Protein Kinase CK2 Controls CaV2.1-Dependent Calcium Currents and Insulin Release in Pancreatic β-cells
Abstract
:1. Introduction
2. Results
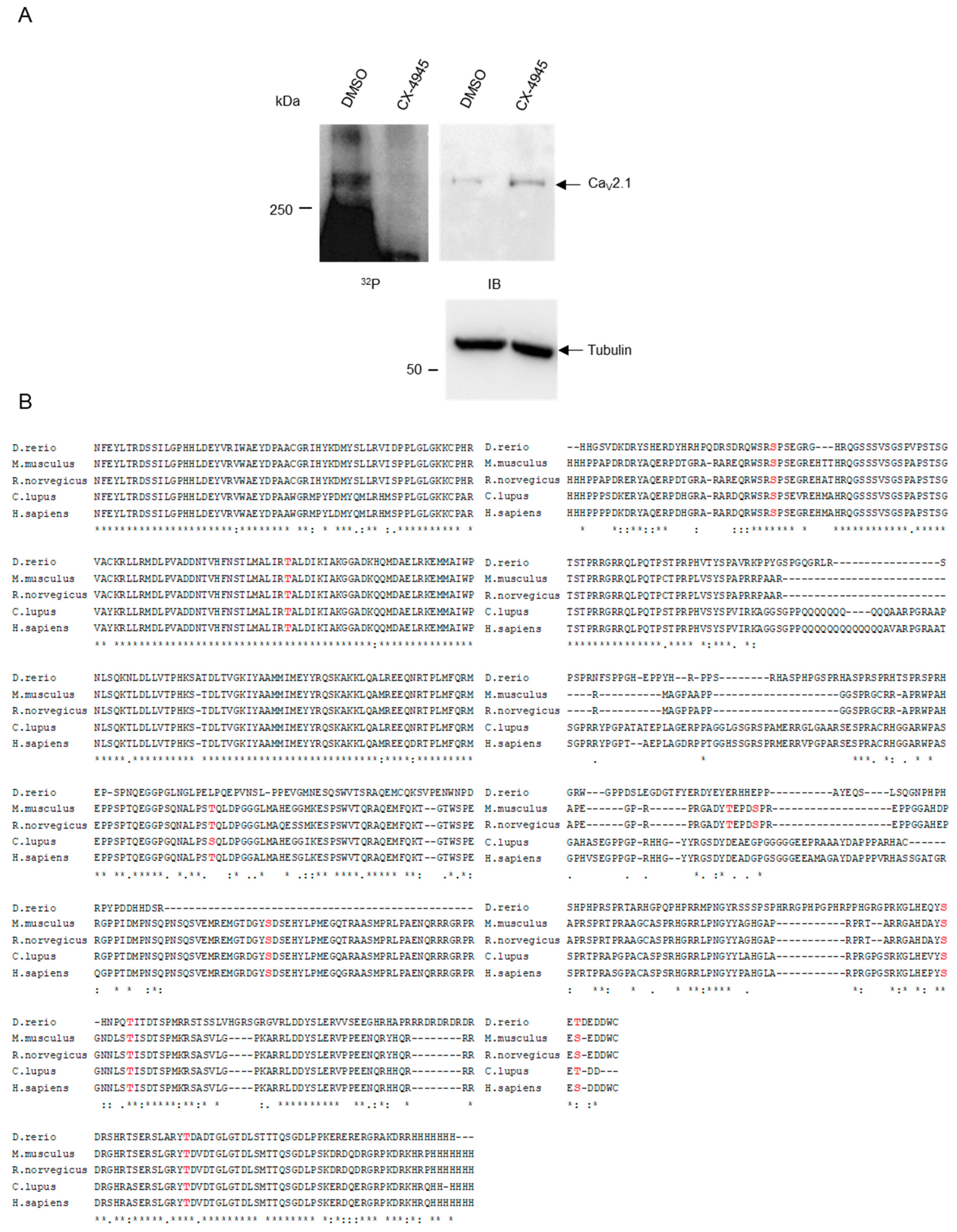
2.1. CK2 Phosphorylates CaV2.1 at Serine Residues S2362 and S2364
2.2. CK2 Interacts with C-Terminal Residues of CaV2.1.
2.3. Inhibition of CK2 Activity in INS-1 Cells Leads to a Rise in Cytosolic Ca2+-Concentration.
2.4. The Rise in Cytosolic Ca2+ after Pharmacological Inhibition of CK2 is Dependent on the Presence of CaV2.1.
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Treatment of Cells
4.2. siRNA Transfection
4.3. Immunoblot
4.4. Immunoprecipitation
4.5. Insulin Secretion
4.6. Calcium Imaging
4.7. Plasmids
4.8. GST Pull-Down Assay
4.9. In Vitro Phosphorylation
4.10. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CaM | Calmodulin |
| DMSO | Dimethyl sulfoxide |
| SDS | Sodium dodecylsulfate |
| PVDF | Polyvinylidene difluoride |
| KRBH | Krebs–Ringer bicarbonate HEPES buffer |
References
- Fu, Z.; Gilbert, E.R.; Liu, D. Regulation of insulin synthesis and secretion and pancreatic Beta-cell dysfunction in diabetes. Curr. Diabetes Rev. 2013, 9, 25–53. [Google Scholar] [CrossRef] [PubMed]
- Ampofo, E.; Nalbach, L.; Menger, M.D.; Montenarh, M.; Götz, C. Protein kinase CK2-A putative target for the therapy of diabetes mellitus. Int. J. Mol. Sci. 2019, 20, 4398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faust, M.; Montenarh, M. Subcellular localization of protein kinase CK2: A key to its function? Cell Tissue Res. 2000, 301, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Boldyreff, B.; Meggio, F.; Pinna, L.A.; Issinger, O.-G. Protein kinase CK2 structure-function relationship:Effects of the b subunit on reconstitution and activity. Cell Mol. Biol. Res. 1994, 40, 391–399. [Google Scholar] [PubMed]
- Heriche, J.K.; Lebrin, F.; Rabilloud, T.; LeRoy, D.; Chambaz, E.M.; Goldberg, Y. Regulation of protein phosphatase 2A by direct interaction with casein kinase 2alpha. Science 1997, 276, 952–955. [Google Scholar] [CrossRef]
- Guerra, B. Protein kinase CK2 subunits are positive regulators of AKT kinase. Int. J. Oncol. 2006, 28, 685–693. [Google Scholar] [CrossRef] [Green Version]
- Shehata, M.; Schnabl, S.; Demirtas, D.; Hilgarth, M.; Hubmann, R.; Ponath, E.; Badrnya, S.; Lehner, C.; Hoelbl, A.; Duechler, M.; et al. Reconstitution of PTEN activity by CK2 inhibitors and interference with the PI3-K/Akt cascade counteract the antiapoptotic effect of human stromal cells in chronic lymphocytic leukemia. Blood 2010, 116, 2513–2521. [Google Scholar] [CrossRef]
- Wang, S.; Jones, K.A. CK2 controls the recruitment of Wnt regulators to target genes in vivo. Curr. Biol. 2006, 16, 2239–2244. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Wang, H.Y. Casein kinase 2 Is activated and essential for Wnt/beta-catenin signaling. J. Biol. Chem. 2006, 281, 18394–18400. [Google Scholar] [CrossRef] [Green Version]
- Ponce, D.P.; Yefi, R.; Cabello, P.; Maturana, J.L.; Niechi, I.; Silva, E.; Galindo, M.; Antonelli, M.; Marcelain, K.; Armisen, R.; et al. CK2 functionally interacts with AKT/PKB to promote the beta-catenin-dependent expression of survivin and enhance cell survival. Mol. Cell Biochem. 2011, 356, 127–132. [Google Scholar] [CrossRef]
- Ponce, D.P.; Maturana, J.L.; Cabello, P.; Yefi, R.; Niechi, I.; Silva, E.; Armisen, R.; Galindo, M.; Antonelli, M.; Tapia, J.C. Phosphorylation of AKT/PKB by CK2 is necessary for the AKT-dependent up-regulation of beta-catenin transcriptional activity. J. Cell Physiol. 2011, 226, 1953–1959. [Google Scholar] [CrossRef] [PubMed]
- Ampofo, E.; Sokolowsky, T.; Götz, C.; Montenarh, M. Functional interaction of protein kinase CK2 and activating transcription factor 4 (ATF4), a key player in the cellular stress response. Biochim. Biophys. Acta. Mol. Cell Res. 2013, 1833, 439–451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Götz, C.; Montenarh, M. Protein kinase CK2 in the ER stress response. Ad. Biol. Chem. 2013, 3, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Montenarh, M. Protein kinase CK2 in DNA damage and repair. Transl. Cancer Res. 2016, 5, 49–63. [Google Scholar]
- Montenarh, M. Protein kinase CK2 and angiogenesis. Adv. Clin. Exp. Med. 2014, 23, 153–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Götz, C.; Montenarh, M. Protein kinase CK2 in development and differentiation. Biomed. Rep. 2016, 6, 127–133. [Google Scholar] [CrossRef]
- Al-Quobaili, F.; Montenarh, M. CK2 and the regulation of the carbohydrate metabolism. Metabolism 2012, 61, 1512–1517. [Google Scholar] [CrossRef]
- Lezmy, J.; Lipinsky, M.; Khrapunsky, Y.; Patrich, E.; Shalom, L.; Peretz, A.; Fleidervish, I.A.; Attali, B. M-current inhibition rapidly induces a unique CK2-dependent plasticity of the axon initial segment. Proc. Natl. Acad. Sci. USA 2017, 114, E10234–E10243. [Google Scholar] [CrossRef] [Green Version]
- Xu, M.; Cooper, E.C. An Ankyrin-G N-terminal Gate and Protein Kinase CK2 Dually Regulate Binding of Voltage-gated Sodium and KCNQ2/3 Potassium Channels. J. Biol. Chem. 2015, 290, 16619–16632. [Google Scholar] [CrossRef] [Green Version]
- Brechet, A.; Fache, M.P.; Brachet, A.; Ferracci, G.; Baude, A.; Irondelle, M.; Pereira, S.; Leterrier, C.; Dargent, B. Protein kinase CK2 contributes to the organization of sodium channels in axonal membranes by regulating their interactions with ankyrin G. J. Cell Biol. 2008, 183, 1101–1114. [Google Scholar] [CrossRef] [Green Version]
- Stocker, M.; Pedarzani, P. Differential distribution of three Ca(2+)-activated K(+) channel subunits, SK1, SK2, and SK3, in the adult rat central nervous system. Mol. Cell Neurosci. 2000, 15, 476–493. [Google Scholar] [CrossRef] [PubMed]
- Brunet, S.; Emrick, M.A.; Sadilek, M.; Scheuer, T.; Catterall, W.A. Phosphorylation Sites in the Hook Domain of Cabeta Subunits Differentially Modulate Ca1.2 Channel Function. J. Mol. Cell. Cardiol. 2015, 87, 248–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kashihara, T.; Nakada, T.; Kojima, K.; Takeshita, T.; Yamada, M. Angiotensin II activates CaV 1.2 Ca2+ channels through beta-arrestin2 and casein kinase 2 in mouse immature cardiomyocytes. J. Physiol. 2017, 595, 4207–4225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braun, M.; Ramracheya, R.; Bengtsson, M.; Zhang, Q.; Karanauskaite, J.; Partridge, C.; Johnson, P.R.; Rorsman, P. Voltage-gated ion channels in human pancreatic beta-cells: Electrophysiological characterization and role in insulin secretion. Diabetes 2008, 57, 1618–1628. [Google Scholar] [CrossRef] [Green Version]
- Meng, R.; Götz, C.; Montenarh, M. The role of protein kinase CK2 in the regulation of the insulin production of pancreatic islets. Biochem. Biophys. Res. Commun. 2010, 401, 203–206. [Google Scholar] [CrossRef]
- Spohrer, S.; Gross, R.; Nalbach, L.; Schwind, L.; Stumpf, H.; Menger, M.D.; Ampofo, E.; Montenarh, M.; Götz, C. Functional interplay between the transcription factors USF1 and PDX-1 and protein kinase CK2 in pancreatic b-cells. Sci. Rep. 2017, 7, 16367. [Google Scholar] [CrossRef] [Green Version]
- Rossi, M.; Ruiz, D.A., I; Barella, L.F.; Sakamoto, W.; Zhu, L.; Cui, Y.; Lu, H.; Rebholz, H.; Matschinsky, F.M.; Doliba, N.M.; et al. CK2 acts as a potent negative regulator of receptor-mediated insulin release in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2015, 112, E6818–E6824. [Google Scholar] [CrossRef] [Green Version]
- Kahle, J.J.; Gulbahce, N.; Shaw, C.A.; Lim, J.; Hill, D.E.; Barabasi, A.L.; Zoghbi, H.Y. Comparison of an expanded ataxia interactome with patient medical records reveals a relationship between macular degeneration and ataxia. Hum. Mol. Genet. 2011, 20, 510–527. [Google Scholar] [CrossRef]
- Marin, O.; Meggio, F.; Marchiori, F.; Borin, G.; Pinna, L.A. Site specificity of casein kinase-2 (TS) from rat liver cytosol. A study with model peptide substrates. Eur. J. Biochem. 1986, 160, 239–244. [Google Scholar] [CrossRef]
- Kuenzel, E.A.; Mulligan, J.A.; Sommercorn, J.; Krebs, E.G. Substrat specificity determinants for casein kinase II as deduced from studies with synthetic peptides. J. Biol. Chem. 1987, 262, 9136–9140. [Google Scholar]
- Sarno, S.; Boldyreff, B.; Marin, O.; Guerra, B.; Meggio, F.; Issinger, O.G.; Pinna, L.A. Mapping the residues of protein kinase CK2 implicated in substrate recognition: Mutagenesis of conserved basic residues in the alpha-subunit. Biochem. Biophys. Res. Commun. 1995, 206, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Poletto, G.; Vilardell, J.; Marin, O.; Pagano, M.A.; Cozza, G.; Sarno, S.; Falques, A.; Itarte, E.; Pinna, L.A.; Meggio, F. The regulatory beta subunit of protein kinase CK2 contributes to the recognition of the substrate consensus sequence. a study with an eIF2beta-derived peptide. Biochemistry 2008, 47, 8317–8325. [Google Scholar] [CrossRef]
- Melloul, D. Transcription factors in islet development and physiology: Role of PDX-1 in beta-cell function. Ann. N. Y. Acad. Sci. 2004, 1014, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Read, M.L.; Clark, A.R.; Docherty, K. The helix-loop-helix transcription factor USF (upstream stimulating factor) binds to a regulatory sequence of the human insulin gene enhancer. Biochem. J. 1993, 295, 233–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klein, S.; Meng, R.; Montenarh, M.; Götz, C. The phosphorylation of PDX-1 by protein kinase CK2 is crucial for its stability. Pharmaceuticals (Basel) 2016, 10, pii2. [Google Scholar] [CrossRef] [Green Version]
- Schmitt, B.M.; Ampofo, E.; Stumpf, H.; Montenarh, M.; Götz, C. The stability of CREB3/Luman is regulated by protein kinase CK2 phosphorylation. Biochem. Biophys. Res. Commun. 2020, 523, 639–644. [Google Scholar] [CrossRef]
- Meng, R.; Al-Quobaili, F.; Müller, I.; Götz, C.; Thiel, G.; Montenarh, M. CK2 phosphorylation of Pdx-1 regulates its transcription factor activity. Cell Mol. Life Sci. 2010, 67, 2481–2489. [Google Scholar] [CrossRef]
- Welker, S.; Götz, C.; Servas, C.; Laschke, M.W.; Menger, M.D.; Montenarh, M. Glucose regulates protein kinase CK2 in pancreatic ß-cells and its interaction with PDX-1. Int. J. Biochem. Cell Biol. 2013, 45, 2786–2795. [Google Scholar] [CrossRef]
- Lan, Y.C.; Wang, Y.H.; Chen, H.H.; Lo, S.F.; Chen, S.Y.; Tsai, F.J. Effects of Casein Kinase 2 Alpha 1 Gene Expression on Mice Liver Susceptible to Type 2 Diabetes Mellitus and Obesity. Int. J. Med. Sci. 2020, 17, 13–20. [Google Scholar] [CrossRef] [Green Version]
- Iori, E.; Ruzzene, M.; Zanin, S.; Sbrignadello, S.; Pinna, L.A.; Tessari, P. Effects of CK2 inhibition in cultured fibroblasts from Type 1 Diabetic patients with or without nephropathy. Growth Factors 2015, 33, 259–266. [Google Scholar] [CrossRef]
- Yang, S.N.; Berggren, P.O. The role of voltage-gated calcium channels in pancreatic beta-cell physiology and pathophysiology. Endocr. Rev. 2006, 27, 621–676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ligon, B.; Boyd, A.E., III; Dunlap, K. Class A calcium channel variants in pancreatic islets and their role in insulin secretion. J. Biol. Chem. 1998, 273, 13905–13911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sher, E.; Giovannini, F.; Codignola, A.; Passafaro, M.; Giorgi-Rossi, P.; Volsen, S.; Craig, P.; Davalli, A.; Carrera, P. Voltage-operated calcium channel heterogeneity in pancreatic beta cells: Physiopathological implications. J. Bioenerg. Biomembr. 2003, 35, 687–696. [Google Scholar] [CrossRef] [PubMed]
- Catterall, W.A. Structure and regulation of voltage-gated Ca2+ channels. Annu. Rev. Cell Dev. Biol. 2000, 16, 521–555. [Google Scholar] [CrossRef]
- Takiyama, Y.; Sakoe, K.; Namekawa, M.; Soutome, M.; Esumi, E.; Ogawa, T.; Ishikawa, K.; Mizusawa, H.; Nakano, I.; Nishizawa, M. A Japanese family with spinocerebellar ataxia type 6 which includes three individuals homozygous for an expanded CAG repeat in the SCA6/CACNL1A4 gene. J. Neurol. Sci. 1998, 158, 141–147. [Google Scholar] [CrossRef]
- Meggio, F.; Pinna, L.A. One-thousand-and-one substrates of protein kinase CK2? FASEB J. 2003, 17, 349–368. [Google Scholar] [CrossRef]
- Humphrey, S.J.; Yang, G.; Yang, P.; Fazakerley, D.J.; Stockli, J.; Yang, J.Y.; James, D.E. Dynamic adipocyte phosphoproteome reveals that Akt directly regulates mTORC2. Cell Metab. 2013, 17, 1009–1020. [Google Scholar] [CrossRef] [Green Version]
- Jahn, H.; Nastainczyk, W.; Rohrkasten, A.; Schneider, T.; Hofmann, F. Site-specific phosphorylation of the purified receptor for calcium-channel blockers by cAMP- and cGMP-dependent protein kinases, protein kinase C, calmodulin-dependent protein kinase II and casein kinase II. Eur. J. Biochem. 1988, 178, 535–542. [Google Scholar] [CrossRef]
- Fu, Y.; Westenbroek, R.E.; Scheuer, T.; Catterall, W.A. Basal and beta-adrenergic regulation of the cardiac calcium channel CaV1.2 requires phosphorylation of serine 1700. Proc. Natl. Acad. Sci. USA 2014, 111, 16598–16603. [Google Scholar] [CrossRef] [Green Version]
- Fu, Y.; Westenbroek, R.E.; Scheuer, T.; Catterall, W.A. Phosphorylation sites required for regulation of cardiac calcium channels in the fight-or-flight response. Proc. Natl. Acad. Sci. USA 2013, 110, 19621–19626. [Google Scholar] [CrossRef] [Green Version]
- Mochida, S. Presynaptic Calcium Channels. Int. J. Mol. Sci. 2019, 20, e2217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, A.; Wong, S.T.; Gallagher, D.; Li, B.; Storm, D.R.; Scheuer, T.; Catterall, W.A. Ca2+/calmodulin binds to and modulates P/Q-type calcium channels. Nature 1999, 399, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Pate, P.; Mochca-Morales, J.; Wu, Y.; Zhang, J.Z.; Rodney, G.G.; Serysheva, I.I.; Williams, B.Y.; Anderson, M.E.; Hamilton, S.L. Determinants for calmodulin binding on voltage-dependent Ca2+ channels. J. Biol. Chem. 2000, 275, 39786–39792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, A.; Scheuer, T.; Catterall, W.A. Ca2+/calmodulin-dependent facilitation and inactivation of P/Q-type Ca2+ channels. J. Neurosci. 2000, 20, 6830–6838. [Google Scholar] [CrossRef] [Green Version]
- Meggio, F.; Brunati, A.M.; Pinna, L.A. Polycation-dependent, Ca2+-antagonized phosphorylation of calmodulin by casein kinase-2 and a spleen tyrosine protein kinase. FEBS Lett. 1987, 215, 241–246. [Google Scholar] [CrossRef] [Green Version]
- Sacks, D.B.; Davis, H.W.; Crimmins, D.L.; McDonald, J.M. Insulin-stimulated phosphorylation of calmodulin. Biochem. J. 1992, 286, 211–216. [Google Scholar] [CrossRef] [Green Version]
- Marin, O.; Meggio, F.; Pinna, L.A. Structural features underlying the unusual mode of calmodulin phosphorylation by protein kinase CK2: A study with synthetic calmodulin fragments. Biochem. Biophys. Res. Commun. 1999, 256, 442–446. [Google Scholar] [CrossRef]
- Simms, B.A.; Zamponi, G.W. Trafficking and stability of voltage-gated calcium channels. Cell Mol. Life Sci. 2012, 69, 843–856. [Google Scholar] [CrossRef]
- Lübbert, M.; Goral, R.O.; Satterfield, R.; Putzke, T.; van den Maagdenberg, A.M.; Kamasawa, N.; Young, S.M., Jr. A novel region in the CaV2.1 alpha1 subunit C-terminus regulates fast synaptic vesicle fusion and vesicle docking at the mammalian presynaptic active zone. Elife 2017, 6, e28412. [Google Scholar] [CrossRef] [Green Version]
- Kaeser, P.S.; Deng, L.; Wang, Y.; Dulubova, I.; Liu, X.; Rizo, J.; Sudhof, T.C. RIM proteins tether Ca2+ channels to presynaptic active zones via a direct PDZ-domain interaction. Cell 2011, 144, 282–295. [Google Scholar] [CrossRef] [Green Version]
- Maximov, A.; Sudhof, T.C.; Bezprozvanny, I. Association of neuronal calcium channels with modular adaptor proteins. J. Biol. Chem. 1999, 274, 24453–24456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hohmeier, H.E.; Mulder, H.; Chen, G.; Henkel-Rieger, R.; Prentki, M.; Newgard, C.B. Isolation of INS-1-derived cell lines with robust ATP-sensitive K+ channel-dependent and -independent glucose-stimulated insulin secretion. Diabetes 2000, 49, 424–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Servas, C.; Kiehlmeier, S.; Hach, J.; Götz, C.; Montenarh, M. The mammalian STE20-like kinase 1 (MST1) is a substrate for the apoptosis inhibiting protein kinase CK2. Cell Signal 2017, 36, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Nastainczyk, W.; Issinger, O.G.; Guerra, B. Epitope analysis of the MAb 1AD9 antibody detection site in human protein kinase CK2alpha-subunit. Hybrid Hybridomics 2003, 22, 87–90. [Google Scholar] [CrossRef]
- Merglen, A.; Theander, S.; Rubi, B.; Chaffard, G.; Wollheim, C.B.; Maechler, P. Glucose sensitivity and metabolism-secretion coupling studied during two-year continuous culture in INS-1E insulinoma cells. Endocrinology 2004, 145, 667–678. [Google Scholar] [CrossRef]
- Richards, K.S.; Swensen, A.M.; Lipscombe, D.; Bommert, K. Novel CaV2.1 clone replicates many properties of Purkinje cell CaV2.1 current. Eur. J. Neurosci. 2007, 26, 2950–2961. [Google Scholar] [CrossRef]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scheuer, R.; Philipp, S.E.; Becker, A.; Nalbach, L.; Ampofo, E.; Montenarh, M.; Götz, C. Protein Kinase CK2 Controls CaV2.1-Dependent Calcium Currents and Insulin Release in Pancreatic β-cells. Int. J. Mol. Sci. 2020, 21, 4668. https://doi.org/10.3390/ijms21134668
Scheuer R, Philipp SE, Becker A, Nalbach L, Ampofo E, Montenarh M, Götz C. Protein Kinase CK2 Controls CaV2.1-Dependent Calcium Currents and Insulin Release in Pancreatic β-cells. International Journal of Molecular Sciences. 2020; 21(13):4668. https://doi.org/10.3390/ijms21134668
Chicago/Turabian StyleScheuer, Rebecca, Stephan Ernst Philipp, Alexander Becker, Lisa Nalbach, Emmanuel Ampofo, Mathias Montenarh, and Claudia Götz. 2020. "Protein Kinase CK2 Controls CaV2.1-Dependent Calcium Currents and Insulin Release in Pancreatic β-cells" International Journal of Molecular Sciences 21, no. 13: 4668. https://doi.org/10.3390/ijms21134668





