossRef]
- Boudh, S.; Tiwari, S.; Singh, J.S. Microbial-Mediated Lindane Bioremediation. In Agro-Environmental Sustainability: Volume 2: Managing Environmental Pollution; Singh, J.S., Seneviratne, G., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 213–233. ISBN 978-3-319-49727-3. [Google Scholar]
- Vargas Aguirre, C.F.; Rivera Páez, F.A.; Escobar Vargas, S. Effect of arbuscular mycorrhizae and mercury on Lactuca sativa (Asteraceae) seedling morpho—Histology. Environ. Exp. Bot. 2018, 156, 197–202. [Google Scholar] [CrossRef]
- Begon, M.; Harper, J.L.; Townsend, C.R. Ecology. Individuals, Populations and Communities; Blackwell Scientific Publications: Oxford, UK, 1986; ISBN 0-632-01337-0. [Google Scholar]
- Schulz, S.; Brankatschk, R.; Dümig, A.; Kögel-Knabner, I.; Schloter, M.; Zeyer, J. The role of microorganisms at different stages of ecosystem development for soil formation. Biogeosciences 2013, 10, 3983–3996. [Google Scholar] [CrossRef] [Green Version]
- Nicolson, T.H. Vesicular arbuscular mycorrhiza a universal plant symbiosis. Sci. Prog. 1967, 55, 561–581. [Google Scholar]
- Marks, G.C.; Kozlowski, T.T. Ectomycorrhizae, Their Ecology and Physiology; Academic Press: New York, NY, USA, 1973; ISBN 0-12-472850-2. [Google Scholar]
- Smith, S.; Read, D. Mycorrhizal Symbiosis, 3rd ed.; Academic Press: London, UK, 2008; ISBN 978-0-08-055934-6. [Google Scholar]
- Luginbuehl, L.H.; Menard, G.N.; Kurup, S.; Erp, H.V.; Radhakrishnan, G.V.; Breakspear, A.; Oldroyd, G.E.D.; Eastmond, P.J. Fatty acids in arbuscular mycorrhizal fungi are synthesized by the host plant. Science 2017, 356, 1175–1178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gianiazzi, S.; Schüepp, H. (Eds.) Impact of Arbuscular Mycorrhizas on Sustainable Agriculture and Natural Ecosystems; Advances in Life Sciences; Birkhäuser Basel: Basel, Switzerland, 1994; ISBN 978-3-0348-9654-2. [Google Scholar]
- Leung, H.-M.; Wang, Z.-W.; Ye, Z.-H.; Yung, K.-L.; Peng, X.-L.; Cheung, K.-C. Interactions between Arbuscular Mycorrhizae and Plants in Phytoremediation of Metal-Contaminated Soils: A Review. Pedosphere 2013, 23, 549–563. [Google Scholar] [CrossRef]
- Coninx, L.; Martinova, V.; Rineau, F. Chapter Four—Mycorrhiza-Assisted Phytoremediation. In Advances in Botanical Research; Cuypers, A., Vangronsveld, J., Eds.; Phytoremediation; Academic Press: London, UK, 2017; Volume 83, pp. 127–188. [Google Scholar]
- Sylvia, D.M.; Fuhrmann, J.J.; Hartel, P.G.; Zuberer, D.A. Principles and Applications of Soil Microbiology, 2nd ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2004; ISBN 978-0-13-094117-6. [Google Scholar]
- Cabral, L.; Soares, C.R.F.S.; Giachini, A.J.; Siqueira, J.O. Arbuscular mycorrhizal fungi in phytoremediation of contaminated areas by trace elements: Mechanisms and major benefits of their applications. World J. Microbiol. Biotechnol. 2015, 31, 1655–1664. [Google Scholar] [CrossRef] [PubMed]
- Barea, J.-M.; Pozo, M.J.; Azcón, R.; Azcón-Aguilar, C. Microbial co-operation in the rhizosphere. J. Exp. Bot. 2005, 56, 1761–1778. [Google Scholar] [CrossRef] [Green Version]
- Gosling, P.; Hodge, A.; Goodlass, G.; Bending, G.D. Arbuscular mycorrhizal fungi and organic farming. Agric. Ecosyst. Environ. 2006, 113, 17–35. [Google Scholar] [CrossRef]
- Li, H.; Hu, T.; Fu, J. Identification of genes associated with adaptation to NaCl toxicity in perennial ryegrass (Lolium perenne L.). Ecotoxicol. Environ. Saf. 2012, 79, 153–162. [Google Scholar] [CrossRef]
- Hasegawa, P.M.; Bressan, R.A.; Zhu, J.-K.; Bohnert, H.J. Plant Cellular and Molecular Responses to High Salinity. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2000, 51, 463–499. [Google Scholar] [CrossRef] [Green Version]
- Cummins, I.; Dixon, D.P.; Freitag-Pohl, S.; Skipsey, M.; Edwards, R. Multiple roles for plant glutathione transferases in xenobiotic detoxification. Drug Metab. Rev. 2011, 43, 266–280. [Google Scholar] [CrossRef]
- Kumar, S.; Trivedi, P.K. Glutathione S-Transferases: Role in Combating Abiotic Stresses Including Arsenic Detoxification in Plants. Front. Plant Sci. 2018, 9. [Google Scholar] [CrossRef] [Green Version]
- Frova, C. Glutathione transferases in the genomics era: New insights and perspectives. Biomol. Eng. 2006, 23, 149–169. [Google Scholar] [CrossRef]
- Gutiérrez Ginés, M.J.; Hernández, A.J.; Pastor Piñeiro, J. Estudio del comportamiento de Lolium perenne L. en suelos del Centro de España contaminados por metales pesados. In Control de la Degradación y uso Sostenible del Suelo; Universidad de Valencia: Valencia, Spain, 2011; ISBN 978-84-615-1679-7. [Google Scholar]
- Coleman, J.; Blake-Kalff, M.; Davies, E. Detoxification of xenobiotics by plants: Chemical modification and vacuolar compartmentation. Trends Plant Sci. 1997, 2, 144–151. [Google Scholar] [CrossRef] [Green Version]
- Kubik, C.; Sawkins, M.; Meyer, W.A.; Gaut, B.S. Genetic Diversity in Seven Perennial Ryegrass (Lolium perenne L.) Cultivars Based on SSR Markers. Crop Sci. 2001, 41, 1565–1572. [Google Scholar] [CrossRef]
- Xing, Y.; Frei, U.; Schejbel, B.; Asp, T.; Lübberstedt, T. Nucleotide diversity and linkage disequilibrium in 11 expressed resistance candidate genes in Lolium perenne. BMC Plant Biol. 2007, 7, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Attar, A.F.; Nickless, G. Response surface methodology for an investigation of the influence of selenium, cadmium and mercury on the growth of Lolium perenne seedings. Chemosphere 1988, 17, 1851–1861. [Google Scholar] [CrossRef]
- Wong, M.H.; Bradshaw, A.D. A Comparison of the Toxicity of Heavy Metals, Using Root Elongation of Rye Grass, Lolium perenne. New Phytol. 1982, 91, 255–261. [Google Scholar] [CrossRef]
- Reis, A.T.; Rodrigues, S.M.; Araújo, C.; Coelho, J.P.; Pereira, E.; Duarte, A.C. Mercury contamination in the vicinity of a chlor-alkali plant and potential risks to local population. Sci. Total Environ. 2009, 407, 2689–2700. [Google Scholar] [CrossRef]
- Jensen, L.B.; Muylle, H.; Arens, P.; Andersen, C.H.; Holm, P.B.; Ghesquiere, M.; Julier, B.; Lübberstedt, T.; Nielsen, K.K.; Riek, J.D.; et al. Development and mapping of a public reference set of SSR markers in Lolium perenne L. Mol. Ecol. Notes 2005, 5, 951–957. [Google Scholar] [CrossRef]
- King, J.; Thorogood, D.; Edwards, K.J.; Armstead, I.P.; Roberts, L.; Skøt, K.; Hanley, Z.; King, I.P. Development of a Genomic Microsatellite Library in Perennial Ryegrass (Lolium perenne) and its Use in Trait Mapping. Ann. Bot. 2008, 101, 845–853. [Google Scholar] [CrossRef]
- Jia, H.; Hou, D.; O’Connor, D.; Pan, S.; Zhu, J.; Bolan, N.S.; Mulder, J. Exogenous phosphorus treatment facilitates chelation-mediated cadmium detoxification in perennial ryegrass (Lolium perenne L.). J. Hazard. Mater. 2020, 389, 121849. [Google Scholar] [CrossRef]
- Ju, X.H.; Tang, S.; Jia, Y.; Guo, J.; Ding, Y.; Song, Z.; Zhao, Y. Determination and characterization of cysteine, glutathione and phytochelatins (PC2–6) in Lolium perenne L. exposed to Cd stress under ambient and elevated carbon dioxide using HPLC with fluorescence detection. J. Chromatogr. B 2011, 879, 1717–1724. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Habibul, N.; Hu, Y.-Y.; Meng, F.-L.; Zhang, X.; Sheng, G.-P. Mixture toxicity and uptake of 1-butyl-3-methylimidazolium bromide and cadmium co-contaminants in water by perennial ryegrass (Lolium perenne L.). J. Hazard. Mater. 2020, 386, 121972. [Google Scholar] [CrossRef] [PubMed]
- Cepero de García, M.C.; Restrepo, S.; Franco-Molano, A.E.; Cárdenas, M.; Vargas, N.V. Biología de Hongos, 1st ed.; Universidad de los Andes: Bogotá, Colombia, 2012; ISBN 978-958-695-701-4. [Google Scholar]
- Haidouti, C. Inactivation of mercury in contaminated soils using natural zeolites. Sci. Total Environ. 1997, 208, 105–109. [Google Scholar] [CrossRef]
- Pitombeira de Figueirêdo, L.; Daam, M.A.; Mainardi, G.; Mariën, J.; Espíndola, E.L.G.; van Gestel, C.A.M.; Roelofs, D. The use of gene expression to unravel the single and mixture toxicity of abamectin and difenoconazole on survival and reproduction of the springtail Folsomia candida. Environ. Pollut. 2019, 244, 342–350. [Google Scholar] [CrossRef] [PubMed]
- United States Environmental Protection Agency. EPA Method 3051A: Microwave Assisted Acid Digestion of Sediments, Sludges, and Oils. Available online: https://www.epa.gov/esam/us-epa-method-3051a-microwave-assisted-acid-digestion-sediments-sludges-and-oils (accessed on 24 March 2020).
- Bellion, M.; Courbot, M.; Jacob, C.; Blaudez, D.; Chalot, M. Extracellular and cellular mechanisms sustaining metal tolerance in ectomycorrhizal fungi. FEMS Microbiol. Lett. 2006, 254, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Daghino, S.; Martino, E.; Perotto, S. Model systems to unravel the molecular mechanisms of heavy metal tolerance in the ericoid mycorrhizal symbiosis. Mycorrhiza 2016, 26, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Tarraf, W.; Ruta, C.; Tagarelli, A.; De Cillis, F.; De Mastro, G. Influence of arbuscular mycorrhizae on plant growth, essential oil production and phosphorus uptake of Salvia officinalis L. Ind. Crops Prod. 2017, 102, 144–153. [Google Scholar] [CrossRef]
- Azcón, R.; Ambrosano, E.; Charest, C. Nutrient acquisition in mycorrhizal lettuce plants under different phosphorus and nitrogen concentration. Plant Sci. 2003, 165, 1137–1145. [Google Scholar] [CrossRef]
- Schnepf, A.; Jones, D.; Roose, T. Modelling Nutrient Uptake by Individual Hyphae of Arbuscular Mycorrhizal Fungi: Temporal and Spatial Scales for an Experimental Design. Bull. Math. Biol. 2011, 73, 2175–2200. [Google Scholar] [CrossRef] [Green Version]
- Steliga, T.; Kluk, D. Application of Festuca arundinacea in phytoremediation of soils contaminated with Pb, Ni, Cd and petroleum hydrocarbons. Ecotoxicol. Environ. Saf. 2020, 194, 110409. [Google Scholar] [CrossRef]
- Morgan, J.A.W.; Bending, G.D.; White, P.J. Biological costs and benefits to plant–microbe interactions in the rhizosphere. J. Exp. Bot. 2005, 56, 1729–1739. [Google Scholar] [CrossRef] [Green Version]
- Shri, M.; Kumar, S.; Chakrabarty, D.; Trivedi, P.K.; Mallick, S.; Misra, P.; Shukla, D.; Mishra, S.; Srivastava, S.; Tripathi, R.D.; et al. Effect of arsenic on growth, oxidative stress, and antioxidant system in rice seedlings. Ecotoxicol. Environ. Saf. 2009, 72, 1102–1110. [Google Scholar] [CrossRef] [PubMed]
- Martinoia, E.; Grill, E.; Tommasini, R.; Kreuz, K.; Amrhein, N. ATP-dependent glutathione S -conjugate “export” pump in the vacuolar membrane of plants. Nature 1993, 364, 247–249. [Google Scholar] [CrossRef]
- Xi, Y.; Jiao, W.; Cao, J.; Jiang, W. Effects of chlorogenic acid on capacity of free radicals scavenging and proteomic changes in postharvest fruit of nectarine. PLoS ONE 2017, 12, e0182494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, H.M.; Neighbors, S.M. Soybean metabolism of chlorimuron ethyl: Physiological basis for soybean selectivity. Pestic. Biochem. Physiol. 1987, 29, 112–120. [Google Scholar] [CrossRef]
- Dixon, D.P.; Davis, B.G.; Edwards, R. Functional Divergence in the Glutathione Transferase Superfamily in Plants identification of two classes with putative functions in redox homeostasis in arabidopsis thaliana. J. Biol. Chem. 2002, 277, 30859–30869. [Google Scholar] [CrossRef] [Green Version]
- Gullner, G.; Uotila, M.; Kömives, T. Responses of Glutathione and Glutathione S-Transferase to Cadmium and Mercury Exposure in Pedunculate Oak (Quercus robur) Leaf Discs. Bot. Acta 1998, 111, 62–65. [Google Scholar] [CrossRef]



| Treatment | Amount of Soil (g) | Amount of Mycorrhiza (g) |
|---|---|---|
| B (control) | 1000 * | - |
| BM (control with mycorrhizae) | 667 | 333 |
| H (mercury) | 1000 | - |
| HM (mercury with mycorrhizae) | 667 | 333 |
| Parameter | Result |
|---|---|
| Aluminum (mg/kg wb) | 43,351 |
| Barium (mg/kg wb) | 240 |
| Calcium (mg/kg wb) | 7490 |
| Zinc (mg/kg wb) | 61.5 |
| Iron (mg/kg wb) | 19,573 |
| Magnesium (mg/kg wb) | 1795 |
| Manganese (mg/kg wb) | 778 |
| Potassium (mg/kg wb) | 1457 |
| Sodium (mg/kg wb) | 322 |
| pH (mg/kg wb) | 6.07 |
| Electrical conductivity (μS/cm) | 4.3 |
| Field capacity (%) | 85 |
| Organic total carbon (TOC) (%) | 24.5 |
| Moisture content (%) | 63 |
| Particle size (μm) | 150–300 |
| Nitrogen (% db) | 0.054 |
| Bulk density (g/cm3) | 1.26 |
| Name | Direction | Sequence | Size (pb) | Temperature Annealing (°C) |
|---|---|---|---|---|
| GST | (5’-3’) | CTACAGAGCCACGCCGTCATCG | 193 | 62 |
| (3’-5’) | CAGCGTGGATCTGGGGTGCT | |||
| TBP-1 | (5’-3’) | GCAGATATTCTTGATCCCGCTTT | 69 | 60 |
| (3’-5’) | CGGATGAGGGAACTCAATCTTT |
| Treatment | Matrix | Concentration Initial | Concentration Final | Units |
|---|---|---|---|---|
| B | S | <0.10 | <0.10 | mg/kg |
| B | T | <0.10 | mg/kg | |
| B | R | <0.10 | mg/kg | |
| BM | S | <0.10 | <0.10 | mg/kg |
| BM | T | <0.10 | mg/kg | |
| BM | R | <0.10 | mg/kg | |
| H | S | 1.19 | 0.29 | mg/kg |
| H | T | 0.75 | mg/kg | |
| H | R | 0.12 | mg/kg | |
| HM | S | 1.20 | 0.41 | mg/kg |
| HM | T | 0.28 | mg/kg | |
| HM | R | 0.49 | mg/kg |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leudo, A.M.; Cruz, Y.; Montoya-Ruiz, C.; Delgado, M.d.P.; Saldarriaga, J.F. Mercury Phytoremediation with Lolium perenne-Mycorrhizae in Contaminated Soils. Sustainability 2020, 12, 3795. https://doi.org/10.3390/su12093795
Leudo AM, Cruz Y, Montoya-Ruiz C, Delgado MdP, Saldarriaga JF. Mercury Phytoremediation with Lolium perenne-Mycorrhizae in Contaminated Soils. Sustainability. 2020; 12(9):3795. https://doi.org/10.3390/su12093795
Chicago/Turabian StyleLeudo, Ana M., Yuby Cruz, Carolina Montoya-Ruiz, María del Pilar Delgado, and Juan F. Saldarriaga. 2020. "Mercury Phytoremediation with Lolium perenne-Mycorrhizae in Contaminated Soils" Sustainability 12, no. 9: 3795. https://doi.org/10.3390/su12093795
APA StyleLeudo, A. M., Cruz, Y., Montoya-Ruiz, C., Delgado, M. d. P., & Saldarriaga, J. F. (2020). Mercury Phytoremediation with Lolium perenne-Mycorrhizae in Contaminated Soils. Sustainability, 12(9), 3795. https://doi.org/10.3390/su12093795