Bioactive Compounds and Metabolites from Grapes and Red Wine in Breast Cancer Chemoprevention and Therapy
Abstract
:1. Introduction
2. Anticancer Effects Produced by Grapes and Seed Extracts
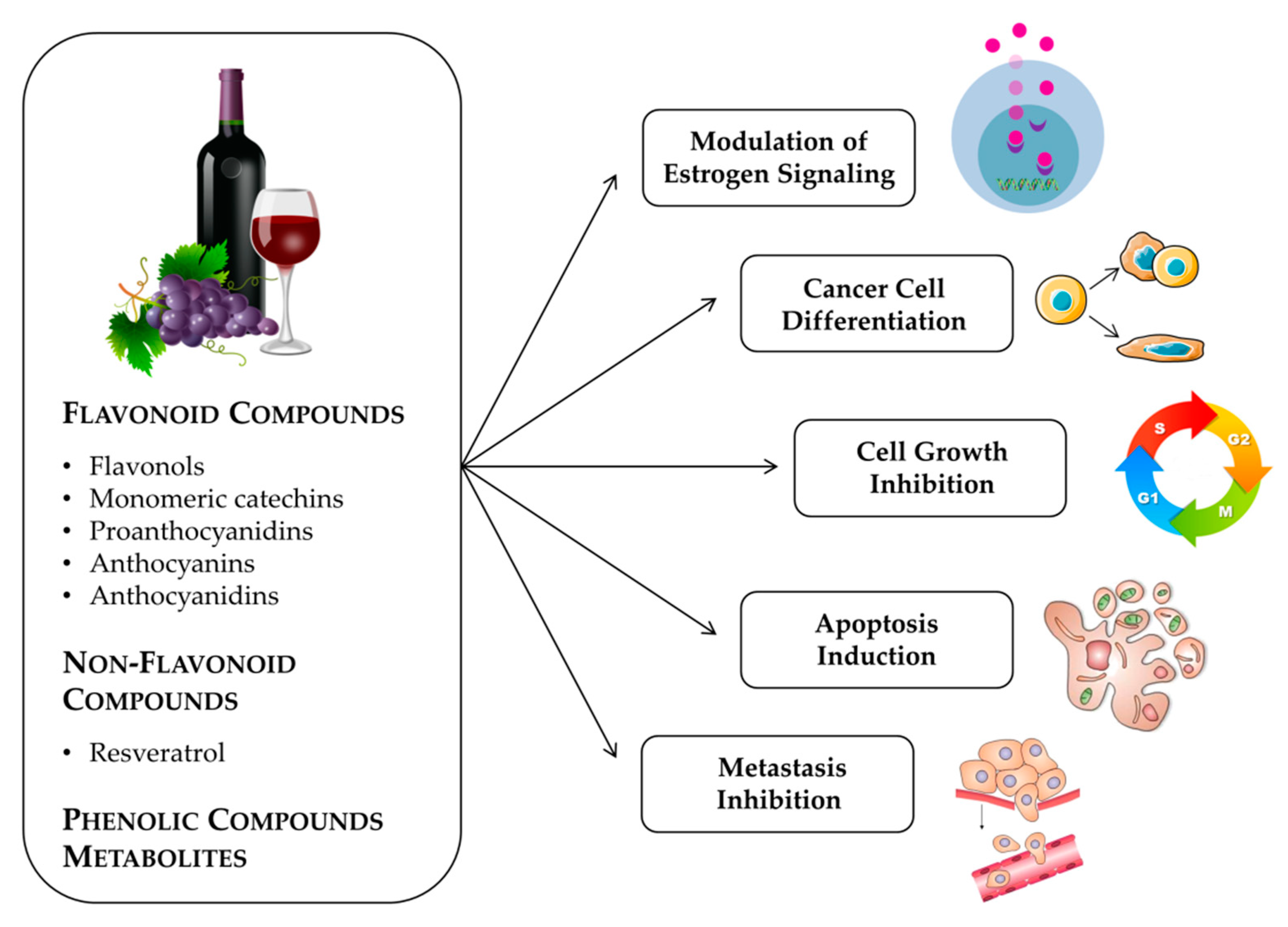
3. Flavonoid Compounds
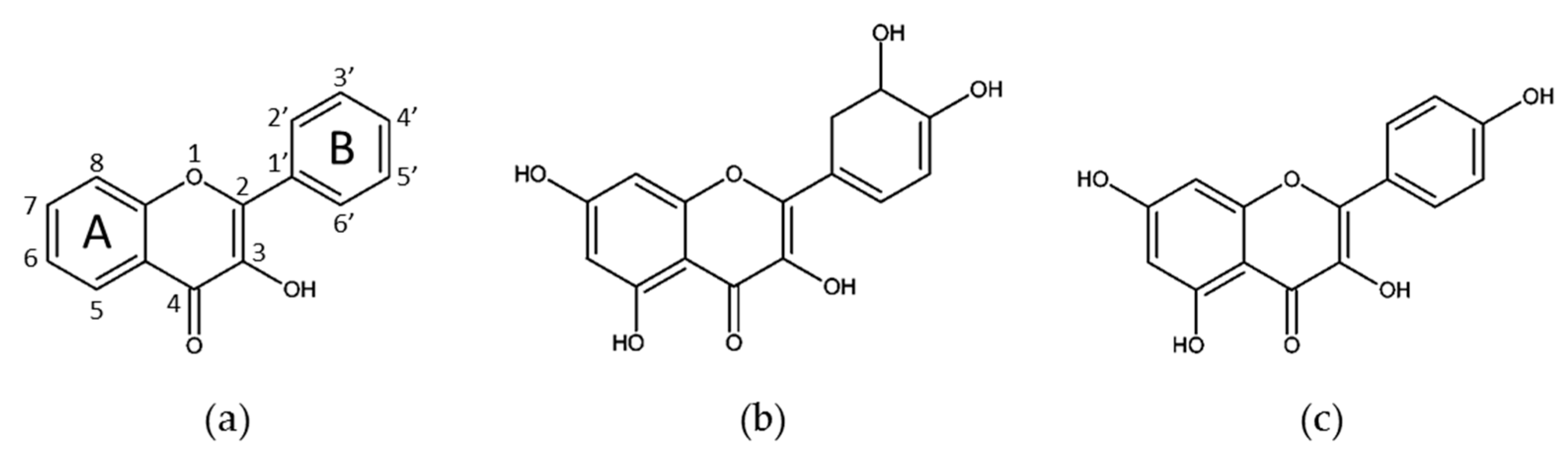
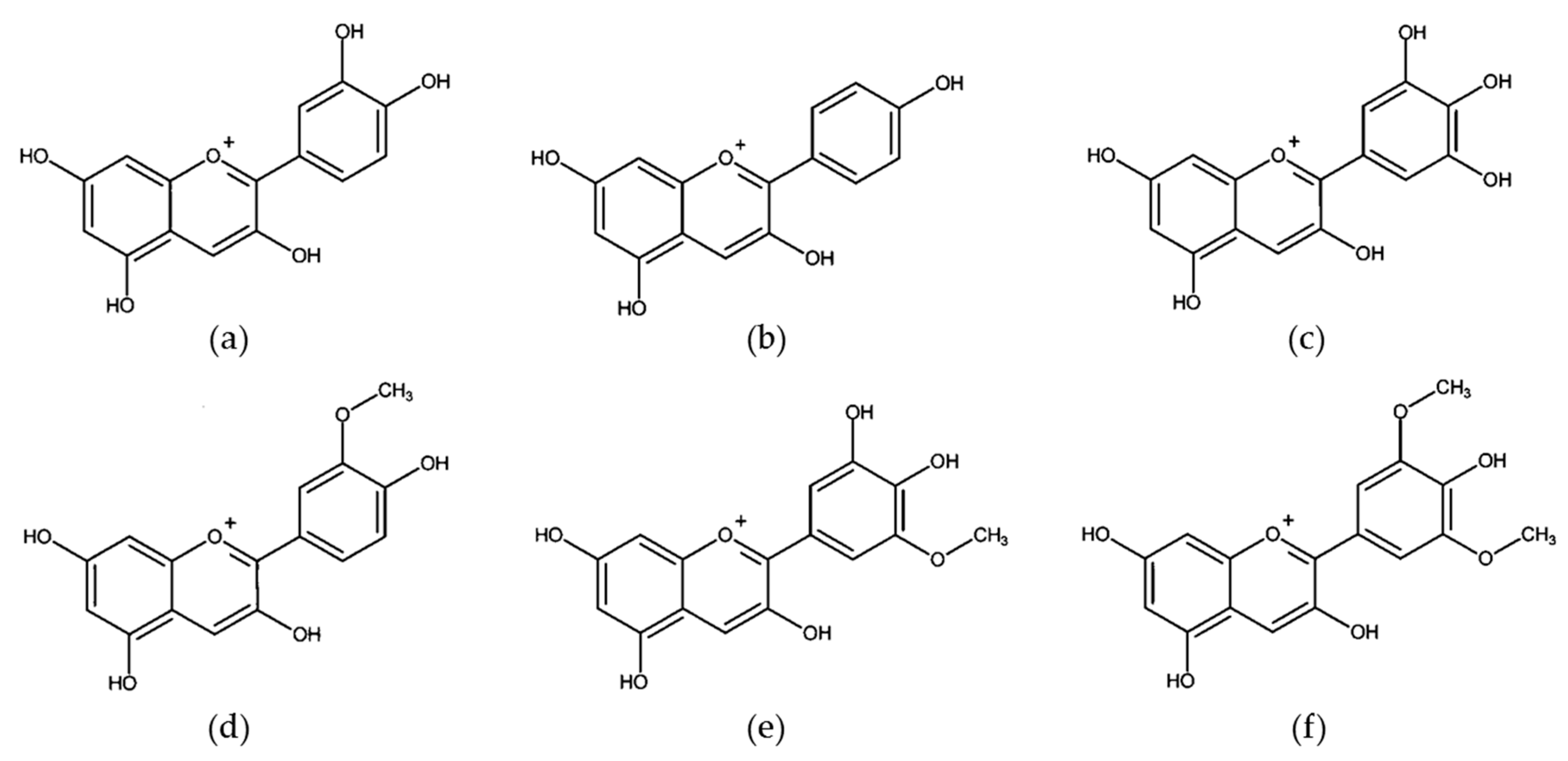
3.1. Flavonols
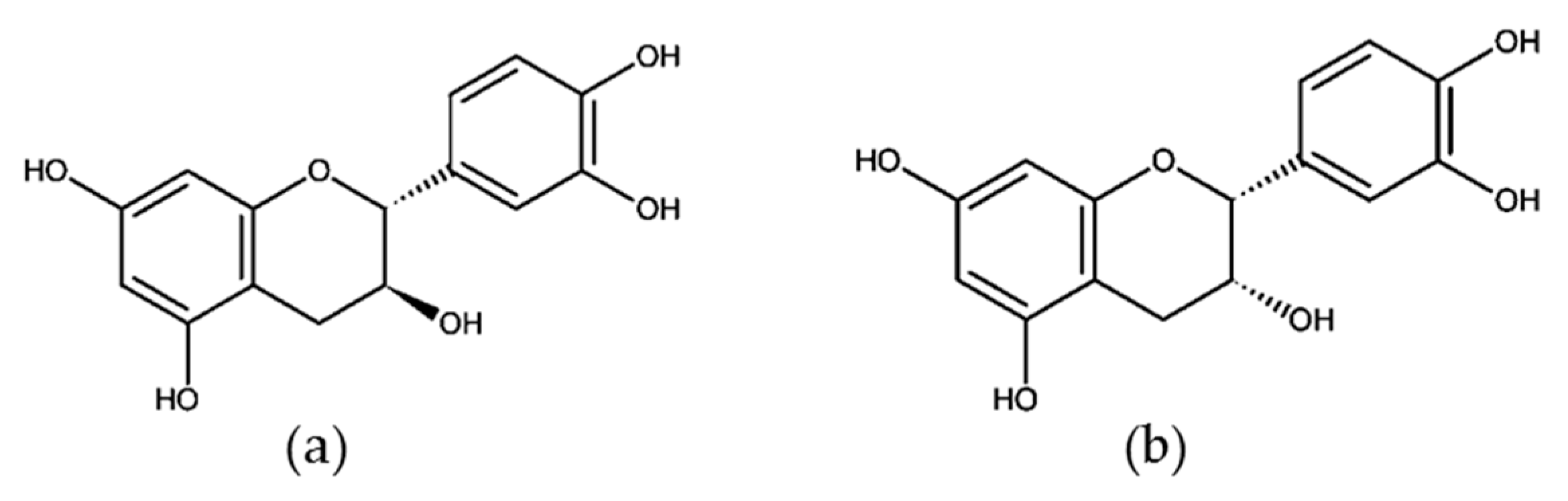
3.2. Monomeric Catechins and Proanthocyanidins
3.3. Anthocyanins and Anthocyanidins
4. Non-Flavonoid Phenolic Compounds
Resveratrol
5. Grape and Wine Metabolites and Breast Cancer (In Vitro and In Vivo Studies)
5.1. Resveratrol Metabolites
5.2. Catechins Metabolites
5.3. Anthocyanins Metabolites
5.4. Quercetin Metabolites
5.5. Metabolites and Breast Cancer Patients
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- The International Agency for Research on Cancer (IARC) Report, World Health Organization (WHO). Latest Global Cancer Data: Cancer Burden Rises to 18.1 Million New Cases and 9.6 Million Cancer Deaths in 2018; IARC: Lyon, France, 2018. [Google Scholar]
- Teixeira, A.; Baenas, N.; Dominguez-Perles, R.; Barros, A.; Rosa, E.; Moreno, D.A.; Garcia-Viguera, C. Natural bioactive compounds from winery by-products as health promoters: A review. Int. J. Mol. Sci. 2014, 15, 15638–15678. [Google Scholar] [CrossRef] [Green Version]
- Amor, S.; Châlons, P.; Aires, V.; Delmas, D. Polyphenol Extracts from Red Wine and Grapevine: Potential Effects on Cancers. Diseases 2018, 6, 106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holliday, D.L.; Speirs, V. Choosing the right cell line for breast cancer research. Breast Cancer Res. 2011, 13, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agarwal, C.; Sharma, Y.; Zhao, J.; Agarwal, R. A polyphenolic fraction from grape seeds causes irreversible growth inhibition of breast carcinoma MDA-MB468 cells by inhibiting mitogen- activated protein kinases activation and inducing G1 arrest and differentiation. Clin. Cancer Res. 2000, 6, 2921–2930. [Google Scholar] [PubMed]
- Leone, A.; Longo, C.; Gerardi, C.; Trosko, J.E. Pro-apoptotic effect of grape seed extract on MCF-7 involves transient increase of gap junction intercellular communication and Cx43 up-regulation: A mechanism of chemoprevention. Int. J. Mol. Sci. 2019, 20, 3244. [Google Scholar] [CrossRef] [Green Version]
- Dinicola, S.; Pasqualato, A.; Cucina, A.; Coluccia, P. Grape seed extract suppresses MDA-MB231 breast cancer cell migration and invasion. Eur. J. Nutr. 2014, 53, 421–431. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, K.; Chen, S.; Wen, W. Grape seed extract inhibits VEGF expression via reducing HIF-1α protein expression. Carcinogenesis 2009, 30, 636–644. [Google Scholar] [CrossRef] [Green Version]
- Eng, E.T.; Williams, D.; Mandava, U.; Kirma, N.; Tekmal, R.R.; Chen, S. Anti-Aromatase Chemicals in Red Wine. Ann. N. Y. Acad. Sci. 2006, 963, 239–246. [Google Scholar] [CrossRef]
- Kijima, I.; Phung, S.; Hur, G.; Kwok, S.L.; Chen, S. Grape seed extract is an aromatase inhibitor and a suppressor of aromatase expression. Cancer Res. 2006, 66, 5960–5967. [Google Scholar] [CrossRef] [Green Version]
- Sharma, G.; Tyagi, A.K.; Singh, R.P.; Chan, D.C.F.; Agarwal, R. Synergistic anti-cancer effects of grape seed extract and conventional cytotoxic agent doxorubicin against human breast carcinoma cells. Breast Cancer Res. Treat. 2004, 85, 1–12. [Google Scholar] [CrossRef]
- Yousef, M.I.; Mahdy, M.A.; Abdou, H.M. The potential protective role of grape seed proanthocyanidin extract against the mixture of carboplatin and thalidomide induced hepatotoxicity and cardiotoxicity in male rats. Prev. Med. Commun. Health 2020, 2, 1–7. [Google Scholar] [CrossRef]
- Makris, D.P.; Kallithraka, S.; Kefalas, P. Flavonols in grapes, grape products and wines: Burden, profile and influential parameters. J. Food Compos. Anal. 2006, 19, 396–404. [Google Scholar] [CrossRef]
- Simonetti, P.; Pietta, P.; Testolin, G. Polyphenol content and total antioxidant potential of selected Italian wines. J. Agric. Food Chem. 1997, 45, 1152–1155. [Google Scholar] [CrossRef]
- Gardner, P.T.; McPhail, D.B.; Crozier, A.; Duthie, G.G. Electron spin resonance (ESR) spectroscopic assessment of the contribution of quercetin and other flavonols to the antioxidant capacity of red wines. J. Sci. Food Agric. 1999, 79, 1011–1014. [Google Scholar] [CrossRef]
- McDonald, M.S.; Hughes, M.; Burns, J.; Lean, M.E.J.; Matthews, D.; Crozier, A. Survey of the Free and Conjugated Myricetin and Quercetin Content of Red Wines of Different Geographical Origins. J. Agric. Food Chem. 1998, 46, 368–375. [Google Scholar] [CrossRef]
- Dabeek, W.M.; Marra, M.V. Dietary quercetin and kaempferol: Bioavailability and potential cardiovascular-related bioactivity in humans. Nutrients 2019, 11, 2288. [Google Scholar] [CrossRef] [Green Version]
- Kennedy, J.A. Grape and wine phenolics: Observations and recent findings. Ciencia e Investigación Agraria 2008, 35, 107–120. [Google Scholar] [CrossRef]
- Fernández de Simón, B.; Hernández, T.; Cadahía, E.; Dueñas, M.; Estrella, I. Phenolic compounds in a Spanish red wine aged in barrels made of Spanish, French and American oak wood. Eur. Food Res. Technol. 2003, 216, 150–156. [Google Scholar] [CrossRef]
- Castellari, M.; Piermattei, B.; Arfelli, G.; Amati, A. Influence of aging conditions on the quality of red Sangiovese wine. J. Agric. Food Chem. 2001, 49, 3672–3676. [Google Scholar] [CrossRef]
- Makris, D.P.; Rossiter, J.T. Heat-induced, metal-catalyzed oxidative degradation of quercetin and rutin (quercetin 3-O-rhamnosylglucoside) in aqueous model systems. J. Agric. Food Chem. 2000, 48, 3830–3838. [Google Scholar] [CrossRef]
- Hashemzaei, M.; Far, A.D.; Yari, A.; Heravi, R.E.; Tabrizian, K.; Taghdisi, S.M.; Sadegh, S.E.; Tsarouhas, K.; Kouretas, D.; Tzanakakis, G.; et al. Anticancer and apoptosis-inducing effects of quercetin in vitro and in vivo. Oncol. Rep. 2017, 38, 819–828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imran, M.; Salehi, B.; Sharifi-Rad, J.; Gondal, T.A.; Saeed, F.; Imran, A.; Shahbaz, M.; Fokou, P.V.T.; Arshad, M.U.; Khan, H.; et al. Kaempferol: A key emphasis to its anticancer potential. Molecules 2019, 24, 2277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franke, A.A.; Custer, L.J.; Wilkens, L.R.; Le Marchand, L.; Nomura, A.M.Y.; Goodman, M.T.; Kolonel, L.N. Liquid chromatographic-photodiode array mass spectrometric analysis of dietary phytoestrogens from human urine and blood. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2002, 777, 45–59. [Google Scholar] [CrossRef]
- Wang, L.; Lee, I.M.; Zhang, S.M.; Blumberg, J.B.; Buring, J.E.; Sesso, H.D. Dietary intake of selected flavonols, flavones, and flavonoid-rich foods and risk of cancer in middle-aged and older women. Am. J. Clin. Nutr. 2009, 89, 905–912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mokbel, K.; Mokbel, K. Chemoprevention of Breast Cancer with Vitamins and Micronutrients: A Concise Review. In Vivo 2019, 33, 983–997. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Yang, Y.; An, Y.; Fang, G. The mechanism of anticancer action and potential clinical use of kaempferol in the treatment of breast cancer. Biomed. Pharmacother. 2019, 117, 109086. [Google Scholar] [CrossRef]
- Tang, S.M.; Deng, X.T.; Zhou, J.; Li, Q.P.; Ge, X.X.; Miao, L. Pharmacological basis and new insights of quercetin action in respect to its anti-cancer effects. Biomed. Pharmacother. 2020, 121, 109604. [Google Scholar] [CrossRef]
- Barve, A.; Chen, C.; Hebbar, V.; Desiderioa, J.; Constance Lay-Lay Saw, A.-N.K. Metabolism, oral bioavailability and pharmacokinetics of chemopreventive kaempferol in rats. Biopharm. Drug Dispos. 2009, 30, 356–365. [Google Scholar] [CrossRef] [Green Version]
- Zabela, V.; Sampath, C.; Oufir, M.; Moradi-Afrapoli, F.; Butterweck, V.; Hamburger, M. Pharmacokinetics of dietary kaempferol and its metabolite 4-hydroxyphenylacetic acid in rats. Fitoterapia 2016, 115, 189–197. [Google Scholar] [CrossRef] [Green Version]
- Lamson, D.W.; Brignall, M.S. Antioxidants and cancer III: Quercetin. Altern. Med. Rev. 2000, 5, 196–208. [Google Scholar]
- Cai, X.; Fang, Z.; Dou, J.; Yu, A.; Zhai, G. Bioavailability of Quercetin: Problems and Promises. Curr. Med. Chem. 2013, 20, 2572–2582. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, R.; Qian, J.; Sun, J.; Li, G.; Shen, J.; Xie, Y. The combination therapy of doxorubicin and quercetin on multidrug resistant breast cancer and their sequential delivery by reduction-sensitive hyaluronic acid-based conjugate/D-α-tocopheryl polyethylene glycol 1000 succinate mixed micelles. Mol. Pharm. 2020, 17, 1415–1427. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Balasubramanian, V.; Bhat, C.; Vahermo, M.; Mäkilä, E.; Kemell, M.; Fontana, F.; Janoniene, A.; Petrikaite, V.; Salonen, J.; et al. Quercetin-Based Modified Porous Silicon Nanoparticles for Enhanced Inhibition of Doxorubicin-Resistant Cancer Cells. Adv. Healthc. Mater. 2017, 6, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lv, L.; Liu, C.; Chen, C.; Yu, X.; Chen, G.; Shi, Y.; Qin, F.; Ou, J.; Qiu, K.; Li, G. Quercetin and doxorubicin co-encapsulated biotin receptortargeting nanoparticles for minimizing drug resistance in breast cancer. Oncotarget 2016, 7, 32184–32199. [Google Scholar] [CrossRef]
- Kubatka, P.; Kapinová, A.; Kello, M.; Kruzliak, P.; Kajo, K.; Výbohová, D.; Mahmood, S.; Murin, R.; Viera, T.; Mojžiš, J.; et al. Fruit peel polyphenols demonstrate substantial anti-tumour effects in the model of breast cancer. Eur. J. Nutr. 2015, 55, 955–965. [Google Scholar] [CrossRef]
- Leigh Ackland, M.; Van De Waarsenburg, S.; Jones, R. Synergistic antiproliferation action of the flavonols quercetin and kaempferol in cultured human cancer cell lines. In Vivo 2005, 19, 69–76. [Google Scholar]
- Yi, X.; Zuo, J.; Tan, C.; Xian, S.; Luo, C.; Chen, S.; Yu, L.; Luo, Y. Kaempferol, a flavonoid compound from gynura medica induced apoptosis and growth inhibition in MCF-7 breast cancer cell. Afr. J. Tradit. Complement. Altern. Med. 2016, 13, 210–215. [Google Scholar] [CrossRef] [Green Version]
- Choi, E.J.; Ahn, W.S. Kaempferol induced the apoptosis via cell cycle arrest in human breast cancer MDA-MB-453 cells. Nutr. Res. Pract. 2008, 2, 322–325. [Google Scholar] [CrossRef] [Green Version]
- Kang, G.Y.; Lee, E.R.; Kim, J.H.; Jung, J.W.; Lim, J.; Kim, S.K.; Cho, S.G.; Kim, K.P. Downregulation of PLK-1 expression in kaempferol-induced apoptosis of MCF-7 cells. Eur. J. Pharmacol. 2009, 611, 17–21. [Google Scholar] [CrossRef]
- Li, S.; Yan, T.; Deng, R.; Jiang, X.; Xiong, H.; Wang, Y.; Yu, Q.; Wang, X.; Chen, C.; Zhu, Y. Low dose of kaempferol suppresses the migration and invasion of triple-negative breast cancer cells by downregulating the activities of RhoA and Rac1. OncoTargets Ther. 2017, 10, 4809–4819. [Google Scholar] [CrossRef] [Green Version]
- Zhu, L.; Xue, L. Kaempferol suppresses proliferation and induces cell cycle arrest, apoptosis, and DNA damage in breast cancer cells. Oncol. Res. 2019, 27, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Ranganathan, S.; Halagowder, D.; Sivasithambaram, N.D. Quercetin suppresses twist to induce apoptosis in MCF-7 breast cancer cells. PLoS ONE 2015, 10, 1–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kabała-Dzik, A.; Rzepecka-Stojko, A.; Kubina, R.; Iriti, M.; Wojtyczka, R.D.; Buszman, E.; Stojko, J. Flavonoids, bioactive components of propolis, exhibit cytotoxic activity and induce cell cycle arrest and apoptosis in human breast cancer cells MDA-MB-231 and MCF-7—A comparative study. Cell. Mol. Biol. 2018, 64, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, B.W.; Lee, E.R.; Min, H.; Jeong, H.S.; Ahn, J.Y.; Kim, J.H.; Choi, H.Y.; Choi, H.; Eun, Y.K.; Se, P.P.; et al. Sustained ERK activation is involved in the kaempferol-induced apoptosis of breast cancer cells and is more evident under 3-D culture condition. Cancer Biol. Ther. 2008, 7, 1080–1089. [Google Scholar] [CrossRef] [Green Version]
- Liao, W.; Chen, L.; Ma, X.; Jiao, R.; Li, X.; Wang, Y. Protective effects of kaempferol against reactive oxygen species-induced hemolysis and its antiproliferative activity on human cancer cells. Eur. J. Med. Chem. 2016, 114, 24–32. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, M.; Yu, L.; Zhao, Y.; He, N.; Yang, X. Antitumor activities of quercetin and quercetin-5′,8-disulfonate in human colon and breast cancer cell lines. Food Chem. Toxicol. 2012, 50, 1589–1599. [Google Scholar] [CrossRef]
- Wu, Q.; Needs, P.W.; Lu, Y.; Kroon, P.A.; Ren, D.; Yang, X. Different antitumor effects of quercetin, quercetin-3′-sulfate and quercetin-3-glucuronide in human breast cancer MCF-7 cells. Food Funct. 2018, 9, 1736–1746. [Google Scholar] [CrossRef]
- Niazvand, F.; Orazizadeh, M.; Khorsandi, L.; Abbaspour, M.; Mansouri, E.; Khodadadi, A. Effects of Quercetin-Loaded Nanoparticles on MCF-7 Human Breast Cancer Cells. Medicina 2019, 55, 114. [Google Scholar] [CrossRef] [Green Version]
- Chou, C.C.; Yang, J.S.; Lu, H.F.; Ip, S.W.; Lo, C.; Wu, C.C.; Lin, J.P.; Tang, N.Y.; Chung, J.G.; Chou, M.J.; et al. Quercetin-mediated cell cycle arrest and apoptosis involving activation of a caspase cascade through the mitochondrial pathway in human breast cancer MCF-7 cells. Arch. Pharm. Res. 2010, 33, 1181–1191. [Google Scholar] [CrossRef]
- Chien, S.Y.; Wu, Y.C.; Chung, J.G.; Yang, J.S.; Lu, H.F.; Tsou, M.F.; Wood, W.; Kuo, S.J.; Chen, D.R. Quercetin-induced apoptosis acts through mitochondrial- and caspase-3-dependent pathways in human breast cancer MDA-MB-231 cells. Hum. Exp. Toxicol. 2009, 28, 493–503. [Google Scholar] [CrossRef]
- Balakrishnan, S.; Mukherjee, S.; Das, S.; Bhat, F.A.; Raja Singh, P.; Patra, C.R.; Arunakaran, J. Gold nanoparticles–conjugated quercetin induces apoptosis via inhibition of EGFR/PI3K/Akt–mediated pathway in breast cancer cell lines (MCF-7 and MDA-MB-231). Cell Biochem. Funct. 2017, 35, 217–231. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.W.; Hou, W.C.; Shen, S.C.; Juan, S.H.; Ko, C.H.; Wang, L.M.; Chen, Y.C. Quercetin inhibition of tumor invasion via suppressing PKCδ/ERK/AP-1-dependent matrix metalloproteinase-9 activation in breast carcinoma cells. Carcinogenesis 2008, 29, 1807–1815. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhu, F.; Lubet, R.A.; De Luca, A.; Grubbs, C.; Ericson, M.E.; D’Alessio, A.; Normanno, N.; Dong, Z.; Bode, A.M. Quercetin-3-methyl ether inhibits lapatinib-sensitive and -resistant breast cancer cell growth by inducing G2/M arrest and apoptosis. Mol. Carcinog. 2013, 52, 134–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khorsandi, L.; Orazizadeh, M.; Niazvand, F.; Abbaspour, M.R.; Mansouri, E.; Khodadadi, A. Quercetin induces apoptosis and necroptosis in MCF-7 breast cancer cells. Bratislava Med. J. 2017, 118, 123–128. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.; Zhang, Y.; Jiang, X.; Zhang, H.; Gao, Z.; Li, Y.; Fu, R.; Li, L.; Li, J.; Cui, H.; et al. ROS-mediated activation and mitochondrial translocation of CaMKII contributes to Drp1-dependent mitochondrial fission and apoptosis in triple-negative breast cancer cells by isorhamnetin and chloroquine. J. Exp. Clin. Cancer Res. 2019, 38, 1–16. [Google Scholar] [CrossRef]
- Qiu, J.; Zhang, T.; Zhu, X.; Yang, C.; Wang, Y.; Zhou, N.; Ju, B.; Zhou, T.; Deng, G.; Qiu, C. Hyperoside induces breast cancer cells apoptosis via ROS-mediated NF-κB signaling pathway. Int. J. Mol. Sci. 2020, 21, 131. [Google Scholar] [CrossRef] [Green Version]
- Steiner, J.L.; Davis, J.M.; McClellan, J.L.; Enos, R.T.; Carson, J.A.; Fayad, R.; Nagarkatti, M.; Nagarkatti, P.S.; Altomare, D.; Creek, K.E.; et al. Dose-dependent benefits of quercetin on tumorigenesis in the C3(1)/SV40Tag transgenic mouse model of breast cancer. Cancer Biol. Ther. 2014, 15, 1456–1467. [Google Scholar] [CrossRef]
- Phromnoi, K.; Yodkeeree, S.; Anuchapreeda, S.; Limtrakul, P. Inhibition of MMP-3 activity and invasion of the MDA-MB-231 human invasive breast carcinoma cell line by bioflavonoids. Acta Pharmacol. Sin. 2009, 30, 1169–1176. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Yang, D.; Zhao, Y.; Qiu, Y.; Cao, X.; Yu, Y.; Guo, H.; Gu, X.; Yin, X. Inhibitory Effects of Isorhamnetin on the Invasion of Human Breast Carcinoma Cells by Downregulating the Expression and Activity of Matrix Metalloproteinase-2/9. Nutr. Cancer 2015, 67, 1191–1200. [Google Scholar] [CrossRef]
- Balakrishnan, S.; Bhat, F.A.; Raja Singh, P.; Mukherjee, S.; Elumalai, P.; Das, S.; Patra, C.R.; Arunakaran, J. Gold nanoparticle–conjugated quercetin inhibits epithelial–mesenchymal transition, angiogenesis and invasiveness via EGFR/VEGFR-2-mediated pathway in breast cancer. Cell Prolif. 2016, 49, 678–697. [Google Scholar] [CrossRef]
- Oh, S.J.; Kim, O.; Lee, J.S.; Kim, J.A.; Kim, M.R.; Choi, H.S.; Shim, J.H.; Kang, K.W.; Kim, Y.C. Inhibition of angiogenesis by quercetin in tamoxifen-resistant breast cancer cells. Food Chem. Toxicol. 2010, 48, 3227–3234. [Google Scholar] [CrossRef] [PubMed]
- Brusselmans, K.; Vrolix, R.; Verhoeven, G.; Swinnen, J.V. Induction of cancer cell apoptosis by flavonoids is associated with their ability to inhibit fatty acid synthase activity. J. Biol. Chem. 2005, 280, 5636–5645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanikoglu, A.; Kucuksayan, E.; Hanikoglu, F.; Ozben, T.; Menounou, G.; Sansone, A.; Chatgilialoglu, C.; Di Bella, G.; Ferreri, C. Effects of somatostatin, curcumin, and quercetin on the fatty acid profile of breast cancer cell membranes. Can. J. Physiol. Pharmacol. 2020, 98, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Kuiper, G.G.J.M.; Lemmen, J.G.; Carlsson, B.; Corton, J.C.; Safe, S.H.; Van Der Saag, P.T.; Van Der Burg, B.; Gustafsson, J.Å. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor β. Endocrinology 1998, 139, 4252–4263. [Google Scholar] [CrossRef] [PubMed]
- Hung, H. Inhibition of Estrogen Receptor Alpha Expression and Function in MCF-7 Cells by Kaempferol. J. Cell. Physiol. 2004, 198, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Yiğitaslan, S.; Erol, K.; Özatik, F.Y.; Özatik, O.; Şahin, S.; Çengelli, Ç. Estrogen-like activity of quercetin in female rats. Erciyes Tip Derg. 2016, 38, 53–58. [Google Scholar] [CrossRef]
- Puranik, N.V.; Srivastava, P.; Bhatt, G.; John Mary, D.J.S.; Limaye, A.M.; Sivaraman, J. Determination and analysis of agonist and antagonist potential of naturally occurring flavonoids for estrogen receptor (ERα) by various parameters and molecular modelling approach. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.J.; Choo, W.H.; Kim, H.R.; Chung, K.H.; Oh, S.M. Inhibitory aromatase effects of flavonoids from ginkgo biloba extracts on estrogen biosynthesis. Asian Pac. J. Cancer Prev. 2015, 16, 6317–6325. [Google Scholar] [CrossRef] [Green Version]
- Kao, Y.C.; Zhou, C.; Sherman, M.; Laughton, C.A.; Chen, S. Molecular basis of the inhibition of human aromatase (estrogen synthetase) by flavone and isoflavone phytoestrogens: A site-directed mutagenesis study. Environ. Health Perspect. 1998, 106, 85–92. [Google Scholar] [CrossRef]
- van Meeuwen, J.A.; ter Burg, W.; Piersma, A.H.; van den Berg, M.; Sanderson, J.T. Mixture effects of estrogenic compounds on proliferation and pS2 expression of MCF-7 human breast cancer cells. Food Chem. Toxicol. 2007, 45, 2319–2330. [Google Scholar] [CrossRef]
- Resende, F.A.; de Oliveira, A.P.S.; de Camargo, M.S.; Vilegas, W.; Varanda, E.A. Evaluation of Estrogenic Potential of Flavonoids Using a Recombinant Yeast Strain and MCF7/BUS Cell Proliferation Assay. PLoS ONE 2013, 8, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Seung, M.O.; Yeon, P.K.; Kyu, H.C. Biphasic effects of kaempferol on the estrogenicity in human breast cancer cells. Arch. Pharm. Res. 2006, 29, 354–362. [Google Scholar]
- Kim, S.H.; Hwang, K.A.; Choi, K.C. Treatment with kaempferol suppresses breast cancer cell growth caused by estrogen and triclosan in cellular and xenograft breast cancer models. J. Nutr. Biochem. 2016, 28, 70–82. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.A.; Choi, K.C.; Hwang, K.A. Treatment with phytoestrogens reversed triclosan and bisphenol A-induced anti-apoptosis in breast cancer cells. Biomol. Ther. 2018, 26, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.A.; Choi, K.C.; Hwang, K.A. Kaempferol, a phytoestrogen, suppressed triclosan-induced epithelial-mesenchymal transition and metastatic-related behaviors of MCF-7 breast cancer cells. Environ. Toxicol. Pharmacol. 2017, 49, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Mei, Y.Q.; Kai, L.; Qun, S.; Qingyue, L.; Jinghui, H.; Fangxuan, H.; Ren-Wang, J. Reversal of multidrug resistance in cancer by multi-functional flavonoids. Front. Oncol. 2019, 9, 487. [Google Scholar]
- Soo, Y.C.; Min, K.S.; Na, H.K.; Jung, O.J.; Eun, J.G.; Hwa, J.L. Inhibition of P-glycoprotein by natural products in human breast cancer cells. Arch. Pharm. Res. 2005, 28, 823–828. [Google Scholar]
- Li, S.; Yuan, S.; Zhao, Q.; Wang, B.; Wang, X.; Li, K. Quercetin enhances chemotherapeutic effect of doxorubicin against human breast cancer cells while reducing toxic side effects of it. Biomed. Pharmacother. 2018, 100, 441–447. [Google Scholar] [CrossRef]
- Li, S.; Zhao, Q.; Wang, B.; Yuan, S.; Wang, X.; Li, K. Quercetin reversed MDR in breast cancer cells through down-regulating P-gp expression and eliminating cancer stem cells mediated by YB-1 nuclear translocation. Phyther. Res. 2018, 32, 1530–1536. [Google Scholar] [CrossRef]
- Desale, J.P.; Swami, R.; Kushwah, V.; Katiyar, S.S.; Jain, S. Chemosensitizer and docetaxel-loaded albumin nanoparticle: Overcoming drug resistance and improving therapeutic efficacy. Nanomedicine 2018, 13, 2759–2776. [Google Scholar] [CrossRef]
- Kumar, M.; Sharma, G.; Misra, C.; Kumar, R.; Singh, B.; Katare, O.P.; Raza, K. N-desmethyl tamoxifen and quercetin-loaded multiwalled CNTs: A synergistic approach to overcome MDR in cancer cells. Mater. Sci. Eng. C 2018, 89, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Imai, Y.; Tsukahara, S.; Asada, S.; Sugimoto, Y. Phytoestrogens/flavonoids reverse breast cancer resistance protein/ABCG2-mediated multidrug resistance. Cancer Res. 2004, 64, 4346–4352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, X.; Bai, J.; Zhao, S.; Hu, M.; Sun, Y.; Wang, B.; Ji, M.; Jin, J.; Wang, X.; Hu, J.; et al. Evaluation of inhibitory effects of flavonoids on breast cancer resistance protein (BCRP): From library screening to biological evaluation to structure-activity relationship. Toxicol. Vitr. 2019, 61, 104642. [Google Scholar] [CrossRef] [PubMed]
- An, G.; Gallegos, J.; Morris, M.E. The bioflavonoid kaempferol is an Abcg2 substrate and inhibits Abcg2-mediated quercetin efflux. Drug Metab. Dispos. 2011, 39, 426–432. [Google Scholar] [CrossRef] [Green Version]
- Zheng, L.; Zhu, L.; Zhao, M.; Shi, J.; Li, Y.; Yu, J.; Jiang, H.; Wu, J.; Tong, Y.; Liu, Y.; et al. In Vivo Exposure of Kaempferol Is Driven by Phase II Metabolic Enzymes and Efflux Transporters. AAPS J. 2016, 18, 1289–1299. [Google Scholar] [CrossRef]
- Li, Y.; Lu, L.; Wang, L.; Qu, W.; Liu, W.; Xie, Y.; Zheng, H.; Wang, Y.; Qi, X.; Hu, M.; et al. Interplay of Efflux Transporters with Glucuronidation and Its Impact on Subcellular Aglycone and Glucuronide Disposition: A Case Study with Kaempferol. Mol. Pharm. 2018, 15, 5602–5614. [Google Scholar] [CrossRef]
- Yao, L.H.; Jiang, Y.M.; Shi, J.; Tomás-Barberán, F.A.; Datta, N.; Singanusong, R.; Chen, S.S. Flavonoids in food and their health benefits. Plant Foods Hum. Nutr. 2004, 59, 113–122. [Google Scholar] [CrossRef]
- Pastrana-Bonilla, E.; Akoh, C.C.; Sellappan, S.; Krewer, G. Phenolic Content and Antioxidant Capacity of Muscadine Grapes. J. Agric. Food Chem. 2003, 51, 5497–5503. [Google Scholar] [CrossRef]
- Graham, H.N. Green tea composition, consumption, and polyphenol chemistry. Prev. Med. 1992, 21, 334–350. [Google Scholar] [CrossRef]
- Bell, J.R.C.; Donovan, J.L.; Wong, R.; Waterhouse, A.L.; German, J.B.; Walzem, R.L. (+)-Catechin in human plasma after ingestion of a single serving of reconstituted red wine. Am. J. Clin. Nutr. 2000, 71, 103–108. [Google Scholar] [CrossRef]
- Silva, J.M.R.; Rigaud, J.; Cheynier, V.; Cheminat, A.; Moutounet, M. Procyanidin dimers and trimers from grape seeds. Phytochemistry 1991, 30, 1259–1264. [Google Scholar] [CrossRef]
- Prieur, C.; Rigaud, J.; Cheynier, V.; Moutounet, M. Oligomeric and polymeric procyanidins from grape seeds. Phytochemistry 1994, 36, 781–784. [Google Scholar] [CrossRef]
- Kim, H.; Hall, P.; Smith, M.; Kirk, M.; Prasain, J.K.; Barnes, S.; Grubbs, C. Chemoprevention by Grape Seed Extract and Genistein in Carcinogen- induced Mammary Cancer in Rats Is Diet Dependent 1, 2. Int. Res. Conf. Food Nutr. Cancer 2004, 3445–3452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ou, K.; Gu, L. Absorption and metabolism of proanthocyanidins. J. Funct. Foods 2014, 7, 43–53. [Google Scholar] [CrossRef]
- Tsang, C.; Auger, C.; Mullen, W.; Bornet, A.; Rouanet, J.-M.; Crozier, A.; Teissedre, P.-L. The absorption, metabolism and excretion of flavan-3-ols and procyanidins following the ingestion of a grape seed extract by rats. Br. J. Nutr. 2005, 94, 170–181. [Google Scholar] [CrossRef]
- Serra, A.; MacI, A.; Romero, M.P.; Valls, J.; Bladé, C.; Arola, L.; Motilva, M.J. Bioavailability of procyanidin dimers and trimers and matrix food effects in in vitro and in vivo models. Br. J. Nutr. 2010, 103, 944–952. [Google Scholar] [CrossRef] [Green Version]
- Sano, A.; Yamakoshi, J.; Tokutake, S.; Tobe, K.; Kubota, Y.; Kikuchi, M. Procyanidin B1 is detected in human serum after intake of proanthocyanidin-rich grape seed extract. Biosci. Biotechnol. Biochem. 2003, 67, 1140–1143. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.S.; Sang, S.; Lambert, J.D.; Lee, M.J. Bioavailability issues in studying the health effects of plant polyphenolic compounds. Mol. Nutr. Food Res. 2008, 52, 139–151. [Google Scholar] [CrossRef]
- Meng, X.; Sang, S.; Zhu, N.; Lu, H.; Sheng, S.; Lee, M.J.; Ho, C.T.; Yang, C.S. Identification and characterization of methylated and ring-fission metabolites of tea catechins formed in humans, mice, and rats. Chem. Res. Toxicol. 2002, 15, 1042–1050. [Google Scholar] [CrossRef]
- Faria, A.; Calhau, C.; De Freitas, V.; Mateus, N. Procyanidins as antioxidants and tumor cell growth modulators. J. Agric. Food Chem. 2006, 54, 2392–2397. [Google Scholar] [CrossRef]
- Alshatwi, A.A. Catechin hydrate suppresses MCF-7 proliferation through TP53/Caspase-mediated apoptosis. J. Exp. Clin. Cancer Res. 2010, 29, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Damianaki, A.; Bakogeorgou, E.; Kampa, M.; Notas, G.; Hatzoglou, A.; Panagiotou, S.; Gemetzi, C.; Kouroumalis, E.; Martin, P.M.; Castanas, E. Potent inhibitory action of red wine polyphenols on human breast cancer cells. J. Cell. Biochem. 2000, 78, 429–441. [Google Scholar] [CrossRef]
- Singletary, K.W.; Meline, B. Effect of Grape Seed Proanthocyanidins on Colon Aberrant Crypts and Breast Tumors in a Rat Dual-Organ Tumor Model. Nutr. Cancer 2001, 39, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Wahner-roedler, D.L.; Bauer, B.A.; Loehrer, L.L.; Cha, S.S.; Vera, J.; Hoskin, T.L.; Olson, J.E. The Effect of Grape Seed Extract on Estrogen Levels of Postmenopausal Women—A Pilot Study. J. Diet. Suppl. 2016, 11, 184–197. [Google Scholar] [CrossRef] [Green Version]
- Ye, X.; Krohn, R.L.; Liu, W.; Joshi, S.S.; Kuszynski, C.A.; McGinn, T.R.; Bagchi, M.; Preuss, H.G.; Stohs, S.J.; Bagchi, D. The cytotoxic effects of a novel IH636 grape seed proanthocyanidin extract on cultured human cancer cells. Stress Adapt. Prophyl. Treat. 1999, 196, 99–108. [Google Scholar]
- Brooker, S.; Martin, S.; Pearson, A.; Bagchi, D.; Earl, J.; Gothard, L.; Hall, E.; Porter, L.; Yarnold, J. Double-blind, placebo-controlled, randomised phase II trial of IH636 grape seed proanthocyanidin extract (GSPE) in patients with radiation-induced breast induration. Radiother. Oncol. 2006, 79, 45–51. [Google Scholar] [CrossRef]
- Ma, Z.F.; Zhang, H. Phytochemical constituents, health benefits, and industrial applications of grape seeds: A mini-review. Antioxidants 2017, 6, 71. [Google Scholar] [CrossRef] [Green Version]
- Tang, K.; Liu, T.; Han, Y.; Xu, Y.; Li, J.M. The Importance of Monomeric Anthocyanins in the Definition of Wine Colour Properties. S. Afr. J. Enol. Vitic. 2017, 38, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Butelli, E.; Martin, C. Engineering anthocyanin biosynthesis in plants. Curr. Opin. Plant Biol. 2014, 19, 81–90. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef] [Green Version]
- Tsuda, T.; Watanabe, M.; Ohshima, K.; Norinobu, S.; Choi, S.W.; Kawakishi, S.; Osawa, T. Antioxidative Activity of the Anthocyanin Pigments Cyanidin 3-O-β-d-Glucoside and Cyanidin. J. Agric. Food Chem. 1994, 42, 2407–2410. [Google Scholar] [CrossRef]
- Lila, M.A.; Burton-Freeman, B.; Grace, M.; Kalt, W. Unraveling Anthocyanin Bioavailability for Human Health. Annu. Rev. Food Sci. Technol. 2016, 7, 375–393. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.W.; Gong, C.C.; Song, H.F.; Cui, Y.Y. Effects of anthocyanins on the prevention and treatment of cancer. Br. J. Pharmacol. 2017, 174, 1226–1243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hemmlnki, K. DNA adducts, mutations and cancer. Carcinogenesis 1993, 14, 2007–2012. [Google Scholar] [CrossRef]
- Singletary, K.W.; Jung, K.J.; Giusti, M. Anthocyanin-rich grape extract blocks breast cell DNA damage. J. Med. Food 2007, 10, 244–251. [Google Scholar] [CrossRef]
- Syed, D.N.; Afaq, F.; Sarfaraz, S.; Khan, N.; Kedlaya, R.; SAetaluri, V.; Mukhtar, H. Delphinidin inhibits cell proliferation and invasion via modulation of Met receptor phosphorylation. Toxicol. Appl. Pharmacol. 2008, 231, 52–60. [Google Scholar] [CrossRef] [Green Version]
- Jeffers, M.; Schmidt, L.; Nakaigawa, N.; Webb, C.P.; Weirich, G.; Kishida, T.; Zbar, B.; Vande Woude, G.F. Activating mutations for the Met tyrosine kinase receptor in human cancer. Proc. Natl. Acad. Sci. USA 1997, 94, 11445–11450. [Google Scholar] [CrossRef] [Green Version]
- Ding, S.; Merkulova-Rainon, T.; Han, Z.C.; Tobelem, G. HGF receptor up-regulation contributes to the angiogenic phenotype of human endothelial cells and promotes angiogenesis in vitro. Blood 2003, 101, 4816–4822. [Google Scholar] [CrossRef]
- Bae-Jump, V.; Segreti, E.M.; Vandermolen, D.; Kauma, S. Hepatocyte growth factor (HGF) induces invasion of endometrial carcinoma cell lines in vitro. Gynecol. Oncol. 1999, 73, 265–272. [Google Scholar] [CrossRef]
- Jeffers, M.; Rong, S.; Woude, G.F. Enhanced tumorigenicity and invasion-metastasis by hepatocyte growth factor/scatter factor-met signalling in human cells concomitant with induction of the urokinase proteolysis network. Mol. Cell. Biol. 1996, 16, 1115–1125. [Google Scholar] [CrossRef] [Green Version]
- Murray, D.W.; Didier, S.; Chan, A.; Paulino, V.; Van Aelst, L.; Ruggieri, R.; Tran, N.L.; Byrne, A.T.; Symons, M. Guanine nucleotide exchange factor Dock7 mediates HGF-induced glioblastoma cell invasion via Rac activation. Br. J. Cancer 2014, 110, 1307–1315. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Spitzer, E.; Meyer, D.; Sachs, M.; Niemann, C.; Hartmann, G.; Weidner, K.M.; Birchmeier, C.; Birchmeier, W. Sequential requirement of hepatocyte growth factor and neuregulin in the morphogenesis and differentiation of the mammary gland. J. Cell Biol. 1995, 131, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Luo, E.; Liu, X.; Han, B.; Yu, X.; Peng, X. Delphinidin-3-glucoside suppresses breast carcinogenesis by inactivating the Akt/HOTAIR signaling pathway. BMC Cancer 2016, 16, 423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khorkova, O.; Hsiao; Wahlestedt, C. Basic Biology and Therapeutic Implications of lncRNA. Physiol. Behav. 2015, 87, 15–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hajjari, M.; Salavaty, A. HOTAIR: An oncogenic long non-coding RNA in different cancers. Cancer Biol. Med. 2015, 12, 1–9. [Google Scholar] [PubMed]
- Yang, G.; Zhang, S.; Gao, F.; Liu, Z.; Lu, M.; Peng, S.; Zhang, T.; Zhang, F. Osteopontin enhances the expression of HOTAIR in cancer cells via IRF1. Biochim. Biophys. Acta Gene Regul. Mech. 2014, 1839, 837–848. [Google Scholar] [CrossRef]
- Chen, J.; Lin, C.; Yong, W.; Ye, Y.; Huang, Z. Calycosin and genistein induce apoptosis by inactivation of HOTAIR/p-akt signaling pathway in human breast cancer MCF-7 cells. Cell. Physiol. Biochem. 2015, 35, 722–728. [Google Scholar] [CrossRef]
- Zhang, Y.; Vareed, S.K.; Nair, M.G. Human tumor cell growth inhibition by nontoxic anthocyanidins, the pigments in fruits and vegetables. Life Sci. 2005, 76, 1465–1472. [Google Scholar] [CrossRef]
- Afaq, F.; Zaman, N.; Khan, N.; Syed, D.N.; Sarfaraz, S.; Zaid, M.A.; Mukhtar, H. Inhibition of epidermal growth factor receptor signaling pathway by delphinidin, an anthocyanidin in pigmented fruits and vegetables. Int. J. Cancer 2008, 123, 1508–1515. [Google Scholar] [CrossRef]
- Abd El-Rehim, D.M.; Pinder, S.E.; Paish, C.E.; Bell, J.A.; Rampaul, R.S.; Blamey, R.W.; Robertson, J.F.R.; Nicholson, R.I.; Ellis, I.O. Expression and co-expression of the members of the epidermal growth factor receptor (EGFR) family in invasive breast carcinoma. Br. J. Cancer 2004, 91, 1532–1542. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Zhu, Y.; Zhang, W.; Peng, X.; Zhou, J.; Li, F.; Han, B.; Liu, X.; Ou, Y.; Yu, X. Delphinidin induced protective autophagy via mTOR pathway suppression and AMPK pathway activation in HER-2 positive breast cancer cells. BMC Cancer 2018, 18, 342. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Ning, S. Cyanidin-3-glucoside attenuates the angiogenesis of breast cancer via inhibiting STAT3/VEGF pathway. Phyther. Res. 2019, 33, 81–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, L.; Liu, X.; He, J.; Shao, Y.; Liu, J.; Wang, Z.; Xia, L.; Han, T.; Wu, P. Cyanidin-3-glucoside induces mesenchymal to epithelial transition via activating Sirt1 expression in triple negative breast cancer cells. Biochimie 2019, 162, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Samavat, H.; Kurzer, M.S. Estrogen Metabolism and Breast Cancer. Cancer Lett. 2015, 356, 231–243. [Google Scholar] [CrossRef] [Green Version]
- Reis-Filho, J.S.; Tutt, A.N.J. Triple negative tumours: A critical review. Histopathology 2008, 52, 108–118. [Google Scholar] [CrossRef]
- Zhang, X.T.; Kang, L.G.; Ding, L.; Vranic, S.; Gatalica, Z.; Wang, Z.Y. A positive feedback loop of ER-α36/EGFR promotes malignant growth of ER-negative breast cancer cells. Oncogene 2011, 30, 770. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.Y.; Zhang, X.T.; Shen, P.; Loggie, B.W.; Chang, Y.C.; Deuel, T.F. A variant of estrogen receptor-α, hER-α36: Transduction of estrogen- and antiestrogen-dependent membrane-initiated mitogenic signaling. Proc. Natl. Acad. Sci. USA 2006, 103, 9063–9068. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Li, H.; Yang, S.; Ma, W.; Liu, M.; Guo, S.; Zhan, J.; Zhang, H.; Tsang, S.Y.; Zhang, Z.; et al. Cyanidin-3-o-glucoside directly binds to ERa36 and inhibits EGFR-positive triple-negative breast cancer. Oncotarget 2016, 7, 68864. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, I.; Faria, A.; Azevedo, J.; Soares, S.; Calhau, C.; De Freitas, V.; Mateus, N. Influence of anthocyanins, derivative pigments and other catechol and pyrogallol-type phenolics on breast cancer cell proliferation. J. Agric. Food Chem. 2010, 58, 3785–3792. [Google Scholar] [CrossRef]
- Swain, S.M.; Baselga, J.; Kim, S.B.; Ro, J.; Semiglazov, V.; Campone, M.; Ciruelos, E.; Ferrero, J.; Schneeweiss, A.; Heeson, S.; et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N. Engl. J. Med. 2015, 372, 724–734. [Google Scholar] [CrossRef] [Green Version]
- Witzel, I.; Müller, V.; Abenhardt, W.; Kaufmann, M.; Schoenegg, W.; Schneeweis, A.; Jänicke, F. Long-term tumor remission under trastuzumab treatment for HER2 positive metastatic breast cancer—Results from the HER-OS patient registry. BMC Cancer 2014, 14, 806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Xu, J.; Tang, X.; Liu, Y.; Yu, X.; Wang, Z.; Liu, W. Anthocyanins inhibit trastuzumab-resistant breast cancer in vitro and in vivo. Mol. Med. Rep. 2016, 13, 4007–4013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, W.; Xu, J.; Liu, Y.; Yu, X.; Tang, X.; Wang, Z.; Li, X. Anthocyanins potentiate the activity of trastuzumab in human epidermal growth factor receptor 2-positive breast cancer cells in vitro and in vivo. Mol. Med. Rep. 2014, 10, 1921–1926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, T.; Wang, X.N.; Lou, H.X. Natural stilbenes: An overview. Nat. Prod. Rep. 2009, 26, 916–935. [Google Scholar] [CrossRef]
- Signorelli, P.; Ghidoni, R. Resveratrol as an anticancer nutrient: Molecular basis, open questions and promises. J. Nutr. Biochem. 2005, 16, 449–466. [Google Scholar] [CrossRef]
- Langcake, P.; Pryce, R.J. The production of resveratrol by Vitis vinifera and other members of the Vitaceae as a response to infection or injury. Physiol. Plant Pathol. 1976, 9, 77–86. [Google Scholar] [CrossRef]
- Jeandet, P.; Bessis, R.; Gautheron, B. The production of resveratrol (3, 5, 4′-trihydroxystilbene) by grape berries in different developmental stages. Am. J. Enol. Vitic. 1991, 42, 41–46. [Google Scholar]
- Goldberg, D.M.; Yan, J.; Ng, E.; Diamandis, E.P.; Karumanchiri, A.; Soleas, G.; Waterhouse, A.L. A global survey of trans-resveratrol concentrations in commercial wines. Am. J. Enol. Vitic. 1995, 46, 159–165. [Google Scholar]
- Romero-Pérez, A.I.; Lamuela-Raventós, R.M.; Waterhouse, A.L.; De La Torre-Boronat, M.C. Levels of cis- and trans-Resveratrol and Their Glucosides in White and Rosé Vitis vinifera Wines from Spain. J. Agric. Food Chem. 1996, 44, 2124–2128. [Google Scholar] [CrossRef]
- Pace-Asciak, C.; Rounova, O.; Hahn, S.E.; Diamandis, E.P.; Goldberg, D.M. Wines and grape juices as modulators of platelet aggregation in healthy human subjects. Clin. Chim. Acta 1996, 246, 163–182. [Google Scholar] [CrossRef]
- Weiskirchen, S.; Weiskirchen, R. Resveratrol: How Much Wine Do You Have to Drink to Stay Healthy? Adv. Nutr. An Int. Rev. J. 2016, 7, 706–718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldberg, D.M.; Yan, J.; Soleas, G.J. Absorption of three wine-related polyphenols in three different matrices by healthy subjects. Clin. Biochem. 2003, 36, 79–87. [Google Scholar] [CrossRef]
- Walle, T. Bioavailability of resveratrol. Ann. N. Y. Acad. Sci. 2011, 1215, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Sinha, D.; Sarkar, N.; Biswas, J.; Bishayee, A. Resveratrol for breast cancer prevention and therapy: Preclinical evidence and molecular mechanisms. Semin. Cancer Biol. 2016, 40, 209–232. [Google Scholar] [CrossRef] [PubMed]
- Chimento, A.; De Amicis, F.; Sirianni, R.; Sinicropi, M.S.; Puoci, F.; Casaburi, I.; Saturnino, C.; Pezzi, V. Progress to improve oral bioavailability and beneficial effects of resveratrol. Int. J. Mol. Sci. 2019, 20, 1381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nawaz, W.; Zhou, Z.; Deng, S.; Ma, X.; Ma, X.; Li, C.; Shu, X. Therapeutic versatility of resveratrol derivatives. Nutrients 2017, 9, 1188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- da Costa, D.C.F.; Fialho, E.; Silva, J.L. Cancer chemoprevention by resveratrol: The P53 tumor suppressor protein as a promising molecular target. Molecules 2017, 22, 1014. [Google Scholar] [CrossRef] [Green Version]
- Jang, M.; Cai, L.; Udeani, G.O.; Slowing, K.V.; Thomas, C.F.; Beecher, C.W.W.; Fong, H.H.S.; Farnsworth, N.R.; Kinghorn, A.D.; Mehta, R.G.; et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science 1997, 275, 218–220. [Google Scholar] [CrossRef] [Green Version]
- Pezzuto, J.M. Resveratrol: Twenty years of growth, development and controversy. Biomol. Ther. 2019, 27, 1. [Google Scholar] [CrossRef]
- Casanova, F.; Quarti, J.; Da Costa, D.C.F.; Ramos, C.A.; Da Silva, J.L.; Fialho, E. Resveratrol chemosensitizes breast cancer cells to melphalan by cell cycle arrest. J. Cell. Biochem. 2012, 113, 2586–2596. [Google Scholar] [CrossRef]
- Varoni, E.M.; Lo Faro, A.F.; Sharifi-Rad, J.; Iriti, M. Anticancer Molecular Mechanisms of Resveratrol. Front. Nutr. 2016, 3, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ko, J.H.; Sethi, G.; Um, J.Y.; Shanmugam, M.K.; Arfuso, F.; Kumar, A.P.; Bishayee, A.; Ahn, K.S. The role of resveratrol in cancer therapy. Int. J. Mol. Sci. 2017, 18, 2589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ndiaye, M.; Kumar, R.; Ahmad, N. Resveratrol in cancer management: Where are we and where we go from here? Ann. N. Y. Acad. Sci. 2011, 1215, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Levi, F.; Pasche, C.; Lucchini, F.; Ghidoni, R.; Ferraroni, M.; La Vecchia, C. Resveratrol and breast cancer risk. Eur. J. Cancer Prev. 2005, 14, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Levenson, A.S.; Biswas, P.K. Structural insights into Resveratrol’s antagonist and partial agonist actions on estrogen receptor alpha. BMC Struct. Biol. 2013, 13, 27. [Google Scholar] [CrossRef] [Green Version]
- Poschner, S.; Maier-Salamon, A.; Thalhammer, T.; Jäger, W. Resveratrol and other dietary polyphenols are inhibitors of estrogen metabolism in human breast cancer cells. J. Steroid Biochem. Mol. Biol. 2019, 190, 11–18. [Google Scholar] [CrossRef]
- Jordan, V.C. Antiestrogens and Selective Estrogen Receptor Modulators as Multifunctional Medicines. 2. Clinical Considerations and New Agents. J. Med. Chem. 2003, 46, 1081–1111. [Google Scholar] [CrossRef]
- Gehm, B.D.; McAndrews, J.M.; Chien, P.Y.; Jameson, J.L. Resveratrol, a polyphenolic compound found in grapes and wine, is an agonist for the estrogen receptor. Proc. Natl. Acad. Sci. USA 1997, 94, 14138–14143. [Google Scholar] [CrossRef] [Green Version]
- Levenson, A.S.; Gehm, B.D.; Pearce, S.T.; Horiguchi, J.; Simons, L.A.; Ward, J.E.; Jameson, J.L.; Jordan, V.C. Resveratrol acts as an estrogen receptor (ER) agonist in breast cancer cells stably transfected with ERα. Int. J. Cancer 2003, 104, 587–596. [Google Scholar] [CrossRef]
- Lu, R.; Serrero, G. Resveratrol, a natural product derived from grape, exhibits antiestrogenic activity and inhibits the growth of human breast cancer cells. J. Cell. Physiol. 1999, 179, 297–304. [Google Scholar] [CrossRef]
- Bowers, J.L.; Tyulmenkov, V.V.; Jernigan, S.C.; Klinge, C.M. Resveratrol acts as a mixed agonist/antagonist for estrogen receptors α and β. Endocrinology 2000, 141, 3657–3667. [Google Scholar] [CrossRef] [PubMed]
- Gehm, B.D.; Levenson, A.S.; Liu, H.; Lee, E.J.; Amundsen, B.M.; Cushman, M.; Jordan, V.C.; Jameson, J.L. Estrogenic effects of resveratrol in breast cancer cells expressing mutant and wild-type estrogen receptors: Role of AF-1 and AF-2. J. Steroid Biochem. Mol. Biol. 2004, 88, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Simpson, E.R.; Michael, M.D.; Agarwal, V.R.; Hinshelwood, M.M.; Bulun, S.E.; Zhao, Y. Cytochromes P450 11: Expression of the CYP19 (aromatase) gene: An unusual case of alternative promoter usage. FASEB J. 1997, 11, 29–36. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Lee, K.W.; Chan, F.L.; Chen, S.; Leung, L.K. The red wine polyphenol resveratrol displays bilevel inhibition on aromatase in breast cancer cells. Toxicol. Sci. 2006, 92, 71–77. [Google Scholar] [CrossRef] [Green Version]
- Chottanapund, S.; Van Duursen, M.B.M.; Navasumrit, P.; Hunsonti, P.; Timtavorn, S.; Ruchirawat, M.; Van den Berg, M. Anti-aromatase effect of resveratrol and melatonin on hormonal positive breast cancer cells co-cultured with breast adipose fibroblasts. Toxicol. In Vitro 2014, 28, 1215–1221. [Google Scholar] [CrossRef]
- Le Corre, L.; Chalabi, N.; Delort, L.; Bignon, Y.J.; Bernard-Gallon, D.J. Resveratrol and breast cancer chemoprevention: Molecular mechanisms. Mol. Nutr. Food Res. 2005, 49, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.; Caporaso, N. Diet, Genetic Susceptibility and Human Cancer Etiology. J. Nutr. 1999, 129, 556S–559S. [Google Scholar] [CrossRef] [Green Version]
- Pozo-Guisado, E.; Alvarez-Barrientos, A.; Mulero-Navarro, S.; Santiago-Josefat, B.; Fernandez-Salguero, P.M. The antiproliferative activity of resveratrol results in apoptosis in MCF-7 but not in MDA-MB-231 human breast cancer cells: Cell-specific alteration of the cell cycle. Biochem. Pharmacol. 2002, 64, 1375–1386. [Google Scholar] [CrossRef]
- Da Costa, D.C.F.; Casanova, F.A.; Quarti, J.; Malheiros, M.S.; Sanches, D.; dos Santos, P.S.; Fialho, E.; Silva, J.L. Transient Transfection of a Wild-Type p53 Gene Triggers Resveratrol-Induced Apoptosis in Cancer Cells. PLoS ONE 2012, 7, 1–12. [Google Scholar]
- Da Costa, D.C.F.; Campos, N.P.C.; Santos, R.A.; Guedes-da-Silva, F.H.; Martins-Dinis, M.M.D.C.; Zanphorlin, L.; Ramos, C.; Rangel, L.P.; Silva, J.L. Resveratrol prevents p53 aggregation in vitro and in breast cancer cells. Oncotarget 2018, 9, 29112–29122. [Google Scholar] [CrossRef] [Green Version]
- Chang, L.C.; Yu, Y.L. Dietary components as epigenetic-regulating agents against cancer. Biomedicine 2016, 6, 1–8. [Google Scholar] [CrossRef]
- Alamolhodaei, N.S.; Tsatsakis, A.M.; Ramezani, M.; Hayes, A.W.; Karimi, G. Resveratrol as MDR reversion molecule in breast cancer: An overview. Food Chem. Toxicol. 2017, 103, 223–232. [Google Scholar] [CrossRef] [Green Version]
- Singh, C.K.; George, J.; Ahmad, N. Resveratrol-based combinatorial strategies for cancer management. Ann. N. Y. Acad. Sci. 2014, 1290, 113–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chow, H.H.S.; Garland, L.L.; Heckman-Stoddard, B.M.; Hsu, C.H.; Butler, V.D.; Cordova, C.A.; Chew, W.M.; Cornelison, T.L. A pilot clinical study of resveratrol in postmenopausal women with high body mass index: Effects on systemic sex steroid hormones. J. Transl. Med. 2014, 12, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, I.; Pérez-Gregorio, R.; Soares, S.; Mateus, N.; De Freitas, V.; Santos-Buelga, C.; Feliciano, A.S. Wine flavonoids in health and disease prevention. Molecules 2017, 22, 292. [Google Scholar] [CrossRef] [PubMed]
- Arbulu, M.; Sampedro, M.C.; Gómez-Caballero, A.; Goicolea, M.A.; Barrio, R.J. Untargeted metabolomic analysis using liquid chromatography quadrupole time-of-flight mass spectrometry for non-volatile profiling of wines. Anal. Chim. Acta 2015, 858, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Alves Filho, E.G.; Silva, L.M.A.; Ribeiro, P.R.V.; de Brito, E.S.; Zocolo, G.J.; Souza-Leão, P.C.; Marques, A.T.B.; Quintela, A.L.; Larsen, F.H.; Canuto, K.M. 1 H NMR and LC-MS-based metabolomic approach for evaluation of the seasonality and viticultural practices in wines from São Francisco River Valley, a Brazilian semi-arid region. Food Chem. 2019, 289, 558–567. [Google Scholar] [CrossRef] [PubMed]
- Cassino, C.; Tsolakis, C.; Bonello, F.; Gianotti, V.; Osella, D. Wine evolution during bottle aging, studied by 1H NMR spectroscopy and multivariate statistical analysis. Food Res. Int. 2019, 116, 566–577. [Google Scholar] [CrossRef]
- Forester, S.C.; Waterhouse, A.L. Metabolites Are Key to Understanding Health Effects of Wine Polyphenolics. J. Nutr. 2009, 139, 1824S–1831S. [Google Scholar] [CrossRef] [Green Version]
- Jiménez-Girón, A.; Queipo-Ortuño, M.; Boto-Ordóñez, M.; Muñoz-Gonzáles, I.; Sánches-Patán, F.; Monagas, M.; Martín-Álvarez, P.; Murri, M.; Tinahones, J.F.; Abdrés-Lacueva, C.; et al. Comparative Study of Microbial-Derived Phenolic Metabolites in Human Feces after Intake of Gin, Red Wine, and Dealcoholized Red Wine. J. Agric. Food Chem. 2013, 61, 3909–3915. [Google Scholar] [CrossRef]
- Jiménez-Girón, A.; Ibáñez, C.; Cifuentes, A.; Simó, C.; Muñoz-González, I.; Martín-Álvarez, P.J.; Bartolomé, B.; Moreno-Arribas, M.V. Faecal metabolomic fingerprint after moderate consumption of red wine by healthy subjects. J. Proteome Res. 2015, 14, 897–905. [Google Scholar] [CrossRef] [PubMed]
- Gonthier, M.-P.; Cheynier, V.; Donovan, J.L.; Manach, C.; Morand, C.; Mila, I.; Lapierre, C.; Rémésy, C.; Scalbert, A. Microbial Aromatic Acid Metabolites Formed in the Gut Account for a Major Fraction of the Polyphenols Excreted in Urine of Rats Fed Red Wine Polyphenols. J. Nutr. 2003, 133, 461–467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al Ali, S.H.H.; Al-Qubaisi, M.; Hussein, M.Z.; Ismail, M.; Bullo, S. Hippuric acid nanocomposite enhances doxorubicin and oxaliplatin-induced cytotoxicity in MDA-MB231, MCF-7 and Caco2 cell lines. Drug Des. Devel. Ther. 2013, 7, 25. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.N.; Wang, K.Y.; Zhang, X.S.; Yang, C.; Li, X.Y. 4-Hydroxybenzoic acid (4-HBA) enhances the sensitivity of human breast cancer cells to adriamycin as a specific HDAC6 inhibitor by promoting HIPK2/p53 pathway. Biochem. Biophys. Res. Commun. 2018, 504, 812–819. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.C.; Lin, C.C.; Wu, H.C.; Tsao, S.M.; Hsu, C.K. Apoptotic effects of protocatechuic acid in human breast, lung, liver, cervix, and prostate cancer cells: Potential mechanisms of action. J. Agric. Food Chem. 2009, 57, 6468–6473. [Google Scholar] [CrossRef]
- Cartron, E.; Fouret, G.; Carbonneau, M.A.; Lauret, C.; Michel, F.; Monnier, L.; Descomps, B.; Léger, C.L. Red-wine beneficial long-term effect on lipids but not on antioxidant characteristics in plasma in a study comparing three types of wine—Description of two O-methylated derivatives of gallic acid in humans. Free Radic. Res. 2003, 37, 1021–1035. [Google Scholar] [CrossRef]
- Mennen, L.I.; Sapinho, D.; Ito, H.; Bertrais, S.; Galan, P.; Hercberg, S.; Scalbert, A. Urinary flavonoids and phenolic acids as biomarkers of intake for polyphenol-rich foods. Br. J. Nutr. 2006, 96, 191–198. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.L.; Lin, C.S.; Kao, S.H.; Chou, M.C. Gallic acid induces G1 phase arrest and apoptosis of triple-negative breast cancer cell MDA-MB-231 via p38 mitogen-activated protein kinase/p21/p27 axis. Anticancer Drugs 2017, 28, 1150–1156. [Google Scholar] [CrossRef]
- Wang, K.; Zhu, X.; Zhang, K.; Zhu, L.; Zhou, F. Investigation of Gallic Acid Induced Anticancer Effect in Human Breast Carcinoma MCF-7 Cells. J. Biochem. Mol. Toxicol. 2014, 28, 387–393. [Google Scholar] [CrossRef]
- Vitaglione, P.; Sforza, S.; Del Rio, D. Occurrence, Bioavailability, and Metabolism of Resveratrol. In Flavonoids and Related Compounds: Bioavailability and Function, 1st ed.; Spencer, J.P.E., Crozier, A., Eds.; CRC Press: Boca Raton, FL, USA, 2012; pp. 167–182. [Google Scholar]
- Henry-Vitrac, C.; Desmoulière, A.; Girard, D.; Mérillon, J.M.; Krisa, S. Transport, deglycosylation, and metabolism of trans-piceid by small intestinal epithelial cells. Eur. J. Nutr. 2006, 45, 376–382. [Google Scholar] [CrossRef]
- Bode, L.M.; Bunzel, D.; Huch, M.; Cho, G.S.; Ruhland, D.; Bunzel, M.; Bub, A.; Franz, C.M.A.P.; Kulling, S.E. In vivo and in vitro metabolism of trans-resveratrol by human gut microbiota. Am. J. Clin. Nutr. 2013, 97, 295–309. [Google Scholar] [CrossRef] [PubMed]
- Lappano, R.; Rosano, C.; Madeo, A.; Albanito, L.; Plastina, P.; Gabriele, B.; Forti, L.; Stivala, L.A.; Iacopetta, D.; Dolce, V.; et al. Structure-activity relationships of resveratrol and derivatives in breast cancer cells. Mol. Nutr. Food Res. 2009, 53, 845–858. [Google Scholar] [CrossRef] [PubMed]
- Ruotolo, R.; Calani, L.; Fietta, E.; Brighenti, F.; Crozier, A.; Meda, C.; Maggi, A.; Ottonello, S.; Del Rio, D. Anti-estrogenic activity of a human resveratrol metabolite. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 1086–1092. [Google Scholar] [CrossRef] [PubMed]
- Gakh, A.A.; Anisimova, N.Y.; Kiselevsky, M.V.; Sadovnikov, S.V.; Stankov, I.N.; Yudin, M.V.; Rufanov, K.A.; Krasavin, M.Y.; Sosnov, A.V. Dihydro-resveratrol—A potent dietary polyphenol. Bioorganic Med. Chem. Lett. 2010, 20, 6149–6151. [Google Scholar] [CrossRef]
- Giménez-Bastida, J.A.; Ávila-Gálvez, M.Á.; Espín, J.C.; González-Sarrías, A. Conjugated Physiological Resveratrol Metabolites Induce Senescence in Breast Cancer Cells: Role of p53/p21 and p16/Rb Pathways, and ABC Transporters. Mol. Nutr. Food Res. 2019, 63, 1900629. [Google Scholar] [CrossRef]
- Donovan, J.L.; Crespy, V.; Manach, C.; Morand, C.; Besson, C.; Scalbert, A.; Rémésy, C. Catechin Is Metabolized by Both the Small Intestine and Liver of Rats. J. Nutr. 2001, 131, 1753–1757. [Google Scholar] [CrossRef]
- Baba, S.; Osakabe, N.; Natsume, M.; Muto, Y.; Takizawa, T.; Terao, J. In Vivo Comparison of the Bioavailability of (+)-Catechin, (−)-Epicatechin and Their Mixture in Orally Administered Rats. J. Nutr. 2001, 131, 2885–2891. [Google Scholar] [CrossRef] [Green Version]
- Donovan, J.L.; Kasim-Karakas, S.; German, J.B.; Waterhouse, A.L. Urinary excretion of catechin metabolites by human subjects after red wine consumption. Br. J. Nutr. 2002, 87, 31–37. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.H.; Jung, E.A.; Sohng, I.S.; Han, J.A.; Kim, T.H.; Han, M.J. Intestinal bacterial metabolism of flavonoids and its relation to some biological activities. Arch. Pharm. Res. 1998, 21, 17–23. [Google Scholar] [CrossRef]
- Kim, R.K.; Suh, Y.; Yoo, K.C.; Cui, Y.H.; Hwang, E.; Kim, H.J.; Kang, J.S.; Kim, M.J.; Lee, Y.Y.; Lee, S.J. Phloroglucinol suppresses metastatic ability of breast cancer cells by inhibition of epithelial-mesenchymal cell transition. Cancer Sci. 2015, 106, 94–101. [Google Scholar] [CrossRef] [Green Version]
- Kim, R.K.; Uddin, N.; Hyun, J.W.; Kim, C.; Suh, Y.; Lee, S.J. Novel anticancer activity of phloroglucinol against breast cancer stem-like cells. Toxicol. Appl. Pharmacol. 2015, 286, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Fang, J. Bioavailability of anthocyanins. Drug Metab. Rev. 2014, 46, 508–520. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, T.; Horio, F.; Osawa, T. Absorption and metabolism of cyanidin 3-O-β-D-glucoside in rats. FEBS Lett. 1999, 449, 179–182. [Google Scholar] [CrossRef] [Green Version]
- Olivas-Aguirre, F.J.; Rodrigo-García, J.; Martínez-Ruiz, N.D.R.; Cárdenas-Robles, A.I.; Mendoza-Díaz, S.O.; Álvarez-Parrilla, E.; González-Aguilar, G.A.; De La Rosa, L.A.; Ramos-Jiménez, A.; Wall-Medrano, A. Cyanidin-3-O-glucoside: Physical-chemistry, foodomics and health effects. Molecules 2016, 21, 1264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bub, A.; Watzl, B.; Heeb, D.; Rechkemmer, G.; Briviba, K. Malvidin-3-glucoside bioavailability in humans after ingestion of red wine, dealcoholized red wine and red grape juice. Eur. J. Nutr. 2001, 40, 113–120. [Google Scholar] [CrossRef]
- Bitsch, R.; Netzel, M.; Frank, T.; Strass, G.; Bitsch, I. Bioavailability and biokinetics of anthocyanins from red grape juice and red wine. J. Biomed. Biotechnol. 2004, 2004, 293–298. [Google Scholar] [CrossRef] [Green Version]
- Marques, C.; Fernandes, I.; Norberto, S.; Sá, C.; Teixeira, D.; de Freitas, V.; Mateus, N.; Calhau, C.; Faria, A. Pharmacokinetics of blackberry anthocyanins consumed with or without ethanol: A randomized and crossover trial. Mol. Nutr. Food Res. 2016, 60, 2319–2330. [Google Scholar] [CrossRef]
- Czank, C.; Cassidy, A.; Zhang, Q.; Morrison, D.J.; Preston, T.; Kroon, P.A.; Botting, N.P.; Kay, C.D. Human metabolism and elimination of the anthocyanin, cyanidin-3-glucoside: A 13C-tracer study. Am. Clin. Nutr. 2013, 97, 995–1003. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, I.; Marques, F.; De Freitas, V.; Mateus, N. Antioxidant and antiproliferative properties of methylated metabolites of anthocyanins. Food Chem. 2013, 141, 2923–2933. [Google Scholar] [CrossRef]
- Geahlen, R.L.; Koonchanok, N.M.; McLaughlin, J.L.; Pratt, D.E. Inhibition of protein-tyrosine kinase activity by flavanoids and related compounds. J. Nat. Prod. 1989, 52, 982–986. [Google Scholar] [CrossRef]
- Hollman, P.C.; de Vries, J.H.; van Leeuwen, S.D.; Mengelers, M.J.; Katan, Martijn, K.B. Absorption of dietary quercetin glycosides and quercetin healthy ileostomy volunteers. Am. J. Clin. Nutr. 1995, 62, 1276–1282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Booth, A.N.; Murray, C.W.; Jones, F.T.; Deeds, F. The Metabolic Fate of Rutin and Quercetin in the Animal Body. J. Biol. Chem. 1956, 233, 251–257. [Google Scholar]
- Gross, M.; Pfeiffer, M.; Martini, M.; Campbell, D.; Slavin, J.; Potter, J. The quantitation of metabolites of quercetin flavonols in human urine. Cancer Epidemiol. Biomarkers Prev. 1996, 5, 711–720. [Google Scholar] [PubMed]
- Yamazaki, S.; Sakakibara, H.; Takemura, H.; Yasuda, M.; Shimoi, K. Quercetin-3-O-glucronide inhibits noradrenaline binding to α2-adrenergic receptor, thus suppressing DNA damage induced by treatment with 4-hydroxyestradiol and noradrenaline in MCF-10A cells. J. Steroid Biochem. Mol. Biol. 2014, 143, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, S.; Miyoshi, N.; Kawabata, K.; Yasuda, M.; Shimoi, K. Quercetin-3-O-glucuronide inhibits noradrenaline-promoted invasion of MDA-MB-231 human breast cancer cells by blocking β2-adrenergic signaling. Arch. Biochem. Biophys. 2014, 557, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Hadi, N.I.; Jamal, Q.; Iqbal, A.; Shaikh, F.; Somroo, S.; Musharraf, S.G. Serum Metabolomic Profiles for Breast Cancer Diagnosis, Grading and Staging by Gas Chromatography-Mass Spectrometry. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Jové, M.; Collado, R.; Quiles, J.L.; Ramírez-Tortosa, M.C.; Sol, J.; Ruiz-Sanjuan, M.; Fernandez, M.; Cabrera, C.d.l.T.; Ramírez-Tortosa, C.; Granados-Principal, S.; et al. A plasma metabolomic signature discloses human breast cancer. Oncotarget 2017, 8, 19522. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Liu, Y.; Zhang, J.T. A new mechanism of drug resistance in breast cancer cells: Fatty acid synthase overexpression-mediated palmitate overproduction. Mol. Cancer Ther. 2008, 7, 263–270. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.B.; Erickson, J.W.; Fuji, R.; Ramachandran, S.; Gao, P.; Dinavahi, R.; Wilson, K.F.; Ambrosio, A.L.B.; Dias, S.M.G.; Dang, C.V.; et al. Targeting mitochondrial glutaminase activity inhibits oncogenic transformation. Cancer Cell 2010, 18, 207–219. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Li, Y.; Yang, Z.; Xu, W.; Yang, Y.; Tan, X. ROS mediated EGFR/MEK/ERK/HIF-1α Loop Regulates Glucose metabolism in pancreatic cancer. Biochem. Biophys. Res. Commun. 2018, 500, 873–878. [Google Scholar] [CrossRef]
- Cardoso, M.R.; Santos, J.C.; Ribeiro, M.L.; Talarico, M.C.R.; Viana, L.R.; Derchain, S.F.M. A metabolomic approach to predict breast cancer behavior and chemotherapy response. Int. J. Mol. Sci. 2018, 19, 617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomes, L.; Viana, L.; Silva, J.L.; Mermelstein, C.; Atella, G.; Fialho, E. Resveratrol Modifies Lipid Composition of Two Cancer Cell Lines. Biomed Res. Int. 2020, 2020, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ávila-Gálvez, M.Á.; García-Villalba, R.; Martínez-Díaz, F.; Ocaña-Castillo, B.; Monedero-Saiz, T.; Torrecillas-Sánchez, A.; Abellán, B.; González-Sarrías, A.; Espín, J.C. Metabolic Profiling of Dietary Polyphenols and Methylxanthines in Normal and Malignant Mammary Tissues from Breast Cancer Patients. Mol. Nutr. Food Res. 2019, 63, 1801239. [Google Scholar] [CrossRef] [PubMed]

| Dietary Factor/Isolated Compound | Model (Human/Animal/Cancer Cell Lines) | Sample Type | Discriminating Metabolites | Average of Metabolites (Found or Used) | Primary Reference |
|---|---|---|---|---|---|
| Red wine | Human (healthy subjects) | Plasma | catechin gallic acid 4-O-methylgallic acid 3-O-methylgallic acid caffeic acid | 0.13–1.5 µmol/L | [197] |
| Red wine resveratrol in capsules | Human (healthy subjects) | Plasma | trans-resveratrol trans-resveratrol-4′-O-glucuronide trans-resveratrol-3′-O-glucuronide resveratrol resveratrol-O-glucuronide resveratrol-O-sulfate | 0–0.03 µM 0–3.9 µM 0–2.7 µM 0.04–0.23 µM 0.56–2.90 µM 0.75–4.78 µM | [201] |
| Red wine and red grape juice | Human (male healthy subjects) | Plasma Urine | cyanidin 3-glucoside, delphinidin 3-glucoside, malvidin 3-glucoside, peonidin 3-glucoside, petunidin 3-glucoside | 0.42–48.8 ng/mL (maximum) 0.66–86.7 µg/h | [218] |
| Red wine, dealcoholized red wine and grape juice | Human (male healthy subjects) | Plasma Urine | malvidin-3-glucoside | 1.38 nM (maximum) 13.3–27.0 µg | [217] |
| Habitual diets | Human (healthy subjects) | Urine | 3,4-dihydroxyphenylacetic acid m-hydroxyphenylacetic acid homovanillic acid | 0.7 µg/mL 4.8 µg/mL 2.8 µg/mL | [225] |
| Red wine and dealcoholized red wine | Human (healthy subjects) | Urine | catechin (unmethylated conjugates) catechin (methylated conjugates) | 5.32 µmol 1.27 µmol | [210] |
| Wine | Human (healthy subjects) | Urine | gallic acid 4-O-methylgallic acid | 1.6–6.1 µmol/d | [198] |
| Red wine and dealcoholized red wine | Human (healthy adults) | Human feces | 3,5-dihydroxybenzoic acid, protocatechuic acid, 3-O-methylgallic acid, vanillic acid, syringic acid, p-coumaric acid, phenylpropionic acid, 4-hydroxy-5-(phenyl)valeric acid, 2-hydroxyglutaric acid, 2-methylbutyric acid, 2,3-pentanedione, diethylmalonate, 2-phenethyl butyrate, 2-phenylethyl hexanoate, 5-(3′,4′-dihydroxyphenyl)gamma-valerolactone, 3-(3′-hydroxyphenyl)propionic acid, 4-hydroxy-5-(3′-hydroxyphenyl)valeric acid, benzoic acid, 4-hydroxy-5-(phenyl)valeric acid | 0.2–50 µg/g | [191] |
| Catechin | Human (healthy subjects) | Feces | 4-hydroxybenzoic acid 2,4,6-trihydroxybenzoic acid phloroglucinol 4-methoxysalicylic acid | Not determined | [211] |
| Trans-resveratrol | Human (healthy subjects) | Feces | dihydroresveratrol 3,4′-dihydroxy-trans-stilbene 3,4′-dihydroxybibenzyl (lunularin) | 0–86.9 µmol/L 0–11.1 µmol/L 0–79.8 µmol/L | [203] |
| Fried onions, quercetin rutinoside, quercetin aglycone | Human (healthy ileostomy subjects) | Ileostomy effluent urine | quercetin | 37–72 mg 73–275 µg | [223] |
| Isotopically labeled cyanidin-3-glucoside (6,8,10,3′,5′-13C5-C3G) | Human (male healthy subjects) | Serum Urine | 24 labeled metabolites were identified (cyanidin-glucuronide, methyl cyanidin-glucuronide, methyl C3G-glucuronide, protocatechuic acid (PCA), phloroglucinaldehyde, phloroglucinaldehyde, PCA-3-glucuronide, PCA-4-glucuronide, PCA-3-sulfate, PCA-4-sulfate, vanillic acid, isovanillic acid, vanillic acid-glucuronide, isovanillic acid-glucuronide, vanillic acid-sulfate, isovanillic acid-sulfate, methyl 3,4-dihydroxybenzoate, 2-hydroxy-4-methoxybenzoic acid, methyl vanillate, 3,4-dihydroxyphenylacetic acid, 4-hydroxyphenylacetic acid, caffeic acid, ferulic acid, hippuric acid) | 6.11 µmol/L (maximum) 15.69 µmol/L (maximum) | [220] |
| Red wine powder | Animal (male Wistar rats) | Urine Plasma | aromatic acids catechins hippuric acid hippuric acid | 4.7–2790 µg/d 0–8 mg/d 0.6–3 mg/d 60–110 µmol/L | [193] |
| (+)-Catechin | Animal (male Wistar rats) | Plasma | catechin glucuronide catechin glucuronide + sulfate 3′-O-methyl catechin-glucuronide 3′-O-methyl catechin-glucuronide + sulfate | 0.2–2.8 µmol/L 0.1–0.8 µmol/L 0.3–19.3 µmol/L 16.8–38.3 µmol/L | [208] |
| (+)-Catechin (−)-Epicatechin (+)-Catechin + (−)-Epicatechin | Animal (male Sprague-Dawley rats) | Plasma Urine | catechin epicatechin 3′-O-methyl-catechin 3′-O-methyl-epicatechin catechin epicatechin 3′-O-methyl-catechin 3′-O-methyl-epicatechin | 0.15–44.2 µmol.h.L−1 0–41.9 µmol.h.L−1 0–23.0 µmol.h.L−1 0.82–78.3 µmol.h.L−1 0.01–8.85 µmol.h.L−1 0.03–16.6 µmol.h.L−1 0–3.60 µmol.h.L−1 0–9.45 µmol.h.L−1 | [209] |
| Cyanidin 3-O-β-D-glucoside | Animal (male Wistar rats) | Plasma Kidney Liver | cyanidin 3-O-β-d-glucoside protocatechuic acid cyanidin 3-O-β-d-glucoside methylated cyanidin 3-O-β-d-glucoside cyanidin 3-O-β-d-glucoside methylated cyanidin 3-O-β-d-glucoside | 0–0.31 µmol/L 0–2.56 µmol/L 0–3.20 µmol/L 0–1.32 µmol/L 0 µmol/L 0–0.64 µmol/L | [215] |
| Rutin Quercetin | Animal (rabbits) | Urine | 3,4-dihydroxyphenylacetic acid m-hydroxyphenylacetic acid p-hydroxyphenylacetic acid homovanillic acid | Not determined | [224] |
| Phloroglucinol | Animal (athymic Balb/c female nude mice) | Mice | phloroglucinol | 25 mg of phloroglucinol/kg of body | [213] |
| Hippuric acid associated with doxorubicin or oxaliplatin | Cancer cell lines (MDA-MB-231, MCF-7, Caco-2) | Cells | Hippuric acid associated with doxorubicin or oxaliplatin | 0.13–20 µg/mL (IC50) | [194] |
| 4-hydroxybenzoic acid | Cancer cell lines (MCF-7, adriamycin-resistant cells MCF-7/ADM, MDA-MB-231, MDA-MB-468, 4T1) Animal (BALB/c mice) | Cells Tumor | 4-hydroxybenzoic acid | 0–20 µM 2 mg/Kg | [195] |
| Protocatechuic acid | Cancer cell lines (MCF-7, A549, HepG2, HeLa, LNCap) | Cells | protocatechuic acid | 1–8 µmol/L | [196] |
| Gallic acid | Cancer cell lines (MDA-MB-231, HS578T, MCF-7) | Cells | gallic acid | 5–400 µM | [199] |
| Resveratrol Hydrosystilbenes Dihydroresveratrol | Cancer cell lines (MCF-7, MDA-MB-231, BT-474, K-562) | Cells | resveratrol hydrosystilbenes dihydroresveratrol | 1 nM–10 µM | [206] |
| Resveratrol-3-O-sulfate | Cancer cell line (MCF-7) | Cells | resveratrol-3-O-sulfate | 500 nM–100 µM | [205] |
| Resveratrol Resveratrol-3′-O-glucuronide Resveratrol 3′-O-sulfate Resveratrol 4′-O-sulfate Dihydroresveratrol Dihydroresveratrol-3′-O-glucuronide | Cancer cell lines (MCF-7, MDA-MB-231) | Cells | resveratrol resveratrol-3′-O-glucuronide resveratrol 3′-O-sulfate resveratrol 4′-O-sulfate dihydroresveratrol dihydroresveratrol-3′-O-glucuronide | 0.4–10 µmol/L | [207] |
| Phloroglucinol | Cancer cell lines (BT549, MDA-MB-231, MCF-7, SK-BR3, BT549) | Cells | phloroglucinol | 0–100 µM | [212] |
| Delphinidin-3-glucuronide Cyanidin-3-glucuronide Petunidin-3-glucuronide | Cancer cell lines (MKN-28, Caco-2, MCF-7) | Cells | delphinidin-3-glucuronide cyanidin-3-glucuronide petunidin-3-glucuronide | 6.3–100 µM | [221] |
| Quercetin-3-O-glucuronide | Non-tumorigenic cell line (MCF-10A) and cancer cell line (MDA-MB-231) | Cells | quercetin-3-O-glucuronide | 0.01–µM | [226] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferraz da Costa, D.C.; Pereira Rangel, L.; Quarti, J.; Santos, R.A.; Silva, J.L.; Fialho, E. Bioactive Compounds and Metabolites from Grapes and Red Wine in Breast Cancer Chemoprevention and Therapy. Molecules 2020, 25, 3531. https://doi.org/10.3390/molecules25153531
Ferraz da Costa DC, Pereira Rangel L, Quarti J, Santos RA, Silva JL, Fialho E. Bioactive Compounds and Metabolites from Grapes and Red Wine in Breast Cancer Chemoprevention and Therapy. Molecules. 2020; 25(15):3531. https://doi.org/10.3390/molecules25153531
Chicago/Turabian StyleFerraz da Costa, Danielly C., Luciana Pereira Rangel, Julia Quarti, Ronimara A. Santos, Jerson L. Silva, and Eliane Fialho. 2020. "Bioactive Compounds and Metabolites from Grapes and Red Wine in Breast Cancer Chemoprevention and Therapy" Molecules 25, no. 15: 3531. https://doi.org/10.3390/molecules25153531
APA StyleFerraz da Costa, D. C., Pereira Rangel, L., Quarti, J., Santos, R. A., Silva, J. L., & Fialho, E. (2020). Bioactive Compounds and Metabolites from Grapes and Red Wine in Breast Cancer Chemoprevention and Therapy. Molecules, 25(15), 3531. https://doi.org/10.3390/molecules25153531