Antioxidative Action of Ellagic Acid—A Kinetic DFT Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Computational Methods
2.2. Quantum Mechanics-Based Test for Overall, Free-Radical Scavenging Activity
2.2.1. Thermodynamic Considerations
2.2.2. Kinetic Considerations
2.2.3. Relative Antioxidative Activity
3. Results and Discussion
3.1. Thermodynamic Considerations
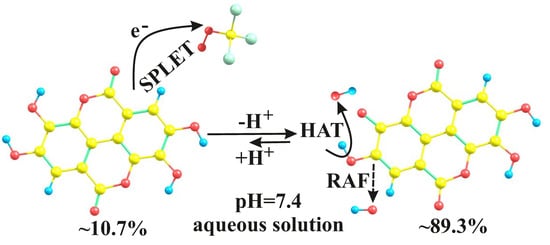
3.2. Kinetic Considerations
3.3. Relative Antioxidative Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Craft, B.D.; Kerrihard, A.L.; Amarowicz, R.; Pegg, R.B. Phenol-Based Antioxidants and the In Vitro Methods Used for Their Assessment. Compr. Rev. Food Sci. Food Saf. 2012, 11, 148–173. [Google Scholar] [CrossRef]
- Verotta, L.; Panzella, L.; Antenucci, S.; Calvenzani, V.; Tomay, F.; Petroni, K.; Caneva, E.; Napolitano, A. Fermented Pomegranate Wastes as Sustainable Source of Ellagic Acid: Antioxidant Properties, Anti-inflammatory Action, and Controlled Release Under Simulated Digestion Conditions. Food Chem. 2018, 246, 129–136. [Google Scholar] [CrossRef]
- Bobinaitė, R.; Viškelis, P.; Venskutonis, P.R. Variation of Total Phenolics, Anthocyanins, Ellagic Acid and Radical Scavenging Capacity in Various Raspberry (Rubus spp.) Cultivars. Food Chem. 2012, 132, 1495–1501. [Google Scholar]
- García-Estévez, I.; Escribano-Bailón, M.T.; Rivas-Gonzalo, J.C.; Alcalde-Eon, C. Validation of a Mass Spectrometry Method To Quantify Oak Ellagitannins in Wine Samples. J. Agric. Food Chem. 2012, 60, 1373–1379. [Google Scholar] [CrossRef]
- González-Sarrías, A.; Miguel, V.; Merino, G.; Lucas, R.; Morales, J.C.; Tomás-Barberán, F.; Álvarez, A.I.; Espín, J.C. The Gut Microbiota Ellagic Acid-Derived Metabolite Urolithin A and Its Sulfate Conjugate Are Substrates for the Drug Efflux Transporter Breast Cancer Resistance Protein (ABCG2/BCRP). J. Agric. Food Chem. 2013, 61, 4352–4359. [Google Scholar] [CrossRef]
- Rogerio, A.P.; Fontanari, C.; Borducchi, É.; Keller, A.C.; Russo, M.; Soares, E.G.; Albuquerque, D.A.; Faccioli, L.H. Anti-inflammatory Effects of Lafoensia pacari and Ellagic Acid in a Murine Model of Asthma. Eur. J. Pharmacol. 2008, 580, 262–270. [Google Scholar] [CrossRef]
- Goodwin, E.C.; Atwood, W.J.; DiMaio, D. High-Throughput Cell-Based Screen for Chemicals That Inhibit Infection by Simian Virus 40 and Human Polyomaviruses. J. Virol. 2009, 83, 5630–5639. [Google Scholar] [CrossRef] [Green Version]
- Nohynek, L.; Alakomi, H.-L.; Kähkönen, M.; Heinonen, M.; M Helander, I.; Oksman-Caldentey, K.-M.; Puupponen-Pimiä, R. Berry Phenolics: Antimicrobial Properties and Mechanisms of Action Against Severe Human Pathogens. Nutr. Cancer 2006, 54, 18–32. [Google Scholar] [CrossRef]
- Lall, R.K.; Syed, D.N.; Adhami, V.M.; Khan, M.I.; Mukhtar, H. Dietary Polyphenols in Prevention and Treatment of Prostate Cancer. Int. J. Mol. Sci. 2015, 16, 3350–3376. [Google Scholar] [CrossRef]
- Santos, I.S.; Ponte, B.M.; Boonme, P.; Silva, A.M.; Souto, E.B. Nanoencapsulation of Polyphenols for Protective Effect Against Colon–rectal Cancer. Biotechnol. Adv. 2013, 31, 514–523. [Google Scholar] [CrossRef]
- Brglez Mojzer, E.; Knez Hrnčič, M.; Škerget, M.; Knez, Ž.; Bren, U. Polyphenols: Extraction Methods, Antioxidative Action, Bioavailability and Anticarcinogenic Effects. Molecules 2016, 21, 901. [Google Scholar] [CrossRef] [PubMed]
- Stoner, G.D.; Mukhtar, H. Polyphenols as Cancer Chemopreventive Agents. J. Cell. Biochem. 1995, 59, 169–180. [Google Scholar] [CrossRef]
- Hostnik, G.; Gladović, M.; Bren, U. Tannin Basic Building Blocks as Potential Scavengers of Chemical Carcinogens: A Computational Study. J. Nat. Prod. 2019, 82, 3279–3287. [Google Scholar] [CrossRef] [PubMed]
- Larrosa, M.; García-Conesa, M.T.; Espín, J.C.; Tomás-Barberán, F.A. Ellagitannins, Ellagic Acid and Vascular Health. Mol. Asp. Med. 2010, 31, 513–539. [Google Scholar] [CrossRef]
- Malik, A.; Afaq, S.; Shahid, M.; Akhtar, K.; Assiri, A. Influence of ellagic acid on prostate cancer cell proliferation: A caspase–dependent pathway. Asian Pac. J. Trop. Med. 2011, 4, 550–555. [Google Scholar] [CrossRef] [Green Version]
- Alkayali, A. Ellagic Acid Food Supplement Prepared from Pomegranate Seed. U.S. Patent US 2006/0280819 A1, 14 December 2006. [Google Scholar]
- Galano, A.; Francisco Marquez, M.; Pérez-González, A. Ellagic Acid: An Unusually Versatile Protector against Oxidative Stress. Chem. Res. Toxicol. 2014, 27, 904–918. [Google Scholar] [CrossRef] [PubMed]
- Priyadarsini, K.I.; Khopde, S.M.; Kumar, S.S.; Mohan, H. Free Radical Studies of Ellagic Acid, a Natural Phenolic Antioxidant. J. Agric. Food Chem. 2002, 50, 2200–2206. [Google Scholar] [CrossRef]
- Hassoun, E.A.; Walter, A.C.; Alsharif, N.Z.; Stohs, S.J. Modulation of TCDD-induced Fetotoxicity and Oxidative Stress in Embryonic and Placental Tissues of C57BL/6J Mice by Vitamin E Succinate and Ellagic Acid. Toxicology 1997, 124, 27–37. [Google Scholar] [CrossRef]
- Marković, Z.; Milenković, D.; Đorović, J.; Dimitrić Marković, J.M.; Lučić, B.; Amić, D. A DFT and PM6 Study of Free Radical Scavenging Activity of Ellagic Acid. Mon. Chemie-Chem. Mon. 2013, 144, 803–812. [Google Scholar] [CrossRef]
- Mazzone, G.; Toscano, M.; Russo, N. Density Functional Predictions of Antioxidant Activity and UV Spectral Features of Nasutin A, Isonasutin, Ellagic Acid, and One of Its Possible Derivatives. J. Agric. Food Chem. 2013, 61, 9650–9657. [Google Scholar] [CrossRef]
- Tiwari, M.K.; Mishra, P.C. Modeling the Scavenging Activity of Ellagic Acid and its Methyl Derivatives Towards Hydroxyl, Methoxy, and Nitrogen Dioxide Radicals. J. Mol. Model. 2013, 19, 5445–5456. [Google Scholar] [CrossRef] [PubMed]
- Galano, A.; Mazzone, G.; Alvarez-Diduk, R.; Marino, T.; Alvarez-Idaboy, J.R.; Russo, N. Food Antioxidants: Chemical Insights at the Molecular Level. Annu. Rev. Food Sci. Technol. 2016, 7, 335–352. [Google Scholar] [CrossRef]
- Leopoldini, M.; Russo, N.; Toscano, M. The Molecular Basis of Working Mechanism of Natural Polyphenolic Antioxidants. Food Chem. 2011, 125, 288–306. [Google Scholar] [CrossRef]
- Lee, C.Y.; Sharma, A.; Semenya, J.; Anamoah, C.; Chapman, K.N.; Barone, V. Computational Study of Ortho-Substituent Effects on Antioxidant Activities of Phenolic Dendritic Antioxidants. Antioxidants 2020, 9, 189. [Google Scholar] [CrossRef] [Green Version]
- Cossi, M.; Rega, N.; Scalmani, G.; Barone, V. Energies, Structures, and Electronic Properties of Molecules in Solution with the C-PCM Solvation Model. J. Comput. Chem. 2003, 24, 669–681. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2013. [Google Scholar]
- Zhao, Y.; Truhlar, D.G. The M06 Suite of Density Functionals for Main Group Thermochemistry, Thermochemical Kinetics, Noncovalent Interactions, Excited States, and Transition Elements: Two New Functionals and Systematic Testing of Four M06-class Functionals and 12 Other Function. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Truhlar, D.G. Density Functionals with Broad Applicability in Chemistry. Acc. Chem. Res. 2008, 41, 157–167. [Google Scholar] [CrossRef]
- Tošović, J.; Marković, S.; Dimitrić Marković, J.M.; Mojović, M.; Milenković, D. Antioxidative Mechanisms in Chlorogenic Acid. Food Chem. 2017, 237, 390–398. [Google Scholar] [CrossRef]
- Tošović, J.; Marković, S. Reactivity of Chlorogenic Acid Toward Hydroxyl and Methyl Peroxy Radicals Relative to Trolox in Nonpolar Media. Theor. Chem. Acc. 2018, 137, 76. [Google Scholar] [CrossRef]
- Tošović, J.; Marković, S. Antioxidative Activity of Chlorogenic Acid Relative to Trolox in Aqueous Solution—DFT Study. Food Chem. 2019, 278, 469–475. [Google Scholar] [CrossRef]
- Marković, S.; Tošović, J. Comparative Study of the Antioxidative Activities of Caffeoylquinic and Caffeic Acids. Food Chem. 2016, 210, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Villuendas-Rey, Y.; Alvarez-Idaboy, J.R.; Galano, A. Assessing the Protective Activity of a Recently Discovered Phenolic Compound Against Oxidative Stress Using Computational Chemistry. J. Chem. Inf. Model. 2015, 55, 2552–2561. [Google Scholar] [CrossRef] [PubMed]
- Glendening, E.D.; Reed, A.E.; Carpenter, J.E.; Weinhold, F. NBO Version 3.1.; ScienceOpen, Inc.: Burlington, MA, USA, 2001. [Google Scholar]
- Galano, A.; Alvarez-Idaboy, J.R. A Computational Methodology for Accurate Predictions of Rate Constants in Solution: Application to the Assessment of Primary Antioxidant Activity. J. Comput. Chem. 2013, 34, 2430–2445. [Google Scholar] [CrossRef] [PubMed]
- Cordova-Gomez, M.; Galano, A.; Alvarez-Idaboy, J.R. Piceatannol, a Better Peroxyl Radical Scavenger than Resveratrol. RSC Adv. 2013, 3, 20209–20218. [Google Scholar] [CrossRef]
- Mazzone, G.; Russo, N.; Toscano, M. Antioxidant Properties Comparative Study of Natural Hydroxycinnamic Acids and Structurally Modified Derivatives: Computational Insights. Comput. Theor. Chem. 2016, 1077, 39–47. [Google Scholar] [CrossRef]
- Marino, T.; Russo, N.; Galano, A. A Deeper Insight on the Radical Scavenger Activity of Two Simple Coumarins Toward OOH Radical. Comput. Theor. Chem. 2016, 1077, 133–138. [Google Scholar] [CrossRef]
- Francisco-Marquez, M.; Galano, A. Detailed Investigation of the Outstanding Peroxyl Radical Scavenging Activity of Two Novel Amino-pyridinol-based Compounds. J. Chem. Inf. Model. 2019, 59, 3494–3505. [Google Scholar] [CrossRef]
- Castañeda-Arriaga, R.; Alvarez-Idaboy, J.R. Lipoic Acid and Dihydrolipoic Acid. A Comprehensive Theoretical Study of Their Antioxidant Activity Supported by Available Experimental Kinetic Data. J. Chem. Inf. Model. 2014, 54, 1642–1652. [Google Scholar]
- Ramis, R.; Ortega-Castro, J.; Caballero, C.; Casasnovas, R.; Cerrillo, A.; Vilanova, B.; Adrover, M.; Frau, J. How Does Pyridoxamine Inhibit the Formation of Advanced Glycation End Products? The Role of Its Primary Antioxidant Activity. Antioxidants 2019, 8, 344. [Google Scholar] [CrossRef] [Green Version]
- Eckart, C. The Penetration of a Potential Barrier by Electrons. Phys. Rev. 1930, 35, 1303–1309. [Google Scholar] [CrossRef]
- Duncan, W.T.; Bell, R.L.; Truong, T.N. TheRate: Program for Ab initio Direct Dynamics Calculations of Thermal and Vibrational-state-selected Rate Constants. J. Comput. Chem. 1998, 19, 1039–1052. [Google Scholar] [CrossRef]
- Marcus, R.A. Electron Transfer Reactions in Chemistry. Theory and Experiment. Rev. Mod. Phys. 1993, 65, 599–610. [Google Scholar] [CrossRef] [Green Version]
- Pan, J.X.; Wang, W.F.; Lin, W.Z.; Lu, C.Y.; Han, Z.H.; Yao, S.D.; Lin, N.Y. Interaction of Hydroxycinnamic Acid Derivatives with the Cl3COO Radical: A Pulse Radiolysis Study. Free. Radic. Res. 1999, 30, 241–245. [Google Scholar] [CrossRef]
- Wang, A.; Lu, Y.; Du, X.; Shi, P.; Zhang, H. A Quantum Chemical Study on the Reactivity of Four Licorice Flavonoids Scavenging ·OOCl 3 C. Struct. Chem. 2019, 30, 1795–1803. [Google Scholar] [CrossRef]
- Aruoma, O.I.; Murcia, A.; Butler, J.; Halliwell, B. Evaluation of the Antioxidant and Prooxidant Actions of Gallic Acid and Its Derivatives. J. Agric. Food Chem. 1993, 41, 1880–1885. [Google Scholar] [CrossRef]
- Alberto, M.E.; Russo, N.; Grand, A.; Galano, A. A Physicochemical Examination of the Free Radical Scavenging Activity of Trolox: Mechanism, Kinetics and Influence of the Environment. Phys. Chem. Chem. Phys. 2013, 15, 4642. [Google Scholar] [CrossRef]




| Mechanism | Position | EA | EA− | ||
|---|---|---|---|---|---|
| HO• | Cl3COO• | HO• | Cl3COO• | ||
| HAT | 1a | −144.0 | −36.3 | ||
| 2a | −144.2 | −36.4 | −167.5 | −59.7 | |
| 1a’ | −150.7 | −42.9 | |||
| 2a’ | −151.8 | −44.0 | |||
| RAF | 1 | −62.6 | 32.8 | −16.9 | / |
| 2 | −47.4 | 39.7 | −66.4 | 8.5 | |
| 3 | −47.9 | 32.9 | −43.4 | 33.7 | |
| 4 | −7.6 | 78.3 | −24.5 | 49.7 | |
| 5 | −11.2 | 66.1 | 0.5 | 66.7 | |
| 6 | −39.9 | 45.9 | −66.8 | 10.8 | |
| 1′ | −61.8 | 31.0 | |||
| 2′ | −47.8 | 42.0 | |||
| 3′ | −45.9 | 48.0 | |||
| 4′ | −6.0 | 75.1 | |||
| 5′ | −6.1 | 65.8 | |||
| 6′ | −40.0 | 50.8 | |||
| SPLET | 1a | −161.6 | −145.1 | ||
| / | 17.6 | 0.7 | −5.6 | −22.5 | |
| SET | / | 127.6 | 110.72 | 17.6 | 0.7 |
| Mechanism | Position | HO• | Cl3COO• | ||||||
|---|---|---|---|---|---|---|---|---|---|
| EA | EA− | EA | EA− | ||||||
| k | k | k | k | ||||||
| HAT | 1a | ~0.0 | 1.91 × 109 | 64.9 | 7.74 × 103 | ||||
| 2a | ~0.0 | 1.91 × 109 | ~0.0 | 1.91 × 109 | 56.7 | 7.54 × 104 | / | / | |
| 1a’ | ~0.0 | 1.91 × 109 | ~0.0 | 1.91 × 109 | 64.9 | 7.74 × 103 | / | / | |
| 2a’ | ~0.0 | 1.91 × 109 | ~0.0 | 1.91 × 109 | 56.7 | 7.54 × 104 | 198.8 | 4.06 × 10−20 | |
| RAF | 1 | 36.9 | 6.17 × 107 | 17.0 | 5.30 × 107 | ||||
| 2 | 40.8 | 1.33 × 107 | ~0.0 | 1.91 × 109 | |||||
| 3 | 39.3 | 2.47 × 107 | 26.8 | 4.60 × 107 | |||||
| 4 | 46.5 | 1.40 × 106 | 9.3 | 3.59 × 107 | |||||
| 5 | 52.1 | 1.58 × 107 | 49.3 | 4.12 × 105 | |||||
| 6 | 40.7 | 1.43 × 107 | ~0.0 | 1.91 × 109 | |||||
| 1′ | 36.9 | 6.17 × 107 | 33.7 | 8.27 × 107 | |||||
| 2′ | 40.8 | 1.33 × 107 | 37.4 | 4.69 × 107 | |||||
| 3′ | 39.3 | 2.47 × 107 | 36.2 | 7.02 × 107 | |||||
| 4′ | 46.5 | 1.40 × 106 | 44.3 | 3.12 × 106 | |||||
| 5′ | 52.1 | 1.58 × 105 | 51.9 | 1.49 × 105 | |||||
| 6′ | 40.7 | 1.43 × 107 | 38.4 | 3.14 × 107 | |||||
| SPLET(I) | 1a | / | / | / | / | ~0.0 | 1.91 × 109 | / | / |
| SPLET(II) | / | / | / | / | / | 0.7 | 1.56 × 109 | / | / |
| SET | / | / | / | / | / | / | / | / | / |
| 9.70 × 109 | 1.59 × 109 | ||||||||
| 8.9 × 109 | 0.84 × 109 | ||||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tošović, J.; Bren, U. Antioxidative Action of Ellagic Acid—A Kinetic DFT Study. Antioxidants 2020, 9, 587. https://doi.org/10.3390/antiox9070587
Tošović J, Bren U. Antioxidative Action of Ellagic Acid—A Kinetic DFT Study. Antioxidants. 2020; 9(7):587. https://doi.org/10.3390/antiox9070587
Chicago/Turabian StyleTošović, Jelena, and Urban Bren. 2020. "Antioxidative Action of Ellagic Acid—A Kinetic DFT Study" Antioxidants 9, no. 7: 587. https://doi.org/10.3390/antiox9070587
APA StyleTošović, J., & Bren, U. (2020). Antioxidative Action of Ellagic Acid—A Kinetic DFT Study. Antioxidants, 9(7), 587. https://doi.org/10.3390/antiox9070587