SIRT1 Activation by Equisetum arvense L. (Horsetail) Modulates Insulin Sensitivity in Streptozotocin Induced Diabetic Rats
Abstract
1. Introduction
2. Results
2.1. Microscopic Examination of Equisetum arvense L.
2.2. Ultra-High Performance Liquid Chromatography (UHPLC) Analysis of Horsetail Extract
2.3. Effect of Horsetail on Body Weight
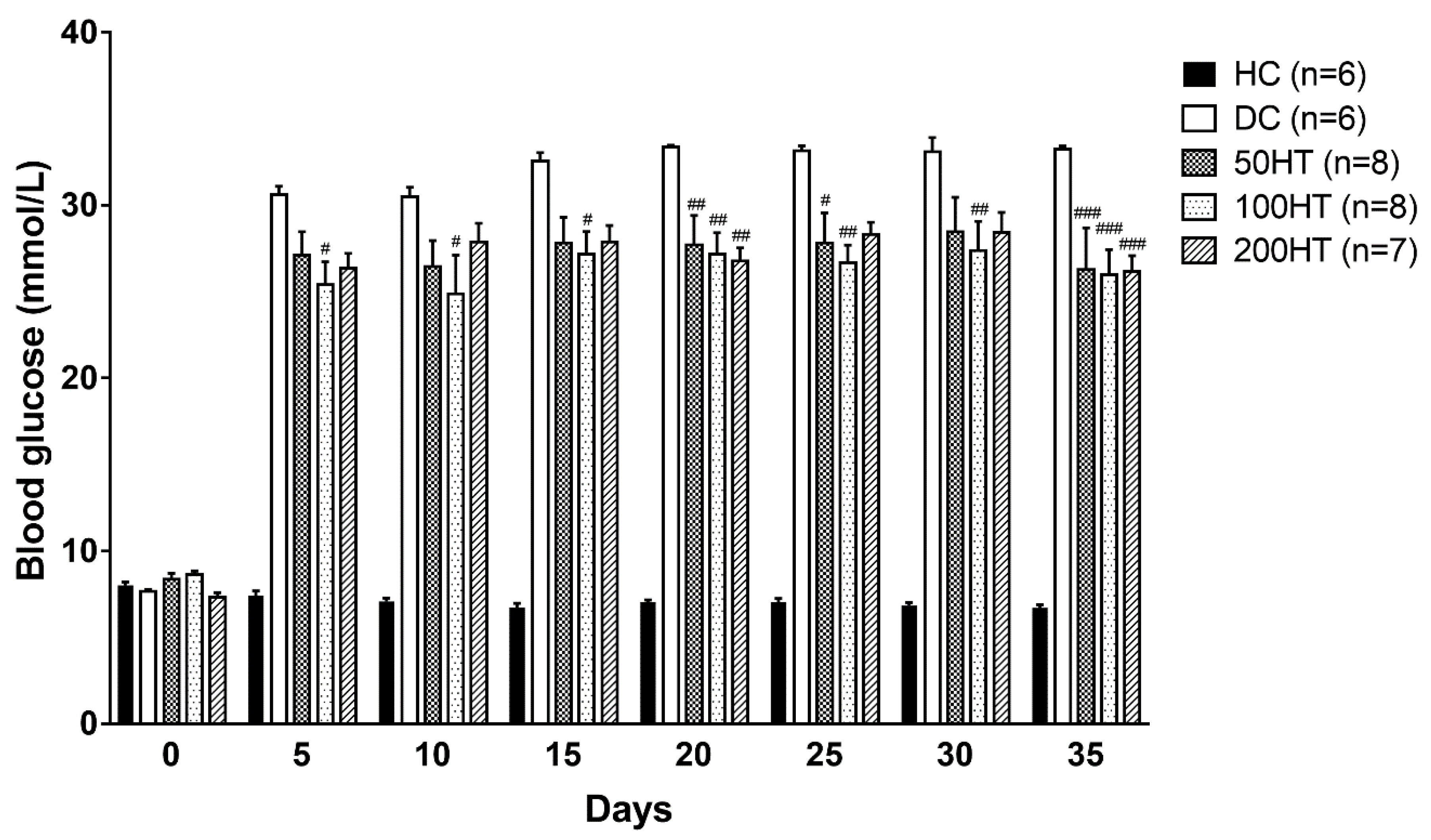
2.4. Effect of Horsetail on Blood Glucose
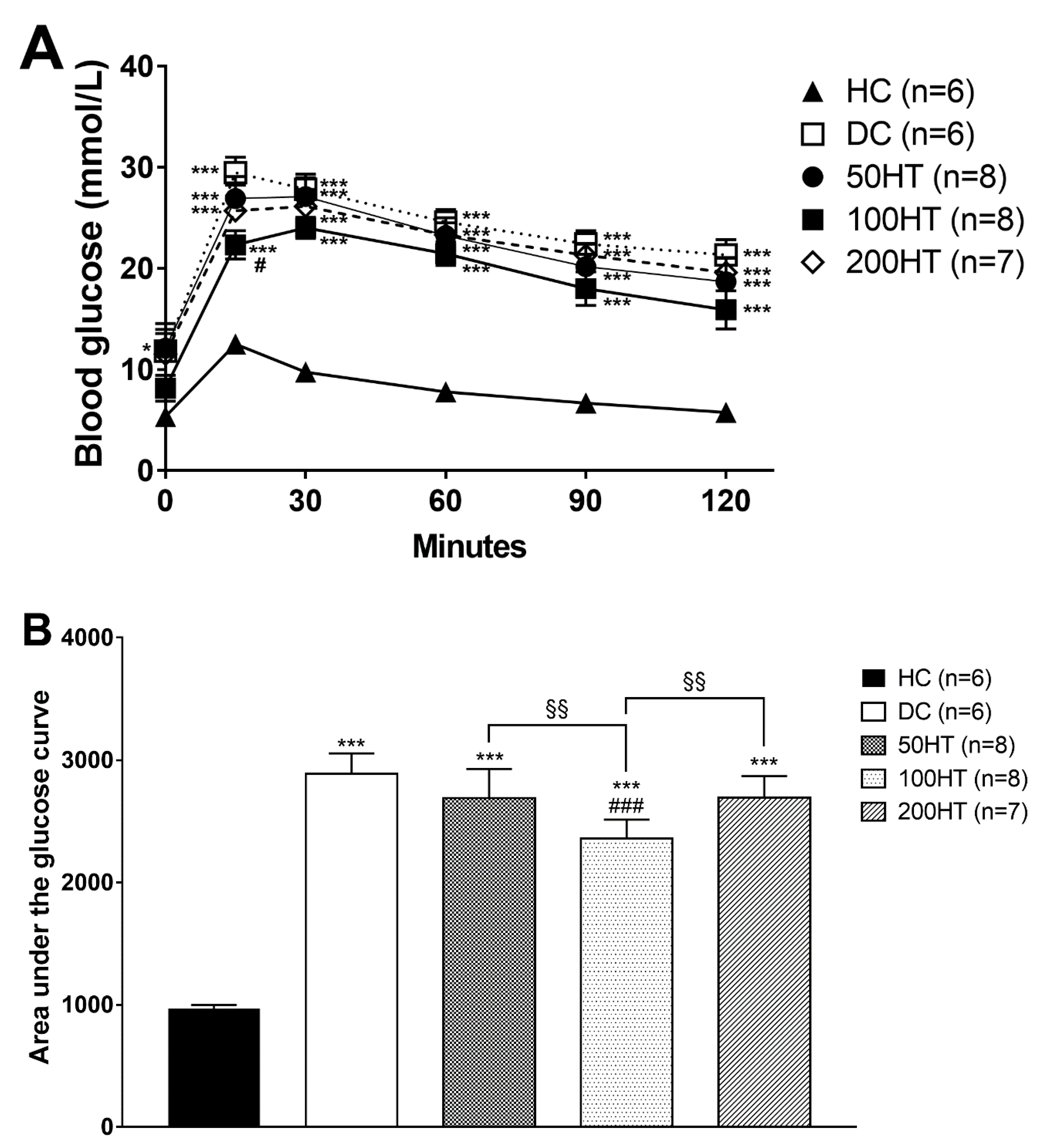
2.5. Effect of Horsetail on Glucose Tolerance
2.6. Effect of Horsetail on Insulin Tolerance
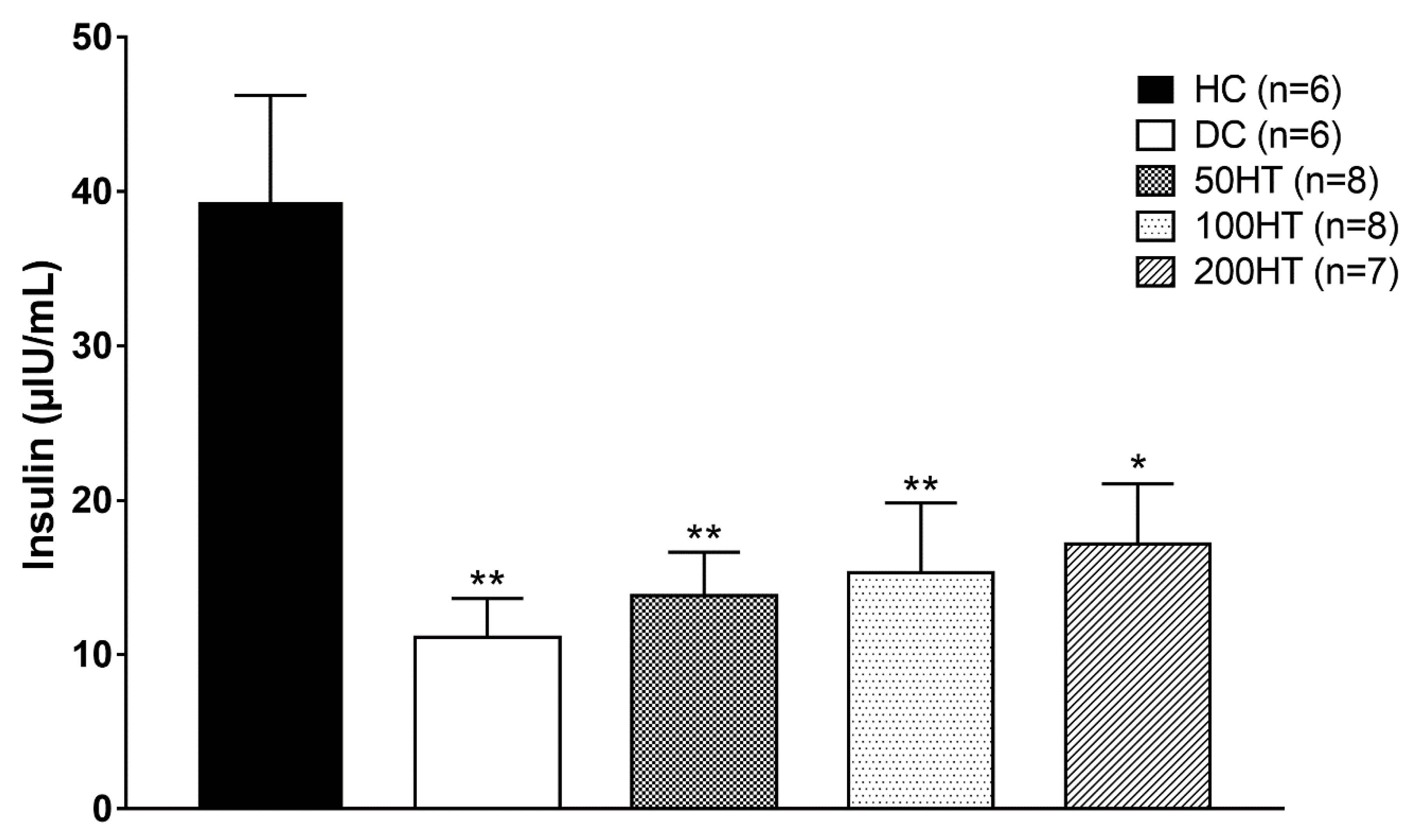
2.7. Effect of Horsetail on Fasting Plasma Insulin
2.8. Effect of Horsetail on Adiposity
2.9. Effect of Horsetail on Heart Weight Index
2.10. Effect of Horsetail on SIRT1 Levels
3. Discussion
4. Materials and Methods
4.1. Ethics
4.2. Animals
4.3. Induction of Diabetes Mellitus
4.4. Microscopic Examination of Equisetum arvense L.
4.5. Preparation of Equisetum arvense L. (horsetail) Extract
4.6. Determination of Phenolic Compounds from Equisetum arvense L. Using UPLC-DAD
4.6.1. Chemicals
4.6.2. Extraction of Phenolic Compounds
4.6.3. UPLC Analysis
4.7. Oral Glucose Tolerance Test (OGTT)
4.8. Insulin Tolerance Test (ITT)
4.9. Samples
4.10. Western Blot
4.11. Statistics
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Europarat; European Department for the Quality of Medicines. European Pharmacopoeia; Council of Europe: Strasbourg, France, 2016. [Google Scholar]
- Pallag, A.; Bungau, S.; Tit, D.M.; Tünde, J.; Sirbu, V.; Honiges, A.; Horhogea, C. Comparative study of polyphenols, flavonoids and chlorophylls in equisetum arvense l. Populations. Rev. Chim. 2016, 67, 530–533. [Google Scholar]
- Pallag, A.; Pasca, M.B.; Tünde, J.; Suciu, R.; Nemeth, S.; Vicas, L. Comparative histo-anatomical researches on the vegetative organs and assessment of antioxidant capacity of two species from equisetum genus. Farmacia 2016, 64, 372–377. [Google Scholar]
- Cetojevic-Simin, D.D.; Canadanovic-Brunet, J.M.; Bogdanovic, G.M.; Djilas, S.M.; Cetkovic, G.S.; Tumbas, V.T.; Stojiljkovic, B.T. Antioxidative and antiproliferative activities of different horsetail (equisetum arvense l.) extracts. J. Med. Food 2010, 13, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Pallag, A.; Tünde, J.; Pasca, M.B.; Sirbu, V.; Honiges, A.; Costuleanu, M. Analysis of phenolic compounds composition by hplc and assessment of antioxidant capacity in equisetum arvense l. Extracts. Rev. Chim. 2016, 67, 1623–1627. [Google Scholar]
- Sandhu, N.S.; Kaur, S.; Chopra, D. Equietum arvense: Pharmacology and phytochemistry-a review. Asian J. Pharm. Clin. Res. 2010, 3, 146–150. [Google Scholar]
- Kukric, Z.; Topalić-Trivunović, L.; Pavicic, S.; Zabic, M.; Matos, S.; Davidovic, A. Total phenolic content, antioxidant and antimicrobial activity of Equisetum arvense L. Chem. Ind. Chem. Eng. Q. 2013, 19, 37–43. [Google Scholar] [CrossRef]
- Pallag, A.; Filip, G.A.; Olteanu, D.; Clichici, S.; Baldea, I.; Jurca, T.; Micle, O.; Vicas, L.; Marian, E.; Soritau, O.; et al. Equisetum arvense l. Extract induces antibacterial activity and modulates oxidative stress, inflammation, and apoptosis in endothelial vascular cells exposed to hyperosmotic stress. Oxid. Med. Cell. Longev. 2018, 2018, 3060525. [Google Scholar] [CrossRef]
- Do Monte, F.H.; dos Santos, J.G., Jr.; Russi, M.; Lanziotti, V.M.; Leal, L.K.; Cunha, G.M. Antinociceptive and anti-inflammatory properties of the hydroalcoholic extract of stems from equisetum arvense l. In mice. Pharmacol. Res. 2004, 49, 239–243. [Google Scholar] [CrossRef]
- Dos Santos, J.G., Jr.; Blanco, M.M.; Do Monte, F.H.; Russi, M.; Lanziotti, V.M.; Leal, L.K.; Cunha, G.M. Sedative and anticonvulsant effects of hydroalcoholic extract of equisetum arvense. Fitoterapia 2005, 76, 508–513. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Inoue, T.; Hamako, J. Crude proteins extracted from equisetum arvense l. Increases the viability of cancer cells in vivo. J. Anal. Bio. Sci. Seibutsu Shiryo Bunseki 2004, 27, 409–412. [Google Scholar]
- Haigis, M.C.; Mostoslavsky, R.; Haigis, K.M.; Fahie, K.; Christodoulou, D.C.; Murphy, A.J.; Valenzuela, D.M.; Yancopoulos, G.D.; Karow, M.; Blander, G.; et al. Sirt4 inhibits glutamate dehydrogenase and opposes the effects of calorie restriction in pancreatic beta cells. Cell 2006, 126, 941–954. [Google Scholar] [CrossRef] [PubMed]
- Liszt, G.; Ford, E.; Kurtev, M.; Guarente, L. Mouse sir2 homolog sirt6 is a nuclear adp-ribosyltransferase. J. Biol. Chem. 2005, 280, 21313–21320. [Google Scholar] [CrossRef] [PubMed]
- Michan, S.; Sinclair, D. Sirtuins in mammals: Insights into their biological function. Biochem. J. 2007, 404, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ghinis, Y.; González-Dávalos, L.; Antaramian, A.; Villarroya, F.; Piña, E.; Shimada, A.; Varela-Echavarría, A.; Mora, O. Effect of resveratrol and lipoic acid on sirtuin-regulated expression of metabolic genes in bovine liver and muscle slice cultures. J. Anim. Sci. 2015, 93, 3820–3831. [Google Scholar] [CrossRef] [PubMed]
- Zakhary, S.M.; Ayubcha, D.; Dileo, J.N.; Jose, R.; Leheste, J.R.; Horowitz, J.M.; Torres, G. Distribution analysis of deacetylase sirt1 in rodent and human nervous systems. Anat. Rec. 2010, 293, 1024–1032. [Google Scholar] [CrossRef] [PubMed]
- Houtkooper, R.H.; Pirinen, E.; Auwerx, J. Sirtuins as regulators of metabolism and healthspan. Nat. Rev. Mol. Cell Biol. 2012, 13, 225–238. [Google Scholar] [CrossRef]
- Haigis, M.C.; Sinclair, D.A. Mammalian sirtuins: Biological insights and disease relevance. Annu. Rev. Pathol. 2010, 5, 253–295. [Google Scholar] [CrossRef]
- Santos, L.; Escande, C.; Denicola, A. Potential modulation of sirtuins by oxidative stress. Oxid. Med. Cell. Longev. 2016, 2016, 9831825. [Google Scholar] [CrossRef]
- Sack, M.N.; Finkel, T. Mitochondrial metabolism, sirtuins, and aging. Cold Spring Harb. Perspect. Biol. 2012, 4. [Google Scholar] [CrossRef]
- Kume, S.; Kitada, M.; Kanasaki, K.; Maegawa, H.; Koya, D. Anti-aging molecule, sirt1: A novel therapeutic target for diabetic nephropathy. Arch. Pharmacal Res. 2013, 36, 230–236. [Google Scholar] [CrossRef]
- Simmons, G.E.; Pruitt, W.M.; Pruitt, K. Diverse roles of sirt1 in cancer biology and lipid metabolism. Int. J. Mol. Sci. 2015, 16, 950–965. [Google Scholar] [CrossRef] [PubMed]
- Ding, R.B.; Bao, J.; Deng, C.X. Emerging roles of sirt1 in fatty liver diseases. Int. J. Biol. Sci. 2017, 13, 852–867. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, M.; Matta, M.J.; Sunderesan, N.R.; Gupta, M.P.; Periasamy, M.; Gupta, M. Resveratrol, an activator of sirt1, upregulates sarcoplasmic calcium atpase and improves cardiac function in diabetic cardiomyopathy. Am. J. Physiol. Heart Circ. Physiol. 2010, 298, 833–843. [Google Scholar] [CrossRef] [PubMed]
- Jia, G.; Hill, M.A.; Sowers, J.R. Diabetic cardiomyopathy: An update of mechanisms contributing to this clinical entity. Circ. Res. 2018, 122, 624–638. [Google Scholar] [CrossRef] [PubMed]
- Bugger, H.; Abel, E.D. Molecular mechanisms of diabetic cardiomyopathy. Diabetologia 2014, 57, 660–671. [Google Scholar] [CrossRef] [PubMed]
- Rubler, S.; Dlugash, J.; Yuceoglu, Y.Z.; Kumral, T.; Branwood, A.W.; Grishman, A. New type of cardiomyopathy associated with diabetic glomerulosclerosis. Am. J. Cardiol. 1972, 30, 595–602. [Google Scholar] [CrossRef]
- Karbasforooshan, H.; Karimi, G. The role of sirt1 in diabetic cardiomyopathy. Biomed. Pharmacother. 2017, 90, 386–392. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, J.H.; Lee, H.Y.; Min, K.J. Sirtuin signaling in cellular senescence and aging. BMB Rep. 2019, 52, 24–34. [Google Scholar]
- Hubbard, B.P.; Sinclair, D.A. Small molecule sirt1 activators for the treatment of aging and age-related diseases. Trends Pharmacol. Sci. 2014, 35, 146–154. [Google Scholar] [CrossRef]
- Park, S.J.; Ahmad, F.; Philp, A.; Baar, K.; Williams, T.; Luo, H.; Ke, H.; Rehmann, H.; Taussig, R.; Brown, A.L.; et al. Resveratrol ameliorates aging-related metabolic phenotypes by inhibiting camp phosphodiesterases. Cell 2012, 148, 421–433. [Google Scholar] [CrossRef]
- Wang, Y.; Liang, X.; Chen, Y.; Zhao, X. Screening sirt1 activators from medicinal plants as bioactive compounds against oxidative damage in mitochondrial function. Oxid. Med. Cell. Longev. 2016, 2016, 9. [Google Scholar] [CrossRef] [PubMed]
- Bonkowski, M.S.; Sinclair, D.A. Slowing ageing by design: The rise of nad+ and sirtuin-activating compounds. Nat. Rev. Mol. Cell Biol. 2016, 17, 679. [Google Scholar] [CrossRef] [PubMed]
- Blaslov, K.; Naranđa, F.S.; Kruljac, I.; Renar, I.P. Treatment approach to type 2 diabetes: Past, present and future. World, J. Diabetes 2018, 9, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Thrasher, J. Pharmacologic management of type 2 diabetes mellitus: Available therapies. Am. J. Med. 2017, 130, S4–S17. [Google Scholar] [CrossRef]
- Maneze, D.; Weaver, R.; Kovai, V.; Salamonson, Y.; Astorga, C.; Yogendran, D.; Everett, B. “Some say no, some say yes”: Receiving inconsistent or insufficient information from healthcare professionals and consequences for diabetes self-management: A qualitative study in patients with type 2 diabetes. Diabetes Res. Clin. Pract. 2019, 156, 107830. [Google Scholar] [CrossRef]
- Muhammad, S.A.; Raza, W.; Nguyen, T.; Bai, B.; Wu, X.; Chen, J. Cellular signaling pathways in insulin resistance-systems biology analyses of microarray dataset reveals new drug target gene signatures of type 2 diabetes mellitus. Front. Physiol. 2017, 8, 13. [Google Scholar] [CrossRef]
- Ma, X.; Chen, Z.; Wang, L.; Wang, G.; Wang, Z.; Dong, X.; Wen, B.; Zhang, Z. The pathogenesis of diabetes mellitus by oxidative stress and inflammation: Its inhibition by berberine. Front. Pharmacol. 2018, 9, 782. [Google Scholar] [CrossRef]
- Wang, Y.; Zhong, J.; Zhang, X.; Liu, Z.; Yang, Y.; Gong, Q.; Ren, B. The role of hmgb1 in the pathogenesis of type 2 diabetes. J. Diabetes Res. 2016, 2016, 11. [Google Scholar] [CrossRef]
- Szabo, K.; Gesztelyi, R.; Lampe, N.; Kiss, R.; Remenyik, J.; Pesti-Asboth, G.; Priksz, D.; Szilvassy, Z.; Juhasz, B. Fenugreek (trigonella foenum-graecum) seed flour and diosgenin preserve endothelium-dependent arterial relaxation in a rat model of early-stage metabolic syndrome. Int. J. Mol. Sci 2018, 19. [Google Scholar] [CrossRef]
- Deyno, S.; Eneyew, K.; Seyfe, S.; Tuyiringire, N.; Peter, E.L.; Muluye, R.A.; Tolo, C.U.; Ogwang, P.E. Efficacy and safety of cinnamon in type 2 diabetes mellitus and pre-diabetes patients: A meta-analysis and meta-regression. Diabetes Res. Clin. Pract. 2019, 156, 107815. [Google Scholar] [CrossRef]
- Eidi, A.; Eidi, M. Antidiabetic effects of sage (salvia officinalis l.) leaves in normal and streptozotocin-induced diabetic rats. Diabetes Metab. Syndr. 2009, 3, 40–44. [Google Scholar] [CrossRef]
- Bulboaca, A.E.; Porfire, A.S.; Tefas, L.R.; Boarescu, P.M.; Bolboaca, S.D.; Stanescu, I.C.; Bulboaca, A.C.; Dogaru, G. Liposomal curcumin is better than curcumin to alleviate complications in experimental diabetic mellitus. Molecules 2019, 24, 846. [Google Scholar] [CrossRef]
- Bulboaca, A.E.; Boarescu, P.M.; Bolboaca, S.D.; Blidaru, M.; Festila, D.; Dogaru, G.; Nicula, C.A. Comparative effect of curcumin versus liposomal curcumin on systemic pro-inflammatory cytokines profile, mcp-1 and rantes in experimental diabetes mellitus. Int. J. Nanomed. 2019, 14, 8961–8972. [Google Scholar] [CrossRef] [PubMed]
- Soleimani, S.; Azarbaizani, F.F.; Nejati, V. The effect of equisetum arvense l. (equisetaceae) in histological changes of pancreatic beta-cells in streptozotocin-induced diabetic in rats. Pak. J. Biol. Sci. 2007, 10, 4236–4240. [Google Scholar]
- Safiyeh, S.; Fathallah, F.B.; Vahid, N.; Hossine, N.; Habib, S.S. Antidiabetic effect of equisetum arvense l. (equisetaceae) in streptozotocin-induced diabetes in male rats. Pak. J. Biol. Sci. 2007, 10, 1661–1666. [Google Scholar]
- Cao, Y.; Jiang, X.; Ma, H.; Wang, Y.; Xue, P.; Liu, Y. Sirt1 and insulin resistance. J. Diabetes Complicat. 2016, 30, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.-H.; Kim, H.-S.; Xiao, C.; Xu, X.; Gavrilova, O.; Deng, C.-X. Hepatic sirt1 deficiency in mice impairs mtorc2/akt signaling and results in hyperglycemia, oxidative damage, and insulin resistance. J. Clin. Investig. 2011, 121, 4477–4490. [Google Scholar] [CrossRef] [PubMed]
- Milne, J.C.; Lambert, P.D.; Schenk, S.; Carney, D.P.; Smith, J.J.; Gagne, D.J.; Jin, L.; Boss, O.; Perni, R.B.; Vu, C.B.; et al. Small molecule activators of sirt1 as therapeutics for the treatment of type 2 diabetes. Nature 2007, 450, 712–716. [Google Scholar] [CrossRef]
- Luu, L.; Dai, F.; Prentice, K.; Huang, X.; Hardy, A.; Hansen, J.; Liu, Y.; Joseph, J.; Wheeler, M. The loss of sirt1 in mouse pancreatic beta cells impairs insulin secretion by disrupting glucose sensing. Diabetologia 2013, 56. [Google Scholar] [CrossRef] [PubMed]
- Moynihan, K.A.; Grimm, A.A.; Plueger, M.M.; Bernal-Mizrachi, E.; Ford, E.; Cras-Meneur, C.; Permutt, M.A.; Imai, S. Increased dosage of mammalian sir2 in pancreatic beta cells enhances glucose-stimulated insulin secretion in mice. Cell Metab. 2005, 2, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Bordone, L.; Motta, M.C.; Picard, F.; Robinson, A.; Jhala, U.S.; Apfeld, J.; McDonagh, T.; Lemieux, M.; McBurney, M.; Szilvasi, A.; et al. Sirt1 regulates insulin secretion by repressing ucp2 in pancreatic beta cells. PLoS Biol. 2006, 4, e31. [Google Scholar] [CrossRef]
- Prud’homme, G.J.; Glinka, Y.; Udovyk, O.; Hasilo, C.; Paraskevas, S.; Wang, Q. Gaba protects pancreatic beta cells against apoptosis by increasing sirt1 expression and activity. Biochem. Biophys. Res. Commun. 2014, 452, 649–654. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Liu, W.; Liu, B.; Zhang, B.; Li, W.; Xu, Y. Sirt1 suppresses cardiomyocyte apoptosis in diabetic cardiomyopathy: An insight into endoplasmic reticulum stress response mechanism. Int. J. Cardiol. 2015, 191, 36–45. [Google Scholar] [CrossRef] [PubMed]
- D’Onofrio, N.; Servillo, L.; Balestrieri, M.L. Sirt1 and sirt6 signaling pathways in cardiovascular disease protection. Antioxid. Red. Signal. 2018, 28, 711–732. [Google Scholar] [CrossRef]
- Fishman, S.L.; Sonmez, H.; Basman, C.; Singh, V.; Poretsky, L. The role of advanced glycation end-products in the development of coronary artery disease in patients with and without diabetes mellitus: A review. Mol. Med. 2018, 24, 59. [Google Scholar] [CrossRef]
- Kertesz, A.; Bombicz, M.; Priksz, D.; Balla, J.; Balla, G.; Gesztelyi, R.; Varga, B.; Haines, D.D.; Tosaki, A.; Juhasz, B. Adverse impact of diet-induced hypercholesterolemia on cardiovascular tissue homeostasis in a rabbit model: Time-dependent changes in cardiac parameters. Int. J. Mol. Sci. 2013, 14, 19086–19108. [Google Scholar] [CrossRef]
- Duan, J.; Yin, Y.; Wei, G.; Cui, J.; Zhang, E.; Guan, Y.; Yan, J.; Guo, C.; Zhu, Y.; Mu, F.; et al. Chikusetsu saponin iva confers cardioprotection via sirt1/erk1/2 and homer1a pathway. Sci. Rep. 2015, 5, 18123. [Google Scholar] [CrossRef]
- Vahtola, E.; Louhelainen, M.; Merasto, S.; Martonen, E.; Penttinen, S.; Aahos, I.; Kyto, V.; Virtanen, I.; Mervaala, E. Forkhead class o transcription factor 3a activation and sirtuin1 overexpression in the hypertrophied myocardium of the diabetic goto-kakizaki rat. J. Hypertens. 2008, 26, 334–344. [Google Scholar] [CrossRef]
- Yuan, Q.; Zhou, Q.-Y.; Liu, D.; Yu, L.; Zhan, L.; Li, X.-J.; Peng, H.-Y.; Zhang, X.-L.; Yuan, X.-C. Advanced glycation end-products impair na+/k+-atpase activity in diabetic cardiomyopathy: Role of the adenosine monophosphate-activated protein kinase/sirtuin 1 pathway. Clin. Exp. Pharmacol. Physiol. 2014, 41, 127–133. [Google Scholar] [CrossRef]
- Thirunavukkarasu, M.; Penumathsa, S.V.; Koneru, S.; Juhasz, B.; Zhan, L.; Otani, H.; Bagchi, D.; Das, D.K.; Maulik, N. Resveratrol alleviates cardiac dysfunction in streptozotocin-induced diabetes: Role of nitric oxide, thioredoxin, and heme oxygenase. Free Radic. Biol. Med. 2007, 43, 720–729. [Google Scholar] [CrossRef]
- Penumathsa, S.V.; Thirunavukkarasu, M.; Koneru, S.; Juhasz, B.; Zhan, L.; Pant, R.; Menon, V.P.; Otani, H.; Maulik, N. Statin and resveratrol in combination induces cardioprotection against myocardial infarction in hypercholesterolemic rat. J. Mol. Cell. Cardiol. 2007, 42, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Juhasz, B.; Kertesz, A.; Balla, J.; Balla, G.; Szabo, Z.; Bombicz, M.; Priksz, D.; Gesztelyi, R.; Varga, B.; Haines, D.D.; et al. Cardioprotective effects of sour cherry seed extract (scse) on the hypercholesterolemic rabbit heart. Curr. Pharm. Des. 2013, 19, 6896–6905. [Google Scholar] [CrossRef] [PubMed]
- Juhasz, B.; Thirunavukkarasu, M.; Pant, R.; Zhan, L.; Penumathsa, S.V.; Secor, E.R., Jr.; Srivastava, S.; Raychaudhuri, U.; Menon, V.P.; Otani, H.; et al. Bromelain induces cardioprotection against ischemia-reperfusion injury through akt/foxo pathway in rat myocardium. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, 1365–1370. [Google Scholar] [CrossRef]
- Erdal, N.; Gurgul, S.; Kavak, S.; Yildiz, A.; Emre, M. Deterioration of bone quality by streptozotocin (stz)-induced type 2 diabetes mellitus in rats. Biol. Trace Elem. Res. 2011, 140, 342–353. [Google Scholar] [CrossRef] [PubMed]
- Sadek, K.M.; Lebda, M.A.; Nasr, S.M.; Shoukry, M. Spirulina platensis prevents hyperglycemia in rats by modulating gluconeogenesis and apoptosis via modification of oxidative stress and mapk-pathways. Biomed. Pharmacother. 2017, 92, 1085–1094. [Google Scholar] [CrossRef]
- Kolniak-Ostek, J.; Oszmiański, J. Characterization of phenolic compounds in different anatomical pear (pyrus communis l.) parts by ultra-performance liquid chromatography photodiode detector-quadrupole/time of flight-mass spectrometry (uplc-pda-q/tof-ms). Int. J. Mass Spectrom. 2015, 392, 154–163. [Google Scholar] [CrossRef]
- Lee, S.; Keirsey, K.I.; Kirkland, R.; Grunewald, Z.I.; Fischer, J.G.; de La Serre, C.B. Blueberry supplementation influences the gut microbiota, inflammation, and insulin resistance in high-fat-diet-fed rats. J. Nutr. 2018, 148, 209–219. [Google Scholar] [CrossRef]
- Badale, A.; Pallag, A.; Kozma, M.; Hegedus, C.; Kovacs, D.; Hajnalka, G.; Zdrîncă, M.; Magyar, I.; Marc, F.; Nemeth, S.; et al. Fenugreek seed and its active agent diosgenin treatment effects on different metabolic parameters in rats. Farmacia 2019, 67, 92. [Google Scholar] [CrossRef]
Sample Availability: Not available. |








| No | RT (min) | Compound | Concentration of Phenolic Compounds (µg g−1 Dry Mass) |
|---|---|---|---|
| 1 | 4.18 | Chlorogenic acid | 1.735 |
| 2 | 4.35 | Ferulic acid | 0.355 |
| 3 | 4.624 | Quercetin-3-O-glucoside | 27.13 |
| 4 | 4.810 | Quercetin-3-O-rutinoside | 36.52 |
| Day | HC | DC | 50HT | 100HT | 200HT | ||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 242.83 ± 4.46 | 252.17 ± 4.85 | 243.29 ± 5.41 | 240.25 ± 5.87 | 236.43 ± 10.70 | ||||
| 3 | 279.17 ± 4.51 | 245.50 ± 3.36 | * | 248.14 ± 4.70 | 260.50 ± 4.92 | 256.43 ± 6.54 | |||
| 6 | 306.17 ± 5.25 | 262.50 ± 5.99 | ** | 255.43 ± 5.94 | *** | 275.13 ± 5.28 | * | 265.86 ± 8.75 | ** |
| 10 | 322.33 ± 5.58 | 277.33 ± 4.39 | ** | 283.14 ± 5.99 | ** | 296.75 ± 6.07 | 291.71 ± 9.29 | ||
| 13 | 339.50 ± 7.89 | 280.83 ± 3.26 | *** | 297.57 ± 5.76 | ** | 310.25 ± 5.84 | 302.86 ± 9.85 | * | |
| 17 | 358.50 ± 5.77 | 268.67 ± 5.45 | *** | 308.43 ± 7.03 | *** ## | 316.25 ± 6.23 | ** ### | 317.86 ± 10.72 | ** ### |
| 20 | 377.50 ± 6.05 | 275.00 ± 6.28 | *** | 301.43 ± 8.07 | *** | 316.38 ± 7.41 | *** ## | 313.71 ± 12.79 | *** ## |
| 24 | 396.83 ± 6.35 | 276.67 ± 7.83 | *** | 318.57 ± 8.99 | *** ## | 332.38 ± 8.20 | *** ### | 329.86 ± 13.16 | *** ### |
| 30 | 409.83 ± 6.31 | 286.83 ± 7.60 | *** | 318.43 ± 9.36 | *** | 338.63 ± 9.11 | *** ### | 329.71 ± 15.59 | *** ## |
| 33 | 424.00 ± 5.56 | 296.67 ± 7.43 | *** | 323.00 ± 8.84 | *** | 345.38 ± 7.56 | *** ### | 334.86 ± 13.29 | *** # |
| 38 | 450.17 ± 6.52 | 296.50 ± 8.92 | *** | 333.14 ± 8.80 | *** # | 354.38 ± 9.00 | *** ### | 342.00 ± 12.33 | *** ## |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hegedűs, C.; Muresan, M.; Badale, A.; Bombicz, M.; Varga, B.; Szilágyi, A.; Sinka, D.; Bácskay, I.; Popoviciu, M.; Magyar, I.; et al. SIRT1 Activation by Equisetum arvense L. (Horsetail) Modulates Insulin Sensitivity in Streptozotocin Induced Diabetic Rats. Molecules 2020, 25, 2541. https://doi.org/10.3390/molecules25112541
Hegedűs C, Muresan M, Badale A, Bombicz M, Varga B, Szilágyi A, Sinka D, Bácskay I, Popoviciu M, Magyar I, et al. SIRT1 Activation by Equisetum arvense L. (Horsetail) Modulates Insulin Sensitivity in Streptozotocin Induced Diabetic Rats. Molecules. 2020; 25(11):2541. https://doi.org/10.3390/molecules25112541
Chicago/Turabian StyleHegedűs, Csaba, Mariana Muresan, Andrea Badale, Mariann Bombicz, Balázs Varga, Anna Szilágyi, Dávid Sinka, Ildikó Bácskay, Mihaela Popoviciu, Ioan Magyar, and et al. 2020. "SIRT1 Activation by Equisetum arvense L. (Horsetail) Modulates Insulin Sensitivity in Streptozotocin Induced Diabetic Rats" Molecules 25, no. 11: 2541. https://doi.org/10.3390/molecules25112541
APA StyleHegedűs, C., Muresan, M., Badale, A., Bombicz, M., Varga, B., Szilágyi, A., Sinka, D., Bácskay, I., Popoviciu, M., Magyar, I., Szarvas, M. M., Szőllősi, E., Németh, J., Szilvássy, Z., Pallag, A., & Kiss, R. (2020). SIRT1 Activation by Equisetum arvense L. (Horsetail) Modulates Insulin Sensitivity in Streptozotocin Induced Diabetic Rats. Molecules, 25(11), 2541. https://doi.org/10.3390/molecules25112541








