Microbial Etiology and Prevention of Dental Caries: Exploiting Natural Products to Inhibit Cariogenic Biofilms
Abstract
:1. Introduction
1.1. Chemical Agents
1.2. Natural Products, Plant Extracts, and Probiotics
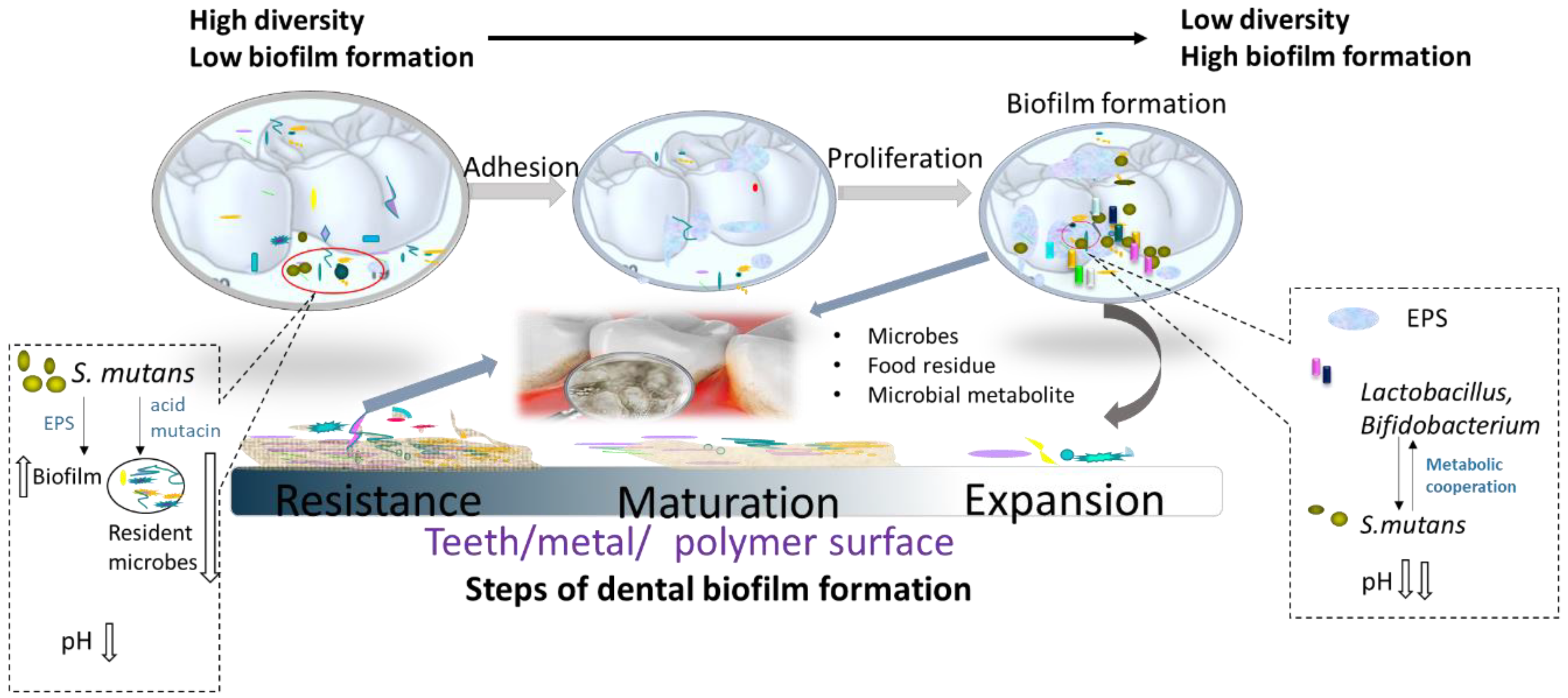
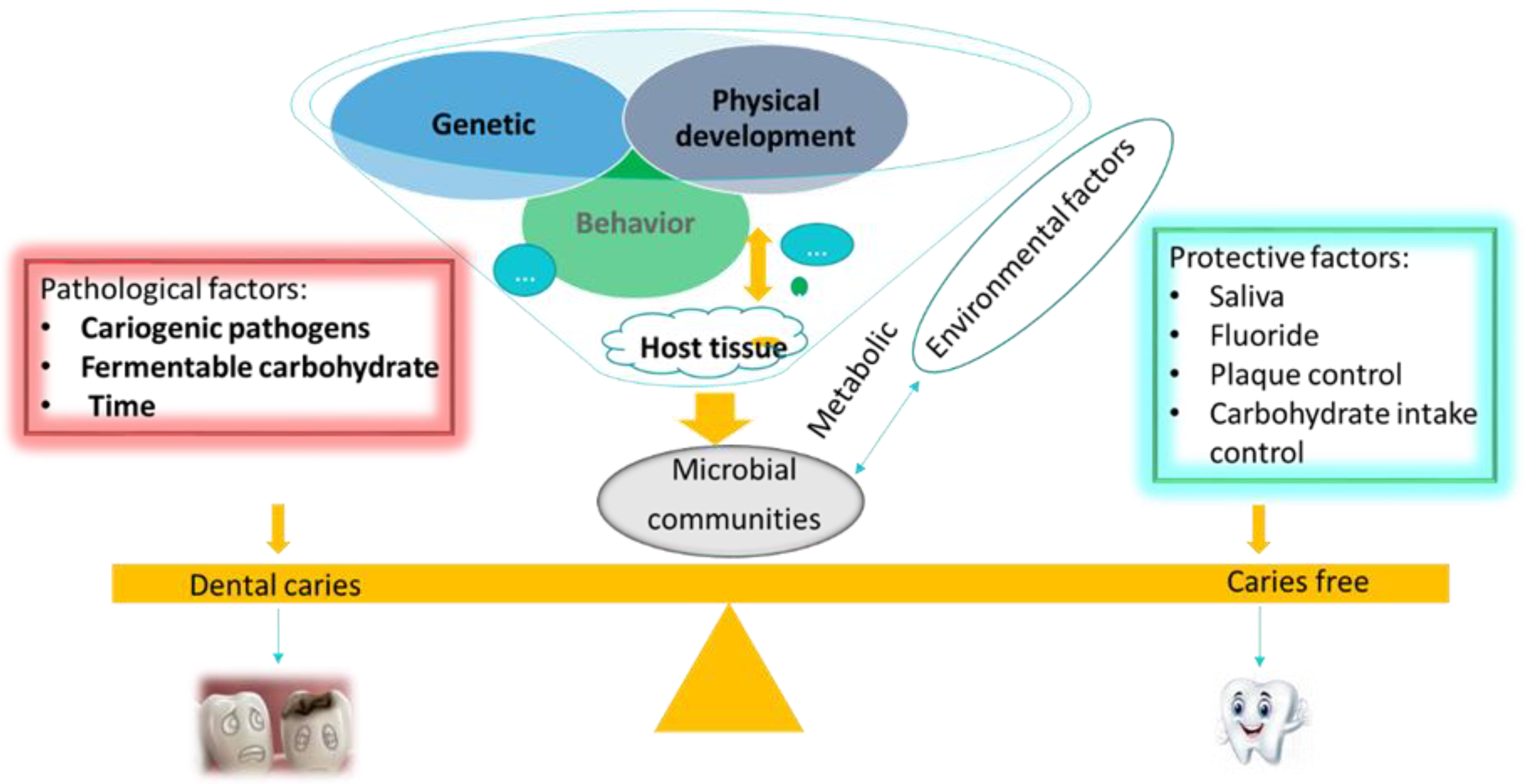
2. Supragingival Microbial Biofilms and Dental Caries
2.1. Oral Microbiota
2.2. Dental Biofilms
2.3. Microbial Etiology of Dental Caries
3. Recent Advances in Natural Antimicrobial Compounds for the Prevention of Dental Caries
3.1. Plant-Derived Cariogenic Biofilm Inhibitors
3.1.1. Effect on Bacterial Growth
3.1.2. Alteration of Initial Adhesion, Aggregation, and Integrity
3.1.3. Modulation of Bacterial Quorum Sensing
3.2. Microbial Cariogenic Biofilm Inhibitors—Probiotics
3.3. Incorporation of Natural Antimicrobials in Caries
4. Conclusion and Future Perspective
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stsepetova, J.; Truu, J.; Runnel, R.; Nommela, R.; Saag, M.; Olak, J.; Nolvak, H.; Preem, J.K.; Oopkaup, K.; Krjutskov, K.; et al. Impact of polyols on oral microbiome of Estonian schoolchildren. BMC Oral Health 2019, 19, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karoly, M.; Gabor, N.; Adam, N.; Andrea, B. Characteristics, diagnosis and treatment of the most common bacterial diseases of the oral cavity. Orvosi Hetilap 2019, 160, 739–746. [Google Scholar]
- Peres, M.A.; Macpherson, L.M.D.; Weyant, R.J.; Daly, B.; Venturelli, R.; Mathur, M.R.; Listl, S.; Celeste, R.K.; Guarnizo-Herreno, C.C.; Kearns, C.; et al. Oral diseases: A global public health challenge. Lancet 2019, 394, 249–260. [Google Scholar] [CrossRef]
- Mosaddad, S.A.; Tahmasebi, E.; Yazdanian, A.; Rezvani, M.B.; Seifalian, A.; Yazdanian, M.; Tebyanian, H. Oral microbial biofilms: An update. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 2005–2019. [Google Scholar] [CrossRef]
- Yadav, K.; Prakash, S. Dental Caries: A microbiological approach. J. Clin. Infect. Dis. Pract. 2017, 2, 1–5. [Google Scholar] [CrossRef]
- Oral Health Database. Available online: https://www.mah.se/CAPP/ (accessed on 14 April 2020).
- Minimal Intervention Dentistry (MID) for Managing Dental Caries. Available online: https://www.fdiworlddental.org/resources/policy-statements-and-resolutions/minimal-intervention-dentistry-mid-for-managing-dental (accessed on 10 June 2020).
- Yon, M.J.Y.; Gao, S.S.; Chen, K.J. Medical model in caries management. J. Dent. 2019, 7, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Maliky, M.A.; Frentzen, M.; Meister, J. Laser-assisted prevention of enamel caries: A 10-year review of the literature. Laser Med. Sci. 2019, 35, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Featherstone, J.D.; Domejean-Orliaguet, S.; Jenson, L.; Wolff, M.; Young, D.A. Caries risk assessment in practice for age 6 through adult. CDA 2007, 35, 703. [Google Scholar]
- What is Caries? Available online: https://www.acffglobal.org/for-patients/what-is-caries/ (accessed on 10 June 2020).
- Do Nascimento, C.; Pita, M.S.; de Souza Santos, E.; Monesi, N.; Pedrazzi, V.; de Albuquerque Junior, R.F.; Ribeiro, R.F. Microbiome of titanium and zirconia dental implants abutments. Dent. Mater. 2016, 32, 93–101. [Google Scholar] [CrossRef]
- Takenaka, S.; Ohsumi, T.; Noiri, Y. Evidence-based strategy for dental biofilms: Current evidence of mouthwashes on dental biofilm and gingivitis. Jpn. Dent. Sci. Rev. 2019, 55, 33–40. [Google Scholar] [CrossRef]
- Camargo, L.; Silva, S.N.; Chambrone, L. Efficacy of toothbrushing procedures performed in intensive care units in reducing the risk of ventilator-associated pneumonia: A systematic review. J. Periodont. Res. 2019, 54, 601–611. [Google Scholar] [CrossRef] [PubMed]
- Daubert, D.M.; Weinstein, B.F. Biofilm as a risk factor in implant treatment. Periodontol. 2000 2019, 81, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.C.C.; Marre, A.T.D.; Almeida, J.S.D.; Lobo, L.A.; Farah, A.; Valenca, A.M.G. Treatment of dental biofilm with a tincture of Bauhinia forficata leaves: An ex-vivo study. Nat. Prod. Res. 2019, 33, 3432–3435. [Google Scholar] [CrossRef] [PubMed]
- Collaerf, B.; Attstrom, R.; De Bruyn, H.; Moverl, R. The effect of delmopinol rinsing on dental plaque formation and gingivitis healing. J. Clin. Periodontol. 1992, 19, 274–280. [Google Scholar] [CrossRef]
- Neilands, J.; Troedsson, U.; Sjodin, T.; Davies, J.R. The effect of delmopinol and fluoride on acid adaptation and acid production in dental plaque biofilms. Arch. Oral Biol. 2014, 59, 318–323. [Google Scholar] [CrossRef]
- Stewart, B.; Shibli, T.A.; Araujo, M.; Figuciredo, L.C.; Panagakos, F.; Matarazzo, F.; Mairink, R.; Onuma, T.; Faveri, M.; Retamal-Valdes, B.; et al. Effects of a toothpaste containing 0.3% triclosan on periodontal parameters of subjects enrolled in a regular maintenance program: A secondary analysis of a 2-year randomized clinical trial. J. Periodont. 2020, 91, 596–605. [Google Scholar] [CrossRef]
- Mei, H.C.; Engels, E.; Vries, J.; Busscher, H.J. Effects of amine fluoride on biofilm growth and salivary pellicles. Caries Res. 2008, 42, 19–27. [Google Scholar]
- Naumova, E.A.; Weber, L.; Pankratz, V.; Czenskowski, V.; Arnold, W.H. Bacterial viability in oral biofilm after tooth brushing with amine fluoride or sodium fluoride. Arch. Oral Biol. 2019, 97, 91–96. [Google Scholar] [CrossRef]
- Madléna, M.; Dombi, C.; Gintner, Z.; Bánóczy, J. Effect of amine fluoride/stannous fluoride toothpaste and mouthrinse on dental plaque accumulation and gingival health. Oral Dis. 2004, 10, 294–297. [Google Scholar] [CrossRef]
- Kaufmann, M.; Bartholmes, P. Purification, characterization and inhibition by fluoride of enolase from Streptococcus mutans DSM320523. Caries Res. 1992, 26, 110–116. [Google Scholar] [CrossRef]
- Sakaue, Y.; Takenaka, S.; Ohsumi, T.; Domon, H.; Terao, Y.; Noiri, Y. The effect of chlorhexidine on dental calculus formation: An in vitro study. BMC Oral Health 2018, 18, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prabakar, J.; John, J.; Arumugham, M.; Kumar, P. Go natural, say no to chemicals—A systematic review on effectiveness of green tea extract containing formulations on dental caries. Asian J. Pharm. Clin. Res. 2019, 12, 63–69. [Google Scholar] [CrossRef]
- Watson, P.S.; Pontefract, H.A.; Devine, D.A.; Shore, R.C.; Nattress, B.R.; Kirkham, J.; Robinson, C. Penetration of fluoride into natural plaque biofilms. J. Dent. Res. 2005, 84, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Ye, Q.; Fan, M.; Liu, C. Antimicrobial effects of the ginsenoside Rh2 on monospecies and multispecies cariogenic biofilms. J. Appl. Microbiol. 2019, 126, 740–751. [Google Scholar] [CrossRef]
- Sharma, D.; Jain, A.; Ahuja, S.; Sachdeva, P. Role of plant extract in the inhibition of dental caries. Int. J. Life Sci. Pharma Res. 2018, 8, L9–L23. [Google Scholar]
- Salehi, B.; López, M.D.; Martínez-López, S.; Victoriano, M.; Sharifi-Rad, J.; Martorell, M.F.; Rodrigues, C.; Martins, N. Stevia rebaudiana Bertoni bioactive effects: From in vivo to clinical trials towards future therapeutic approaches. Phytother. Res. 2019, 33, 2904–2917. [Google Scholar] [CrossRef]
- Chauhan, D.N.; Singh, P.R.; Shah, K.; Chauhan, N.S. Natural oral care in dental therapy: Current and future prospects. Nat. Oral Care Dent. Ther. 2020, 1–29. [Google Scholar] [CrossRef]
- Farid, A.B.; Omar, A.Z. Natural products for dental caries prevention. J. Med. Food 2004, 7, 381–384. [Google Scholar]
- Bodiba, D.C.; Prasad, P.; Srivastava, A.; Crampton, B.; Lall, N.S. Antibacterial activity of Azadirachta indica, Pongamia pinnata, Psidium guajava, and Mangifera indica and their mechanism of action against Streptococcus mutans. Pharmacogn. Mag. 2018, 14, 76. [Google Scholar] [CrossRef]
- Usha, C.; Ramarao, S.; John, B.; Babu, M. Anticariogenicity of Stevia rebaudiana extract when used as a mouthwash in high caries risk patients: Randomized controlled clinical trial. World 2017, 8, 364–369. [Google Scholar] [CrossRef]
- Zschüttig, A.; Zimmermann, K.; Blom, J.; Goesmann, A.; Pöhlmann, C.; Gunzer, F. Identification and characterization of microcin S, a new antibacterial peptide produced by probiotic Escherichia coli G3/10. PLoS ONE 2012, 7, e33351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waghmode, M.; Gunjal, A.; Patil, N. Probiotic sugar confectionery fortified with flax seeds (Linum usitatissimum L.). J. Food Sci. Tech. 2020, 57, 1964–1970. [Google Scholar] [CrossRef] [PubMed]
- Goel, A.; Halami, P.M.; Tamang, J.P. Genome Analysis of Lactobacillus plantarum isolated from some indian fermented foods for bacteriocin production and probiotic marker genes. Front. Microbiol. 2020, 11, 40. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, G.; Ruiz, B.; Faleiros, S.; Vistoso, A.; Marró, M.; Sánchez, J.; Urzúa, I.; Cabello, R. Probiotic compared with standard milk for high-caries children: A cluster randomized trial. J. Dent. Res. 2016, 95, 402–407. [Google Scholar] [CrossRef]
- Miller, W.D. The Micro-Organisms of the Human Mouth: The Local and General Diseases Which are Caused by Them; The S.S. White Dental Mfg.Co.: Philadelphia, PA, USA, 1890; pp. 274–341. [Google Scholar]
- Lu, M.; Xuan, S.; Wang, Z. Oral microbiota: A new view of body health. Food Sci. Hum. Wellness 2019, 8, 8–15. [Google Scholar] [CrossRef]
- Pitts, N.B.; Zero, D.T.; Marsh, P.D.; Ekstrand, K.; Weintraub, J.A.; Ramos-Gomez, F.; Tagami, J.; Twetman, S.; Tsakos, G.; Ismail, A. Dental caries. Nat. Rev. Dis. Prim. 2017, 3, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Razi, M.A.; Qamar, S.; Singhal, A.; Mahajan, A.; Siddiqui, S.; Minz, R.S.M. Role of natural salivary defenses in the maintenance of healthy oral microbiota in children and adolescents. J. Family Med. Prim. Care 2020, 9, 1603. [Google Scholar] [CrossRef] [PubMed]
- Marsh, P.; Zaura, E. Dental biofilm: Ecological interactions in health and disease. J. Clin. Periodontol. 2017, 44, S12–S22. [Google Scholar] [CrossRef]
- Sim, C.P.C.; Dashper, S.G.; Reynolds, E.C. Oral microbial biofilm models and their application to the testing of anticariogenic agents. J. Dent. 2016, 50, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lamont, R.J.; Koo, H.; Hajishengallis, G. The oral microbiota: Dynamic communities and host interactions. Nat. Rev. Microbiol. 2018, 16, 745–759. [Google Scholar] [CrossRef]
- Seminario, A.; Broukal, Z.; Ivancakova, R. Mutans streptococci and the development of dental plaque. Prague Med. Rep. 2005, 106, 349–358. [Google Scholar] [PubMed]
- Widyarman, A.S.; Theodorea, C.F. Effect of reuterin on dual-species biofilm in vitro of Streptococcus mutans and Veillonella parvula. J. Int. Dent. 2019, 12, 77–83. [Google Scholar]
- Fakhruddin, K.S.; Ngo, H.C.; Samaranayake, L.P. Cariogenic microbiome and microbiota of the early primary dentition: A contemporary overview. Oral Dis. 2019, 25, 982–995. [Google Scholar] [CrossRef]
- Liu, J.-f.; Hsu, C.-L.; Chen, L.-R. Correlation between salivary mutans streptococci, lactobacilli and the severity of early childhood caries. J. Dent. Sci. 2019, 14, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Obata, J.; Fujishima, K.; Nagata, E.; Oho, T. Pathogenic mechanisms of cariogenic Propionibacterium acidifaciens. Arch. Oral Biol. 2019, 105, 46–51. [Google Scholar] [CrossRef]
- Bowen, W.H.; Burne, R.A.; Wu, H.; Koo, H. Oral biofilms: Pathogens, matrix, and polymicrobial interactions in microenvironments. Trends Microbiol. 2018, 26, 229–242. [Google Scholar] [CrossRef]
- Amaechi, B.T.; Tenuta, L.M.A.; Ricomini Filho, A.P.; Cury, J.A. Protocols to study dental caries in vitro: Microbial caries models. Methods Mol. Biol. 2019, 1922, 357–368. [Google Scholar]
- Ccahuana-Vasquez, R.A.; Cury, J.A. S. mutans biofilm model to evaluate antimicrobial substances and enamel demineralization. Braz. Oral Res. 2010, 24, 135–141. [Google Scholar] [CrossRef] [Green Version]
- Alshahrani, A.M.; Gregory, R.L. In vitro Cariostatic effects of cinnamon water extract on nicotine-induced Streptococcus mutans biofilm. BMC Complement. Altern. Med. 2020, 20, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.X.; Qin, S.J.; Huang, Y.; Xu, X.Y.; Zhao, J.X.; Zhang, H.; Chen, W. Inhibitory and preventive effects of Lactobacillus plantarum FB-T9 on dental caries in rats. J. Oral Microbiol. 2020, 12, 10. [Google Scholar] [CrossRef] [Green Version]
- Abranches, J.; Zeng, L.; Kajfasz, J.K.; Palmer, S.; Chakraborty, B.; Wen, Z.Z.; Richards, V.P.; Brady, L.J.; Lemos, J.A. Biology of oral streptococci. Microbiol. Spectr. 2018, 6, 12. [Google Scholar] [CrossRef] [PubMed]
- Palmer, S.R.; Ren, Z.; Hwang, G.; Liu, Y.; Combs, A.; Söderström, B.; Lara Vasquez, P.; Khosravi, Y.; Brady, L.J.; Koo, H.; et al. Streptococcus mutans yidC1 and yidC2 impact cell envelope biogenesis, the biofilm matrix, and biofilm biophysical properties. J. Bacteriol. 2019, 201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Q.; Nijampatnam, B.; Hua, Z.; Nguyen, T.; Zou, J.; Cai, X.; Michalek, S.M.; Velu, S.E.; Wu, H. Structure-based discovery of small molecule inhibitors of cariogenic virulence. Sci. Rep-Uk. 2017, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Elgamily, H.; Safy, R.; Makharita, R. Influence of medicinal plant extracts on the growth of oral pathogens streptococcus mutans and lactobacillus acidophilus: An in-vitro study. Open Access Maced. J. Med. Sci. 2019, 7, 2328–2334. [Google Scholar] [CrossRef] [Green Version]
- Philip, N.; Leishman, S.J.; Bandara, H.; Walsh, L.J. Polyphenol-rich cranberry extracts modulate virulence of streptococcus mutans-candida albicans biofilms implicated in the pathogenesis of early childhood caries. Pediatr. Dent. 2019, 41, 56–62. [Google Scholar]
- Manome, A.; Abiko, Y.; Kawashima, J.; Washio, J.; Fukumoto, S.; Takahashi, N. Acidogenic potential of oral bifidobacterium and its high fluoride tolerance. Front. Microbiol. 2019, 10, 1099. [Google Scholar] [CrossRef]
- Rosário Palma, A.L.; Domingues, N.; Barros, P.P.; Brito, G.N.B.; Jorge, A.O.C. Influence of Streptococcus mitis and Streptococcus sanguinis on virulence of Candida albicans: In vitro and in vivo studies. Folia. Microbiol. 2019, 64, 215–222. [Google Scholar] [CrossRef]
- Mira, A.; Buetas, E.; Rosier, B.; Mazurel, D.; Villanueva-Castellote, A.; Llena, C.; Ferrer, M.D. Development of an in vitro system to study oral biofilms in real time through impedance technology: Validation and potential applications. J. Oral Microbiol. 2019, 11, 12. [Google Scholar] [CrossRef]
- Shu, M.; Wong, L.; Miller, J.H.; Sissons, C.H. Development of multi-species consortia biofilms of oral bacteria as an enamel and root caries model system. Arch. Oral Biol. 2000, 45, 27–40. [Google Scholar] [CrossRef]
- Balhaddad, A.A.; Kansara, A.A.; Hidan, D.; Weir, M.D.; Xu, H.H.K.; Melo, M.A.S. Toward dental caries: Exploring nanoparticle-based platforms and calcium phosphate compounds for dental restorative materials. Bioact. Mater. 2019, 4, 43–55. [Google Scholar] [CrossRef]
- Abdalla, M.A.; McGaw, L.J. The pharmacological and nutritional significance of plant-derived natural products: An alternative for animal health. In Ethnoveterinary Medicine; Springer: Cham, Switzerland, 2020; pp. 7–12. [Google Scholar]
- Malvania, E.A.; Sharma, A.S.; Sheth, S.A.; Rathod, S.; Chovatia, N.R.; Kachwala, M.S. In vitro analysis of licorice (glycyrrhiza glabra) root extract activity on streptococcus mutans in comparison to chlorhexidine and fluoride mouthwash. J. Contemp. Dent. Pract. 2019, 20, 1390. [Google Scholar] [CrossRef]
- Ekor, M. The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol. 2014, 4, 177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palhares, R.M.; Drummond, M.G.; Brasil, B.D.; Cosenza, G.P.; Brandão, M.D.; Oliveira, G. Medicinal plants recommended by the world health organization: DNA barcode identification associated with chemical analyses guarantees their quality. PLoS ONE 2015, 10, e0127866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, F.; Wang, D. Novel technologies for the prevention and treatment of dental caries: A patent survey. Expert. Opin.Ther. Pat. 2010, 20, 681–694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosas-Piñón, Y.; Mejía, A.; Díaz-Ruiz, G.; Aguilar, M.I.; Sánchez-Nieto, S.; Rivero-Cruz, J.F. Ethnobotanical survey and antibacterial activity of plants used in the Altiplane region of Mexico for the treatment of oral cavity infections. J. Ethnopharmacol. 2012, 141, 860–865. [Google Scholar] [CrossRef]
- Shad, A.A.; Ahmad, S.; Ullah, R.; AbdEl-Salam, N.M.; Fouad, H.; Rehman, N.U.; Hussain, H.; Saeed, W. Phytochemical and biological activities of four wild medicinal plants. Sci. World J. 2014, 857363. [Google Scholar] [CrossRef] [Green Version]
- Binimeliz, M.F.; Martins, M.L.; Filho, J.C.C.F.; Cabral, L.M.; da Cruz, A.G.; Maia, L.C.; Fonseca-Gonçalves, A. Antimicrobial effect of a cardamom ethanolic extract on oral biofilm: An ex vivo study. Nat. Oral Care. Dent. Ther. 2020, 121–131. [Google Scholar] [CrossRef]
- Yabuta, Y.; Mukoyama, H.; Kaneda, Y.; Kimura, N.; Bito, T.; Ichiyanagi, T.; Ishihara, A.; Watanabe, F. A lemon myrtle extract inhibits glucosyltransferases activity of Streptococcus mutans. Biosci. Biotech. Bioch. 2018, 82, 1584–1590. [Google Scholar] [CrossRef]
- Farkash, Y.; Feldman, M.; Ginsburg, I.; Shalish, M.; Steinberg, D. The effect of Padma-hepaten herbal combination on the caries-inducing properties of Streptococcus mutans on orthodontic surfaces. J. Herb. Med. 2019, 20, 100321. [Google Scholar] [CrossRef]
- Sachdeva, A.; Sharma, A.; Bhateja, S. Emerging trends of herbs and spices in dentistry. Biomed J. Sci. Tech. Res. 2018, 2, 5. [Google Scholar] [CrossRef] [Green Version]
- Ferreira-Filho, J.C.C.; Marre, A.T.d.O.; de Sá Almeida, J.S.; Lobo, L.d.A.; Farah, A.; Romanos, M.T.V.; Maia, L.C.; Valença, A.M.G.; Fonseca-Gonçalves, A. Therapeutic potential of bauhinia forficata link in dental biofilm treatment. J. Med. Food 2020. [Google Scholar] [CrossRef] [PubMed]
- Ardiansyah, S.; Hashiinah, F.; Farida, R.; Puspitawati, R. Javanese turmeric (Curcuma xanthorrhixza roxb.) ethanol extract has inhibitory effect on the development of intermediate phase of candida albicans biofilm. J. Int. Dent. Med Res. 2019, 12, 460–464. [Google Scholar]
- Choi, H.-A.; Cheong, D.-E.; Lim, H.-D.; Kim, W.-H.; Ham, M.-H.; Oh, M.-H.; Wu, Y.; Shin, H.-J.; Kim, G.-J. Antimicrobial and anti-biofilm activities of the methanol extracts of medicinal plants against dental pathogens Streptococcus mutans and Candida albicans. J. Microbiol. Biotechnol. 2017, 27, 1242–1248. [Google Scholar] [CrossRef] [PubMed]
- Alloha, I.B.; Aziz, N.A.L.B.; Faisal, G.G.; Abllah, Z.; Arzmi, M.H. Effects of Eurycoma Longifolia Jack (Tongkat Ali) alcoholic root extract against oral pathogens. Pharmacogn. Res. 2019, 11, 1299–1302. [Google Scholar] [CrossRef] [Green Version]
- Khalid, M.; Hassani, D.; Bilal, M.; Butt, Z.A.; Hamayun, M.; Ahmad, A.; Huang, D.; Hussain, A. Identification of oral cavity biofilm forming bacteria and determination of their growth inhibition by Acacia arabica, Tamarix aphylla L. and Melia azedarach L. medicinal plants. Arch. Oral Biol. 2017, 81, 175–185. [Google Scholar] [CrossRef]
- Ambade, S.V.; Deshpande, N.M. Antimicrobial and antibiofilm activity of essential oil of cymbopogon citratus against oral microflora associated with dental plaque. Eur. J. Med. Plants Res. 2019, 28, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Chatterjee, T.; Das, S. Antimicrobial efficacy of some medicinal plant extract against Streptococcus mutans causing dental caries. J. Med. Plants 2017, 5, 315–317. [Google Scholar]
- Kanth, M.R.; Prakash, A.R.; Sreenath, G.; Reddy, V.S.; Huldah, S. Efficacy of specific plant products on microorganisms causing dental caries. J. Clin. Diagn. Res. 2016, 10, ZM01. [Google Scholar] [CrossRef]
- Patel, D.M.; Chauhan, J.B.; Ishnava, K.B. Studies on the anticariogenic potential of medicinal plant seed and fruit extracts. Nat. Oral Care Dent. Ther. 2020. [Google Scholar] [CrossRef]
- Yang, Y.; Park, B.-I.; Hwang, E.-H.; You, Y.-O. Composition analysis and inhibitory effect of Sterculia lychnophora against biofilm formation by Streptococcus mutans. Evid. Based Complement. Altern. Med. 2016. [Google Scholar] [CrossRef] [Green Version]
- Latti, P.; Subramaniam Ramanarayanan, G. Antifungal efficacy of spice extracts against Candida albicans: An in vitro study. Indian. J. Community Med. 2019, 44, S77. [Google Scholar] [CrossRef]
- Jeong, S.I.; Kim, B.S.; Keum, K.S.; Lee, K.H.; Kang, S.Y.; Park, B.I.; Lee, Y.R.; You, Y.O. Kaurenoic acid from aralia continentalis inhibits biofilm formation of Streptococcus mutans. Evid. Based Complement. Altern. Med. 2013, 160592. [Google Scholar] [CrossRef] [Green Version]
- Limsong, J.; Benjavongkulchai, E.; Kuvatanasuchati, J. Inhibitory effect of some herbal extracts on adherence of Streptococcus mutans. J. Ethnopharmacol. 2004, 92, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.C.; Cho, S.G.; Kim, S.W.; Kim, J.N. Anticariogenic potential of korean native plant extracts against Streptococcus mutans. Planta. Med. 2019, 85, 1242–1252. [Google Scholar] [CrossRef] [Green Version]
- Adyanthaya, A.; Ismail, S.; Sreelakshmi, N. Indian traditional medicinal herbs against dental caries–an unsung past to a bright future. Saudi J. Oral Dent. Res. 2016, 1, 1–6. [Google Scholar]
- Geetha, R.; Thangavelu, L. Anti-bacterial activity of three essential oils-An in vitro study. Int. J. Pharm. Sci. 2019, 10, 1049–1053. [Google Scholar] [CrossRef]
- Philip, N.; Leishman, S.; Walsh, L. Potential role for natural products in dental caries control. Oral Health Prev. Dent. 2019, 17, 479–485. [Google Scholar] [PubMed]
- Ancuceanu, R.; Anghel, A.I.; Ionescu, C.; Hovaneț, M.V.; Cojocaru-Toma, M.; Dinu, M. Clinical trials with herbal products for the prevention of dental caries and their quality: A scoping study. Biomolecules 2019, 9, 884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janakiram, C.; Venkitachalam, R.; Fontelo, P.; Iafolla, T.J.; Dye, B.A. Effectiveness of herbal oral care products in reducing dental plaque & gingivitis—A systematic review and meta-analysis. BMC Complement. Altern. Med. 2020, 20, 43. [Google Scholar]
- Guo, L.; Edlund, A. Targeted antimicrobial peptides: A novel technology to eradicate harmful streptococcus mutans. J. Calif. Dent. Assoc. 2017, 45, 557. [Google Scholar]
- Wasfi, R.; Abd El-Rahman, O.A.; Zafer, M.M.; Ashour, H.M. Probiotic Lactobacillus sp. inhibit growth, biofilm formation and gene expression of caries-inducing Streptococcus mutans. J. Cell. Mol. Med. 2018, 22, 1972–1983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piwat, S.; Pahumunto, N.; Srisommai, P.; Mapaisansin, C.; Teanpaisan, R. Effect of probiotic delivery vehicles for probiotic Lactobacillus rhamnosus SD11 in caries prevention: A clinical study. J. Food Process. Pres. 2019, 43, e14147. [Google Scholar] [CrossRef]
- Zaura, E.; Twetman, S. Critical appraisal of oral pre- and probiotics for caries prevention and care. Caries Res. 2019, 53, 514–526. [Google Scholar] [CrossRef] [PubMed]
- Shah, N. Probiotics and prebiotics. Agro Food Ind. Hi-Tech. 2004, 1, 13–16. [Google Scholar]
- Pradhan, D.; Mallappa, R.H.; Grover, S. Comprehensive approaches for assessing the safety of probiotic bacteria. Food Control 2020, 108, 14. [Google Scholar] [CrossRef]
- Ohshima, T.; Kawai, T.; Maeda, N. Bacterial cell-free probiotics using effective substances produced by probiotic bacteria, for application in the oral cavity. In Prebiotics and Probiotics-Potential Benefits in Human Nutrition and Health; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef] [Green Version]
- Jalasvuori, H.; Haukioja, A.; Tenovuo, J. Probiotic Lactobacillus reuteri strains ATCC PTA 5289 and ATCC 55730 differ in their cariogenic properties in vitro. Arch. Oral Biol. 2012, 57, 1633–1638. [Google Scholar] [CrossRef]
- Hasslöf, P.; Stecksén-Blicks, C. Chapter 10: Probiotic bacteria and dental caries. In The Impact of Nutrition and Diet on Oral Health; Zohoori, F.V., Duckworth, R.M., Eds.; Academic Press: London, UK, 2020; Volume 28, pp. 99–107. [Google Scholar]
- Giacaman, R. Sugars and beyond. The role of sugars and the other nutrients and their potential impact on caries. Oral Dis. 2018, 24, 1185–1197. [Google Scholar] [CrossRef]
- Ramanujam, P.; Poorni, S.; Srinivasan, M.R.; Sureshbabu, N.M. Probiotics in dental caries prevention. Indian J. Nutr. Diet. 2019, 56, 84. [Google Scholar] [CrossRef]
- Villalobos-Delgado, L.H.; Nevárez-Moorillon, G.V.; Caro, I.; Quinto, E.J.; Mateo, J. Natural antimicrobial agents to improve foods shelf life. In Food Quality and Shelf Life; Galanakis, C.M., Ed.; Elsevier Academic Press: Amsterdam, The Netherlands, 2019; pp. 125–157. [Google Scholar]
- Krzyściak, W.; Kościelniak, D.; Papież, M.; Vyhouskaya, P.; Zagórska-Świeży, K.; Kołodziej, I.; Bystrowska, B.; Jurczak, A. Effect of a Lactobacillus salivarius probiotic on a double-species Streptococcus mutans and Candida albicans caries biofilm. Nutrients 2017, 9, 1242. [Google Scholar] [CrossRef] [Green Version]
- Burton, J.P.; Drummond, B.K.; Chilcott, C.N.; Tagg, J.R.; Thomson, W.M.; Hale, J.D.F.; Wescombe, P.A. Influence of the probiotic Streptococcus salivarius strain M18 on indices of dental health in children: A randomized double-blind, placebo-controlled trial. J. Med. Microbiol. 2013, 62, 875–884. [Google Scholar] [CrossRef]
- Lin, X.; Chen, X.; Tu, Y.; Wang, S.; Chen, H. Effect of probiotic lactobacilli on the growth of streptococcus mutans and multispecies biofilms isolated from children with active caries. Med. Sci. Monit. 2017, 23, 4175–4181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nadelman, P.; Monteiro, A.; Balthazar, C.F.; Silva, H.L.A.; Cruz, A.G.; de Almeida Neves, A.; Fonseca-Gonçalves, A.; Maia, L.C. Probiotic fermented sheep’s milk containing Lactobacillus casei 01: Effects on enamel mineral loss and Streptococcus counts in a dental biofilm model. J. Funct. Foods 2019, 54, 241–248. [Google Scholar] [CrossRef]
- Su, P.; Henriksson, A.; Nilsson, C.; Mitchell, H. Synergistic effect of green tea extract and probiotics on the pathogenic bacteria, Staphylococcus aureus and Streptococcus pyogenes. World J. Microbiol. Biotechnol. 2008, 24, 1837–1842. [Google Scholar] [CrossRef]
- Dimitrova, M.; Ivanov, G.; Mihalev, K.; Slavchev, A.; Ivanova, I.; Vlaseva, R. Investigation of antimicrobial activity of polyphenol-enriched extracts against probiotic lactic acid bacteria. Food Sci. Appl. Biotechnol. 2019, 2, 67–73. [Google Scholar] [CrossRef]
| No. | Plants | Extracts & Bioactive Compound | Target Organisms | Biological Activity | Reference |
|---|---|---|---|---|---|
| 1 | Acacia arabica | Ethanol, acetone, and water extract | Strong biofilm-forming strains isolated from patients | Anti-biofilm, antimicrobial | [80] 2017 |
| 2 | Tamarix aphylla L. | ||||
| 3 | Melia azedarach L. | ||||
| 4 | Bauhinia forficata | Tincture | Streptococcus spp. Salivary samples from healthy volunteers | Anti-biofilm, antimicrobial | [16] 2019 |
| 5 | Bauhinia forficata | Phenolic acids, chlorogenic acids | S. mutans (ATCC 25175) Streptococcus sanguinis (ATCC 10556) Candida albicans (ATCC 22972) Fusubacterium nucleatum (ATCC 25586) Lactobacillus casei (ATCC 393) Prevotella nigrescens (ATCC 33563), Bifidobacterium dentium (ATCC 27534) | Antimicrobial, anti-demineralizing | [76] 2020 |
| 6 | Curcuma xanthrorrhiza | Ethanol extract, xanthorrhizol | C. albicans | Anti-biofilm, antimicrobial | [77] 2019 |
| 7. | Cymbopogon citratus | Lemon Grass Essential Oil | Streptococcus agalactiae, Staphylococcus epidermidisand, Lactobacillus fermentum | Antimicrobial, Anti-biofilm | [81] 2019 |
| 8 | Pongamia pinnata | Methanolic extract | S. mutans MTCC 497, S. mutans MTCC 890 | Antimicrobial | [82] 2017 |
| 9 | Acacia catechu | Methanolic extract | |||
| 10 | Clove | Eugenol, oleic acid, lipids | Microorganisms collected from extracted teeth | Antimicrobial | [83] 2016 |
| 11 | Ginger-garlic paste | Gingerol, allicin | |||
| 12 | Tea tree | Catechins | |||
| 13 | Camellia japonica | Phenolic compound, flavonoid | S. mutans ATCC 25175 Candida albicans NUM961 | Antimicrobial, anti-biofilm, anti-GTase | [78] 2017 |
| 14 | Thuja orientalis | ||||
| 15 | Quercus infecteria | Tannins, cardiac glycosides, sterioids, terpenoids, alkaloids | Lactobacillus casei | Antimicrobial | [84] 2020 |
| 16 | Sterculia lychnophora Hance | Organic acids, glycosides, | S. mutans ATCC 25175 | Antimicrobial, cariogenic properties inhibition | [85] 2016 |
| 17 | Cinnamon bark | Methanol extract, cinnamaldehyde | Candidaalbicans ATCC 2091 | Antimicrobial‘ | [86] 2019 |
| 18 | Cinnamomum burmannii | Water extract | S. mutans UA159 | Antimicrobial, anti-biofilm | [53] 2020 |
| 19 | Licorice Root | Glycyrrhizin | S. mutans ATCC 25175 | Antimicrobial | [66] 2019 |
| 20 | Eurycoma longifolia jack | Ethanol extract, canthin-6-one alkaloids, β-carboline alkaloids, quassinoids | Candida albicans, S. mutans, Lactobacillus casei | Antifungal, Antimicrobial, | [79] 2019 |
| Probiotics | Target Bacteria | Type of Biofilm Model | Bioactive Compound/Action Mechanism | References (Year) |
|---|---|---|---|---|
| L. rhamnosus SD11 | S. mutans lactobacilli | Human oral cavity | Integrate into the bacterial communities of the dental biofilm | [97] 2019 |
| L. salivarius | S. mutans C. albicans | Double species, static | Strong competitor of oral pathogens | [107] 2017 |
| Streptococcus salivarius strain M18 | S. mutans | plaque-disclosing solution | Bacterins | [108] 2013 |
| L. casei ATCC 393, L. reuteri ATCC 23272, L. plantarum ATCC 14917, L. salivarius ATCC 11741 | S. mutans ATCC 25175 | Single specie biofilm, dual-S. mutans–Lactobacillus spp. biofilm, static | Organic acid, peroxide | [96] 2018 |
| L. casei Shirota, L. casei LC01, L. plantarum ST-III L. paracasei LPC37 | S. mutans Streptococcus spp., S. sanguinis | Multi-species biofilm, static | Alteration of the oral microbiota | [109] 2017 |
| L. casei 01 | S. mutans, S. parasanguinis, S. salivarius | Multi-species biofilm, static | Adhere to dental surfaces and integrate into the bacterial communities of the dental biofilm | [110] 2019 |
| Lactobacillus plantarum FB-T9 | S. mutans | Rat oral cavity | FB-T9 is a strong competitor of S. mutans for temporal and spatial niches | [54] 2020 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Daliri, E.B.-M.; Kim, N.; Kim, J.-R.; Yoo, D.; Oh, D.-H. Microbial Etiology and Prevention of Dental Caries: Exploiting Natural Products to Inhibit Cariogenic Biofilms. Pathogens 2020, 9, 569. https://doi.org/10.3390/pathogens9070569
Chen X, Daliri EB-M, Kim N, Kim J-R, Yoo D, Oh D-H. Microbial Etiology and Prevention of Dental Caries: Exploiting Natural Products to Inhibit Cariogenic Biofilms. Pathogens. 2020; 9(7):569. https://doi.org/10.3390/pathogens9070569
Chicago/Turabian StyleChen, Xiuqin, Eric Banan-Mwine Daliri, Namhyeon Kim, Jong-Rae Kim, Daesang Yoo, and Deog-Hwan Oh. 2020. "Microbial Etiology and Prevention of Dental Caries: Exploiting Natural Products to Inhibit Cariogenic Biofilms" Pathogens 9, no. 7: 569. https://doi.org/10.3390/pathogens9070569
APA StyleChen, X., Daliri, E. B.-M., Kim, N., Kim, J.-R., Yoo, D., & Oh, D.-H. (2020). Microbial Etiology and Prevention of Dental Caries: Exploiting Natural Products to Inhibit Cariogenic Biofilms. Pathogens, 9(7), 569. https://doi.org/10.3390/pathogens9070569






