Nutraceutical Compounds Targeting Inflammasomes in Human Diseases
Abstract
:1. Background
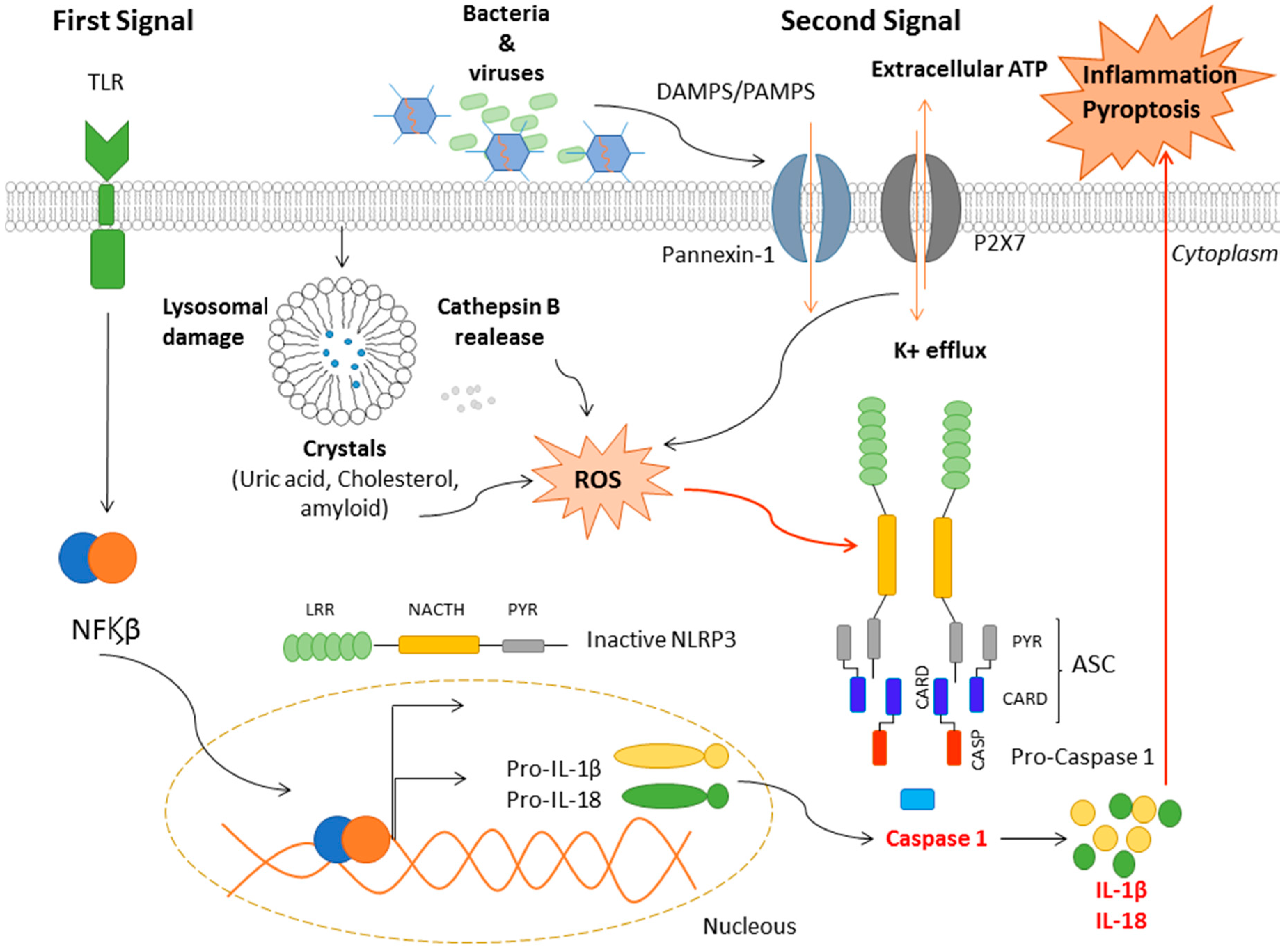
2. Inflammasomes
3. Mechanisms of NLRP3 Activation
4. Nutraceutical Compounds
5. Nutraceutical Compounds, the NLRP3 Inflammasome and Cardiovascular Diseases
6. Nutraceutical Compounds, the NLRP3 Inflammasome and Type 2 Diabetes
7. Nutraceutical Compounds, the NLRP3 Inflammasome and Neurological Diseases
8. Nutraceutical Compounds, the NLRP3 Inflammasome and Cancer
9. Conclusion and Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| IS | immune system |
| ASC | apoptosis-associated speck-like protein |
| PAMPs | pathogen-associated molecular patterns |
| DAMPs | damage-associated molecular patterns |
| PRRs | pattern recognition receptors |
| TLRs | toll-like receptors |
| CLRs | c-type lectin receptors |
| RLRs | retinoic acid inducible gene-I (RIG1)-like receptors |
| OLRs | olygoadenylate synthetase-like receptors |
| CARD | caspase recruitment |
| PYR | pyrin domains |
| ROS | Reactive Oxygen Species |
| CVDs | cardiovascular diseases |
| PUFAs | polyunsaturated fatty acids |
References
- Schroder, K.; Tschopp, J. The inflammasomes. Cell 2010, 140, 821–832. [Google Scholar] [CrossRef] [Green Version]
- Amin, J.; Boche, D.; Rakic, S. What do we know about the inflammasome in humans? Brain Pathol. 2017, 27, 192–204. [Google Scholar] [CrossRef] [Green Version]
- Abderrazak, A.; Syrovets, T.; Couchie, D.; El Hadri, K.; Friguet, B.; Simmet, T.; Rouis, M. NLRP3 inflammasome: From a danger signal sensor to a regulatory node of oxidative stress and inflammatory diseases. Redox Biol. 2015, 4, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Lamkanfi, M.; Dixit, V.M. Inflammasomes and Their Roles in Health and Disease. Annu. Rev. Cell Dev. Biol. 2012, 28, 137–161. [Google Scholar] [CrossRef] [Green Version]
- Kagan, J.C.; Barton, G.M. Emerging principles governing signal transduction by pattern-recognition receptors. Cold Spring Harb. Perspect. Biol. 2014, 7, a016253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mogensen, T.H. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin. Microbiol. Rev. 2009, 22, 240–273. [Google Scholar] [CrossRef] [Green Version]
- Uzman, A. Molecular biology of the cell. Biochem. Mol. Biol. Educ. 2003, 31, 212–214. [Google Scholar] [CrossRef]
- De Zoete, M.R.; Palm, N.W.; Zhu, S.; Flavell, R.A. Inflammasomes. Cold Spring Harb. Perspect. Biol. 2014, 6, a016287. [Google Scholar] [CrossRef] [PubMed]
- Yaribeygi, H.; Mohammadi, M.T.; Rezaee, R.; Sahebkar, A. Fenofibrate improves renal function by amelioration of NOX-4, IL-18, and p53 expression in an experimental model of diabetic nephropathy. J. Cell. Biochem. 2018, 119, 7458–7469. [Google Scholar] [CrossRef]
- Kim, H.Y.; Lee, H.J.; Chang, Y.J.; Pichavant, M.; Shore, S.A.; Fitzgerald, K.A.; Iwakura, Y.; Israel, E.; Bolger, K.; Faul, J.; et al. Interleukin-17-producing innate lymphoid cells and the NLRP3 inflammasome facilitate obesity-associated airway hyperreactivity. Nat. Med. 2014, 20, 54–61. [Google Scholar] [CrossRef] [Green Version]
- Grebe, A.; Hoss, F.; Latz, E. NLRP3 inflammasome and the IL-1 pathway in atherosclerosis. Circ. Res. 2018, 122, 1722–1740. [Google Scholar] [CrossRef] [PubMed]
- Hoseini, Z.; Sepahvand, F.; Rashidi, B.; Sahebkar, A.; Masoudifar, A.; Mirzaei, H. NLRP3 inflammasome: Its regulation and involvement in atherosclerosis. J. Cell. Physiol. 2018, 233, 2116–2132. [Google Scholar] [CrossRef] [PubMed]
- Ference, B.A.; Ginsberg, H.N.; Graham, I.; Ray, K.K.; Packard, C.J.; Bruckert, E.; Hegele, R.A.; Krauss, R.M.; Raal, F.J.; Schunkert, H.; et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement fromthe European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2017, 38, 2459–2472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, F.; Xing, S.; Gong, Z.; Xing, Q. NLRP3 inflammasomes show high expression in Aorta of patients with atherosclerosis. Heart Lung Circ. 2013, 22, 746–750. [Google Scholar] [CrossRef]
- Ising, C.; Venegas, C.; Zhang, S.; Scheiblich, H.; Schmidt, S.V.; Vieira-Saecker, A.; Schwartz, S.; Albasset, S.; McManus, R.M.; Tejera, D.; et al. NLRP3 inflammasome activation drives tau pathology. Nature 2019, 575, 669–673. [Google Scholar] [CrossRef]
- Heneka, M.T.; Kummer, M.P.; Stutz, A.; Delekate, A.; Schwartz, S.; Vieira-Saecker, A.; Griep, A.; Axt, D.; Remus, A.; Tzeng, T.C.; et al. NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature 2013, 493, 674–678. [Google Scholar] [CrossRef]
- Wang, S.; Yuan, Y.H.; Chen, N.H.; Wang, H.B. The mechanisms of NLRP3 inflammasome/pyroptosis activation and their role in Parkinson’s disease. Int. Immunopharmacol. 2019, 67, 458–464. [Google Scholar] [CrossRef]
- Moossavi, M.; Parsamanesh, N.; Bahrami, A.; Atkin, S.L.; Sahebkar, A. Role of the NLRP3 inflammasome in cancer. Mol. Cancer 2018, 17, 158. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Dong, Y.; Ye, M.; Jin, S.; Yang, J.; Joosse, M.E.; Sun, Y.; Zhang, J.; Lazarev, M.; Brant, S.R.; et al. The Pathogenic Role of NLRP3 Inflammasome Activation in Inflammatory Bowel Diseases of Both Mice and Humans. J. Crohn’s Colitis 2017, 11, 737–750. [Google Scholar] [CrossRef]
- Mangan, M.S.J.; Olhava, E.J.; Roush, W.R.; Seidel, H.M.; Glick, G.D.; Latz, E. Targeting the NLRP3 inflammasome in inflammatory diseases. Nat. Rev. Drug Discov. 2018, 17, 588–606. [Google Scholar] [CrossRef]
- De Torre-Minguela, C.; del Castillo, P.M.; Pelegrín, P. The NLRP3 and pyrin inflammasomes: Implications in the pathophysiology of autoinflammatory diseases. Front. Immunol. 2017, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Li, F.; Li, W.-W.; Stary, C.; Clark, J.D.; Xu, S.; Xiong, X. The inflammasome as a target for pain therapy. Br. J. Anaesth. 2016, 117, 693–707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, H.; Callaway, J.B.; Ting, J.P.-Y. Inflammasomes: Mechanism of action, role in disease, and therapeutics. Nat. Med. 2015, 21, 677–687. [Google Scholar] [CrossRef] [Green Version]
- DeBusk, R. Diet-Related Disease, Nutritional Genomics, and Food and Nutrition Professionals. J. Am. Diet. Assoc. 2009, 109, 410–413. [Google Scholar] [CrossRef] [PubMed]
- Martín Ortega, A.M.; Segura Campos, M.R. Bioactive Compounds as Therapeutic Alternatives. In Bioactive Compounds: Health Benefits and Potential Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 247–264. ISBN 9780128147757. [Google Scholar]
- Madalena, D.A.; Pereira, R.N.; Vicente, A.A.; Ramos, Ó.L. New insights on bio-based micro-and nanosystems in food. In Encyclopedia of Food Chemistry; Elsevier: Amsterdam, The Netherlands, 2018; pp. 708–714. ISBN 9780128140451. [Google Scholar]
- Mahfoudhi, N.; Ksouri, R.; Hamdi, S. Nanoemulsions as potential delivery systems for bioactive compounds in food systems: Preparation, characterization, and applications in food industry. In Emulsions; Elsevier: Amsterdam, The Netherlands, 2016; pp. 365–403. [Google Scholar]
- Gul, K.; Singh, A.K.; Jabeen, R. Nutraceuticals and Functional Foods: The Foods for the Future World. Crit. Rev. Food Sci. Nutr. 2016, 56, 2617–2627. [Google Scholar] [CrossRef]
- Pavillard, L.E.; Marín-Aguilar, F.; Bullon, P.; Cordero, M.D. Cardiovascular diseases, NLRP3 inflammasome, and western dietary patterns. Pharmacol. Res. 2018, 131, 44–50. [Google Scholar] [CrossRef]
- Lim, H.; Min, D.S.; Park, H.; Kim, H.P. Flavonoids interfere with NLRP3 inflammasome activation. Toxicol. Appl. Pharmacol. 2018, 355, 93–102. [Google Scholar] [CrossRef]
- Wu, J.; Luo, Y.; Jiang, Q.; Li, S.; Huang, W.; Xiang, L.; Liu, D.; Hu, Y.; Wang, P.; Lu, X.; et al. Coptisine from Coptis chinensis blocks NLRP3 inflammasome activation by inhibiting caspase-1. Pharmacol. Res. 2019, 147, 104348. [Google Scholar] [CrossRef]
- Zhu, T.; Zhang, L.; Ling, S.; Duan, J.; Qian, F.; Li, Y.; Xu, J.-W. Scropolioside B inhibits IL-1β and cytokines expression through NF-κB and inflammasome NLRP3 pathways. Mediat. Inflamm. 2014, 2014, 819053. [Google Scholar] [CrossRef] [Green Version]
- Yan, Y.; Jiang, W.; Spinetti, T.; Tardivel, A.; Castillo, R.; Bourquin, C.; Guarda, G.; Tian, Z.; Tschopp, J.; Zhou, R. Omega-3 Fatty Acids Prevent Inflammation and Metabolic Disorder through Inhibition of NLRP3 Inflammasome Activation. Immunity 2013, 38, 1154–1163. [Google Scholar] [CrossRef] [Green Version]
- Sun, C.; Wang, X.; Zheng, G.; Fan, S.; Lu, J.; Zhang, Z.; Wu, D.; Shan, Q.; Hu, B.; Zheng, Y. Protective effect of different flavonoids against endothelial senescence via NLRP3 inflammasome. J. Funct. Foods 2016, 26, 598–609. [Google Scholar] [CrossRef]
- Ajdukovic, J. The Role of NLRP3 Inflammasome in Cardiovascular Diseases. J. Clin. Exp. Cardiolog. 2015, 6, 1–3. [Google Scholar]
- Zhang, X.; Wang, G.; Gurley, E.C.; Zhou, H. Flavonoid Apigenin Inhibits Lipopolysaccharide-Induced Inflammatory Response through Multiple Mechanisms in Macrophages. PLoS ONE 2014, 9, e107072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López-Franco, O.; Hernández-Vargas, P.; Ortiz-Muñoz, G.; Sanjuán, G.; Suzuki, Y.; Ortega, L.; Blanco, J.; Egido, J.; Gómez-Guerrero, C. Parthenolide Modulates the NF-κB–Mediated Inflammatory Responses in Experimental Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 1864–1870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heinrich, M.; Robles, M.; West, J.E.; Ortiz De Montellano, B.R.; Rodriguez, E. Ethnopharmacology of Mexican asteraceae (compositae). Annu. Rev. Pharmacol. Toxicol. 1998, 38, 539–565. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Gao, X.; Wu, X.; Wu, Z.; Cheng, L.; Zhu, L.; Shen, D.; Tong, X. Parthenolide inhibits LPS-induced inflammatory cytokines through the toll-like receptor 4 signal pathway in THP-1 cells. Acta Biochim. Biophys. Sin. 2015, 47, 368–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juliana, C.; Fernandes-Alnemri, T.; Wu, J.; Datta, P.; Solorzano, L.; Yu, J.-W.; Meng, R.; Quong, A.A.; Latz, E.; Scott, C.P.; et al. Anti-inflammatory compounds parthenolide and Bay 11-7082 are direct inhibitors of the inflammasome. J. Biol. Chem. 2010, 285, 9792–9802. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Ahn, H.; Han, B.-C.; Lee, S.-H.; Cho, Y.-W.; Kim, C.H.; Hong, E.-J.; An, B.-S.; Jeung, E.-B.; Lee, G.-S. Korean red ginseng extracts inhibit NLRP3 and AIM2 inflammasome activation. Immunol. Lett. 2014, 158, 143–150. [Google Scholar] [CrossRef]
- Domiciano, T.P.; Wakita, D.; Jones, H.D.; Crother, T.R.; Verri, W.A.; Arditi, M.; Shimada, K. Quercetin Inhibits Inflammasome Activation by Interfering with ASC Oligomerization and Prevents Interleukin-1 Mediated Mouse Vasculitis. Sci. Rep. 2017, 7, 41539. [Google Scholar] [CrossRef]
- Yu, S.X.; Du, C.T.; Chen, W.; Lei, Q.Q.; Li, N.; Qi, S.; Zhang, X.J.; Hu, G.Q.; Deng, X.M.; Han, W.Y.; et al. Genipin inhibits NLRP3 and NLRC4 inflammasome activation via autophagy suppression. Sci. Rep. 2015, 5, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Chen, X.; Zong, B.; Yuan, H.; Wang, Z.; Wei, Y.; Wang, X.; Liu, G.; Zhang, J.; Li, S.; et al. Gypenosides improve diabetic cardiomyopathy by inhibiting ROS-mediated NLRP3 inflammasome activation. J. Cell. Mol. Med. 2018, 22, 4437–4448. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Chen, M.; Xu, L.; Lv, Z.; Chen, L.; Li, Y.; He, W.F. Morroniside alleviates coxsackievirus B3-induced myocardial damage apoptosis via restraining NLRP3 inflammasome activation. RSC Adv. 2019, 9, 1222–1229. [Google Scholar] [CrossRef] [Green Version]
- Ahn, H.; Lee, G.S. Isorhamnetin and hyperoside derived from water dropwort inhibits inflammasome activation. Phytomedicine 2017, 24, 77–86. [Google Scholar] [CrossRef]
- Chalons, P.; Amor, S.; Courtaut, F.; Cantos-Villar, E.; Richard, T.; Auger, C.; Chabert, P.; Schni-Kerth, V.; Aires, V.; Delmas, D. Study of potential anti-inflammatory effects of red wine extract and resveratrol through a modulation of interleukin-1-beta in macrophages. Nutrients 2018, 10, 1856. [Google Scholar] [CrossRef] [Green Version]
- Deng, Z.Y.; Hu, M.M.; Xin, Y.F.; Gang, C. Resveratrol alleviates vascular inflammatory injury by inhibiting inflammasome activation in rats with hypercholesterolemia and vitamin D2 treatment. Inflamm. Res. 2015, 64, 321–332. [Google Scholar] [CrossRef]
- Dong, W.; Yang, R.; Yang, J.; Yang, J.; Ding, J.; Wu, H.; Zhang, J. Resveratrol pretreatment protects rat hearts from ischemia/reperfusion injury partly via a NALP3 inflammasome pathway. Int. J. Clin. Exp. Pathol. 2015, 8, 8731–8741. [Google Scholar]
- Kong, F.; Ye, B.; Cao, J.; Cai, X.; Lin, L.; Huang, S.; Huang, W.; Huang, Z. Curcumin Represses NLRP3 Inflammasome Activation via TLR4/MyD88/NF-κB and P2X7R Signaling in PMA-Induced Macrophages. Front. Pharmacol. 2016, 7, 369. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Wu, A.; Kwan Law, B.Y.; Liu, C.; Zeng, W.; Ling Qiu, A.C.; Han, Y.; He, Y.; Wai Wong, V.K. The active components derived from Penthorum chinense Pursh protect against oxidative-stress-induced vascular injury via autophagy induction. Free Radic. Biol. Med. 2020, 146, 160–180. [Google Scholar] [CrossRef]
- Qiu, H.; Liu, W.; Lan, T.; Pan, W.; Chen, X.; Wu, H.; Xu, D. Salvianolate reduces atrial fibrillation through suppressing atrial interstitial fibrosis by inhibiting TGF-β1/Smad2/3 and TXNIP/NLRP3 inflammasome signaling pathways in post-MI rats. Phytomedicine 2018, 51, 255–265. [Google Scholar] [CrossRef]
- Li, Y.; Yang, J.; Chen, M.-H.; Wang, Q.; Qin, M.-J.; Zhang, T.; Chen, X.-Q.; Liu, B.-L.; Wen, X.-D. Ilexgenin A inhibits endoplasmic reticulum stress and ameliorates endothelial dysfunction via suppression of TXNIP/NLRP3 inflammasome activation in an AMPK dependent manner. Pharmacol. Res. 2015, 99, 101–115. [Google Scholar] [CrossRef]
- Jang, S., II; Jeong, S., II; Kim, K.J.; Kim, H.J.; Yu, H.H.; Park, R.; Kim, H.M.; You, Y.O. Tanshinone IIA from Salvia miltiorrhiza Inhibits Inducible Nitric Oxide Synthase Expression and Production of TNF-α, IL-1β and IL-6 in Activated RAW 264.7 Cells. Planta Med. 2003, 69, 1057–1059. [Google Scholar]
- Hu, Q.; Wei, B.; Wei, L.; Hua, K.; Yu, X.; Li, H.; Ji, H. Sodium tanshinone IIA sulfonate ameliorates ischemia-induced myocardial inflammation and lipid accumulation in Beagle dogs through NLRP3 inflammasome. Int. J. Cardiol. 2015, 196, 183–192. [Google Scholar] [CrossRef]
- Hu, Q.; Zhang, T.; Yi, L.; Zhou, X.; Mi, M. Dihydromyricetin inhibits NLRP3 inflammasome-dependent pyroptosis by activating the Nrf2 signaling pathway in vascular endothelial cells. BioFactors 2018, 44, 123–136. [Google Scholar] [CrossRef]
- Zhang, B.C.; Li, Z.; Xu, W.; Xiang, C.H.; Ma, Y.F. Luteolin alleviates NLRP3 inflammasome activation and directs macrophage polarization in lipopolysaccharide-stimulated RAW264.7 cells. Am. J. Transl. Res. 2018, 10, 265–273. [Google Scholar]
- Martínez, G.J.; Robertson, S.; Barraclough, J.; Xia, Q.; Mallat, Z.; Bursill, C.; Celermajer, D.S.; Patel, S. Colchicine Acutely Suppresses Local Cardiac Production of Inflammatory Cytokines in Patients With an Acute Coronary Syndrome. J. Am. Heart Assoc. 2015, 4, e002128. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Lu, K.; Wang, Y.; Chen, M.; Zhang, F.; Shen, H.; Yao, D.; Gong, K.; Zhang, Z. Triptolide attenuates pressure overload-induced myocardial remodeling in mice via the inhibition of NLRP3 inflammasome expression. Biochem. Biophys. Res. Commun. 2017, 485, 69–75. [Google Scholar] [CrossRef]
- Lv, D.; Cheng, X.; Tang, L.; Jiang, M. The cardioprotective effect of total flavonoids on myocardial ischemia/reperfusion in rats. BioMed Pharmacother. 2017, 88, 277–284. [Google Scholar] [CrossRef]
- Luo, H.; Fan, Z.; Xiang, D.; Jiang, Z.; Zhang, W.; Gao, L.; Feng, C. The protective effect of umbelliferone ameliorates myocardial injury following ischemia-reperfusion in the rat through suppression NLRP3 inflammasome and upregulating the PPAR-γ. Mol. Med. Rep. 2018, 17, 3404–3410. [Google Scholar] [CrossRef]
- Viljoen, A.; Mncwangi, N.; Vermaak, I. Anti-Inflammatory Iridoids of Botanical Origin. Curr. Med. Chem. 2012, 19, 2104–2127. [Google Scholar] [CrossRef] [Green Version]
- Badorff, C.; Fichtlscherer, B.; Rhoads, R.E.; Zeiher, A.M.; Muelsch, A.; Dimmeler, S.; Knowlton, K.U. Nitric oxide inhibits dystrophin proteolysis by coxsackieviral protease 2A through S-nitrosylation: A protective mechanism against enteroviral cardiomyopathy. Circulation 2000, 102, 2276–2281. [Google Scholar] [CrossRef] [Green Version]
- Fairweather, D.L.; Rose, N.R. Coxsackievirus-induced myocarditis in mice: A model of autoimmune disease for studying immunotoxicity. Methods 2007, 41, 118–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawaguchi, M.; Takahashi, M.; Hata, T.; Kashima, Y.; Usui, F.; Morimoto, H.; Izawa, A.; Takahashi, Y.; Masumoto, J.; Koyama, J.; et al. Inflammasome Activation of Cardiac Fibroblasts Is Essential for Myocardial Ischemia/Reperfusion Injury. Circulation 2011, 123, 594–604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, G.X.; Li, J.M.; Zhou, Y.; Zhao, S.P. Anti-inflammatory and immune suppressive effects of Conus officinalis glucosides in rats. Chin. J. Microbiol. Immunol. 2007, 27, 316–320. [Google Scholar]
- Sung, Y.-H.; Chang, H.-K.; Kim, S.-E.; Kim, Y.-M.; Seo, J.-H.; Shin, M.-C.; Shin, M.-S.; Yi, J.-W.; Shin, D.-H.; Kim, H.; et al. Anti-Inflammatory and Analgesic Effects of the Aqueous Extract of Corni Fructus in Murine RAW 264.7 Macrophage Cells. J. Med. Food 2009, 12, 788–795. [Google Scholar] [CrossRef]
- Swanson, D.; Block, R.; Mousa, S.A. Omega-3 Fatty Acids EPA and DHA: Health Benefits Throughout Life. Adv. Nutr. 2012, 3, 1–7. [Google Scholar] [CrossRef]
- Sun, C.; Fan, S.; Wang, X.; Lu, J.; Zhang, Z.; Wu, D.; Shan, Q.; Zheng, Y. Purple sweet potato color inhibits endothelial premature senescence by blocking the NLRP3 inflammasome. J. Nutr. Biochem. 2015, 26, 1029–1040. [Google Scholar] [CrossRef]
- Calgarotto, A.K.; Maso, V.; Junior, G.C.F.; Nowill, A.E.; Filho, P.L.; Vassallo, J.; Saad, S.T.O. Antitumor activities of Quercetin and Green Tea in xenografts of human leukemia HL60 cells. Sci. Rep. 2018, 8, 3459. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Pan, Y.; Zhang, Q.-Y.; Wang, F.-M.; Kong, L.-D. Quercetin and Allopurinol Ameliorate Kidney Injury in STZ-Treated Rats with Regulation of Renal NLRP3 Inflammasome Activation and Lipid Accumulation. PLoS ONE 2012, 7, e38285. [Google Scholar] [CrossRef] [Green Version]
- Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; Das, S.R.; De Ferranti, S.; Després, J.P.; Fullerton, H.J.; et al. Heart disease and stroke statistics-2016 update a report from the American Heart Association. Circulation 2016, 133, e38–e48. [Google Scholar]
- Shishodia, S. Molecular mechanisms of curcumin action: Gene expression. BioFactors 2013, 39, 37–55. [Google Scholar] [CrossRef]
- Zhang, T.; Chen, Y.-M.; Zhang, G.-L. Novel Neolignan from Penthorum chinense. J. Integr. Plant. Biol. 2007, 49, 1611–1614. [Google Scholar] [CrossRef]
- Wang, M.; Jiang, Y.; Liu, H.L.; Chen, X.Q.; Wu, X.; Zhang, D.Y. A new flavanone from the aerial parts of Penthorum chinense. Nat. Prod. Res. 2014, 28, 70–73. [Google Scholar] [CrossRef] [PubMed]
- Kirii, H.; Niwa, T.; Yamada, Y.; Wada, H.; Saito, K.; Iwakura, Y.; Asano, M.; Moriwaki, H.; Seishima, M. Lack of Interleukin-1β Decreases the Severity of Atherosclerosis in ApoE-Deficient Mice. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 656–660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Razani, B.; Feng, C.; Coleman, T.; Emanuel, R.; Wen, H.; Hwang, S.; Ting, J.P.; Virgin, H.W.; Kastan, M.B.; Semenkovich, C.F. Autophagy links inflammasomes to atherosclerotic progression. Cell Metab. 2012, 15, 534–544. [Google Scholar] [CrossRef] [Green Version]
- Ding, C.; Zhao, Y.; Shi, X.; Zhang, N.; Zu, G.; Li, Z.; Zhou, J.; Gao, D.; Lv, L.; Tian, X.; et al. New insights into salvianolic acid A action: Regulation of the TXNIP/NLRP3 and TXNIP/ChREBP pathways ameliorates HFD-induced NAFLD in rats. Sci. Rep. 2016, 6, 28734. [Google Scholar] [CrossRef]
- Chang, Y.P.; Zhang, H.; Xie, Y.M.; Zeng, X.B.; Hu, J.; Zhuang, Y. Analysis of salvianolate injection combined with usual drugs in treatment of coronary heart disease in real world. Zhongguo Zhongyao Zazhi 2013, 38, 3186–3189. [Google Scholar]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Buriner, M.; Clement, D.L.; Coca, A.; De Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension|European Heart Journal|Oxford Academic. Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef]
- Zhuang, S.; Cheng, T.H.; Shih, N.L.; Liu, J.C.; Chen, J.J.; Hong, H.J.; Chan, P. Tanshinone IIA Induces Heme Oxygenase 1 Expression and Inhibits Cyclic Strain-Induced Interleukin 8 Expression in Vascular Endothelial Cells. Am. J. Chin. Med. 2016, 44, 377–388. [Google Scholar] [CrossRef]
- Park, J.C.; Yu, Y.B.; Lee, J.H. Isolation of steroids and flavonoids from the herb of Oenanthe javanica Dc. Korean J. Pharmacogn. 1993, 24, 244–246. [Google Scholar]
- Ji, G.; Yao, X.; Zang, Z.; Huang, Z. Antiarrhythmic effect of Oenanthe javanica (Bl.) DC. injection. Zhongguo Zhong Yao Za Zhi 1990, 15, 429–431. [Google Scholar]
- Xin-Bo, Y.; Zheng-Ming, H.; Wen-Bin, C.; Ming, Z.; Hong-Yan, C.; Jing-Zhen, Z. Antidiabetic effect of Oenanthe javanica flavone. Acta Pharmacol. Sin. 2000, 21, 239–242. [Google Scholar]
- Ku, S.K.; Kim, T.H.; Lee, S.; Kim, S.M.; Bae, J.S. Antithrombotic and profibrinolytic activities of isorhamnetin-3-O-galactoside and hyperoside. Food Chem. Toxicol. 2013, 53, 197–204. [Google Scholar] [CrossRef]
- Goldberg, D.M.; Soleas, G.J.; Levesque, M. Moderate alcohol consumption: The gentle face of janus. Clin. Biochem. 1999, 32, 505–518. [Google Scholar] [CrossRef]
- Hollingsworth, P.M.; Forrest, L.L.; Spouge, J.L.; Hajibabaei, M.; Ratnasingham, S.; van der Bank, M.; Chase, M.W.; Cowan, R.S.; Erickson, D.L.; Fazekas, A.J.; et al. A DNA barcode for land plants. Proc. Natl. Acad. Sci. USA 2009, 106, 12794–12797. [Google Scholar]
- Bonnefont-Rousselot, D. Resveratrol and cardiovascular diseases. Nutrients 2016, 8, 250. [Google Scholar] [CrossRef]
- Cui, W.X.; Yang, J.; Chen, X.Q.; Mao, Q.; Wei, X.L.; Wen, X.D.; Wang, Q. Triterpenoid-rich fraction from ilex hainanensis merr. attenuates non-alcoholic fatty liver disease induced by high fat diet in rats. Am. J. Chin. Med. 2013, 41, 487–502. [Google Scholar] [CrossRef]
- Qiu, Y.; Tang, L. Roles of the NLRP3 inflammasome in the pathogenesis of diabetic nephropathy. Pharmacol. Res. 2016, 114, 251–264. [Google Scholar] [CrossRef]
- Ding, X.-Q.; Wu, W.-Y.; Jiao, R.-Q.; Gu, T.-T.; Xu, Q.; Pan, Y.; Kong, L.-D. Curcumin and allopurinol ameliorate fructose-induced hepatic inflammation in rats via miR-200a-mediated TXNIP/NLRP3 inflammasome inhibition. Pharmacol. Res. 2018, 137, 64–75. [Google Scholar] [CrossRef]
- Chen, W.; Wang, J.; Luo, Y.; Wang, T.; Li, X.; Li, A.; Li, J.; Liu, K.; Liu, B. Ginsenoside Rb1 and compound K improve insulin signaling and inhibit ER stress-associated NLRP3 inflammasome activation in adipose tissue. J. Ginseng Res. 2016, 40, 351–358. [Google Scholar] [CrossRef] [Green Version]
- Ahsan, H.; Ahad, A.; Iqbal, J.; Siddiqui, W.A. Pharmacological potential of tocotrienols: A review. Nutr. Metab. 2014, 11, 52. [Google Scholar] [CrossRef] [Green Version]
- Samra, Y.A.; Said, H.S.; Elsherbiny, N.M.; Liou, G.I.; El-Shishtawy, M.M.; Eissa, L.A. Cepharanthine and Piperine ameliorate diabetic nephropathy in rats: Role of NF-κB and NLRP3 inflammasome. Life Sci. 2016, 157, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Yin, N.; Liu, W.; Cui, X.; Chen, S.; Wang, E. Curcumin Ameliorates Diabetic Nephropathy by Suppressing NLRP3 Inflammasome Signaling. BioMed Res. Int. 2017, 2017, 1–10. [Google Scholar] [CrossRef]
- Abderrazak, A.; El Hadri, K.; Bosc, E.; Blondeau, B.; Slimane, M.-N.; Buchele, B.; Simmet, T.; Couchie, D.; Rouis, M. Inhibition of the Inflammasome NLRP3 by Arglabin Attenuates Inflammation, Protects Pancreatic β-Cells from Apoptosis, and Prevents Type 2 Diabetes Mellitus Development in ApoE2Ki Mice on a Chronic High-Fat Diet. J. Pharmacol. Exp. Ther. 2016, 357, 487–494. [Google Scholar] [CrossRef] [Green Version]
- Li, A.; Zhang, S.; Li, J.; Liu, K.; Huang, F.; Liu, B. Metformin and resveratrol inhibit Drp1-mediated mitochondrial fission and prevent ER stress-associated NLRP3 inflammasome activation in the adipose tissue of diabetic mice. Mol. Cell. Endocrinol. 2016, 434, 36–47. [Google Scholar] [CrossRef]
- Lu, L.; Lu, Q.; Chen, W.; Li, J.; Li, C.; Zheng, Z. Vitamin D 3 Protects against Diabetic Retinopathy by Inhibiting High-Glucose-Induced Activation of the ROS/TXNIP/NLRP3 Inflammasome Pathway. J. Diabetes Res. 2018, 2018, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Chen, Y.; Song, J.; Hou, F.; Ma, X.; Liu, B.; Huang, F. Mangiferin suppresses endoplasmic reticulum stress in perivascular adipose tissue and prevents insulin resistance in the endothelium. Eur. J. Nutr. 2018, 57, 1563–1575. [Google Scholar] [CrossRef]
- Ma, Q.; Yang, Q.; Chen, J.; Yu, C.; Zhang, L.; Zhou, W.; Chen, M. Salvianolic Acid A Ameliorates Early-Stage Atherosclerosis Development by Inhibiting NLRP3 Inflammasome Activation in Zucker Diabetic Fatty Rats. Molecules 2020, 25, 1089. [Google Scholar] [CrossRef] [Green Version]
- Lalitha, N.; Sadashivaiah, B.; Ramaprasad, T.R.; Singh, S.A. Anti-hyperglycemic activity of myricetin, through inhibition of DPP-4 and enhanced GLP-1 levels, is attenuated by co-ingestion with lectin-rich protein. PLoS ONE 2020, 15, e0231543. [Google Scholar] [CrossRef] [Green Version]
- Zhu, M.-J.; Kang, Y.; Xue, Y.; Liang, X.; García, M.P.G.; Rodgers, D.; Kagel, D.R.; Du, M. Red raspberries suppress NLRP3 inflammasome and attenuate metabolic abnormalities in diet-induced obese mice. J. Nutr. Biochem. 2018, 53, 96–103. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhu, C.; Yang, H.; Deng, J.; Fan, D. Protective effect of ginsenoside Rg5 against kidney injury via inhibition of NLRP3 inflammasome activation and the MAPK signaling pathway in high-fat diet/streptozotocin-induced diabetic mice. Pharmacol. Res. 2020, 155, 104746. [Google Scholar] [CrossRef]
- Eo, H.; Lee, H.-J.; Lim, Y. Ameliorative effect of dietary genistein on diabetes induced hyper-inflammation and oxidative stress during early stage of wound healing in alloxan induced diabetic mice. Biochem. Biophys. Res. Commun. 2016, 478, 1021–1027. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, C.; Ding, X.-Q.; Pan, Y.; Gu, T.-T.; Wang, M.-X.; Liu, Y.-L.; Wang, F.-M.; Wang, S.-J.; Kong, L.-D. Quercetin and allopurinol reduce liver thioredoxin-interacting protein to alleviate inflammation and lipid accumulation in diabetic rats. Br. J. Pharmacol. 2013, 169, 1352–1371. [Google Scholar] [CrossRef]
- Harbis, A.; Perdreau, S.; Vincent-Baudry, S.; Charbonnier, M.; Bernard, M.-C.; Raccah, D.; Senft, M.; Lorec, A.-M.; Defoort, C.; Portugal, H.; et al. Glycemic and insulinemic meal responses modulate postprandial hepatic and intestinal lipoprotein accumulation in obese, insulin-resistant subjects. Am. J. Clin. Nutr. 2004, 80, 896–902. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Shah, G.; Singh, S.; Gohil, P.; Chauhan, K.; Shah, K.; Chorawala, M. Effect of piperine in the regulation of obesity-induced dyslipidemia in high-fat diet rats. Indian J. Pharmacol. 2011, 43, 296. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Su, M.; Zhou, F. Mangiferin inhibits hippocampal NLRP3 inflammasome and exerts antidepressant effects in a chronic mild stress mice model. Behav. Pharmacol. 2017, 28, 356–364. [Google Scholar] [CrossRef]
- Alcocer-Gómez, E.; Casas-Barquero, N.; Williams, M.R.; Romero-Guillena, S.L.; Cañadas-Lozano, D.; Bullón, P.; Sánchez-Alcazar, J.A.; Navarro-Pando, J.M.; Cordero, M.D. Antidepressants induce autophagy dependent-NLRP3-inflammasome inhibition in Major depressive disorder. Pharmacol. Res. 2017, 121, 114–121. [Google Scholar] [CrossRef]
- Li, R.; Wang, X.; Qin, T.; Qu, R.; Ma, S. Apigenin ameliorates chronic mild stress-induced depressive behavior by inhibiting interleukin-1β production and NLRP3 inflammasome activation in the rat brain. Behav. Brain Res. 2016, 296, 318–325. [Google Scholar] [CrossRef]
- Sui, D.; Xie, Q.; Yi, W.; Gupta, S.; Yu, X.; Li, J.; Wang, J.; Wang, J.; Deng, X. Resveratrol Protects against Sepsis-Associated Encephalopathy and Inhibits the NLRP3/IL-1 β Axis in Microglia. Mediat. Inflamm. 2016, 2016, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Li, R.; Wang, X.; Fu, Q.; Ma, S. Umbelliferone ameliorates cerebral ischemia–reperfusion injury via upregulating the PPAR gamma expression and suppressing TXNIP/NLRP3 inflammasome. Neurosci. Lett. 2015, 600, 182–187. [Google Scholar] [CrossRef]
- Yu, C.; He, Q.; Zheng, J.; Li, L.Y.; Hou, Y.H.; Song, F.Z. Sulforaphane improves outcomes and slows cerebral ischemic/reperfusion injury via inhibition of NLRP3 inflammasome activation in rats. Int. Immunopharmacol. 2017, 45, 74–78. [Google Scholar] [CrossRef]
- Yang, F.; Wang, Z.; Wei, X.; Han, H.; Meng, X.; Zhang, Y.; Shi, W.; Li, F.; Xin, T.; Pang, Q.; et al. NLRP3 deficiency ameliorates neurovascular damage in experimental ischemic stroke. J. Cereb. Blood Flow Metab. 2014, 34, 660–667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.; Li, H.; Fang, F.; Deng, X.; Ma, S. Astragaloside IV attenuates cognitive impairments induced by transient cerebral ischemia and reperfusion in mice via anti-inflammatory mechanisms. Neurosci. Lett. 2017, 639, 114–119. [Google Scholar] [CrossRef]
- Cao, G.; Jiang, N.; Hu, Y.; Zhang, Y.; Wang, G.; Yin, M.; Ma, X.; Zhou, K.; Qi, J.; Yu, B.; et al. Ruscogenin attenuates cerebral ischemia-induced blood-brain barrier dysfunction by suppressing TXNIP/NLRP3 inflammasome activation and the MAPK pathway. Int. J. Mol. Sci. 2016, 17, 1418. [Google Scholar] [CrossRef] [Green Version]
- Qiu, J.; Wang, M.; Zhang, J.; Cai, Q.; Lu, D.; Li, Y.; Dong, Y.; Zhao, T.; Chen, H. The neuroprotection of Sinomenine against ischemic stroke in mice by suppressing NLRP3 inflammasome via AMPK signaling. Int. Immunopharmacol. 2016, 40, 492–500. [Google Scholar] [CrossRef]
- Zhang, S.; Jiang, L.; Che, F.; Lu, Y.; Xie, Z.; Wang, H. Arctigenin attenuates ischemic stroke via SIRT1-dependent inhibition of NLRP3 inflammasome. Biochem. Biophys. Res. Commun. 2017, 493, 821–826. [Google Scholar] [CrossRef]
- He, Q.; Li, Z.; Wang, Y.; Hou, Y.; Li, L.; Zhao, J. Resveratrol alleviates cerebral ischemia/reperfusion injury in rats by inhibiting NLRP3 inflammasome activation through Sirt1-dependent autophagy induction. Int. Immunopharmacol. 2017, 50, 208–215. [Google Scholar] [CrossRef]
- Li, Y.; Li, J.; Li, S.; Li, Y.; Wang, X.; Liu, B.; Fu, Q.; Ma, S. Curcumin attenuates glutamate neurotoxicity in the hippocampus by suppression of ER stress-associated TXNIP/NLRP3 inflammasome activation in a manner dependent on AMPK. Toxicol. Appl. Pharmacol. 2015, 286, 53–63. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, X.; Liu, X.; Wang, H.; Xue, J.; Yu, J.; Kang, N.; Wang, X. Chrysophanol Inhibits NALP3 Inflammasome Activation and Ameliorates Cerebral Ischemia/Reperfusion in Mice. Mediat. Inflamm. 2014, 2014, 1–12. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, W.; Zhang, B.; Yin, Y.; Zhang, J.; Huang, D.; Huang, R.; Li, W.; Li, W. Ginsenoside Rg1 protects against neuronal degeneration induced by chronic dexamethasone treatment by inhibiting NLRP-1 inflammasomes in mice. Int. J. Mol. Med. 2017, 40, 1134–1142. [Google Scholar] [CrossRef]
- Che, H.; Li, Q.; Zhang, T.; Wang, D.; Yang, L.; Xu, J.; Yanagita, T.; Xue, C.; Chang, Y.; Wang, Y. Effects of Astaxanthin and Docosahexaenoic-Acid-Acylated Astaxanthin on Alzheimer’s Disease in APP/PS1 Double-Transgenic Mice. J. Agric. Food Chem. 2018, 66, 4948–4957. [Google Scholar] [CrossRef]
- Fraga, V.G.; das Graças Carvalho, M.; Caramelli, P.; de Sousa, L.P.; Gomes, K.B. Resolution of inflammation, n-3 fatty acid supplementation and Alzheimer disease: A narrative review. J. Neuroimmunol. 2017, 310, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Chow, M.T.; Sceneay, J.; Paget, C.; Wong, C.S.F.; Duret, H.; Tschopp, J.; Moller, A.; Smyth, M.J. NLRP3 Suppresses NK Cell-Mediated Responses to Carcinogen-Induced Tumors and Metastases. Cancer Res. 2012, 72, 5721–5732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cătană, C.-S.; Atanasov, A.G.; Berindan-Neagoe, I. Natural products with anti-aging potential: Affected targets and molecular mechanisms. Biotechnol. Adv. 2018, 36, 1649–1656. [Google Scholar] [CrossRef] [PubMed]
- Jhang, J.-J.; Lin, J.-H.; Yen, G.-C. Beneficial Properties of Phytochemicals on NLRP3 Inflammasome-Mediated Gout and Complication. J. Agric. Food Chem. 2018, 66, 765–772. [Google Scholar] [CrossRef]
- Ellis, L.Z.; Liu, W.; Luo, Y.; Okamoto, M.; Qu, D.; Dunn, J.H.; Fujita, M. Green tea polyphenol epigallocatechin-3-gallate suppresses melanoma growth by inhibiting inflammasome and IL-1β secretion. Biochem. Biophys. Res. Commun. 2011, 414, 551–556. [Google Scholar] [CrossRef] [Green Version]
- Shin, H.-Y.; Kim, S.-H.; Jeong, H.-J.; Kim, S.-Y.; Shin, T.-Y.; Um, J.-Y.; Hong, S.-H.; Kim, H.-M. Epigallocatechin-3-Gallate Inhibits Secretion of TNF-α, IL-6 and IL-8 through the Attenuation of ERK and NF-κB in HMC-1 Cells. Int. Arch. Allergy Immunol. 2007, 142, 335–344. [Google Scholar] [CrossRef]
- Tsai, P.-Y.; Ka, S.-M.; Chang, J.-M.; Chen, H.-C.; Shui, H.-A.; Li, C.-Y.; Hua, K.-F.; Chang, W.-L.; Huang, J.-J.; Yang, S.-S.; et al. Epigallocatechin-3-gallate prevents lupus nephritis development in mice via enhancing the Nrf2 antioxidant pathway and inhibiting NLRP3 inflammasome activation. Free Radic. Biol. Med. 2011, 51, 744–754. [Google Scholar] [CrossRef]
- Umeda, D.; Yano, S.; Yamada, K.; Tachibana, H. Green Tea Polyphenol Epigallocatechin-3-gallate Signaling Pathway through 67-kDa Laminin Receptor. J. Biol. Chem. 2008, 283, 3050–3058. [Google Scholar] [CrossRef] [Green Version]
- Fan, S.; Wang, Y.; Lu, J.; Zheng, Y.; Wu, D.; Li, M.; Hu, B.; Zhang, Z.; Cheng, W.; Shan, Q. Luteoloside Suppresses Proliferation and Metastasis of Hepatocellular Carcinoma Cells by Inhibition of NLRP3 Inflammasome. PLoS ONE 2014, 9, e89961. [Google Scholar] [CrossRef]
- Lu, X.; Liu, T.; Chen, K.; Xia, Y.; Dai, W.; Xu, S.; Xu, L.; Wang, F.; Wu, L.; Li, J.; et al. Isorhamnetin: A hepatoprotective flavonoid inhibits apoptosis and autophagy via P38/PPAR-α pathway in mice. BioMed Pharmacother. 2018, 103, 800–811. [Google Scholar] [CrossRef]
- Miller, J.M.; Thompson, J.K.; MacPherson, M.B.; Beuschel, S.L.; Westbom, C.M.; Sayan, M.; Shukla, A. Curcumin: A Double Hit on Malignant Mesothelioma. Cancer Prev. Res. 2014, 7, 330–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, M.; Fan, X.; Yuan, B.; Takagi, N.; Liu, S.; Han, X.; Ren, J.; Liu, J. Berberine inhibits NLRP3 Inflammasome pathway in human triple-negative breast cancer MDA-MB-231 cell. BMC Complement. Altern. Med. 2019, 19, 216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teng, J.-F.; Mei, Q.-B.; Zhou, X.-G.; Tang, Y.; Xiong, R.; Qiu, W.-Q.; Pan, R.; Law, B.Y.-K.; Wong, V.K.-W.; Yu, C.-L.; et al. Polyphyllin VI Induces Caspase-1-Mediated Pyroptosis via the Induction of ROS/NF-κB/NLRP3/GSDMD Signal Axis in Non-Small Cell Lung Cancer. Cancers 2020, 12, 193. [Google Scholar] [CrossRef] [Green Version]
- Xie, J.; Zhuan, B.; Wang, H.; Wang, Y.; Wang, X.; Yuan, Q.; Yang, Z. Huaier extract suppresses non-small cell lung cancer progression through activating NLRP3-dependent pyroptosis. Anat. Rec. 2019, ar.24307. [Google Scholar] [CrossRef] [PubMed]
- Yue, E.; Tuguzbaeva, G.; Chen, X.; Qin, Y.; Li, A.; Sun, X.; Dong, C.; Liu, Y.; Yu, Y.; Zahra, S.M.; et al. Anthocyanin is involved in the activation of pyroptosis in oral squamous cell carcinoma. Phytomedicine 2019, 56, 286–294. [Google Scholar] [CrossRef]
- Dumont, A.; de Rosny, C.; Kieu, T.-L.-V.; Perrey, S.; Berger, H.; Fluckiger, A.; Muller, T.; Pais de Barros, J.-P.; Pichon, L.; Hichami, A.; et al. Docosahexaenoic acid inhibits both NLRP3 inflammasome assembly and JNK-mediated mature IL-1β secretion in 5-fluorouracil-treated MDSC: Implication in cancer treatment. Cell Death Dis. 2019, 10, 485. [Google Scholar] [CrossRef]
- Yuldashev, M.P. Cynaroside content of the plants Ferula varia and F. foetida. Chem. Nat. Compd. 1997, 33, 597–598. [Google Scholar] [CrossRef]
- Hu, C.; Kitts, D.D. Luteolin and luteolin-7-O-glucoside from dandelion flower suppress iNOS and COX-2 in RAW264.7 cells. Mol. Cell. Biochem. 2004, 265, 107–113. [Google Scholar] [CrossRef]
- Sun, X.; Sun, G.; Wang, M.; Xiao, J.; Sun, X. Protective effects of cynaroside against H2O2-induced apoptosis in H9c2 cardiomyoblasts. J. Cell. Biochem. 2011, 112, 2019–2029. [Google Scholar] [CrossRef]
- Xiong, J.; Li, S.; Wang, W.; Hong, Y.; Tang, K.; Luo, Q. Screening and identification of the antibacterial bioactive compounds from Lonicera japonica Thunb. leaves. Food Chem. 2013, 138, 327–333. [Google Scholar] [CrossRef]

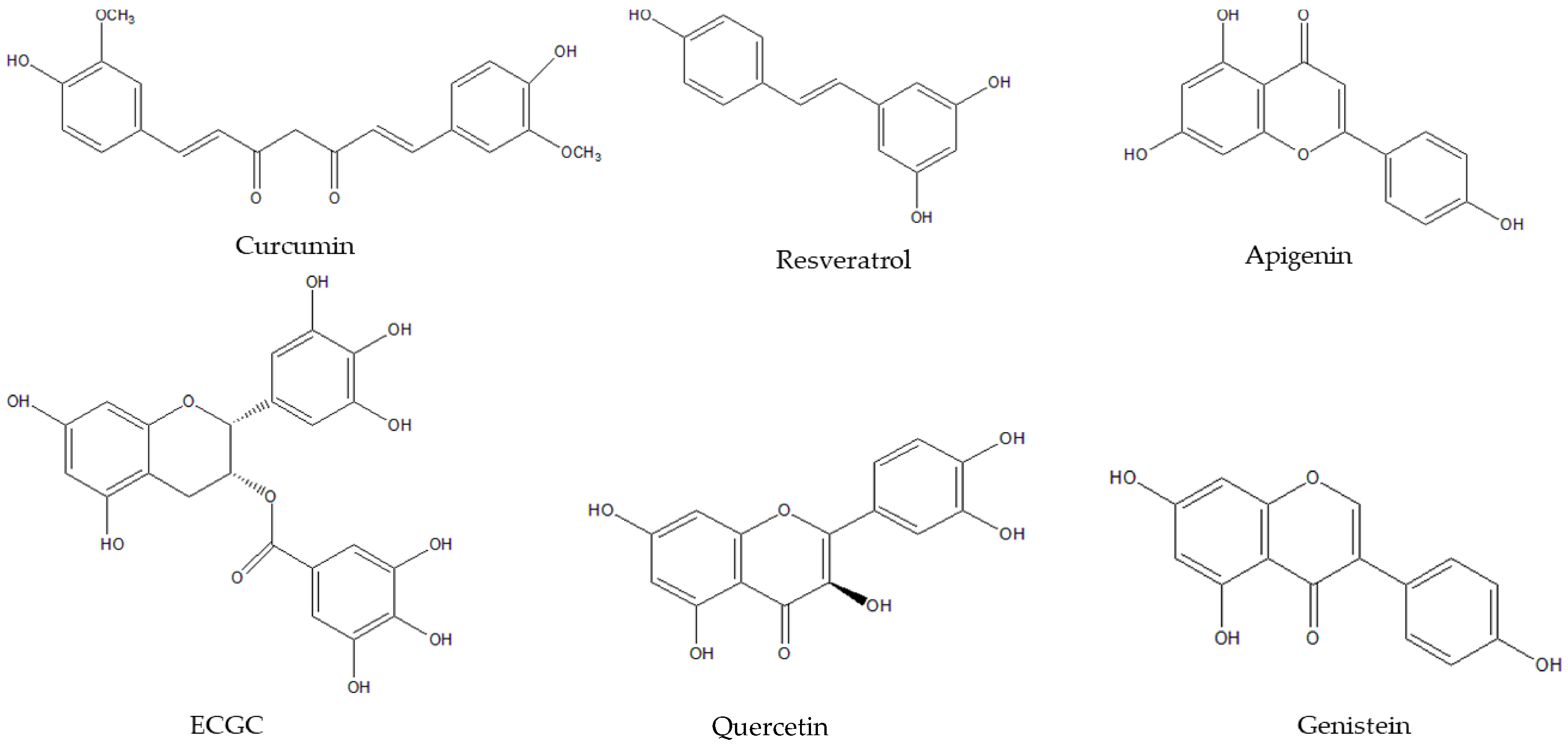
| Nutraceutical Compound | Classification/Source | Overall Role in Inflammasomes | Experimental Model | Molecular Mechanism | Ref. |
|---|---|---|---|---|---|
| Apigenin | Flavonoid/Citrus fruits, vegetables | NLRP3, AIM2 inhibitor | -Human THP1 cells -Mouse J774A.1 macrophage -HEK-293 cells | 1. Syk/Pyk2 pathway interruption 2. Inhibits ERK1/2 and NFϏβ activation in macrophages 3. Inhibits oligomerization of ASC and interferes with its assembly in the cytoplasm 4. No activation of caspase 1 | [30,36] |
| Parthenolide | Sesquiterpene lactone/Tanacetum parthenium (L.) Sch. Bip. | NLRP3, NALP1, NLRC4 inhibitor | -LPS-induced inflammation in NG5 cell line mouse bone marrow cells | 1. Inhibits NFϏβ 2. Inhibits oligomerization and ASC recruitments 3. Inhibits NLRP3 and caspase 1 | [40] |
| Scropoloside B | Iridoids glycosides/Scrophularia dentata Royle ex Benth. | NLRP3 inhibitor | -HEK293 cells -Human THP1 cells | 1. Inhibits NFϏβ 2. Decreases the expression of NLRP3 and Il-1β | [32] |
| Catapol | Iridoids glycosides/Rehmannia glutinosa (Gaertn.) Libosch. ex Fisch. & C.A. Mey | NLRP3 inhibitor | -HEK293 cells -Human THP1 cells | 1.Decreases the expression of NLRP3 | [32] |
| Rh1 and Rg3 | Ginsenoside/Panax ginseng C.A.Mey. | NLRP3, AIM2 inhibitor | -LPS-induced inflammation in bone marrow-derived macrophages (BMDMs) and THP-1 cells -LPS-induced inflammation in Male C57BL/6 mice (8-week-old) | 1.Inhibits the NLRP3 and AIM2 expression 2.Inhibits ASC pyroptosome formation 3.Inhibits caspase 1 activation and the secretion of IL-1 | [41] |
| DHA | ω-3FAs/Fish, Crustaceans, Molluscs, Eggs | NLRP3, NLRPb1 inhibitor | -Mouse model -Human THP1 cells | 1. Decreases the expression of genes involved in the inflammatory pathways of NFϏβ 2. Inhibits the activation of caspase 1 and the release of IL-1β | [33] |
| PSPC | Flavonoid/Fruits, vegetables, leaves and grains | NLRP3 inhibitor | -Male ICR mice -HUVECs | 1. Suppress ROS level 2. Downregulation of pro-caspase1 | [34] |
| Quercetin | Flavonoids/Fruits, vegetables, leaves, and grains | NLRP3, AIM2 inhibitor | -Vasculitis model in C57BL/6 mice | 1. Impaired expression of caspase-1 and IL-1β 2. Prevention of ASC oligomerization | [42] |
| Puerarin and Troxerutin | Isoflavone/Root of Pueraria lobata (Willd.) Ohwi Flavonoid/Sophora japonica L. | NLRP3 inhibitor | -HUVECs cells | 1. Decreases NLPR3, Il-1B and casapase-1 levels | [34] |
| Genipin | Iridoids glycosides/Gardenia jasminoides J.Ellis | NLRP3, NLRC4 inhibitor | -Mouse model -BMDMs cells | 1. Inhibits NLRP3 and NLRC4 inflammasomes 2. Decreases Il-1β, caspase-1 and ASC protein levels | [43] |
| Gypenoside | Triterpenoid saponin/Gynostemma pentaphylla (Thunb.) Makino | NLRP3 inhibitor | -H9C2 cells -SD rats | 1. Inhibits NLRP3 inflammasome 2. Decreases Il-1β and IL-18 protein levels | [44] |
| Morroniside | Iridoid glycoside/Cornus officinalis Siebold & Zucc. | NLRP3 inhibitor | -SD rats | 1. Inhibits NLRP3 2. Downregulation of ASC, caspase-3, Il-1β and IL-18 | [45] |
| Isorhamnetin and Hyperoside | Flavonoids/Water dropwort Oenanthe javanica (Blume) DC. | NLPR3, AIM-2 inhibitor | -Bone marrow-derived macrophages (BMDMs) form C57BL/6 mice -THP1 cells | 1.Decreases the of Il-1β, IL-18 and caspase-1 secretion | [46] |
| Resveratrol | Stilbene (flavonoid)/Skin of grapes, blueberries, raspberries, cmulberries and red wine | NLRP3 inhibitor | -J774A.1 cells -Raw 264.7 cells -Sprague–Dawley rat | 1. Decreases the secretion of Il-1β 2. Decreases the ACS and NLRP3 proteins 1. Suppresses NFϏβ and inhibits NLRP3 1.Supresses IL-1β and IL-18 2.Decreases NLRP3 and caspase-1 expression | [47] [48] [49] |
| Curcumin | Polyphenol/Curcuma longa L. roots | NLRP3 inhibitor | -THP1 cells -PMA-induced macrophages | 1. Decreases NLRP3 expression and Il-1β and caspase 1 secretion through the inhibition of TLR4/MyD88/ NFϏβ signalling and P2X7R expression | [50] |
| Thonningianin A | Polyphenol/Penthorum chinense Pursh | NLRP3 inhibitor | -ApoE-KO mice | 1. Decreases NLRP3 and Il-1β expression | [51] |
| Salvianolato | Polyphenol/Salvia miltiorrhiza Bunge | NLRP3 inhibitor | -SPF Sprague-Dawley rats | 1. Decreases NLRP3, pro-caspase1, caspase-1, Il-1β, IL-18 and TXNIP expression | [52] |
| Ilexgenin A | Triterpenoid/Ilex hainanensis Merr. | NLRP3 inhibitor | -EA.hy-926 cells -Primary rat vascular endothelial cells (VECs) | 1. Decreases the TXNIP/NLRP3 activation under ER stress condition | [53] |
| Tanshinone IIA and sodium tanshinone IIA | Diterpenoid/Salvia miltiorrhiza Bunge | NLRP3 inhibitor | -RAW264.7 macrophages -Beagle dogs | 1. Decreases IL-1β levels 1. Inhibits the generation of ROS and TXNIP 2. Decreases the NLRP3 activation and the secretion of IL1-β and IL-18 | [54] [55] |
| Dihydromyricetin | Flavonoid/Ampelopsis grossedentata (Hand.-Mazz.) W.T.Wang | NLRP3 inhibitor | -HUVECs | 1. Attenuates NLRP3 inflammasome | [56] |
| Luteolin | Flavonoid/Reseda luteola L. | NLRP3 inhibitor | -RAW264.7 cells | 1. Inhibits NLRP3 inflammasome 2. Decreases TNF-α and IL-6 levels | [57] |
| Colchicine | Alkaloid/Colchicum autumnale L. | NLRP3 inhibitor | -ACS patients | 1. Suppresses NLRP3 inflammasome 2. Decreases Il-1β, IL-6 and IL-18 levels | [58] |
| Triptolide | Diterpenoid/Tripterygiumwilfordii Hook F. | NLRP3 inhibitor | -C57/BL6 mice | 1. Inhibits the NLRP3 inflammasome 2. Inhibits IL-1β, IL-18, MCP-1 and VCAM-1 release | [59] |
| Total flavones | Flavonoids/Abelmoschus manihot (L.) Medic | NLRP3 inhibitor | -I/R Rats | 1. Inhibits NLRP3 inflammasome 2. Decreases the IL-1β, IL-6 and TNF-α levels | [60] |
| Umbelliferone | Phenolic coumarin/Rutaceae and Umbelliferae | NLRP3 inhibitor | -Sprague-Dawley rats | 1. Inhibits the NLRP3 inflammasome and IL-6 and TNF-α levels | [61] |
| Nutraceutical Compound | Classification/Source | Overall Role in Inflammasomes | Experimental Model | Molecular Mechanism | Ref. |
|---|---|---|---|---|---|
| Ginsenoside Rb1 and Ginsenoside CK | Triterpene saponins/Panax ginseng C.A.Mey. root | NLRP3 inhibitor | -3T3-L1 adipocyte cells -Mouse model | 1. Inhibits NLRP3 inflammasome 2. Attenuation of TXNIP expression 3. Reduction in IL-1β expression 4. Reduction in IRS-1 phosphorylation and PI3K and AKT activation | [92] |
| γ-Tocotrienol | Isomers unsaturated Vitamin E/Fruits, vegetables, nuts, meats, cooking oils and some grains | NLRP3 inhibitor | -Mouse model of type 2 diabetes | 1. Inhibits NFϏβ 2. Inhibits NLRP3 activation | [93] |
| DHA | Omega 3 Fatty acids (ω-3FAs)/Animal and plant origin | NLRP3, NLRP1b inhibitor | -Human THP1 cells -Mouse model | 1. Decreases the expression of genes involved in the NFϏβ inflammatory pathways 2. Inhibits caspase1 activation and thus inhibits IL-1β release | [33] |
| PiperineCepharanthine | Alkaloid/Black pepper/Stephania cepharantha “Hayata” | NLRP3 inhibitor | -Diabetic nephropathy model in adult male (SD rats) | 1. Decreases the levels of oxidative stress and activation of NFKβ 2. Decreases levels of TXNIP and NLRP3 mRNA and proteins in kidney tissues 3. Increase insulin-like growth factor-I (IGF-1) | [94] |
| Curcumin | Flavonoid/Curcuma longa L. | NLRP3 inhibitor | -C57BL/KsJ db/db (diabetic) mice model HK-2 cells | 1. Decreases the NLRP3 i, capase1 and IL-1B expression | [95] |
| Arglabin | Sesquiterpene lactone/Artemisia glabella Kar. & Kir. | NLRP3 inhibitor | -INS-1 cells -ApoE2Ki mice | 1. Degrading NLRP3 and pro-IL-1β, pro-caspase 1 and ASC | [96] |
| Resveratrol | Stilbene/Skin of grapes, blueberries, raspberries and mulberries | NLRP3 inhibitor | -3T3-L1 adipocytes -Streptozotocin-induced diabetic mice (ICR male mice) | 1. Decreases TXNIP levels and inhibits cleavage caspase-1 induction 2. Reduced release of IL-1β | [97] |
| Vitamin D3 | Cholecalciferol/Fish, beef, cheese, egg yolk | NLRP3 inhibitor | -HRMECs -Streptozotocin-induced SD rats | 1. Decreases the TXNIP levels 2. Decreases NLRP3 activation | [98] |
| Mangiferin | Naturally occurring glucosylxanthone/Mango | NLRP3 inhibitor | -Perivascular adipose tissue isolated from male SD rats and from high-fat diet feeding in mice | 1. Decreased levels of TXNIP and inhibition of cleaved caspase-1induction 2. Reduced release of IL-1β | [99] |
| Salvianolic acid A | Propanoic acid/Salvia miltiorrhiza Bunge | NLRP3 inhibitor | -Male Zucker diabetic fatty rats | 1. Inhibits NFϏβ 2. Inhibits NLRP3 activation | [100] |
| Myricetin | Flavonoid/Horsegram seed coat (Macrotyloma uniflorum (Lam.) Verdc.) | NLRP3 inhibitor | -Streptozotocin-induced diabetic male Wistar rats | 1. Decreases the expression of NLRP3, ASC and Caspase-1 | [101] |
| Polyphenols | Polyphenol/Freeze-dried red raspberry | NLRP3 inhibitor | -High-fat diet feeding C57BL/6 mice | 1. Decreases NLRP3 and caspase-1 levels 2. Decreases IL-1β and IL-18 production | [102] |
| Ginsenoside Rg5 | Ginsenoside/Panax ginseng C.A.Mey. | NLRP3 inhibitor | -High-fat diet/streptozotocin-induced diabetic mice (C57BL/6 mice) | 1. Decreases the expression of NLRP3, ASC and Caspase-1 2. Decreases the expression of IL-1β and IL-18 3. Decreases of NFϏβ and P38 MAPK phosphorylation | [103] |
| Genistein | Isoflavone/Legumes | NLRP3 activator | -Alloxan-induced diabetic ICR mice | 1. Restored expression levels of NLRP3, ASC and Caspase-1 2. Improves the levels of NF-Ϗβ, NFϏβ, TNFα COX2 and iNOS | [104] |
| Curcumin+Allopurinol | Flavonoid/Curcuma longa L. | NLRP3 inhibitor | -BRL-3A cells and -Human HepG2 cells exposed to high fructose -Fructose-fed rat (Male SD rats) | 1. Decreases overexpression of TXNIP via up-regulating miR-200a | [91] |
| Quercetin+Allopurinol | Flavonoid/Found in many fruits, vegetables, leaves and grains | NLRP3 inhibitor | -BRL-3A and -Human HepG2 exposed to high glucose -Streptozotocin-induced diabetic rats (Male SD rats) | 1. Decreases overexpression of TXNIP 2. Reduces expression of IL-1β 3. Modulates the expression of proteins involved in lipid metabolism | [105] |
| Nutraceutical Compound | Classification/Source | Overall Role in Inflammasomes | Experimental Model | Molecular Mechanism | Ref. |
|---|---|---|---|---|---|
| Magniferin | Poliphenol of C-glucosylxanthone/Mangifera indica L. (Mango tree) | NLRP3 inhibitor | -CMS in mice | 1. Inhibits hippocampal NLRP3 inflammasome 2. Inhibits caspase-1/Il-1β axis 3. Decreases the ASC expression 4. Decreases the Il-18 production | [108] |
| Apigenin | Flavone/Citrus fruits, vegetables | NLRP3 inhibitor | -Rat model of chronic unpredictable mild stress (CUMS) | 1. Increases the expression levels of PPARγ 2. Decreases the NLPR3, Il-1B and casapase-1 levels | [110] |
| Resveratrol | Stilbene/Skin of grapes, blueberries, raspberries and mulberries | NLRP3 inhibitor | -C57BL/6 mouse model -Mouse BV2 cells -MCAO-injury rats | 1. Decreases the NLRP3 generation via activation of SIRT1 2. Downregulates the level of IL-1β and IL-18 3. Decreases the NFϏβ levels | [111] |
| Umbelliferone | 7-hydroxycoumarin/Plants: Rutaceae and Apiaceae families Carrot, coriander, garden angelica | NLRP3 inhibitor | -Rat model of ischemic reperfusion (SD rats) | 1. Decreases the TXNIP expression 2. Increases the PPARγ levels 3. Inhibits NLRP3 inflammasome | [112] |
| Sulphoraphane | Isothiocyanate/Broccoli, Brussels sprouts, cabbages | NLRP3 inhibitor | -Brain ischemia/reperfusion injury model in adult male (SD rats) | 1. Suppresses I/R-induced NLRP3 inflammasome expression 2. Downregulation of cleaved caspase-1 3. Reducing IL-1β and IL-18 expression | [113] |
| Curcumin | Pigment from tumeric/Curcuma longa L. | NLRP3 inhibitor | -INS-1 cells -ApoE2Ki mice | 1. Inhibits hippocampal NLRP3 inflammasome 2. Downregulates TXNIP/NLRP3 with the regulation of AMPK activity | [120] |
| Rg1 | Ginsenoside/Panax ginseng C.A. Mey Panax japonicus (T.Nees) C.A. Mey. | NLRP1 inhibitor | -ICR mice | 1. Reduces expression levels of NLRP1, caspase 1 and 5, ASC and IL-1β and IL-18 2. Increases expression of the glucocorticoid receptors | [122] |
| Astragaloside-IV | Astragalus membranaceus (Fisch.) Bunge | NLRP3 inhibitor | -ICR mice | 1. Attenuates NLRP3 2. Decreases IL-1β and TNF-α levels 3. Decreases NFϏβ translation | [115] |
| Ruscogenin | Steroidal sapognin/Ophiopogon japonicus (Thunb.) Ker Gawl | NLRP3 inhibitor | -bEnd.3 cells-C57BL/6J mice | 1. Inhibits NLRP3, IL-1β, caspase-1 and TXNIP expression | [116] |
| Sinomenine | Alkaloid/Sinomenium acutum (Thunb.) Rehder & E.H.Wilson | NLRP3 inhibitor | -MCAO mice model -OGD cell model (Primary mixed glial cells) | 1. Inhibits the NLRP3 via AMPK pathway 2. Inhibits ASC and caspase-1 | [117] |
| Arctigenin | Lignan/Arctium lappa L. | NLRP3 inhibitor | -MCAO-injury rats -OGD-injury EX527 cells | 1. Decreases the NLRP3 generation via activation of SIRT1 2. Downregulates the level of IL-1β and IL-18 | [118] |
| Asthaxantin | Carotenoid/marine organisms, such as crab, salmon, shrimp, krill and microalgae | NLRP3 inhibitor | -PSEN1(APP/PS1) double-transgenic mice | 1. Decreases the ASC expression 2. Reduces the IL-1β and TNF-α levels | [123] |
| Chrysophano | Anthraquinone/Rheum genus | NLRP3 inhibitor | -MCAO Male CD1 mice | 1. Decreases the NLRP3 and ASC expression 2. Reduces the IL-1β and caspase 1 expression | [121] |
| Nutraceutical Compound | Classification/Source | Overall Role in Inflammasomes | Experimental Model | Molecular Mechanism | Ref. |
|---|---|---|---|---|---|
| ECGC | Phenol/Green tea | NLRP3 inhibitor | -HMC | 1. Inhibitory effect on proliferation 2. Suppresses NFϏβ activity 3. Decreases IL-1β secretion 4. Decreases caspase-1 activation | [128] |
| Luteoloside | Taraxacum officinale (L.) Weber ex F.H.Wigg. and Cynara scolymus L. | NLRP3 inhibitor | -HCC | 1. Inhibition of cell migration and invasion 2. Suppresses proliferation and metastasis 3. Downregulates the expression level of caspase 1 and IL-1β | [132] |
| Isorhamnetin | Flavonoid/Hippophae rhamnoides L. | NLRP3, AIM2 inhibitor | -BMDMs | 1. Downregulates the expression of pro-inflammatory cytokines 2. Attenuates the secretion of IL-1β resulting from NLRP3, NLRC4, and AIM2 inflammasome activation | [133] |
| Curcumin | Polyphenol/Curcuma longa L. | NLRP3 activator | -Malignant mesothelioma cells | 1. Activates NLRP3 inflammasome 2. Activates the expression of caspase-1 3. Attenuates the expression of NFϏβ, TLR and IL-1β | [134] |
| Berberine | Alkaloid/Chinese herbs | NLRP3 | -Triple-negative breast MDA-MB-231 cancer cells | 1. Reduces pro-caspase-1, caspase-1, IL-1β, P2X7 and ASC expression | [135] |
| Polyphyllin VI | Saponin/Trillium tschonoskii Maxim. | NLRP3 activator | -Non-Small-Cell Lung A549 and H1299 cancer cells | 1. Activation of caspase-1 via the induction of the ROS/NFϏβ /NLRP3/GSDMD signal axis 2. Upregulates NLRP3 inflammasome | [136] |
| Huaier extract | A kind of fungus/Trametes robiniophila Murr. | NLRP3 activator | -Non-Small-Cell Lung H520 -H358 cancer cells | 1. Upregulates NLRP3 2. Activation of caspase-1, IL-1β, and IL-18 | [137] |
| Anthocyanins | Natural pigment widely found in colored plants | NLRP3 activator | -Oral squamous HaCaT, Tca8113 -SCC15 cancer cells | 1. Upregulates NLRP3 2. Activation of caspase-1 and IL-1β | [138] |
| DHA | ω-3FAs/Fish, Crustaceans, Molluscs, Eggs | NLRP3 | -Myeloid-derived suppressor cells | 1. Reduction in IL-1β secretion, inhibition of JNK pathway through β-arrestin-2 activation | [139] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castejón-Vega, B.; Giampieri, F.; Alvarez-Suarez, J.M. Nutraceutical Compounds Targeting Inflammasomes in Human Diseases. Int. J. Mol. Sci. 2020, 21, 4829. https://doi.org/10.3390/ijms21144829
Castejón-Vega B, Giampieri F, Alvarez-Suarez JM. Nutraceutical Compounds Targeting Inflammasomes in Human Diseases. International Journal of Molecular Sciences. 2020; 21(14):4829. https://doi.org/10.3390/ijms21144829
Chicago/Turabian StyleCastejón-Vega, Beatriz, Francesca Giampieri, and José M. Alvarez-Suarez. 2020. "Nutraceutical Compounds Targeting Inflammasomes in Human Diseases" International Journal of Molecular Sciences 21, no. 14: 4829. https://doi.org/10.3390/ijms21144829





