Effects of Maternal Grape Juice Intake on Unfolded Protein Response in the Mammary Glands of Offspring of High Fat Diet Fed Rat Dams
Abstract
:1. Introduction
2. Methods
2.1. Maternal Dietary and Fluid Exposures
2.2. Offspring′s Mammary Gland Morphology
2.3. Immunohistochemistry (IHC) for Ki67 in Mammary Glands
2.4. Total Protein Extraction
2.5. Western Blotting
2.6. Evaluation of mRNA Expression
2.7. Statistical Analyzes
3. Results
3.1. Effects of Maternal Grape Juice Intake on Biomarkers of Increased Breast Cancer Risk in the Offspring′s Mammary Glands: TEB, Cell Proliferation and Apoptosis
3.2. Effects of Maternal Grape Juice Intake on Unfolded Protein Response in the Offspring′s Mammary Glands
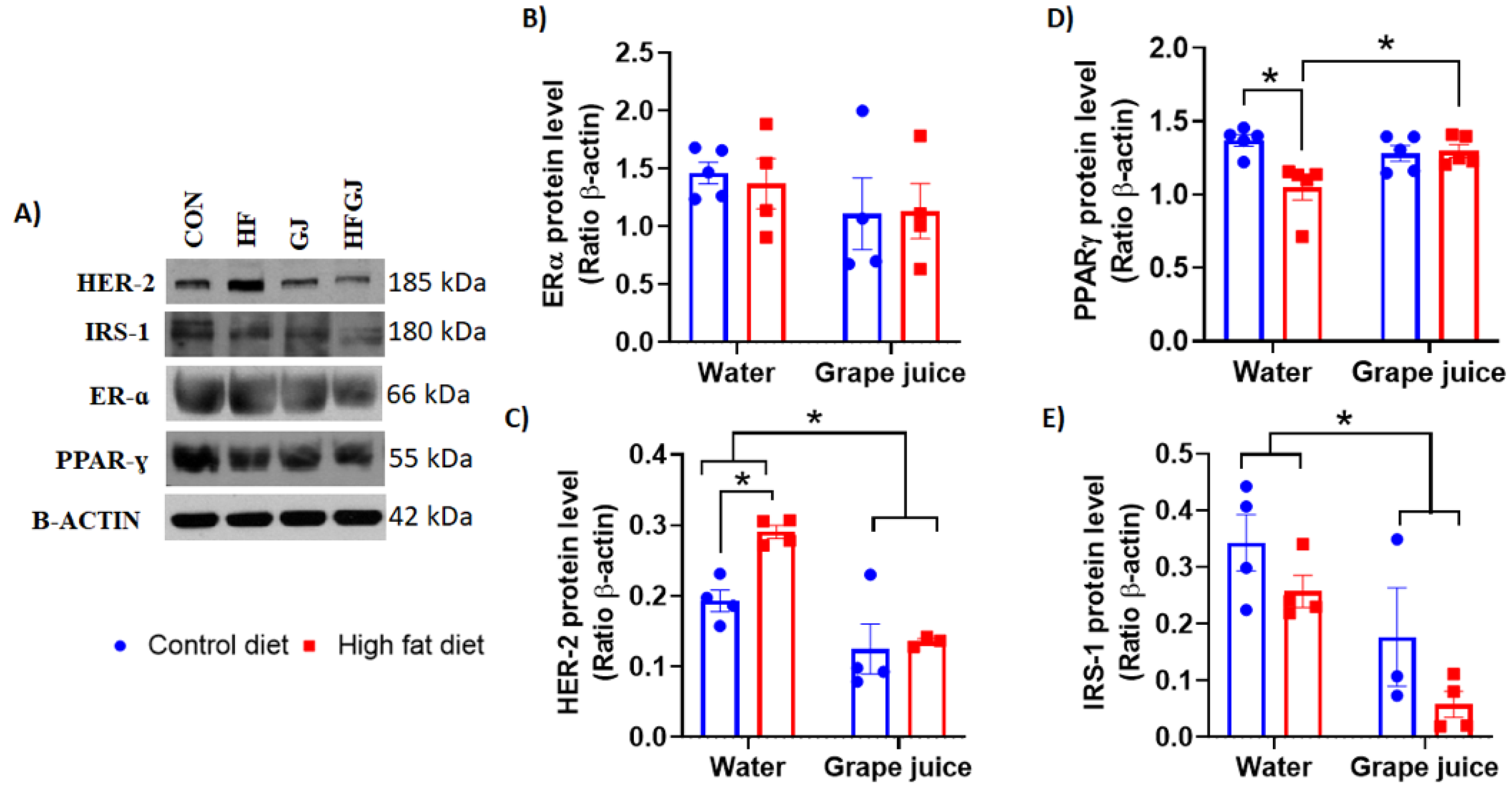
3.3. Effects of Maternal Grape Juice Intake on Erα, Her2, Pparγ and Irs1 Levels in the Offspring′s Mammary Glands
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics 2019. CA. Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef]
- Ziegler, E.E.; Filer, L.J. Conocimientos actuales sobre nutrición. Rev. Esp. Salud Publica 1998, 72, 379–380. [Google Scholar] [CrossRef] [Green Version]
- Stanford, J.L.; Herrinton, L.J.; Schwartz, S.M.; Weiss, N.S. Breast cancer incidence in Asian migrants to the United States and their descendants. Epidemiology 1995, 6, 181–183. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Han, S.; Zhu, J.; Sun, X.; Ji, C.; Guo, X. Pre-Pregnancy Body Mass Index in Relation to Infant Birth Weight and Offspring Overweight/Obesity: A Systematic Review and Meta-Analysis. PLoS ONE 2013, 8, e61627. [Google Scholar] [CrossRef] [Green Version]
- Michels, K.B.; Trichopoulos, D.; Robins, J.M.; Rosner, B.A.; Manson, J.E.; Hunter, D.J.; Colditz, G.A.; Hankinson, S.E.; Speizer, F.E.; Willett, W.C. Birthweight as a risk factor for breast cancer. Lancet 1996, 348, 1542–1546. [Google Scholar] [CrossRef]
- Michels, K.B.; Xue, F. Role of birthweight in the etiology of breast cancer. Int. J. Cancer 2006, 119, 2007–2025. [Google Scholar] [CrossRef]
- dos Silva, I.S.; De Stavola, B.; McCormack, V. Birth Size and Breast Cancer Risk: Re-analysis of Individual Participant Data from 32 Studies. PLoS Med. 2008, 5, e193. [Google Scholar] [CrossRef]
- Hilakivi-Clarke, L.; Clarke, R.; Lippman, M. The influence of maternal diet on breast cancer risk among female offspring. Nutrition 1999, 15, 392–401. [Google Scholar] [CrossRef]
- De Assis, S.; Warri, A.; Cruz, M.I.; Laja, O.; Tian, Y.; Zhang, B.; Wang, Y.; Huang, T.H.-M.; Hilakivi-Clarke, L. High-fat or ethinyl-oestradiol intake during pregnancy increases mammary cancer risk in several generations of offspring. Nat. Commun. 2012, 3, 1053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, N.M.; de Oliveira Andrade, F.; Jin, L.; Zhang, X.; Macon, M.; Cruz, M.I.; Benitez, C.; Wehrenberg, B.; Yin, C.; Wang, X.; et al. Maternal intake of high n-6 polyunsaturated fatty acid diet during pregnancy causes transgenerational increase in mammary cancer risk in mice. Breast Cancer Res. 2017, 19, 77. [Google Scholar] [CrossRef]
- Walker, B.E. Tumors in female offspring of control and diethylstilbestrol-exposed mice fed high-fat diets. J. Natl. Cancer Inst. 1990, 82, 50–54. [Google Scholar] [CrossRef]
- Luijten, M.; Thomsen, A.R.; van den Berg, J.A.H.; Wester, P.W.; Verhoef, A.; Nagelkerke, N.J.D.; Adlercreutz, H.; van Kranen, H.J.; Piersma, A.H.; Sorensen, I.K.; et al. Effects of Soy-Derived Isoflavones and a High-Fat Diet on Spontaneous Mammary Tumor Development in Tg.NK (MMTV/c-neu) Mice. Nutr. Cancer 2004, 50, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Hilakivi-Clarke, L.; Clarke, R.; Onojafe, I.; Raygada, M.; Cho, E.; Lippman, M. A maternal diet high in n − 6 polyunsaturated fats alters mammary gland development, puberty onset, and breast cancer risk among female rat offspring. Proc. Natl. Acad. Sci. USA 1997, 94, 9372–9377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Assis, S.; Hilkavi-Clarke, L. Timing of Dietary Estrogenic Exposures and Breast Cancer Risk. Ann. N. Y. Acad. Sci. 2006, 1089, 14–35. [Google Scholar] [CrossRef] [PubMed]
- Mendes-da-Silva, C.; Lemes, S.F.; da Silva Baliani, T.; Versutti, M.D.; Torsoni, M.A. Increased expression of Hes5 protein in Notch signaling pathway in the hippocampus of mice offspring of dams fed a high-fat diet during pregnancy and suckling. Int. J. Dev. Neurosci. 2015, 40, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Montales, M.T.E.; Melnyk, S.B.; Simmen, F.A.; Simmen, R.C.M. Maternal metabolic perturbations elicited by high-fat diet promote Wnt-1-induced mammary tumor risk in adult female offspring via long-term effects on mammary and systemic phenotypes. Carcinogenesis 2014, 35, 2102–2112. [Google Scholar] [CrossRef]
- Gallardo, J.M.; Gómez-López, J.; Medina-Bravo, P.; Juárez-Sánchez, F.; Contreras-Ramos, A.; Galicia-Esquivel, M.; Sánchez-Urbina, R.; Klünder-Klünder, M. Maternal obesity increases oxidative stress in the newborn. Obesity 2015, 23, 1650–1654. [Google Scholar] [CrossRef]
- Wu, Z.; Zhao, J.; Xu, H.; Lyv, Y.; Feng, X.; Fang, Y.; Xu, Y. Maternal quercetin administration during gestation and lactation decrease endoplasmic reticulum stress and related inflammation in the adult offspring of obese female rats. Eur. J. Nutr. 2014, 53, 1669–1683. [Google Scholar] [CrossRef]
- Melo, A.M.; Benatti, R.O.; Ignacio-Souza, L.M.; Okino, C.; Torsoni, A.S.; Milanski, M.; Velloso, L.A.; Torsoni, M.A. Hypothalamic endoplasmic reticulum stress and insulin resistance in offspring of mice dams fed high-fat diet during pregnancy and lactation. Metabolism 2014, 63, 682–692. [Google Scholar] [CrossRef]
- Soeda, J.; Mouralidarane, A.; Cordero, P.; Li, J.; Nguyen, V.; Carter, R.; Kapur, S.R.; Pombo, J.; Poston, L.; Taylor, P.D.; et al. Maternal obesity alters endoplasmic reticulum homeostasis in offspring pancreas. J. Physiol. Biochem. 2016, 72, 281–291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGrath, E.; Logue, S.; Mnich, K.; Deegan, S.; Jäger, R.; Gorman, A.; Samali, A. The Unfolded Protein Response in Breast Cancer. Cancers (Basel) 2018, 10, 344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chevet, E.; Hetz, C.; Samali, A. Endoplasmic Reticulum Stress-Activated Cell Reprogramming in Oncogenesis. Cancer Discov. 2015, 5, 586–597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K. IRE1-mediated unconventional mRNA splicing and S2P-mediated ATF6 cleavage merge to regulate XBP1 in signaling the unfolded protein response. Genes Dev. 2002, 16, 452–466. [Google Scholar] [CrossRef] [Green Version]
- Sengupta, S.; Sharma, C.G.N.; Jordan, V.C. Estrogen regulation of X-box binding protein-1 and its role in estrogen induced growth of breast and endometrial cancer cells. Horm. Mol. Biol. Clin. Investig. 2010, 2. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Warri, A.; Jin, L.; Zwart, A.; Riggins, R.B.; Fang, H.-B.; Clarke, R. NF-κB Signaling Is Required for XBP1 (Unspliced and Spliced)-Mediated Effects on Antiestrogen Responsiveness and Cell Fate Decisions in Breast Cancer. Mol. Cell. Biol. 2015, 35, 379–390. [Google Scholar] [CrossRef] [Green Version]
- Lacerda, D.d.S.; Santos, C.F.; Oliveira, A.S.; Zimmermann, R.; Schneider, R.; Agostini, F.; Dani, C.; Funchal, C.; Gomez, R. Antioxidant and hepatoprotective effects of an organic grapevine leaf (Vitis labrusca L.) extract in diabetic rats. RSC Adv. 2014, 4, 52611–52619. [Google Scholar] [CrossRef]
- Parmar, J.H.; Cook, K.L.; Shajahan-Haq, A.N.; Clarke, P.A.G.; Tavassoly, I.; Clarke, R.; Tyson, J.J.; Baumann, W.T. Modelling the effect of GRP78 on anti-oestrogen sensitivity and resistance in breast cancer. Interface Focus 2013, 3, 20130012. [Google Scholar] [CrossRef] [Green Version]
- Godfrey, K.M.; Reynolds, R.M.; Prescott, S.L.; Nyirenda, M.; Jaddoe, V.W.V.; Eriksson, J.G.; Broekman, B.F.P. Influence of maternal obesity on the long-term health of offspring. Lancet Diabetes Endocrinol. 2017, 5, 53–64. [Google Scholar] [CrossRef] [Green Version]
- Menichini, D.; Longo, M.; Facchinetti, F. Maternal interventions to improve offspring outcomes in rodent models of diet-induced obesity: A review. J. Matern. Neonatal Med. 2019, 32, 2943–2949. [Google Scholar] [CrossRef]
- Seeram, N.P.; Aviram, M.; Zhang, Y.; Henning, S.M.; Feng, L.; Dreher, M.; Heber, D. Comparison of Antioxidant Potency of Commonly Consumed Polyphenol-Rich Beverages in the United States. J. Agric. Food Chem. 2008, 56, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Castilla, P.; Echarri, R.; Dávalos, A.; Cerrato, F.; Ortega, H.; Teruel, J.L.; Lucas, M.F.; Gómez-Coronado, D.; Ortuño, J.; Lasunción, M.A. Concentrated red grape juice exerts antioxidant, hypolipidemic, and antiinflammatory effects in both hemodialysis patients and healthy subjects. Am. J. Clin. Nutr. 2006, 84, 252–262. [Google Scholar] [CrossRef]
- Buchner, I.; Medeiros, N.; Lacerda, D.S.; Normann, C.A.B.M.; Gemelli, T.; Rigon, P.; Wannmacher, C.M.D.; Henriques, J.A.P.; Dani, C.; Funchal, C. Hepatoprotective and antioxidant potential of organic and conventional grape juices in rats fed a high-fat diet. Antioxidants 2014, 3, 323–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodrigues, A.D.; Scheffel, T.B.; Scola, G.; Dos Santos, M.T.; Fank, B.; De Freitas, S.C.V.; Dani, C.; Vanderlinde, R.; Henriques, J.A.P.; Coitinho, A.S.; et al. Neuroprotective and anticonvulsant effects of organic and conventional purple grape juices on seizures in Wistar rats induced by pentylenetetrazole. Neurochem. Int. 2012, 60, 799–805. [Google Scholar] [CrossRef] [Green Version]
- Dani, C.; Andreazza, A.C.; Gonçalves, C.A.; Kapizinski, F.; Henriques, J.A.P.; Salvador, M. Grape juice increases the BDNF levels but not alter the S100B levels in hippocampus and frontal cortex from male wistar rats. An. Acad. Bras. Cienc. 2017, 89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dani, C.; Pasquali, M.A.B.; Oliveira, M.R.; Umezu, F.M.; Salvador, M.; Henriques, J.A.P.; Moreira, J.C.F. Protective effects of purple grape juice on carbon tetrachloride-induced oxidative stress in brains of adult Wistar rats. J. Med. Food 2008, 11. [Google Scholar] [CrossRef] [PubMed]
- Cardozo, M.G.; Medeiros, N.; Dos Santos Lacerda, D.; De Almeida, D.C.; Henriques, J.A.P.; Dani, C.; Funchal, C. Effect of chronic treatment with conventional and organic purple grape juices (Vitis labrusca) on rats fed with high-fat diet. Cell. Mol. Neurobiol. 2013, 33. [Google Scholar] [CrossRef]
- Shufelt, C.; Merz, C.N.B.; Yang, Y.; Kirschner, J.; Polk, D.; Stanczyk, F.; Paul-Labrador, M.; Braunstein, G.D. Red versus white wine as a nutritional aromatase inhibitor in premenopausal women: A pilot study. J. Womens. Health (Larchmt) 2012, 21, 281–284. [Google Scholar] [CrossRef] [Green Version]
- Gonçalves, L.; Bortolato, G.; Dario Braccini Neto, R.; Rocha Frusciante, M.; Funchal, C.; Dani, C.; Gonçalves, L.K.; Bortolato, G.; Dario Braccini Neto, R.; Rocha Frusciante, M.; et al. Grape Juice Consumption with or without High Fat Diet during Pregnancy Reduced the Weight Gain and Improved Lipid Profile and Oxidative Stress Levels in Liver and Serum from Wistar Rats. Beverages 2018, 4, 78. [Google Scholar] [CrossRef] [Green Version]
- Hilakivi-Clarke, L. Nutritional modulation of terminal end buds: Its relevance to breast cancer prevention. Curr. Cancer Drug Targets 2007, 7, 465–474. [Google Scholar] [CrossRef]
- Brentnall, M.; Rodriguez-Menocal, L.; De Guevara, R.L.; Cepero, E.; Boise, L.H. Caspase-9, caspase-3 and caspase-7 have distinct roles during intrinsic apoptosis. BMC Cell Biol. 2013, 14, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.-W.; Yu, H.-R.; Sheen, J.-M.; Tiao, M.-M.; Tain, Y.-L.; Lin, I.-C.; Lin, Y.-J.; Chang, K.-A.; Tsai, C.-C.; Huang, L.-T. A maternal high-fat diet during pregnancy and lactation, in addition to a postnatal high-fat diet, leads to metabolic syndrome with spatial learning and memory deficits: Beneficial effects of resveratrol. Oncotarget 2017, 8, 111998–112013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dani, C.; Oliboni, L.S.; Umezu, F.M.; Pasquali, M.A.B.; Salvador, M.; Moreira, J.C.F.; Henriques, J.A.P. Antioxidant and Antigenotoxic Activities of Purple Grape Juice—Organic and Conventional—in Adult Rats. J. Med. Food 2009, 12, 1111–1118. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.D.; Scheffel, T.B.; Scola, G.; dos Santos, M.T.; Fank, B.; Dani, C.; Vanderlinde, R.; Henriques, J.A.P.; Coitinho, A.S.; Salvador, M. Purple grape juices prevent pentylenetetrazol-induced oxidative damage in the liver and serum of Wistar rats. Nutr. Res. 2013, 33, 120–125. [Google Scholar] [CrossRef] [Green Version]
- Russo, J.; Russo, I.H. Biological and molecular bases of mammary carcinogenesis. Lab. Investig. 1987, 57, 112–137. [Google Scholar]
- Russo, I.H.; Russo, J. Physiological bases of breast cancer prevention. Eur. J. Cancer Prev. 1993, 2 (Suppl. 3), 101–111. [Google Scholar] [CrossRef]
- Paine, I.S.; Lewis, M.T. The Terminal End Bud: The Little Engine that Could. J. Mammary Gland Biol. Neoplasia 2017, 22, 93–108. [Google Scholar] [CrossRef] [Green Version]
- Hilakivi-Clarke, L.; de Assis, S.; Warri, A.; Luoto, R. Pregnancy hormonal environment and mother’s breast cancer risk. Horm. Mol. Biol. Clin. Investig. 2012, 9, 11–23. [Google Scholar] [CrossRef]
- Penault-Llorca, F.; Radosevic-Robin, N. Ki67 assessment in breast cancer: An update. Pathology 2017, 49, 166–171. [Google Scholar] [CrossRef]
- Abukhdeir, A.M.; Park, B.H. p21 and p27: Roles in carcinogenesis and drug resistance. Expert Rev. Mol. Med. 2008, 10, e19. [Google Scholar] [CrossRef] [Green Version]
- Dressler, A.C.; Hudelist, G.; Fink-Retter, A.; Gschwantler-Kaulich, D.; Pfeiler, G.; Rosner, M.; Hengstschläger, M.; Singer, C.F. Tuberin and p27 expression in breast cancer patients with or without BRCA germline mutations. J. Cancer Res. Clin. Oncol. 2013, 139, 1349–1355. [Google Scholar] [CrossRef] [PubMed]
- Levine, A.J. P53, the Cellular Gatekeeper for Growth and Division. Cell 1997, 88, 323–331. [Google Scholar] [CrossRef] [Green Version]
- Lubos, E.; Loscalzo, J.; Handy, D.E. Glutathione Peroxidase-1 in Health and Disease: From Molecular Mechanisms to Therapeutic Opportunities. Antioxid. Redox Signal. 2011, 15, 1957–1997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naiki-Ito, A.; Asamoto, M.; Hokaiwado, N.; Takahashi, S.; Yamashita, H.; Tsuda, H.; Ogawa, K.; Shirai, T. Gpx2 Is an Overexpressed Gene in Rat Breast Cancers Induced by Three Different Chemical Carcinogens. Cancer Res. 2007, 67, 11353–11358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, G.; Zhong, W.-C.; Bi, Y.-H.; Tao, S.-Y.; Zhu, H.; Zhu, H.-X.; Xu, A.-M. The Prognosis Of Peroxiredoxin Family In Breast Cancer. Cancer Manag. Res. 2019, 11, 9685–9699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chakrabarti, A.; Chen, A.W.; Varner, J.D. A review of the mammalian unfolded protein response. Biotechnol. Bioeng. 2011, 108, 2777–2793. [Google Scholar] [CrossRef] [Green Version]
- Gregor, M.F.; Yang, L.; Fabbrini, E.; Mohammed, B.S.; Eagon, J.C.; Hotamisligil, G.S.; Klein, S. Endoplasmic Reticulum Stress Is Reduced in Tissues of Obese Subjects After Weight Loss. Diabetes 2009, 58, 693–700. [Google Scholar] [CrossRef] [Green Version]
- Hosogai, N.; Fukuhara, A.; Oshima, K.; Miyata, Y.; Tanaka, S.; Segawa, K.; Furukawa, S.; Tochino, Y.; Komuro, R.; Matsuda, M.; et al. Adipose Tissue Hypoxia in Obesity and Its Impact on Adipocytokine Dysregulation. Diabetes 2007, 56, 901–911. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Guo, Y.; Tang, J.; Jiang, J.; Chen, Z. New insights into the roles of CHOP-induced apoptosis in ER stress. Acta Biochim. Biophys. Sin. (Shanghai) 2014, 46, 629–640. [Google Scholar] [CrossRef] [Green Version]
- Desvergne, B.; Wahli, W. Peroxisome Proliferator-Activated Receptors: Nuclear Control of Metabolism. Endocr. Rev. 1999, 20, 649–688. [Google Scholar] [CrossRef] [Green Version]
- Fenner, M.H.; Elstner, E. Peroxisome proliferator-activated receptor-γ ligands for the treatment of breast cancer. Expert Opin. Investig. Drugs 2005, 14, 557–568. [Google Scholar] [CrossRef] [PubMed]
- Vidal-Puig, A.J.; Considine, R.V.; Jimenez-Liñan, M.; Werman, A.; Pories, W.J.; Caro, J.F.; Flier, J.S. Peroxisome proliferator-activated receptor gene expression in human tissues. Effects of obesity, weight loss, and regulation by insulin and glucocorticoids. J. Clin. Invest. 1997, 99, 2416–2422. [Google Scholar] [CrossRef] [PubMed]
- Grommes, C.; Landreth, G.E.; Heneka, M.T. Antineoplastic effects of peroxisome proliferatoractivated receptor γ agonists. Lancet Oncol. 2004, 5, 419–429. [Google Scholar] [CrossRef]
- Apostoli, A.J.; Skelhorne-Gross, G.E.A.; Rubino, R.E.; Peterson, N.T.; Di Lena, M.A.; Schneider, M.M.; SenGupta, S.K.; Nicol, C.J.B. Loss of PPARγ expression in mammary secretory epithelial cells creates a pro-breast tumorigenic environment. Int. J. Cancer 2014, 134, 1055–1066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burfoot, M.S.; Rogers, N.C.; Watling, D.; Smith, J.M.; Pons, S.; Paonessaw, G.; Pellegrini, S.; White, M.F.; Kerr, I.M. Janus kinase-dependent activation of insulin receptor substrate 1 in response to interleukin-4, oncostatin M, and the interferons. J. Biol. Chem. 1997, 272, 24183–24190. [Google Scholar] [CrossRef] [Green Version]
- Chan, B.T.-Y.; Lee, A.V. Insulin Receptor Substrates (IRSs) and Breast Tumorigenesis. J. Mammary Gland Biol. Neoplasia 2008, 13, 415–422. [Google Scholar] [CrossRef] [Green Version]
- Trojanek, J.; Ho, T.; Croul, S.; Wang, J.Y.; Chintapalli, J.; Koptyra, M.; Giordano, A.; Khalili, K.; Reiss, K. IRS-1–Rad51 nuclear interaction sensitizes JCV T-antigen positive medulloblastoma cells to genotoxic treatment. Int. J. Cancer 2006, 119, 539–548. [Google Scholar] [CrossRef]
- Toaldo, I.M.; Cruz, F.A.; Alves, T.D.L.; De Gois, J.S.; Borges, D.L.G.; Cunha, H.P.; Da Silva, E.L.; Bordignon-Luiz, M.T. Bioactive potential of Vitis labrusca L. grape juices from the Southern Region of Brazil: Phenolic and elemental composition and effect on lipid peroxidation in healthy subjects. Food Chem. 2015, 173, 527–535. [Google Scholar] [CrossRef] [Green Version]
- Saleh, A.; ElFayoumi, H.M.; Youns, M.; Barakat, W. Rutin and orlistat produce antitumor effects via antioxidant and apoptotic actions. Naunyn. Schmiedebergs. Arch. Pharmacol. 2019, 392, 165–175. [Google Scholar] [CrossRef]
- Romanos-Nanclares, A.; Sánchez-Quesada, C.; Gardeazábal, I.; Martínez-González, M.Á.; Gea, A.; Toledo, E. Phenolic Acid Subclasses, Individual Compounds, and Breast Cancer Risk in a Mediterranean Cohort: The SUN Project. J. Acad. Nutr. Diet. 2020, 120, 1002–1015. [Google Scholar] [CrossRef]
- Segovia, S.A.; Vickers, M.H.; Gray, C.; Reynolds, C.M. Maternal Obesity, Inflammation, and Developmental Programming. Biomed Res. Int. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Broadney, M.M.; Chahal, N.; Michels, K.A.; McLain, A.C.; Ghassabian, A.; Lawrence, D.A.; Yeung, E.H. Impact of parental obesity on neonatal markers of inflammation and immune response. Int. J. Obes. 2017, 41, 30–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dani, C.; Gonçalves, L.K.; Proença, I.T.; Andrade, F.d.O.; Hilakivi-Clarke, L. Effects of Maternal Grape Juice Intake on Unfolded Protein Response in the Mammary Glands of Offspring of High Fat Diet Fed Rat Dams. Nutrients 2020, 12, 2253. https://doi.org/10.3390/nu12082253
Dani C, Gonçalves LK, Proença IT, Andrade FdO, Hilakivi-Clarke L. Effects of Maternal Grape Juice Intake on Unfolded Protein Response in the Mammary Glands of Offspring of High Fat Diet Fed Rat Dams. Nutrients. 2020; 12(8):2253. https://doi.org/10.3390/nu12082253
Chicago/Turabian StyleDani, Caroline, Luciana Kneib Gonçalves, Isabel Teixeira Proença, Fabia de Oliveira Andrade, and Leena Hilakivi-Clarke. 2020. "Effects of Maternal Grape Juice Intake on Unfolded Protein Response in the Mammary Glands of Offspring of High Fat Diet Fed Rat Dams" Nutrients 12, no. 8: 2253. https://doi.org/10.3390/nu12082253




