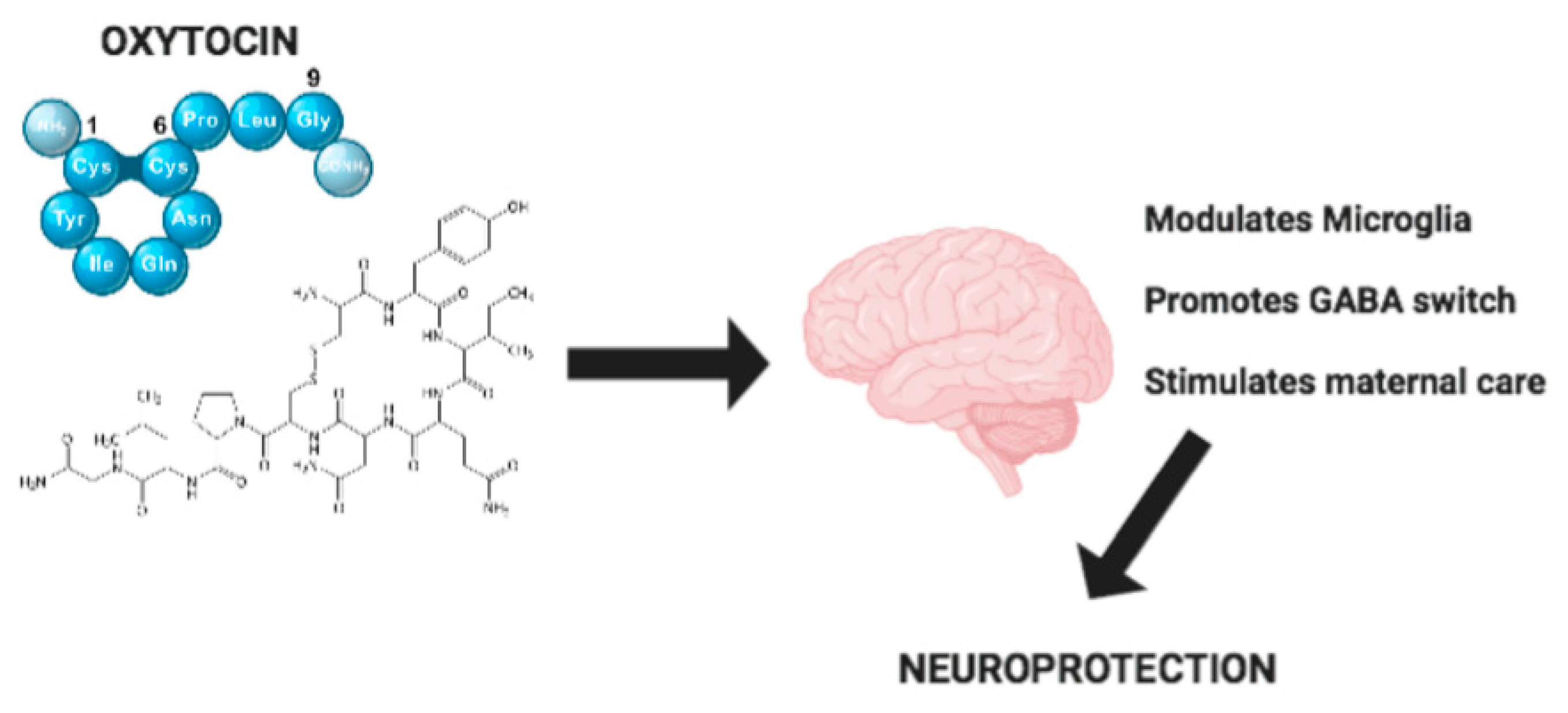
Hypothalamic Neuropeptide Brain Protection: Focus on Oxytocin
Abstract
1. Introduction
2. Oxytocin-System: Oxytocin Synthesis, Release and its Receptor
3. The Role of Microglia in Neuroinflammation
4. Effect of Oxytocin on Microglia
5. Effect of Oxytocin in Brain
6. Strategies to Enhance OTX Delivery
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Oliveira-Pelegrin, G.R.; Saia, R.S.; Cárnio, E.C.; Rocha, M.J. Oxytocin affects nitric oxide and cytokine production by sepsis-sensitized macrophages. Neuroimmunomodulation 2013, 20, 5–71. [Google Scholar] [CrossRef]
- Tarawneh, R.; Galvin, J.E. Potential future neuroprotective therapies for neurodegenerative disorders and stroke. Clin. Geriatr. Med. 2010, 26, 125–147. [Google Scholar] [CrossRef] [PubMed]
- van Tilborg, E.; de Theije, C.G.M.; van Hal, M.; Wagenaar, N.; de Vries, L.S.; Benders, M.J.; Rowitch, D.H.; Nijboer, C.H. Origin and dynamics of oligodendrocytes in the developing brain: Implications for perinatal white matter injury. Glia 2018, 66, 221–238. [Google Scholar] [CrossRef] [PubMed]
- Van Bulck, M.; Sierra-Magro, A.; Alarcon-Gil, J.; Perez-Castillo, A.; Morales-Garcia, J.A. Novel Approaches for the Treatment of Alzheimer’s and Parkinson’s Disease. Int. J. Mol. Sci. 2019, 20, 719. [Google Scholar] [CrossRef]
- Szeto, J.Y.Y.; Lewis, S.J.G. Current Treatment Options for Alzheimer’s Disease and Parkinson’s Disease Dementia. Curr. Neuropharmacol. 2016, 14, 326–338. [Google Scholar] [CrossRef] [PubMed]
- Olff, M.; Frijling, J.L.; Kubzansky, L.D.; Bradley, B.; Ellenbogen, M.A.; Cardoso, C.; Bartz, J.A.; Yee, J.R.; van Zuiden, M. The role of oxytocin in social bonding, stress regulation and mental health: An update on the moderating effects of context and interindividual differences. Psychoneuroendocrinology 2013, 38, 1883–1894. [Google Scholar] [CrossRef] [PubMed]
- Petersson, M.; Wiberg, U.; Lundeberg, T.; Uvnas-Moberg, K. Oxytocin decreases carrageenan induced inflammation in rats. Peptides 2001, 22, 1479–1484. [Google Scholar] [CrossRef]
- Iseri, S.O.; Sener, G.; Saglam, B.; Gedik, N.; Ercan, F.; Yegen, B.C. Oxytocin ameliorates oxidative colonic inflammation by a neutrophil-dependent mechanism. Peptides 2005, 26, 483–491. [Google Scholar] [CrossRef]
- Guillou, M.D.; Camier, M.; Clamagirand, C. Evidence for the presence of pro oxytocin/neurophysin-converting enzyme in the human ovary. J. Endocrinol. 1994, 142, 345–352. [Google Scholar] [CrossRef]
- Brownstein, M.J.; Russell, J.T.; Gainer, H. Synthesis, transport, and release of posterior pituitary hormones. Science 1980, 207, 373–378. [Google Scholar] [CrossRef]
- Chatterjee, O.; Patil, K.; Sahu, A.; Gopalakrishnan, L.; Mol, P.; Advani, J.; Mukherjee, S.; Christopher, R.; Prasad, T.S. An overview of the oxytocin-oxytocin receptor signaling network. J. Cell Commun. Signal 2016, 10, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Gimpl, G.; Fahrenholz, F. The oxytocin receptor system: Structure, function, and regulation. Physiol. Rev. 2001, 81, 629–683. [Google Scholar] [CrossRef] [PubMed]
- Arrowsmith, S.; Wray, S. Oxytocin: Its mechanism of action and receptor signalling in the myometrium. J. Neuroendocrinol. 2014, 26, 356–369. [Google Scholar] [CrossRef] [PubMed]
- Chini, B.; Mouillac, B.; Ala, Y.; Balestre, M.N.; Trumpp-Kallmeyer, S.; Hoflack, J.; Elands, J.; Hibert, M.; Manning, M.; Jard, S. Tyr115 is the key residue for determining agonist selectivity in the V1a vasopressin receptor. EMBO J. 1995, 14, 2176–2182. [Google Scholar] [CrossRef] [PubMed]
- Fanelli, F.; Barbier, P.; Zanchetta, D.; de Benedetti, P.G.; Chini, B. Activation mechanism of human oxytocin receptor: A combined study of experimental and computer-simulated mutagenesis. Mol. Pharmacol. 1999, 56, 214–225. [Google Scholar] [CrossRef] [PubMed]
- Favre, N.; Fanelli, F.; Missotten, M.; Nichols, A.; Wilson, J.; di Tiani, M.; Rommel, C.; Scheer, A. The DRY motif as a molecular switch of the human oxytocin receptor. Biochemistry 2005, 44, 9990–10008. [Google Scholar] [CrossRef]
- Frantz, M.C.; Rodrigo, J.; Boudier, L.; Durroux, T.; Mouillac, B.; Hibert, M. Subtlety of the structure-affinity and structure efficacy relationships around a nonpeptide oxytocin receptor agonist. J. Med. Chem. 2010, 53, 1546–1562. [Google Scholar] [CrossRef] [PubMed]
- Busnelli, M.; Kleinau, G.; Muttenthaler, M.; Stoev, S.; Manning, M.; Bibic, L.; Howell, L.A.; McCormick, P.J.; Di Lascio, S.; Braida, D.; et al. Design and characterization of Superpotent bivalent ligands targeting oxytocin receptor dimers via a channel-like structure. J. Med. Chem. 2016, 59, 7152–7166. [Google Scholar] [CrossRef]
- Young, L.J.; Muns, S.; Wang, Z.; Insel, T.R. Changes in oxytocin receptor mRNA in rat brain during pregnancy and the effects of estrogen and interleukin-6. J. Neuroendocrinol. 1997, 9, 859–865. [Google Scholar] [CrossRef]
- Dogra, Y.; Suri, V.; Aggarwal, N.; Dogra, R.K. Induction of labor with oxytocin in pregnancy with low-risk heart disease: A randomized controlled trial. Turk. J. Obstet. Gynecol. 2019, 16, 213–218. [Google Scholar] [CrossRef]
- Adan, R.A.; Van Leeuwen, F.W.; Sonnemans, M.A.; Brouns, M.; Hoffman, G.; Verbalis, J.G.; Burbach, J.P. Rat oxytocin receptor in brain, pituitary, mammary gland, and uterus: Partial sequence and immunocytochemical localization. Endocrinology 1995, 136, 4022–4028. [Google Scholar] [CrossRef] [PubMed]
- Insel, T.R.; Shapiro, L.E. Oxytocin receptor distribution reflects social organization in monogamous and polygamous voles. Proc. Natl. Acad. Sci. USA 1992, 89, 5981–5985. [Google Scholar] [CrossRef] [PubMed]
- Krzysztof, M.; Wilczyński, A.S.; Małgorzata, J.K. Systematic Review of Literature on Single-Nucleotide Polymorphisms Within the Oxytocin and Vasopressin Receptor Genes in the Development of Social Cognition Dysfunctions in Individuals Suffering from Autism Spectrum Disorder. Front. Psychiatry 2019, 10, 380. [Google Scholar]
- Jurek, B.; Neumann, I.D. The Oxytocin Receptor: From Intracellular Signaling to Behavior. Physiol. Rev. 2018, 98, 1805–1908. [Google Scholar] [CrossRef] [PubMed]
- Freeman, S.M.; Smith, A.L.; Goodman, M.M.; Bales, K.L. Selective localization of oxytocin receptors and vasopressin 1a receptors in the human brainstem. Soc. Neurosci. 2017, 12, 113–123. [Google Scholar] [CrossRef]
- Mizumoto, Y.; Kimura, T.; Ivell, R.A. genomic element within the third intron of the human oxytocin receptor gene may be implicated in transcription suppression. Mol. Cell Endocrinol. 1997, 135, 129–138. [Google Scholar] [CrossRef]
- Quintana, D.S.; Rokicki, J.; van der Meer, D.; Alnæs, D.; Kaufmann, T.; Córdova-Palomera, A.; Dieset, I.; Andreassen, O.A.; Westlye, L.T. Oxytocin pathway gene networks in the human brain. Nat. Commun. 2019, 10, 668. [Google Scholar] [CrossRef]
- Miller, T.V.; Caldwell, H.K. Oxytocin during development: Possible organizational effects on behavior. Front. Endocrinol. 2015, 6, 76. [Google Scholar] [CrossRef]
- Johnson, Z.V.; Young, L.J. Oxytocin and vasopressin neural networks: Implications for social behavioral diversity and translational neuroscience. Neurosci. Biobehav. Rev. 2017, 76, 87–98. [Google Scholar] [CrossRef]
- Tracy, L.M.; Georgiou-Karistianis, N.; Gibson, S.J.; Giummarra, M.J. Oxytocin and the modulation of pain experience: Implications for chronic pain management. Neurosci. Biobehav. Rev. 2015, 55, 53–67. [Google Scholar] [CrossRef]
- Leslie, M.; Silva, P.; Paloyelis, Y.; Blevins, J.; Treasure, J. A systematic review and quantitative meta-analysis of the effects of oxytocin on feeding. J. Neuroendocrinol. 2018, 30. [Google Scholar] [CrossRef] [PubMed]
- Napso, T.; Yong, H.E.J.; Lopez-Tello, J.; Sferruzzi-Perri, A.N. The Role of Placental Hormones in Mediating Maternal Adaptations to Support Pregnancy and Lactation. Front. Physiol. 2018, 17, e1091. [Google Scholar] [CrossRef] [PubMed]
- Page, K.; McCool, W.F.; Guidera, M. Examination of the pharmacology of oxytocin and clinical guidelines for use in labor. J. Midwifery Womens Health 2017, 62, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Churchland, P.S.; Winkielman, P. Modulating social behavior with oxytocin: How does it work? What does it mean? Horm. Behav. 2012, 61, 392–399. [Google Scholar] [CrossRef]
- Zik, J.B.; Roberts, D.L. The many faces of oxytocin: Implications for psychiatry. Psychiatry Res. 2015, 226, 31–37. [Google Scholar] [CrossRef]
- Bordt, E.A.; Smith, C.J.; Demarest, T.G.; Bilbo, S.D.; Kingsbury, M.A. Mitochondria, Oxytocin, and Vasopressin: Unfolding the Inflammatory Protein Response. Neurotox. Res. 2018, 36, 239–256. [Google Scholar] [CrossRef]
- Walum, H.; Young, L.J. The neural mechanisms and circuitry of the pair bond. Nat. Rev. Neurosci. 2018, 19, 643–654. [Google Scholar] [CrossRef]
- Sapolsky, R.M. Doubled-Edged Swords in the Biology of Conflict. Front. Psychol. 2018, 9, 2625. [Google Scholar] [CrossRef]
- Ellis, J.A.; Brown, C.M.; Barger, B.; Carlson, N.S. Influence of Maternal Obesity on Labor Induction: A Systematic Review and Meta-Analysis. J. Midwifery Womens Health 2019, 64, 55–67. [Google Scholar] [CrossRef]
- Viteri, O.A.; Sibai, B.M. Challenges and Limitations of Clinical Trials on Labor Induction: A Review of the Literature. AJP Rep. 2018, 8, 4. [Google Scholar]
- Menassa, D.A.; Gomez-Nicola, D. Microglial Dynamics During Human Brain Development. Front. Immunol. 2018, 24, 1014. [Google Scholar] [CrossRef] [PubMed]
- Bondy, S.C. Aspects of the immune system that impact brain function. J. Neuroimmunol. 2020, 340, 577167. [Google Scholar] [CrossRef] [PubMed]
- Alliot, F.; Godin, I.; Pessac, B. Microglia derive from progenitors, originating from the yolk sac, and which proliferate in the brain. Dev. Brain Res. 1999, 117, 145–152. [Google Scholar] [CrossRef]
- Kierdorf, K.; Erny, D.; Goldmann, T.; Sander, V.; Schulz, C.; Perdiguero, E.G.; Wieghofer, P.; Heinrich, A.; Riemke, P.; Hölscher, C.; et al. Microglia emerge from erythromyeloid precursors via Pu.1-and Irf8-dependent pathways. Nat. Neurosci. 2013, 16, 273–280. [Google Scholar] [CrossRef]
- Ginhoux, F.; Lim, S.; Hoeffel, G.; Low, D.; Huber, T. Origin and differentiation of microglia. Front. Cell. Neurosci. 2013, 7, 45. [Google Scholar] [CrossRef]
- Wolf, S.A.; Boddeke, H.; Kettenmann, H. Microglia in Physiology and Disease. Annu. Rev. Physiol. 2017, 79, 619–643. [Google Scholar] [CrossRef]
- García-Revilla, J.; Alonso-Bellido, I.M.; Burguillos, M.A.; Herrera, A.J.; Espinosa-Oliva, A.M.; Ruiz, R.; Cruz-Hernández, L.; García-Domínguez, I.; Roca-Ceballos, M.A.; Santiago, M.; et al. Reformulating Pro-Oxidant Microglia in Neurodegeneration. J. Clin. Med. 2019, 8, 1719. [Google Scholar] [CrossRef]
- Akhmetzyanova, E.; Kletenkov, K.; Mukhamedshina, Y.; Rizvanov, A. Different Approaches to Modulation of Microglia Phenotypes After Spinal Cord Injury. Front. Syst. Neurosci. 2019, 27, 37. [Google Scholar] [CrossRef]
- Block, M.L.; Zecca, L.; Hong, J.S. Microglia-mediated neurotoxicity: Uncovering the molecular mechanisms. Nat. Rev. Neurosci. 2007, 8, 57–69. [Google Scholar] [CrossRef]
- Cherry, J.D.; Olschowka, J.A.; O’Banion, M.K. Neuroinflammation and M2 microglia: The good, the bad, and the inflamed. J. Neuroinflamm. 2014, 11, 98. [Google Scholar] [CrossRef]
- Martinez, F.O.; Gordon, S. The M1 and M2 paradigm of macrophage activation: Time for reassessment. F1000prime Rep. 2014, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Franco, R.; Fernandez-Suarez, D. Alternatively activated microglia and macrophages in the central nervous system. Prog. Neurobiol. 2015, 131, 65–86. [Google Scholar] [CrossRef] [PubMed]
- Peferoen, L.A.; Vogel, D.Y.; Ummenthum, K.; Breur, M.; Heijnen, P.D.; Gerritsen, W.H. Activation status of human microglia is dependent on lesion formation stage and remyelination in multiple sclerosis. J. Neuropathol. Exp. Neurol. 2015, 74, 48–63. [Google Scholar] [CrossRef] [PubMed]
- Peress, N.S.; Fleit, H.B.; Perillo, E.; Kuljis, R.; Pezzullo, C. Identification of FcgRI, II and III on normal human brain ramified microglia and on microglia in senile plaques in Alzheimer’s disease. J. Neuroimmunol. 1993, 48, 71–79. [Google Scholar] [CrossRef]
- Wilcock, D.M. Neuroinflammatory phenotypes and their roles in Alzheimer’s disease. Neurodegener. Dis. 2014, 13, 183–185. [Google Scholar] [CrossRef]
- Wu, S.Y.; Watabe, K. The roles of microglia/macrophages in tumor progression of brain cancer and metastatic disease. Front. Biosci. 2017, 22, 1805. [Google Scholar] [CrossRef]
- Scott, A.; Khan, K.; Cook, J.; Duronio, V. What is “inflammation”? Are we ready to move beyond Celsus? Br. J. Sports Med. 2004, 38, 248–249. [Google Scholar] [CrossRef]
- Fullerton, J.N.; Gilroy, D.W. Resolution of inflammation: A new therapeutic frontier. Nat. Rev. Drug Discov. 2016, 15, 551–567. [Google Scholar] [CrossRef]
- Nathan, C.; Ding, A. Nonresolving inflammation. Cell 2010, 140, 871–882. [Google Scholar] [CrossRef]
- Tansey, M.G.; McCoy, M.K.; Frank-Cannon, T.C. Neuroinflammatory mechanisms in Parkinson’s disease: Potential environmental triggers, pathways, and targets for early therapeutic intervention. Exp. Neurol. 2007, 208, 1–25. [Google Scholar] [CrossRef]
- Block, M.L.; Hong, J.S. Microglia and inflammation-mediated neurodegeneration: Multiple triggers with a common mechanism. Prog. Neurobiol. 2005, 76, 77–98. [Google Scholar] [CrossRef]
- Liu, W.; Tang, Y.; Feng, J. Cross talk between activation of microglia and astrocytes in pathological conditions in the central nervous system. Life Sci. 2011, 89, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Millington, C.; Sonego, S.; Karunaweera, N.; Rangel, A.; Aldrich-Wright, J.R.; Campbell, I.L.; Gyengesi, E.; Münch, G. Chronic neuroinflammation in Alzheimer’s disease: New perspectives on animal models and promising candidate drugs. Biomed Res. Int. 2014. [Google Scholar] [CrossRef] [PubMed]
- Panaro, M.A.; Lofrumento, D.D.; Saponaro, C.; De Nuccio, F.; Cianciulli, A.; Mitolo, V.; Nicolardi, G. Expression of TLR4 and CD14 in the central nervous system (CNS) in a MPTP mouse model of Parkinson’s-like disease. Immunopharmacol. Immunotoxicol. 2008, 30, 729–740. [Google Scholar] [CrossRef] [PubMed]
- Kempuraj, D.T.; Thangavel, R.; Natteru, P.A.; Selvakumar, G.P.; Saeed, D.; Zahoor, H.; Zaheer, S.; Iyer, S.S.; Zaheer, A. Neuroinflammation Induces Neurodegeneration. J. Neurol. Neurosurg. Spine 2016, 1, 1. [Google Scholar]
- Perry, V.H.; Teeling, J. Microglia and macrophages of the central nervous system: The contribution of microglia priming and systemic inflammation to chronic neurodegeneration. Semin. Immunopathol. 2013, 35, 601–612. [Google Scholar] [CrossRef]
- Sofroniew, M.V.; Vinters, H.V. Astrocytes: Biology and pathology. Acta Neuropathol. 2010, 119, 7–35. [Google Scholar] [CrossRef]
- Lofrumento, D.D.; Saponaro, C.; Cianciulli, A.; De Nuccio, F.; Mitolo, V.; Nicolardi, G.; Panaro, M.A. MPTP-induced neuroinflammation increases the expression of pro-inflammatory cytokines and their receptors in mouse brain. Neuroimmunomodulation 2011, 18, 79–88. [Google Scholar] [CrossRef]
- Amor, S.; Puentes, F.; Baker, D.; van der Valk, P. Inflammation in neurodegenerative diseases. Immunology 2010, 129, 154–169. [Google Scholar] [CrossRef]
- Sapp, E.; Kegel, K.B.; Aronin, N.; Hashikawa, T.; Uchiyama, Y.; Tohyama, K.; Bhide, P.G.; Vonsattel, J.P.; DiFiglia, M. Early and progressive accumulation of reactive microglia in the Huntington disease brain. J. Neuropathol. Exp. Neurol. 2001, 60, 161–172. [Google Scholar] [CrossRef]
- McGeer, P.; Itagaki, S.; Boyes, B.; McGeer, E. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson’s and Alzheimer’s disease brains. Neurology 1988, 38, 1285. [Google Scholar] [CrossRef] [PubMed]
- Cianciulli, A.; Calvello, R.; Porro, C.; Trotta, T.; Panaro, M.A. Understanding the role of SOCS signaling in neurodegenerative diseases: Current and emerging concepts. Cytokine Growth Factor Rev. 2017, 37, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Porro, C.; Cianciulli, A.; Trotta, T.; Lofrumento, D.D.; Panaro, M.A. Curcumin Regulates Anti-Inflammatory Responses by JAK/STAT/SOCS Signaling Pathway in BV-2 Microglial Cells. Biology 2019, 8, 51. [Google Scholar] [CrossRef] [PubMed]
- Cianciulli, A.; Porro, C.; Calvello, R.; Trotta, T.; Lofrumento, D.D.; Panaro, M.A. Microglia Mediated Neuroinflammation: Focus on PI3K Modulation. Biomolecules 2020, 10, 137. [Google Scholar] [CrossRef] [PubMed]
- Panaro, M.A.; Corrado, A.; Benameur, T.; Paolo, C.F.; Cici, D.; Porro, C. The Emerging Role of Curcumin in the Modulation of TLR-4 Signaling Pathway: Focus on Neuroprotective and Anti-Rheumatic Properties. Int. J. Mol. Sci. 2020, 21, 2299. [Google Scholar] [CrossRef] [PubMed]
- Cianciulli, A.; Salvatore, R.; Porro, C.; Trotta, T.; Panaro, M.A. Folic Acid Is Able to Polarize the Inflammatory Response in LPS Activated Microglia by Regulating Multiple Signaling Pathways. Mediat. Inflamm. 2016. [Google Scholar] [CrossRef] [PubMed]
- Porro, C.; Cianciulli, A.; Calvello, R.; Panaro, M.A. Reviewing the Role of Resveratrol as a Natural Modulator of Microglial Activities. Curr. Pharm. Des. 2015, 21, 5277–5291. [Google Scholar] [CrossRef]
- Trotta, T.; Panaro, M.A.; Prifti, E.; Porro, C. Modulation of Biological Activities in Glioblastoma Mediated by Curcumin. Nutr. Cancer 2019, 71, 1241–1253. [Google Scholar] [CrossRef]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Münch, A.E.; Chung, W.S.; Peterson, T.C.; et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef]
- Beck, A.; Penner, R.; Fleig, A. Lipopolysaccharide-induced down-regulation of Ca2+ release-activated Ca2+ currents (I CRAC) but not Ca2+-activated TRPM4-like currents (I CAN) in cultured mouse microglial cells. J. Physiol. 2008, 586, 427–439. [Google Scholar] [CrossRef]
- Campagno, K.; Lu, W.; Albalawi, F.; Cenaj, A.; Tso, H.Y.; Mitchell, C. Modulation of Microglia by P2X and P2Y Receptors. FASEB J. 2019, 33, 501.1. [Google Scholar]
- ElAli, A.; Rivest, S. Microglia Ontology and Signaling. Front. Cell Dev. Biol. 2016, 4, 72. [Google Scholar] [CrossRef] [PubMed]
- Mizoguchi, Y.; Monji, A. Microglial Intracellular Ca2+ Signaling in Synaptic Development and its Alterations in Neurodevelopmental Disorders. Front. Cell. Neurosci. 2017. [Google Scholar] [CrossRef] [PubMed]
- Fumagalli, M.; Lombardi, M.; Gressens, P.; Verderio, C. How to reprogram microglia toward beneficial functions. Glia 2018, 66, 2531–2549. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, M.; Leng, G. Dendritic peptide release and peptide-dependent behaviours. Nat. Rev. Neurosci. 2006, 7, 126–136. [Google Scholar] [CrossRef]
- Zheng, J.J.; Li, S.J.; Zhang, X.; Di Miao, W.Y.; Zhang, D.; Yao, H.; Yu, X. Oxytocin mediates early experience-dependent cross-modal plasticity in the sensory cortices. Nat. Neurosci. 2014, 17, 391–399. [Google Scholar] [CrossRef]
- Marlin, B.J.; Froemke, R.C. Oxytocin modulation of neural circuits for social behavior. Dev. Neurobiol. 2017, 77, 169–189. [Google Scholar] [CrossRef]
- Lin, Y.T.; Chen, C.C.; Huang, C.C.; Nishimori, K.; Hsu, K.S. Oxytocin stimulates hippocampal neurogenesis via oxytocin receptor expressed in CA3 pyramidal neurons. Nat. Commun. 2017, 14, 537. [Google Scholar] [CrossRef]
- Stoop, R.; Yu, X. Special issue on: “Oxytocin in development and plasticity”. Dev. Neurobiol. 2017, 77, 125–127. [Google Scholar] [CrossRef]
- Katherine, A.; Blackmore, J.K.J.; Colin, N.; Young, A. Novel Oxytocin Expressing Microglia Population in the Brain Subfornical Organ. FASEB J. 2018, 32, 598. [Google Scholar]
- Szeto, A.; Sun-Suslow, N.; Mendez, A.J.; Hernandez, R.I.; Wagner, K.V.; McCabe, P.M. Regulation of the macrophage oxytocin receptor in response to inflammation. Am. J. Physiol. Endocrinol. Metabol. 2017, 312, E183–E189. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Liu, S.; Bai, X.; Gao, Y.; Liu, G.; Wang, X.; Liu, D.; Li, T.; Hao, A.; Wang, Z. Oxytocin inhibits lipopolysaccharide-induced inflammation in microglial cells and attenuates microglial activation in lipopolysaccharide-treated mice. J. Neuroinflammat. 2016, 13, 77. [Google Scholar] [CrossRef] [PubMed]
- Zinni, M.; Colella, M.; Batista Novais, A.R.; Baud, O.; Mairesse, J. Modulating the Oxytocin System During the Perinatal Period: A New Strategy for Neuroprotection of the Immature Brain? Front. Neurol. 2018, 9, 229. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Leak, R.K.; Hu, X. Neurotransmitter receptors on microglia. Stroke Vasc. Neurol. 2016, 1, 52–58. [Google Scholar] [CrossRef]
- Inoue, T.; Yamakage, H.; Tanaka, M.; Kusakabe, T.; Shimatsu, A.; Satoh-Asahara, N. Oxytocin Suppresses Inflammatory Responses Induced by Lipopolysaccharide through Inhibition of the eIF-2α–ATF4 Pathway in Mouse Microglia. Cells 2019, 8, 527. [Google Scholar] [CrossRef]
- Amini-Khoei, H.; Mohammadi-Asl, A.; Amiri, S.; Hosseini, M.J.; Momeny, M.; Hassanipour, M.; Rastegar, M.; Haj-Mirzaian, A.; Mirzaian, A.H.; Sanjarimoghaddam, H.; et al. Oxytocin mitigated the depressive-like behaviors of maternal separation stress through modulating mitochondrial function and neuroinflammation. Prog. Neuropsychopharmacol. Biol. Psychiatry 2017, 2, 169–178. [Google Scholar] [CrossRef]
- Karelina, K.; Stuller, K.A.; Jarrett, B.; Zhang, N.; Wells, J.; Norman, G.J.; DeVries, A.C. Oxytocin mediates social neuroprotection after cerebral ischemia. Stroke 2011, 42, 3606–3611. [Google Scholar] [CrossRef]
- Galloway, D.A.; Phillips, A.E.M.; Owen, D.R.J.; Moore, C.S. Phagocytosis in the Brain: Homeostasis and Disease. Front. Immunol. 2019, 10, 790. [Google Scholar] [CrossRef]
- Li, Q.; Barres, B. Microglia and macrophages in brain homeostasis and disease. Nat. Rev. Immunol. 2018, 18, 225–242. [Google Scholar] [CrossRef]
- Mairesse, J.; Zinni, M.; Pansiot, J.; Hassan-Abdi, R.; Demene, C.; Colella, M.; Charriaut- Marlangue, C.; Rideau Batista Novais, A.; Tanter, M.; Maccari, S.; et al. Oxytocin receptor agonist reduces perinatal brain damage by targeting microglia. Glia 2019, 67, 345–359. [Google Scholar] [CrossRef]
- Kingsbury, M.A.; Bilbo, S.D. The inflammatory event of birth: How oxytocin signaling may guide the development of the brain and gastrointestinal system. Front. Neuroendocrinol. 2019, 55, 100794. [Google Scholar] [CrossRef] [PubMed]
- Tyzio, R.; Cossart, R.; Khalilov, I.; Minlebaev, M.; Hübner, C.A.; Represa, A.; Ben-Ari, Y.; Khazipov, R. Maternal oxytocin triggers a transient inhibitory switch in GABA signaling in the fetal brain during delivery. Science 2006, 314, 1788–1792. [Google Scholar] [CrossRef]
- Valeeva, G.; Valiullina, F.; Khazipov, R. Excitatory actions of GABA in the intact neonatal rodent hippocampus in vitro. Front. Cell Neurosci. 2013, 7, 20. [Google Scholar] [CrossRef] [PubMed]
- Tyzio, R.; Nardou, R.; Ferrari, D.C.; Tsintsadze, T.; Shahrokhi, A.; Eftekhari, S.; Lemonnier, E.; Lozovaya, N.; Burnashev, N.; Ben-Ari, Y. Oxytocin-mediated GABA inhibition during delivery attenuates autism pathogenesis in rodent offspring. Science 2014, 343, 675–679. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Nomura, T.; Xu, J.; Contractor, A. The developmental switch in GABA polarity is delayed in fragile X mice. J. Neurosci. 2014, 34, 446–450. [Google Scholar] [CrossRef]
- Curia, G.; Papouin, T.; Séguéla, P.; Avoli, M. Downregulation of tonic GABAergic inhibition in a mouse model of fragile X syndrome. Cereb. Cortex 2009, 19, 1515–1520. [Google Scholar] [CrossRef]
- Malek, A.; Blann, E.; Mattison, D.R. Human placental transport of oxytocin. J. Matern. Fetal Med. 1996, 5, 245–255. [Google Scholar]
- Vargas, D.L.; Nascimbene, C.; Krishnan, C.; Zimmerman, A.W.; Pardo, C.A. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann. Neurol. 2005, 57, 67–81. [Google Scholar] [CrossRef]
- Takano, T. Role of microglia in autism: Recent advances. Dev. Neurosci. 2015, 37, 195–202. [Google Scholar] [CrossRef]
- Koyama, R.; Ikegaya, Y. Microglia in the pathogenesis of autism spectrum disorders. Neurosci. Res. 2015, 100, 1–5. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, S.; Liu, X.; Zheng, Y.; Li, L.; Meng, S. Oxytocin improves animal behaviors and ameliorates oxidative stress and inflammation in autistic mice. Biomed. Pharmacother. 2018, 107, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Teng, B.L.; Nikolova, V.D.; Riddick, N.V.; Agster, K.L.; Crowley, J.J.; Baker, L.K.; Koller, B.H.; Pedersen, C.A.; Jarstfer, M.B.; Moy, S.S. Reversal of social deficits by subchronic oxytocin in two autism mouse models. Neuropharmacology 2016, 105, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Teng, B.; Nonneman, R.J.; Agster, K.L.; Nikolova, V.D.; Davis, T.T.; Riddick, N.V.; Baker, L.K.; Pedersen, C.A.; Jarstfer, M.B.; Moy, S.S. Prosocial effects of oxytocin in two mouse models of autism spectrum disorders. Neuropharmacology 2013, 72, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Moy, S.S.; Teng, B.L.; Nikolova, V.D.; Riddick, N.V.; Simpson, C.D.; Van Deusen, A.; Janzen, W.P.; Sassano, M.F.; Pedersen, C.A.; Jarstfer, M.B. Prosocial effects of an oxytocin metabolite, but not synthetic oxytocin receptor agonists, in a mouse model of autism. Neuropharmacology 2019, 144, 301–311. [Google Scholar] [CrossRef]
- Angulo, M.A.; Butler, M.G.; Cataletto, M.E. Prader-Willi syndrome: A review of clinical, genetic, and endocrine findings. J. Endocrinol. Investig. 2015, 38, 1249–1263. [Google Scholar] [CrossRef]
- Bennett, J.A.; Germani, T.; Haqq, A.M.; Zwaigenbaum, L. Autism spectrum disorder in Prader-Willi syndrome: A systematic review. Am. J. Med. Genet. A 2015, 167, 2936–2944. [Google Scholar] [CrossRef]
- Höybye, C. Endocrine and metabolic aspects of adult Prader-Willi syndrome with special emphasis on the effect of growth hormone treatment. Growth Horm. IGF Res. 2004, 14, 1–15. [Google Scholar] [CrossRef]
- Tauber, M.; Boulanouar, K.; Diene, G.; Çabal-Berthoumieu, S.; Ehlinger, V.; Fichaux-Bourin, P.; Molinas, C.; Faye, S.; Valette, M.; Pourrinet, J.; et al. The use of oxytocin to improve feeding and social skills in infants with prader-willi syndrome. Pediatrics 2017, 139, e20162976. [Google Scholar] [CrossRef]
- Einfeld, S.L.; Smith, E.; McGregor, I.S.; Steinbeck, K.; Taffe, J.; Rice, L.J.; Horstead, S.K.; Rogers, N.; Hodge, M.A.; Guastella, A.J. A double-blind randomized controlled trial of oxytocin nasal spray in Prader Willi syndrome. Am. J. Med. Genet. A 2014, 164, 2232–2239. [Google Scholar] [CrossRef]
- Miller, J.L.; Tamura, R.; Butler, M.G.; Kimonis, V.; Sulsona, C.; Gold, J.A.; Driscoll, D.J. Oxytocin treatment in children with Prader–Willi syndrome: A double-blind, placebo-controlled, crossover study. Am. J. Med. Genet. Part A 2017, 173, 1243–1250. [Google Scholar] [CrossRef]
- Rodenas-Cuadrado, P.; Ho, J.; Vernes, S.C. Shining a light on CNTNAP2: Complex functions to complex disorders. Eur. J. Hum. Genet. 2014, 22, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Strauss, K.A.; Puffenberger, E.G.; Huentelman, M.J.; Gottlieb, S.; Dobrin, S.E.; Parod, J.M.; Stephan, D.A.; Morton, D.H. Recessive symptomatic focal epilepsy and mutant contactin-associated protein-like 2. N. Engl. J. Med. 2006, 354, 1370–1377. [Google Scholar] [CrossRef] [PubMed]
- Elia, J.; Gai, X.; Xie, H.M.; Perin, J.C.; Geiger, E.; Glessner, J.T.; D’arcy, M.; deBerardinis, R.; Frackelton, E.; Kim, C.; et al. Rare structural variants found in attention-deficit hyperactivity disorder are preferentially associated with neurodevelopmental genes. Mol. Psychiatry 2010, 15, 637–646. [Google Scholar] [CrossRef]
- Peñagarikano, O.; Lázaro, M.T.; Lu, X.H.; Gordon, A.; Dong, H.; Lam, H.A.; Peles, E.; Maidment, N.T.; Murphy, N.P.; Yang, X.W.; et al. Exogenous and evoked oxytocin restores social behavior in the Cntnap2 mouse model of autism. Sci. Transl. Med. 2015, 21, 271. [Google Scholar]
- Etehadi Moghadam, S.; Azami Tameh, A.; Vahidinia, Z.; Atlasi, M.A.; Hassani Bafrani, H.; Naderian, H. Neuroprotective Effects of Oxytocin Hormone after an Experimental Stroke Model and the Possible Role of Calpain-1. J. Stroke Cerebrovasc. Dis. 2018, 27, 724–732. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, Y.; Pappas, C.; Tajiri, N.; Borlongan, C.V. Oxytocin modulates GABAAR subunits to confer neuroprotection in stroke in vitro. Sci. Rep. 2016, 6, 35659. [Google Scholar] [CrossRef] [PubMed]
- Tanyeri, G.; Celik, O.; Erbas, O.; Oltulu, F.; Yilmaz Dilsiz, O. The effectiveness of different neuroprotective agents in facial nerve injury: An experimental study. Laryngoscope 2015, 125, 356–364. [Google Scholar] [CrossRef]
- Matsuzaki, M.; Matsushita, H.; Tomizawa, K.; Matsui, H. Oxytocin: A therapeutic target for mental disorders. J. Physiol. Sci. 2012, 62, 441–444. [Google Scholar] [CrossRef]
- Frijling, J.L. Preventing PTSD with oxytocin: Effects of oxytocin administration on fear neurocircuitry and PTSD symptom development in recently trauma-exposed individuals. Eur. J. Psychotraumatol. 2017, 8, 1302652. [Google Scholar] [CrossRef]
- Frasch, A.; Zetzsche, T.; Steiger, A.; Jirikowski, G.F. Reduction of plasma oxytocin levels in patients suffering from major depression. Adv. Exp. Med. Biol. 1995, 395, 257–258. [Google Scholar]
- Zetzsche, T.; Frasch, A.; Jirikowski, G.F.; Murck, H.; Steiger, A. Nocturnal oxytocin secretion is reduced in major depression. Biol. Psychiatry 1996, 39, 584. [Google Scholar] [CrossRef]
- Arletti, R.; Bertolini, A. Oxytocin acts as an antidepressant in two animal models of depression. Life Sci. 1987, 41, 1725–1730. [Google Scholar] [CrossRef]
- Matsushita, H.; Matsuzaki, M.; Han, X.J.; Nishiki, T.I.; Ohmori, I.; Michiue, H.; Matsui, H.; Tomizawa, K. Antidepressant-like effect of sildenafil through oxytocin-dependent cyclic AMP response element-binding protein phosphorylation. Neuroscience 2012, 200, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Shi, C.; Li, X.; Zhang, P.; Liu, B.; Wang, H.; Wang, Y.; Yang, Y.; Wu, Y.; Li, H.; et al. Injection of oxytocin into paraventricular nucleus reverses depressive-like behaviors in the postpartum depression rat model. Behav. Brain Res. 2018, 336, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Scantamburlo, G.; Hansenne, M.; Fuchs, S.; Pitchot, W.; MareÅL chal, P.; Pequeux, C.; Ansseau, M.; Legros, J.J. Plasma oxytocin levels and anxiety in patients with major depression. Psychoneuroendocrinology 2007, 32, 407–410. [Google Scholar] [CrossRef] [PubMed]
- Eapen, V.; Dadds, M.; Barnett, B.; Kohlhoff, J.; Khan, F.; Radom, N.; Silove, D.M. Separation anxiety, attachment and inter-personal representations: Disentangling the role of oxytocin in the perinatal period. PLoS ONE 2014, 9, e107745. [Google Scholar] [CrossRef] [PubMed]
- Guastella, A.J.; Howard, A.L.; Dadds, M.R.; Mitchell, P.; Carson, D.S. A randomized controlled trial of intranasal oxytocin as an adjunct to exposure therapy for social anxiety disorder. Psychoneuroendocrinology 2009, 34, 917–923. [Google Scholar] [CrossRef]
- Arcelus, J.; Mitchell, A.J.; Wales, J.; Nielsen, S. Mortality rates in patients with anorexia nervosa and other eating disorders. A meta-analysis of 36 studies. Arch. Gen. Psychiatry 2011, 68, 724–731. [Google Scholar] [CrossRef]
- Liu, C.M.; Kanoski, S.E. Homeostatic and non-homeostatic controls of feeding behavior: Distinct vs. common neural systems. Physiol. Behav. 2018, 193, 223–231. [Google Scholar] [CrossRef]
- Zingg, H.H.; Laporte, S.A. The oxytocin receptor. Trends Endocrinol. Metab. 2003, 14, 222–227. [Google Scholar] [CrossRef]
- Spetter, M.S.; Hallschmid, M. Current findings on the role of oxytocin in the regulation of food intake. Physiol. Behav. 2017, 1, 31–39. [Google Scholar] [CrossRef]
- Takayanagi, Y.; Kasahara, Y.; Onaka, T.; Takahashi, N.; Kawada, T.; Nishimori, K. Oxytocin receptor-deficient mice developed late-onset obesity. Neuroreport. Jun. 2008, 9, 951–955. [Google Scholar] [CrossRef]
- Arletti, R.; Benelli, A.; Bertolini, A. Influence of oxytocin on feeding behavior in the rat. Peptides 1998, 1, 89–93. [Google Scholar] [CrossRef]
- Peris, J.; MacFadyen, K.; Smith, J.A.; de Kloet, A.D.; Wang, L.; Krause, E.G. Oxytocin receptors are expressed on dopamine and glutamate neurons in the mouse ventral tegmental area that project to nucleus accumbens and other mesolimbic targets. J. Comp. Neurol. 2017, 525, 1094–1108. [Google Scholar] [CrossRef]
- Klement, J.; Ott, V.; Rapp, K.; Brede, S.; Piccinini, F.; Cobelli, C.; Hallschmid, M. Oxytocin improves ß-Cell responsivity and glucose tolerance in healthy men. Diabetes 2017, 2, 264–271. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, C.; Chen, Q.; Chen, X.; Xu, Z.; Wu, J.; Cai, D. Treatment of obesity and diabetes using oxytocin or analogs in patients and mouse models. PLoS ONE 2013, 5, 61477. [Google Scholar] [CrossRef]
- Acevedo, S.F.; Valencia, C.; Lutter, M.; McAdams, C.J. Severity of eating disorder symptoms related to oxytocin receptor polymorphisms in anorexia nervosa. Psychiatry Res. 2015, 3, 641–648. [Google Scholar] [CrossRef]
- Micali, N.; Crous-Bou, M.; Treasure, J.; Lawson, E.A. Association between oxytocin receptor genotype, maternal care, and eating disorder behaviours in a community sample of women. Eur. Eat Disord. Rev. 2017, 1, 19–25. [Google Scholar] [CrossRef]
- Fenstermacher, J.; Gross, P.; Sposito, N.; Acuff, V.; Pettersen, S.; Gruber, K. Structural and Functional Variations in Capillary Systems within the Braina. Ann. N. Y. Acad. Sci. 1988, 529, 21–30. [Google Scholar] [CrossRef]
- Kniesel, U.; Wolburg, H. Tight junctions of the blood–brain barrier. Cell. Mol. Neurobiol. 2000, 20, 57–76. [Google Scholar] [CrossRef]
- Zhang, E.Y.; Knipp, G.T.; Ekins, S.; Swaan, P.W. Structural biology and function of solute transporters: Implications for identifying and designing substrates. Drug Metab. Rev. 2002, 34, 709–750. [Google Scholar] [CrossRef] [PubMed]
- Bak, A.; Leung, D.; Barrett, S.E.; Forster, S.; Minnihan, E.C.; Leithead, A.W.; Cunningham, J.; Toussaint, N.; Crocker, L.S. Physicochemical and formulation developability assessment for therapeutic peptide delivery—A primer. AAPS J. 2015, 17, 144–155. [Google Scholar] [PubMed]
- Bruno, B.J.; Miller, G.D.; Lim, C.S. Basics and recent advances in peptide and protein drug delivery. Ther. Deliv. 2013, 4, 1443–1467. [Google Scholar] [CrossRef] [PubMed]
- Hua, S.; Vaughan, B. In vitro comparison of liposomal drug delivery systems targeting the oxytocin receptor: A potential novel treatment for obstetric complications. Int. J. Nanomed. 2019, 14, 2191–2206. [Google Scholar] [CrossRef]
- Hua, S. Synthesis and in vitro characterization of oxytocin receptor targeted PEGylated immunoliposomes for drug delivery to the uterus. J. Liposome Res. 2019, 29, 357–367. [Google Scholar] [CrossRef]
- Zaman, R.U.; Mulla, N.S.; Braz Gomes, K.; D’Souza, C.; Murnane, K.S.; D’Souza, M.J. Nanoparticle formulations that allow for sustained delivery and brain targeting of the neuropeptide oxytocin. Int. J. Pharm. 2018, 5, 698–706. [Google Scholar] [CrossRef]
- Gourdon, B.; Chemin, C.; Moreau, A.; Arnauld, T.; Delbos, J.M.; Péan, J.M.; Declèves, X. Influence of PLA-PEG nanoparticles manufacturing process on intestinal transporter PepT1 targeting and oxytocin transport. Eur. J. Pharm. Biopharm. 2018, 129, 122–133. [Google Scholar] [CrossRef]
- Lee, M.R.; Scheidweiler, K.B.; Diao, X.X.; Akhlaghi, F.; Cummins, A.; Huestis, M.A.; Leggio, L.; Averbeck, B.B. Oxytocin by intranasal and intravenous routes reaches the cerebrospinal fluid in rhesus macaques: Determination using a novel oxytocin assay. Mol. Psychiatry 2018, 23, 115–122. [Google Scholar] [CrossRef]
- Neumann, I.D.; Maloumby, R.; Beiderbeck, D.I.; Lukas, M.; Landgraf, R. Increased brain and plasma oxytocin after nasal and peripheral administration in rats and mice. Psychoneuroendocrinology 2013, 38, 1985–1993. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Liang, M.; Munesue, S.; Deguchi, K.; Harashima, A.; Furuhara, K.; Yuhi, T.; Zhong, J.; Akther, S.; Goto, H.; et al. Vascular RAGE transports oxytocin into the brain to elicit its maternal bonding behaviour in mice. Commun. Biol. 2019, 2, 76. [Google Scholar] [CrossRef]
- Ji, H.; Su, W.; Zhou, R.; Feng, J.; Lin, Y.; Zhang, Y.; Wang, X.; Chen, X.; Li, J. Intranasal oxytocin administration improves depression like behaviors in adult rats that experienced neonatal maternal deprivation. Behav. Pharmacol. 2016, 27, 689–696. [Google Scholar] [CrossRef] [PubMed]
- Gainer, H. Cell-specific gene expression in oxytocin and vasopressin magnocellular neurons. Adv. Exp. Med. Biol. 1998, 449, 15–27. [Google Scholar] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panaro, M.A.; Benameur, T.; Porro, C. Hypothalamic Neuropeptide Brain Protection: Focus on Oxytocin. J. Clin. Med. 2020, 9, 1534. https://doi.org/10.3390/jcm9051534
Panaro MA, Benameur T, Porro C. Hypothalamic Neuropeptide Brain Protection: Focus on Oxytocin. Journal of Clinical Medicine. 2020; 9(5):1534. https://doi.org/10.3390/jcm9051534
Chicago/Turabian StylePanaro, Maria Antonietta, Tarek Benameur, and Chiara Porro. 2020. "Hypothalamic Neuropeptide Brain Protection: Focus on Oxytocin" Journal of Clinical Medicine 9, no. 5: 1534. https://doi.org/10.3390/jcm9051534
APA StylePanaro, M. A., Benameur, T., & Porro, C. (2020). Hypothalamic Neuropeptide Brain Protection: Focus on Oxytocin. Journal of Clinical Medicine, 9(5), 1534. https://doi.org/10.3390/jcm9051534





