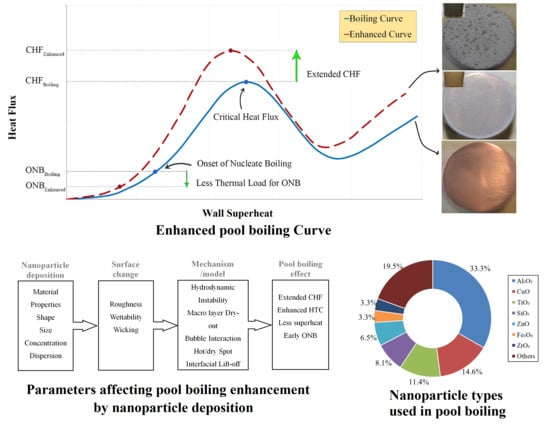
Recent Advances in the Critical Heat Flux Amelioration of Pool Boiling Surfaces Using Metal Oxide Nanoparticle Deposition
Abstract
:1. Introduction
1.1. Pool Boiling Phenomenon
1.2. CHF Importance in Energy Applications
1.3. CHF Enhancement Methods
1.3.1. CHF Enhancement by Ameliorating Fluid Properties with Nanoparticles
1.3.2. CHF Enhancement by Modifying Surface Characteristics
1.3.3. CHF Enhancement by Changing Flow Channel Structures
1.3.4. CHF Enhancement by Integration of Hybrid Approaches
1.4. Previous Review Studies on CHF Enhancement
1.5. Nanoparticle Deposition Method
1.6. Objectives of the Present Review
2. Key Factors of Metal Oxide Nanoparticle Deposition for CHF Enhancement
2.1. Nanoparticle Material
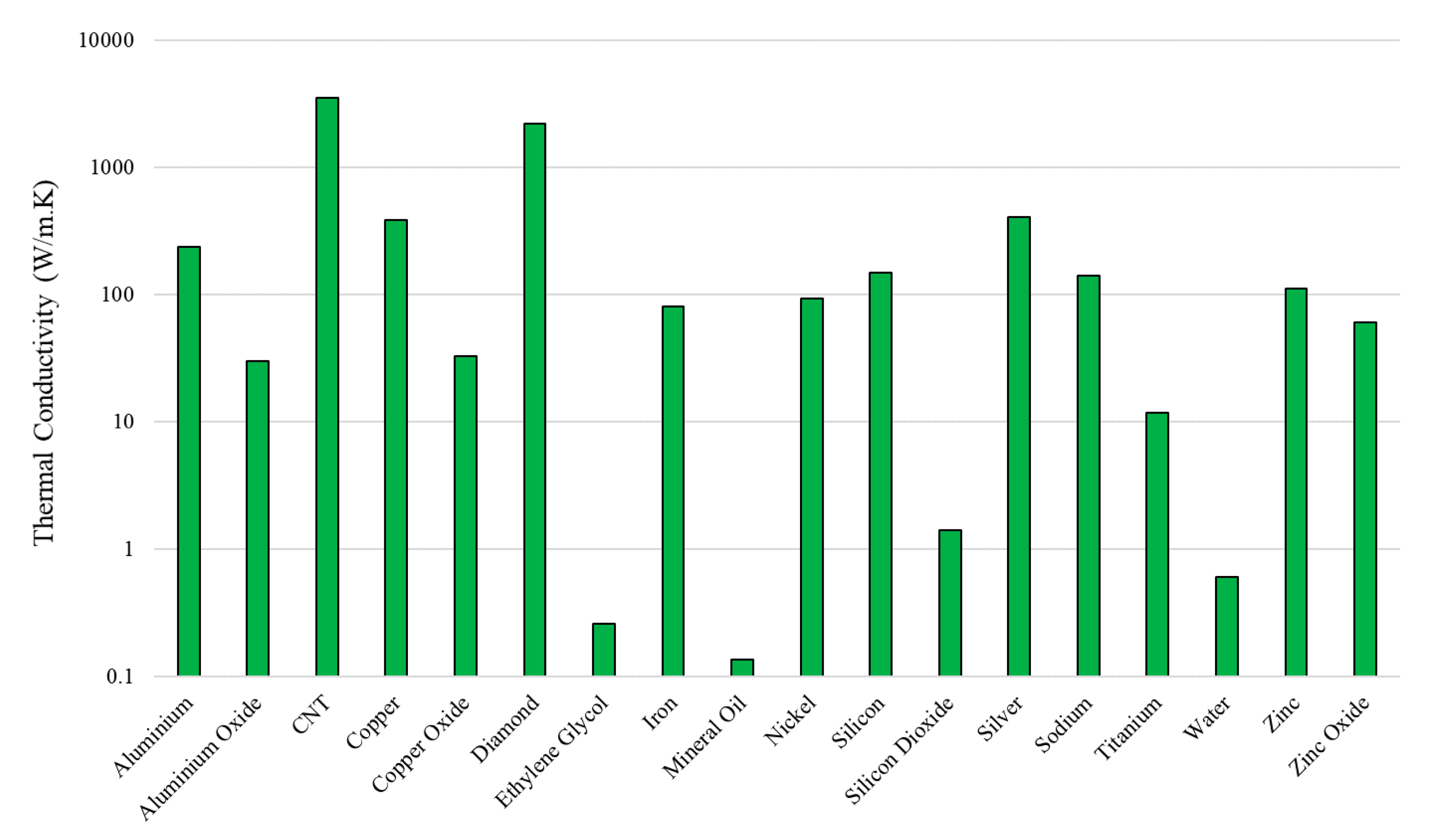
2.2. Nanoparticle Thermo-Physical Properties
- Empirical and analytical studies to establish a database for the nanofluid viscosity.
- Application of more accurate models and/or correlations for the simulation or analysis of the nanofluid heat transfer.
2.3. Nanoparticle Shape and Size
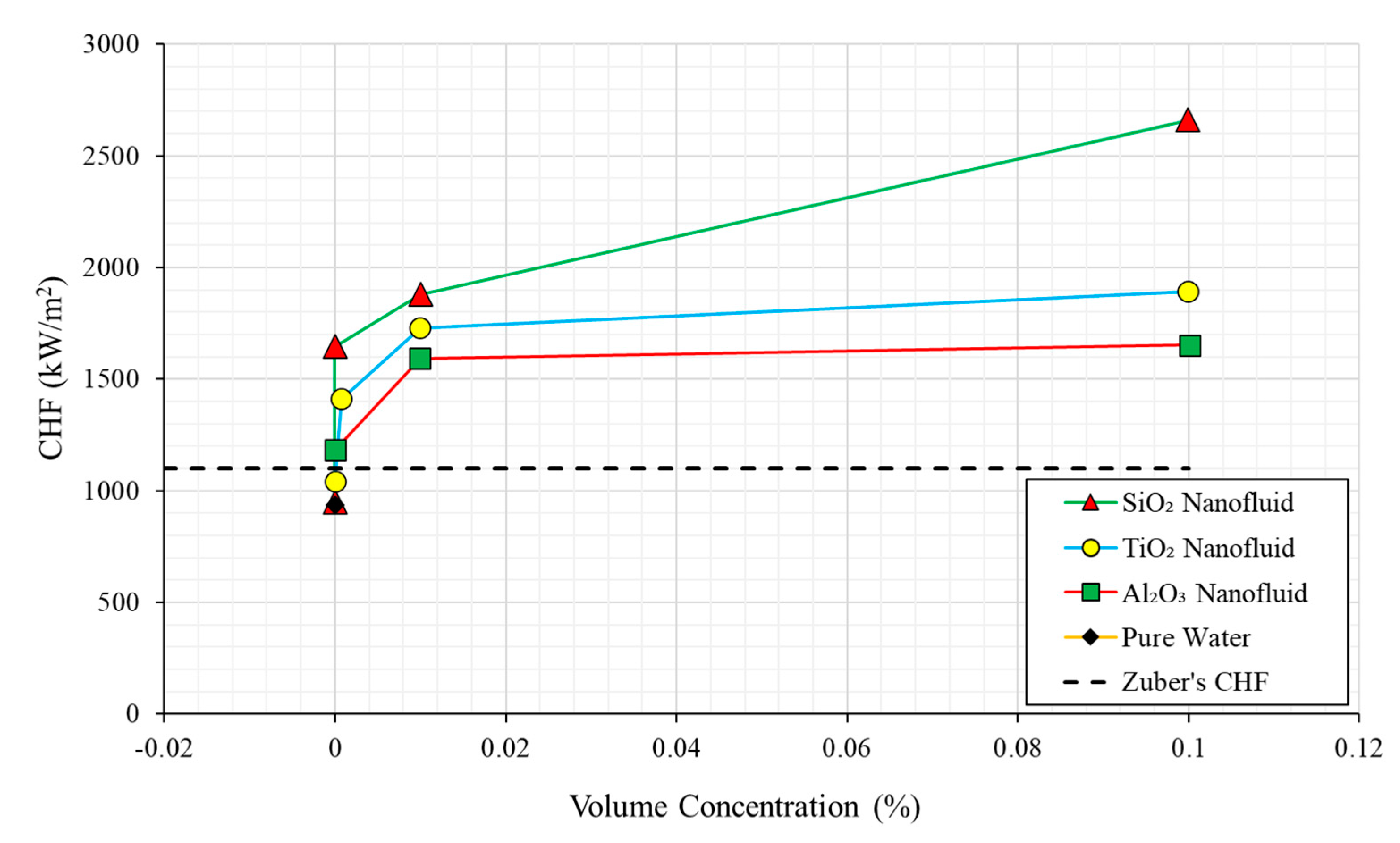
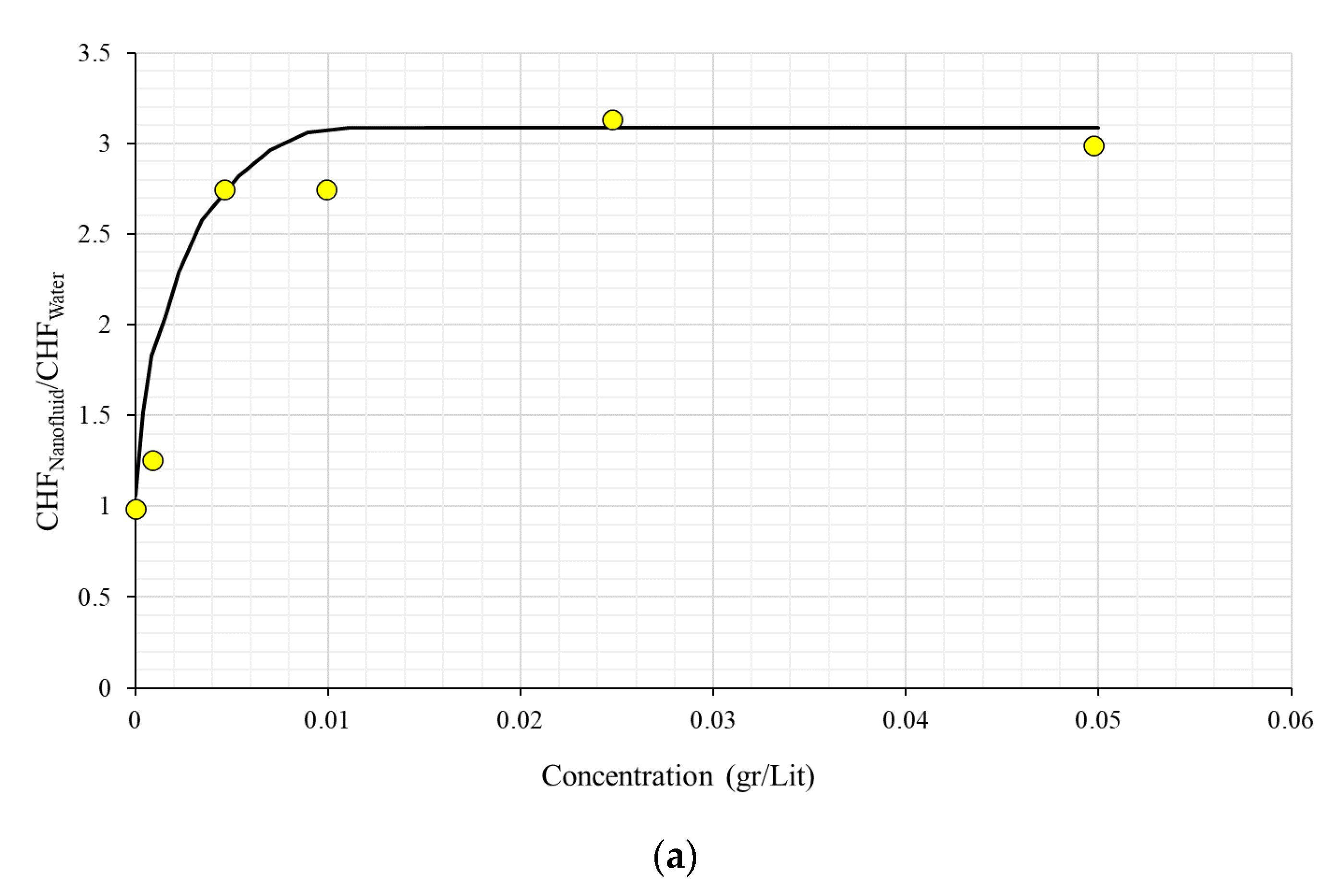
2.4. Nanoparticle Concentration
2.5. Nanoparticle Dispersion Method
- Changing the pH of the solution via the addition of acid to keep the nanoparticles away from their isoelectric point;
- The addition of surfactants and/or dispersants;
- Stabilization through the use of ultrasonic vibration or electrostatic stabilization.
3. Key Surface Factors of Deposited Nanoparticle for CHF Enhancement
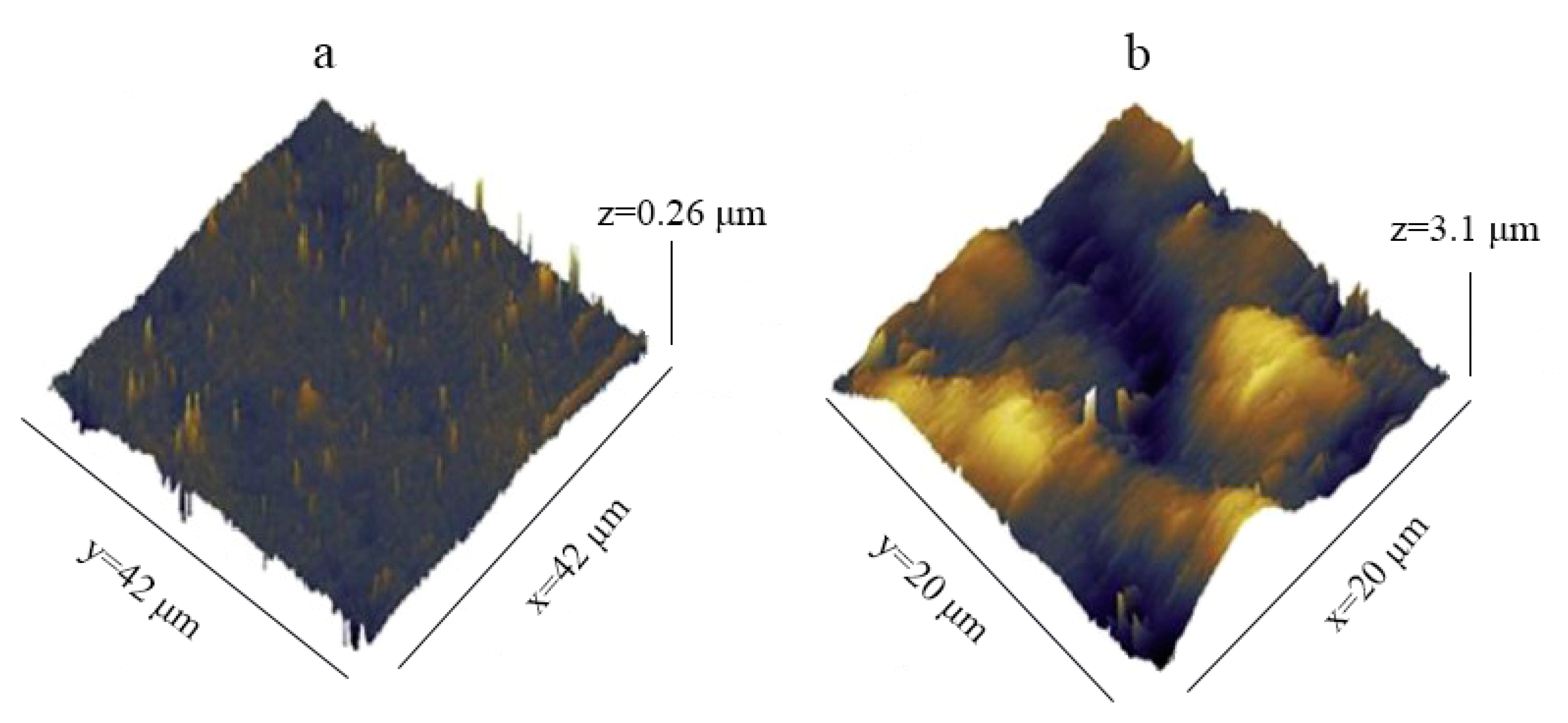
3.1. Surface Roughness
3.2. Surface Wettability
3.3. Capillary Wicking
4. Mechanisms of CHF Enhancement
4.1. Hydrodynamic Instability Model
4.2. Macrolayer Dry-Out Model
4.3. Bubble Interaction Model
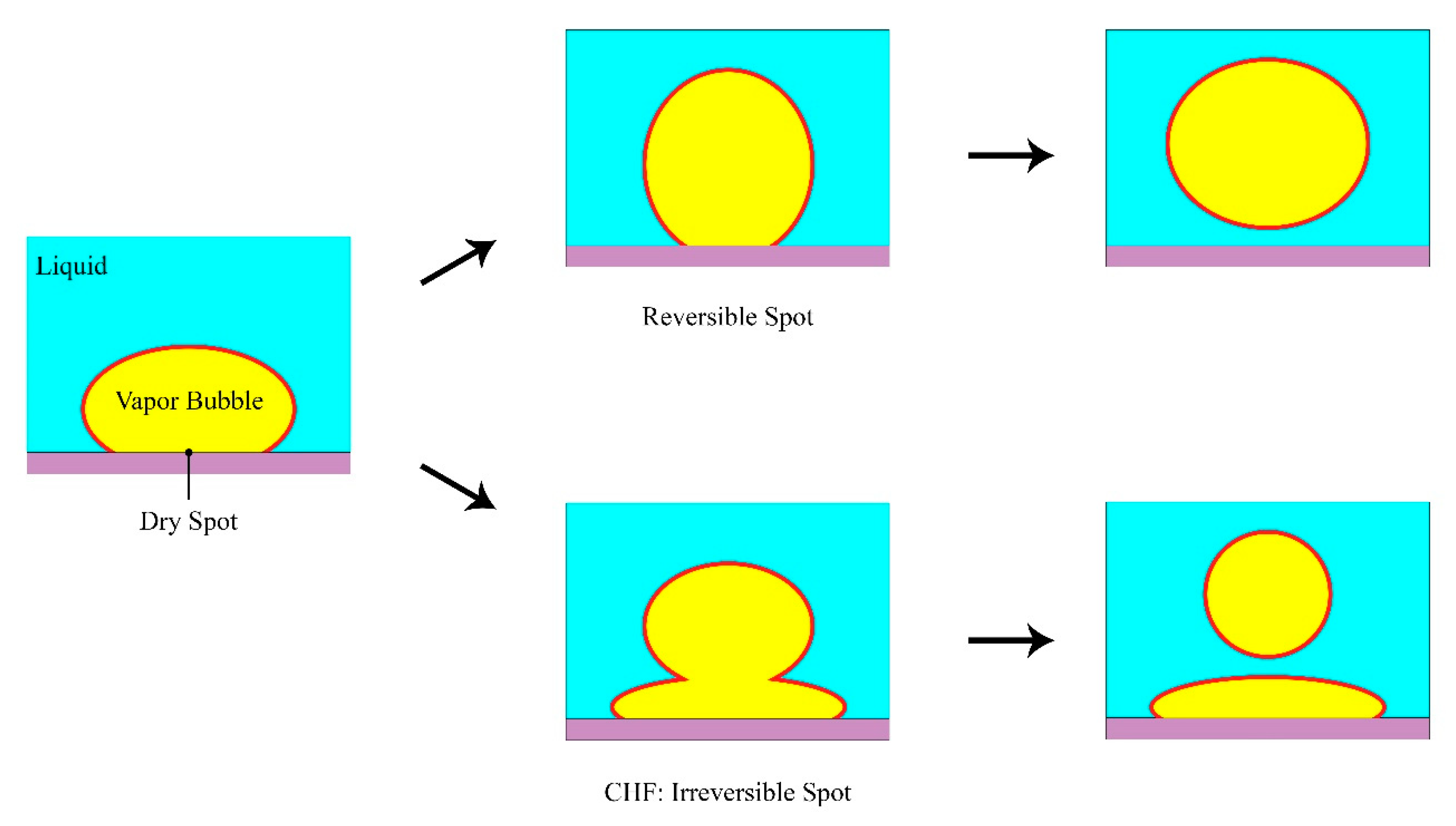
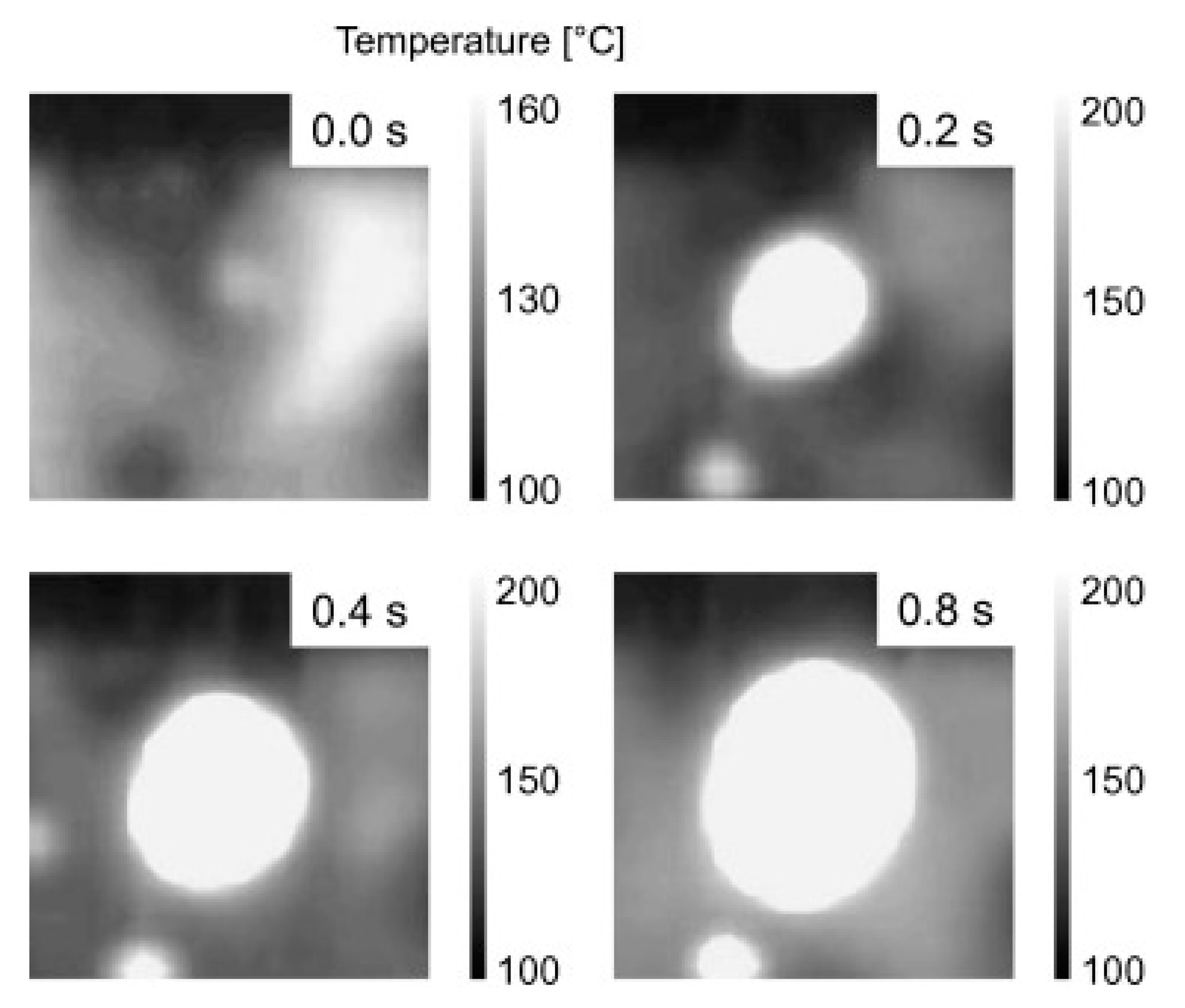
4.4. Hot/Dry Spot Model
4.4.1. Yagov Model
4.4.2. Theofanous and Dinh Formulation
4.4.3. Other Observations
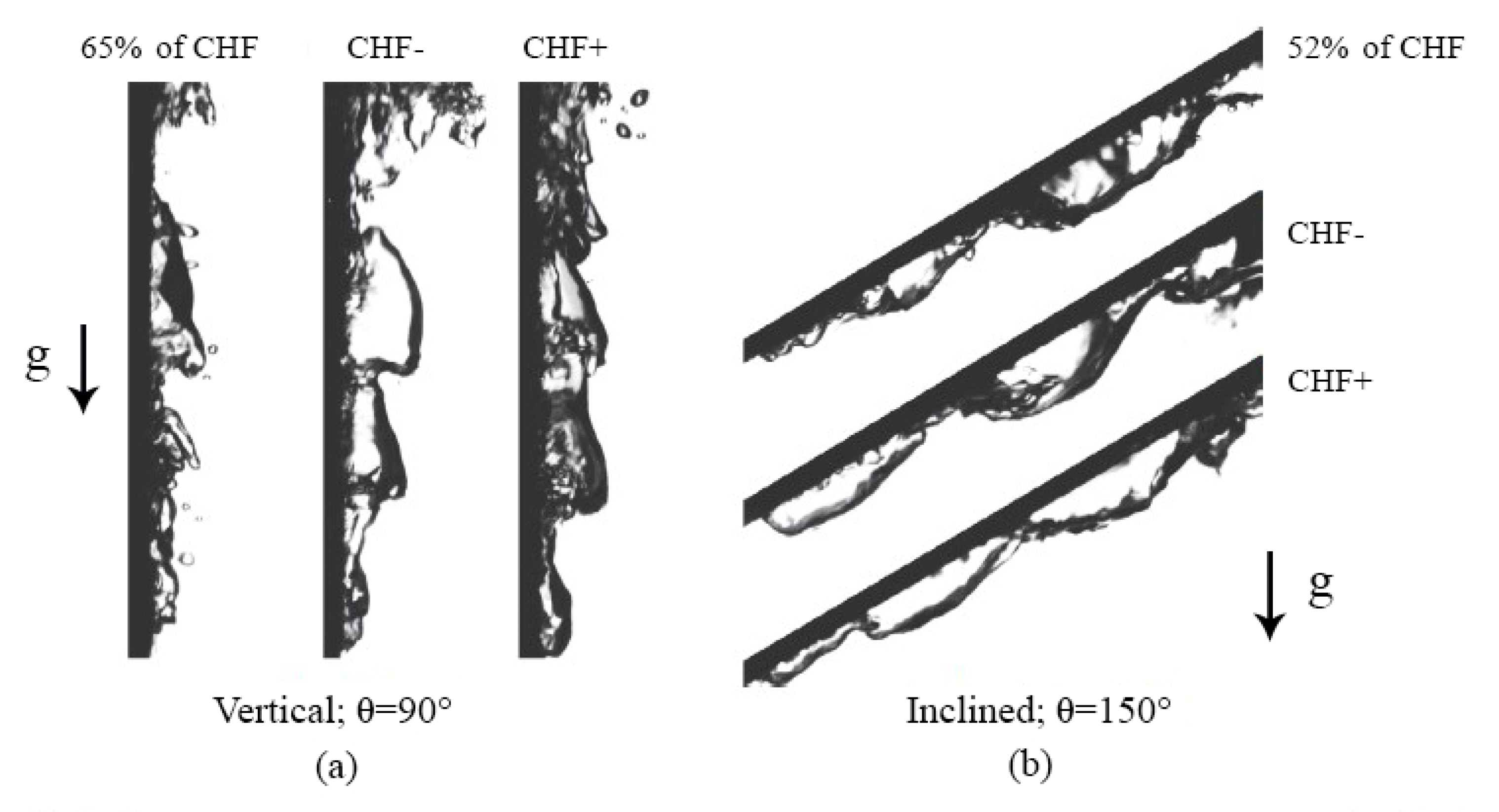
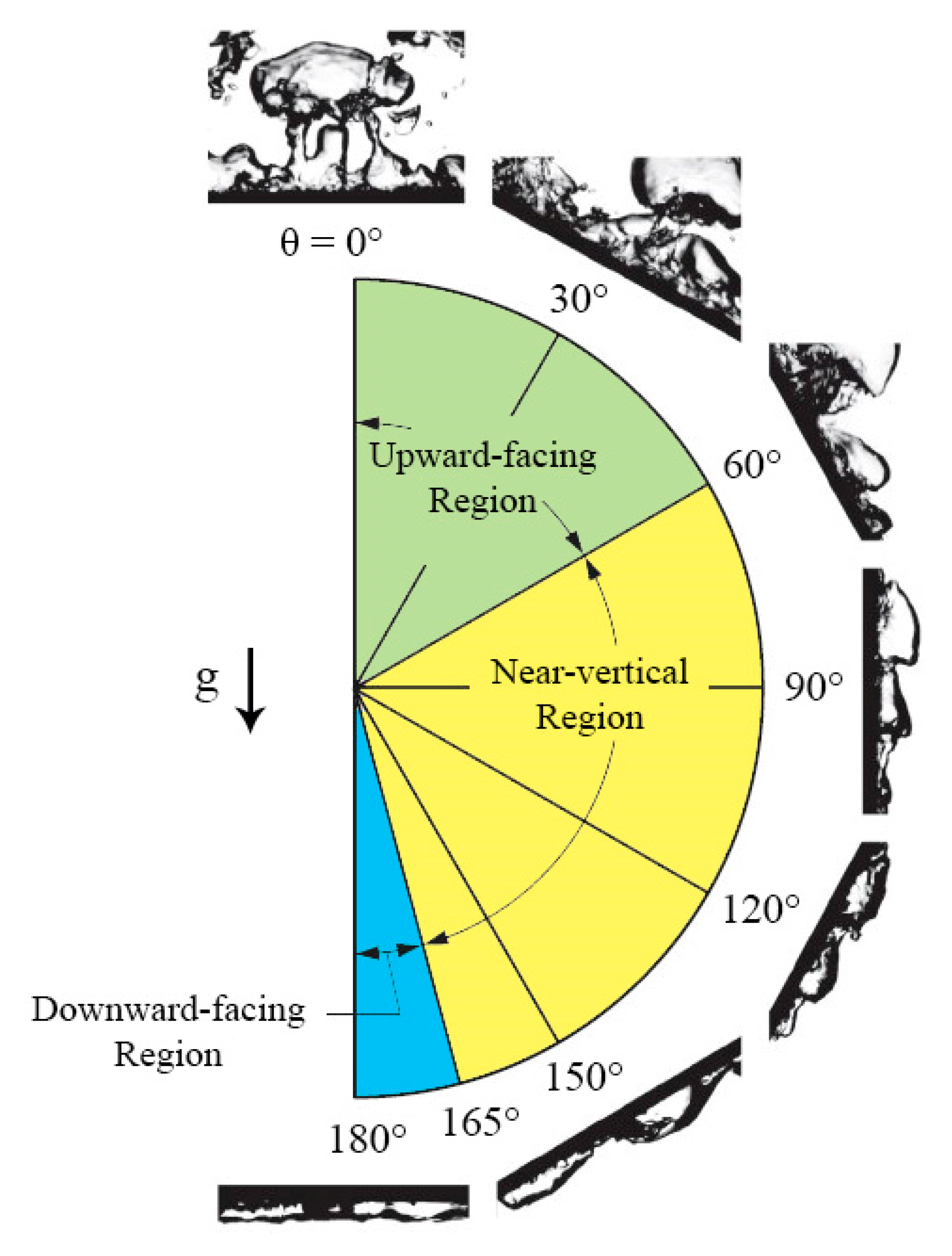
4.5. Interfacial Lift-Off Model
4.5.1. Vertical and Near-Vertical Orientations
4.5.2. Horizontal, Upward-Facing Orientation
5. Challenges and Suggestions
- (1)
- Despite the relative maturity of nanofluid-related studies, issues such as long-period stability, probable erosion and required maintenance measures as well as cleaning procedures have remained obstacles to nanofluid commercialization. In this context, studies to extend the available predictive correlations or numerical simulation tools for CHF prediction are highly encouraged. Furthermore, the next generation of nanofluids, including shell-core nanofluids, microfluidics and hybrids of more than two nanofluids, are interesting topics owing to their phase-change characteristics and optimized thermo-physical properties, which may result in higher heat transfer efficiency and outstanding stability.
- (2)
- Regarding the depositing tendency of nanofluids during boiling, texture fabrications and coating particles on the surface have been intensively explored to improve CHF. The probable micro/nanostructure detachment or failure during the boiling process needs to be further addressed experimentally. Moreover, in the case of applying nanotubes or nanowires as the coating substance, the choking issue must be avoided by the systematic optimization of their diameter and length. Besides, the complexity of the surface properties’ relationships should be addressed to further clarify the fundamental mechanisms of CHF amelioration.
- (3)
- In spite of huge improvements to surface modifications for flow boiling, a considerable gap still remains between the studies and engineering applications, particularly considering the size of system. Large engineering scales with different shapes were explored by micro-channels, making the coating process an obstacle in practical engineering scales.
- (4)
- The literature review showed that the integration of hybrid and hierarchical structures can significantly enhance the CHF. It is, however, accompanied by difficulties for separately investigating the impact of surface features on CHF amelioration. Putting the safe CHF enhancement as the major objective, the integration of advanced approaches, such as acoustic methods, magnetic fields and other creative techniques through novel preparation methods and materials are highly recommended.
- (5)
- Nanofluid stability has remained one of the major challenges. Instead of experimental studies with varying mass fractions, various approaches have been tested (i.e., pH control, surfactants and surface functionalization) to prevent from nanoparticles’ agglomeration and sedimentation, which are, albeit, at a research level. In this regard, the industrial application of nanofluids will be realized only when their long-term physical and chemical stabilities are guaranteed at the mass production level.
- (6)
- Nanoparticle size is another important factor in the heat transfer field. The use of smaller nanoparticles is recommended. Thus, cost-effective synthesis methods should be established to prepare relatively small nanoparticles.
- (7)
- The relative size effect of nanoparticles in the liquid phase requires further examination to prevent from particle clustering.
- (8)
- To the best of our knowledge, a limited number of studies have attributed the CHF amelioration to alternations in nanofluids’ thermal transport properties. Therefore, a database including the thermal transport characteristics along with the detailed specification of nanoparticle sizes and dispersion stability with/without surfactant is recommended, in which the promising nanofluids are prioritized.
- (9)
- Regarding the key role of the deposited layer on the heating surface in CHF amelioration, the thickness should be optimized to induce the maximum latency in the CHF occurrence. Moreover, the stability of the deposited layer should be experimentally checked in several replications.
- (10)
- The synergetic impact of surface wettability and capillary wicking should be addressed to mechanistically elucidate the CHF enhancement for various particle sizes and concentrations.
- (11)
- Application of nano-coats on the heating surface by the physical/chemical vapor deposition method is a promising approach compared to nanofluids. This technique, however, demands extensive investigations regarding its stability and optimal thickness to examine the delay in the CHF occurrence.
- (12)
- The bubble dynamics should be empirically and numerically examined to determine the exact share of deposited layer and nanoparticle in the CHF amelioration.
- (13)
- Regarding the significance of pressure, the irreversible growth of dry patches should be explored at various pressure levels to elucidate the CHF enhancement in nanoparticles deposited layer.
6. Concluding Remarks
- (1)
- Besides the innovative dimensional analysis-based Kutateladze’s CHF formulation, five different CHF mechanisms have been widely employed: bubble interference, hydrodynamic instability, macrolayer dry-out, hot/dry spot, and interfacial lift-off, among which the Zuber’s hydrodynamic instability theory has gained the highest popularity owing to its mechanistic formulation and theoretical attractiveness. Lately, the theoretical-based interfacial lift-off mechanism has been widely confirmed by experimental results that can deal with various surface orientations.
- (2)
- The impacts of thermo-physical properties, concentration, shape and size on CHF have been extensively addressed in heat transfer field. Accordingly, CHF rises with wall thickness enhancement but reaches an asymptotic value beyond a thickness threshold. Thus, the data corresponding to wall thicknesses beyond this threshold are highly essential. Altogether, a limited amount of data covers the entire relevant parameters of the fluids with various thermal features. A severe shortage is also felt regarding the horizontal, downward-facing surface orientations reflecting the need for more advanced strategies to plan the future studies including the micro-photographic analysis of near-wall interfacial phenomena to confirm or reject the introduced CHF mechanism.
- (3)
- Considering the complexity of the CHF phenomenon and its dependence on a diverse range of factors, the available data should be combined in a comprehensive database to assess diverse models and correlations. Such database will be expanded by the inclusion of new data to fill the vacancies regarding the relevant parameters.
- (4)
- Nanofluids can undoubtedly improve the CHF, which can be attributed to the enhanced surface wettability upon nanoparticles deposition. The data regarding the impact of nanofluids on nucleate boiling HTC are, however, contradictory, which may be due to involvement of numerous complex factors, such as the type of liquid, initial surface roughness, and heat flux in addition to nanoparticle type, size, concentration, preparation and functionalization procedure, which can substantially affect the thermo-physical characteristics of the nanofluid and surface characteristics (i.e., surface finish, active nucleation site density, wettability and changes in the triple line). Such complexities can definitely restrict the theoretical attempts to model the nanofluid boiling.
- (5)
- Bath quenching the metal portion of a nanofluid is related to the cooling rates similar to, or even weaker than, those of the base liquid. The cooling rate can be accelerated by successive quenching as it will thicken the nanoparticle layer on the surface. At elevated surface temperatures, this layer will further destabilize the vapor film, leading to the premature disruption of the film boiling pattern.
- (6)
- The nanofluid-induced nucleate boiling enhancement can be attributed to nanoparticle deposition on the surface, resulting in capillary wicking in the porous layer and enhancement in surface wettability and bubble dynamics. These impacts are competing with each other depending on the size of nanoparticles relative to the surface roughness.
- (7)
- Although the nanofluids have shown high potentials in improving the boiling performance, some practical issues should be closely considered prior to the utilization of nanofluids in practical cooling purposes, among which the clustering, sedimentation and precipitation of nanoparticles, the clogging of intricate features, the heating surface erosion of the temporal variation of cooling performance, and the cost of quality can be mentioned.
- (8)
- Despite the improving impacts of nanofluids on the thermal conductivity of the boiling fluid, the majority of their usefulness is rooted in their surface modification. Other approaches of surface modifications (such as micro/nano studs, nanotube/nanowire arrays, microporous structure, and nanoparticle pre-deposition) are capable of offering comparable or even better heat transfer enhancements with no practical problems relevant to use of nanofluid boiling.
Author Contributions
Funding
Conflicts of Interest
Nomenclature
| Channel half-spacing (m) | |
| Specific heat capacity | |
| Interfacial friction factor | |
| Gravitational acceleration | |
| Heat transfer coefficient | |
| Latent heat of evaporation | |
| Superficial velocity | |
| Channel height | |
| Rise of liquid | |
| Active nucleation site density | |
| Pressure | |
| Heat transfer | |
| Local radius of curvature of the interface | |
| Micro/nano structured surface (m) | |
| Individual gas constant | |
| Temperature | |
| Velocity | |
| Greek symbols | |
| Wavelength | |
| Mean vapor layer thickness | |
| Liquid macrolayer thickness | |
| Contact angle | |
| Thermal conductivity | |
| Dynamic viscosity | |
| Kinematic viscosity | |
| Density | |
| Liquid vapor interface tension | |
| Time (s) | |
| Concentration | |
| Subscript | |
| Base fluid | |
| Critical | |
| Departure | |
| Fluid | |
| Gas | |
| High pressure | |
| Liquid | |
| Low pressure | |
| Nano-fluid | |
| Saturation | |
| Subcooled | |
| Vapor | |
| Wall | |
| Ambient fluid | |
| Abbreviation | |
| Atomic force microscopy | |
| Critical heat flux | |
| Computer numerical control | |
| Carbon nano tube | |
| Departure from nucleate boiling | |
| Ethylene glycol | |
| Heat transfer coefficient | |
| Magnetite-water nanofluids | |
| Onset of nucleate boiling | |
| Prandtl number | |
| Polyvinyl alcohol | |
| Reduced graphene oxide | |
| Sodium lauryl benzene sulphonate | |
| Sodium lauryl sulfate | |
| Transmission electron microscopy | |
| Thermal interface material | |
| Tri-sodium phosphate | |
References
- Moghadasi, H.; Saffari, H.; Malekian, N. Experimental and semi-analytical investigation of heat transfer in nucleate pool boiling by considering surface structuring methods. Exp. Heat Transf. 2020, 1–21. [Google Scholar] [CrossRef]
- Kumar, A.; Hung, K.-S.; Wang, C.-C. Nucleate pool boiling heat transf. from high-flux tube with dielectric Fluid HFE-7200. Energies 2020, 13, 2313. [Google Scholar] [CrossRef]
- Swain, S.; Swain, A.; Kar, S.P. Influence of different surface coatings on pool boiling heat transfer enhancement: A brief review. Mater. Today Proc. 2020, 26, 1903–1907. [Google Scholar] [CrossRef]
- Mehdikhani, A.; Moghadasi, H.; Saffari, H. An exp. investigation of pool boiling augmentation using four-step electrodeposited micro/nanostructured porous surface in distilled water. Int. J. Mech. Sci. 2020, 187, 105924. [Google Scholar] [CrossRef]
- Orman, Ł.J.; Radek, N.; Pietraszek, J.; Szczepaniak, M. Analysis of enhanced pool boiling heat transfer on laser—Textured surfaces. Energies 2020, 13, 2700. [Google Scholar] [CrossRef]
- Liang, G.; Chen, Y.; Yang, H.; Li, D.; Shen, S. Nucleate boiling heat transfer and critical heat flux (CHF) from micro-pit surfaces. Int. J. Heat Mass Transf. 2020, 152, 119510. [Google Scholar] [CrossRef]
- Hernandez, R.; Folsom, C.P.; Woolstenhulme, N.E.; Jensen, C.B.; Bess, J.D.; Gorton, J.P.; Brown, N.R. Review of pool boiling critical heat flux (CHF) and heater rod design for CHF experiments in TREAT. Prog. Nucl. Energy 2020, 123, 103303. [Google Scholar] [CrossRef]
- Todreas, N.E.; Kazimi, M.S. Nuclear Systems Volume I: Thermal Hydraulic Fundamentals; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Tetreault-Friend, M.; Azizian, R.; Bucci, M.; McKrell, T.; Buongiorno, J.; Rubner, M.; Cohen, R. Critical heat flux maxima resulting from the controlled morphology of nanoporous hydrophilic surface layers. Appl. Phys. Lett. 2016, 108, 243102. [Google Scholar] [CrossRef]
- Choi, K.-Y.; Moon, S.-K.; Chun, S.-Y.; Park, J.-K.; Hwang, D.-H.; Baek, W.-P.; Park, S.-K.; Lee, C.-Y. Critical heat flux tests for an application of the three-pin fuel test loop in HANARO. Heat Transf. Eng. 2008, 29, 685–694. [Google Scholar] [CrossRef]
- Guo, Z.-Y.; Wu, X.-B. Thermal drag and critical heat flux for natural convection of air in vertical parallel plates. Heat Transf. Eng. 1993, 115, 124–129. [Google Scholar] [CrossRef]
- Wu, J.; Zhao, J. A review of nanofluid heat transfer and critical heat flux enhancement—research gap to engineering application. Prog. Nucl. Energy 2013, 66, 13–24. [Google Scholar] [CrossRef]
- Yu, W.; France, D.; Wambsganss, M.; Hull, J. Two-phase pressure drop, boiling heat transfer, and critical heat flux to water in a small-diameter horizontal tube. Int. J. Multiph. Flow 2002, 28, 927–941. [Google Scholar] [CrossRef]
- Dhillon, N.S.; Buongiorno, J.; Varanasi, K.K. Critical heat flux maxima during boiling crisis on textured surfaces. Nat. Commun. 2015, 6, 8247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, S.; Beni, M.S.; Cai, J.; Zhao, J. Review of critical-heat-flux enhancement methods. Int. Commun. Heat Mass Transf. 2018, 122, 275–289. [Google Scholar] [CrossRef]
- June Zhang, B.; Kim, K.J. Effect of liquid uptake on critical heat flux utilizing a three dimensional, interconnected alumina nano porous surfaces. Appl. Phys. Lett. 2012, 101, 054104. [Google Scholar] [CrossRef]
- Sarangi, S.; Weibel, J.A.; Garimella, S.V. Effect of particle size on surface-coating enhancement of pool boiling heat transfer. Int. J. Heat Mass Transf. 2015, 81, 103–113. [Google Scholar] [CrossRef] [Green Version]
- Ji, X.; Xu, J.; Zhao, Z.; Yang, W. Pool boiling heat transfer on uniform and non-uniform porous coating surfaces. Exp. Therm. Fluid Sci. 2013, 48, 198–212. [Google Scholar] [CrossRef]
- Ahmadi, A.A.; Khodabandeh, E.; Moghadasi, H.; Malekian, N.; Akbari, O.A.; Bahiraei, M. Numerical study of flow and heat transfer of water-Al 2 O 3 nanofluid inside a channel with an inner cylinder using Eulerian–Lagrangian approach. Therm. Anal. Calorim. 2018, 132, 651–665. [Google Scholar] [CrossRef]
- Malekian, S.; Fathi, E.; Malekian, N.; Moghadasi, H.; Moghimi, M. Analytical and numerical investigations of unsteady graphene oxide nanofluid flow between two parallel plates. Int. J. Thermophys. 2018, 39, 100. [Google Scholar] [CrossRef]
- Farhangmehr, V.; Moghadasi, H.; Asiaei, S. A nanofluid MHD flow with heat and mass transfers over a sheet by nonlinear boundary conditions: Heat and mass transfers enhancement. J. Cent. South. Univ. 2019, 26, 1205–1217. [Google Scholar] [CrossRef]
- Aminian, E.; Moghadasi, H.; Saffari, H. Magnetic field effects on forced convection flow of a hybrid nanofluid in a cylinder filled with porous media: A numerical study. J. Therm. Anal. Calorim. 2020, 1–13. [Google Scholar] [CrossRef]
- Moghadasi, H.; Aminian, E.; Saffari, H.; Mahjoorghani, M.; Emamifar, A. Numerical analysis on laminar forced convection improvement of hybrid nanofluid within a U-Bend pipe in porous media. Int. J. Mech. Sci. 2020, 179, 105659. [Google Scholar] [CrossRef]
- Takabi, B.; Gheitaghy, A.M.; Tazraei, P. Hybrid water-based suspension of Al2O3 and Cu nanoparticles on laminar convection effectiveness. J. Thermophys. Heat. Trans. 2016, 30, 523–532. [Google Scholar] [CrossRef]
- Raveshi, M.R.; Keshavarz, A.; Mojarrad, M.S.; Amiri, S. Experimental investigation of pool boiling heat transfer enhancement of alumina–water–ethylene glycol nanofluids. Exp. Therm. Fluid Sci. 2013, 44, 805–814. [Google Scholar] [CrossRef]
- Yagnem, A.R.; S, V. Heat transfer enhancement studies in pool boiling using hybrid nanofluids. Thermochim. Acta 2019, 672, 93–100. [Google Scholar] [CrossRef]
- Yang, X.; Liu, Z.-H. A kind of nanofluid consisting of surface-functionalized nanoparticles. Nanosc. Res. Lett. 2010, 5, 1324. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.-F.; Liu, Z.-H. Pool boiling heat transfer of functionalized nanofluid under sub-atmospheric pressures. Int. J. Therm. Sci. 2011, 50, 2402–2412. [Google Scholar] [CrossRef]
- Kiyomura, I.S.; Manetti, L.L.; Da Cunha, A.; Ribatski, G.; Cardoso, E.M. An analysis of the effects of nanoparticles deposition on characteristics of the heating surface and ON pool boiling of water. Int. J. Heat Mass Transf. 2017, 106, 666–674. [Google Scholar] [CrossRef] [Green Version]
- Cao, Z.; Liu, B.; Preger, C.; Wu, Z.; Zhang, Y.; Wang, X.; Messing, M.E.; Deppert, K.; Wei, J.; Sundén, B. Pool boiling heat transfer of FC-72 on pin-fin silicon surfaces with nanoparticle deposition. Int. J. Heat Mass Transf. 2018, 126, 1019–1033. [Google Scholar] [CrossRef]
- Khan, S.A.; Atieh, M.A.; Koç, M. Micro-nano scale surface coating for nucleate boiling heat transfer: A critical review. Energies 2018, 11, 3189. [Google Scholar] [CrossRef] [Green Version]
- Saffari, H.; Fathalizadeh, H.; Moghadasi, H.; Alipour, S.; Hosseinalipour, S.M. Experimental study of pool boiling enhancement for surface structuring with inclined intersected mesochannels using WEDM method on copper surfaces. Therm. Anal. Calorim. 2020, 139, 1849–1861. [Google Scholar] [CrossRef]
- Yao, Z.; Lu, Y.W.; Kandlikar, S.G. Effects of nanowire height on pool boiling performance of water on silicon chips. Int. J. Therm. Sci. 2011, 50, 2084–2090. [Google Scholar] [CrossRef]
- Shi, B.; Wang, Y.-B.; Chen, K. Pool boiling heat transfer enhancement with copper nanowire arrays. Appl. Therm. Eng. 2015, 75, 115–121. [Google Scholar] [CrossRef]
- Gheitaghy, A.M.; Saffari, H.; Ghasimi, D.; Ghasemi, A. Effect of electrolyte temperature on porous electrodeposited copper for pool boiling enhancement. Appl. Therm. Eng. 2017, 113, 1097–1106. [Google Scholar] [CrossRef]
- Gheitaghy, A.M.; Saffari, H.; Zhang, G.Q. Effect of nanostructured microporous surfaces on pool boiling augmentation. Heat Trans. Eng. 2019, 40, 762–771. [Google Scholar] [CrossRef]
- El-Genk, M.S.; Ali, A.F. Enhancement of saturation boiling of PF-5060 on microporous copper dendrite surfaces. Heat Transf. 2010, 132, 071501. [Google Scholar] [CrossRef]
- Seo, G.H.; Hwang, H.; Yoon, J.; Yeo, T.; Son, H.H.; Jeong, U.; Jeun, G.; Choi, W.; Kim, S.J. Enhanced critical heat flux with single-walled carbon nanotubes bonded on metal surfaces. Exp. Therm. Fluid Sci. 2015, 60, 138–147. [Google Scholar] [CrossRef]
- Dharmendra, M.; Suresh, S.; Sujith Kumar, C.S.; Yang, Q. Pool boiling heat transfer enhancement using vertically aligned carbon nanotube coatings on a copper substrate. Appl. Therm. Eng. 2016, 99, 61–71. [Google Scholar] [CrossRef]
- Xu, P.; Li, Q.; Xuan, Y. Enhanced boiling heat transfer on composite porous surface. Int. J. Heat Mass Transf. 2015, 80, 107–114. [Google Scholar] [CrossRef]
- Liter, S.G.; Kaviany, M. Pool-boiling CHF enhancement by modulated porous-layer coating: Theory and experiment. Int. J. Heat Mass Transf. 2001, 44, 4287–4311. [Google Scholar] [CrossRef]
- Wu, W.; Du, J.-H.; Hu, X.-J.; Wang, B.-X. Pool boiling heat transfer and simplified one-dimensional model for prediction on coated porous surfaces with vapor channels. Int. J. Heat Mass Transf. 2002, 45, 1117–1125. [Google Scholar] [CrossRef]
- Mori, S.; Okuyama, K. Enhancement of the critical heat flux in saturated pool boiling using honeycomb porous media. Int. J. Multiph. Flow 2009, 35, 946–951. [Google Scholar] [CrossRef]
- Kim, S.; Kim, H.D.; Kim, H.; Ahn, H.S.; Jo, H.; Kim, J.; Kim, M.H. Effects of nano-fluid and surfaces with nano structure on the increase of CHF. Exp. Therm. Fluid Sci. 2010, 34, 487–495. [Google Scholar] [CrossRef]
- Yao, Z.; Lu, Y.W.; Kandlikar, S.G. Pool boiling heat transf. enhancement through nanostructures on silicon microchannels. Nanotechnol. Eng. Med. 2013, 3, 031002. [Google Scholar] [CrossRef]
- Moon, H.W.; Yoon, Y.J.; Park, J.H.; Myung, B.-S.; Kim, D.E. Dynamic wetting and boiling characteristics on micro-structured and micro/nano hierarchically structured surfaces. Exp. Therm. Fluid Sci. 2016, 74, 19–26. [Google Scholar] [CrossRef]
- Jaikumar, A.; Kandlikar, S.G. Pool boiling enhancement through bubble induced convective liquid flow in feeder microchannels. Appl. Phys. Lett. 2016, 108, 041604. [Google Scholar] [CrossRef]
- Jaikumar, A.; Kandlikar, S.G. Ultra-high pool boiling performance and effect of channel width with selectively coated open microchannels. Int. J. Heat Mass Transf. 2016, 95, 795–805. [Google Scholar] [CrossRef] [Green Version]
- Rahman, M.M.; Ölçeroğlu, E.; McCarthy, M. Role of wickability on the critical heat flux of structured superhydrophilic surfaces. Langmuir 2014, 30, 11225–11234. [Google Scholar] [CrossRef]
- Kandlikar, S. Controlling bubble motion over heated surface through evaporation momentum force to enhance pool boiling heat transfer. Appl. Phys. Lett. 2013, 102, 051611. [Google Scholar] [CrossRef] [Green Version]
- Boziuk, T.R.; Smith, M.K.; Glezer, A. Enhanced boiling heat transfer on plain and featured surfaces using acoustic actuation. Int. Commun. Heat Mass Transf. 2017, 108, 181–190. [Google Scholar] [CrossRef] [Green Version]
- Miner, M.J.; Phelan, P.E.; Odom, B.A.; Ortiz, C.A. Experimental measurements of critical heat flux in expanding microchannel arrays. Heat Transf. 2013, 135, 101501. [Google Scholar] [CrossRef]
- Gheitaghy, A.M.; Samimi, A.; Saffari, H. Surface structuring with inclined minichannels for pool boiling improvement. Appl. Therm. Eng. 2017, 126, 892–902. [Google Scholar] [CrossRef]
- Gheitaghy, A.M.; Saffari, H.; Mohebbi, M. Investigation pool boiling heat transfer in U-shaped mesochannel with electrodeposited porous coating. Exp. Therm. Fluid Sci. 2016, 76, 87–97. [Google Scholar] [CrossRef]
- Bai, L.; Zhang, L.; Lin, G.; Peterson, G. Pool boiling with high heat flux enabled by a porous artery structure. Appl. Phys. Lett. 2016, 108, 233901. [Google Scholar] [CrossRef] [Green Version]
- Joshi, S.N.; Dede, E.M. Two-phase jet impingement cooling for high heat flux wide band-gap devices using multi-scale porous surfaces. Appl. Therm. Eng. 2017, 110, 10–17. [Google Scholar] [CrossRef]
- Tan, L.; Chen, C.; Dong, X.; Gong, Z.; Wang, M. Experimental study on CHF of R134a flow boiling in a horizontal helically-coiled tube near the critical pressure. Exp. Therm. Fluid Sci. 2017, 82, 472–481. [Google Scholar] [CrossRef]
- Bergles, A.; Nirmalan, V.; Junkhan, G.; Webb, R. Bibliography on Augmentation of Convective Heat and Mass Transfer-II; Iowa State University of Science and Technology: Ames, IA, USA, 1983. [Google Scholar]
- Webb, R.L.; Bergles, A.; Junkhan, G. Bibliography of US Patents on Augmentation of Convective Heat and Mass Transfer-II; Iowa State University of Science and Technology: Ames, IA, USA, 1983. [Google Scholar]
- Bhavnani, S.; Narayanan, V.; Qu, W.; Jensen, M.; Kandlikar, S.; Kim, J.; Thome, J. Boiling augmentation with micro/nanostructured surfaces: Current status and research outlook. Nanosc. Microsc. Thermophys. Eng. 2014, 18, 197–222. [Google Scholar] [CrossRef]
- Shojaeian, M.; Koşar, A. Pool boiling and flow boiling on micro-and nanostructured surfaces. Exp. Therm. Fluid Sci. 2015, 63, 45–73. [Google Scholar] [CrossRef]
- McCarthy, M.; Gerasopoulos, K.; Maroo, S.C.; Hart, A.J. Materials, fabrication, and manufacturing of micro/nanostructured surfaces for phase-change heat transfer enhancement. Nanosc. Microsc. Thermophys. Eng. 2014, 18, 288–310. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.E.; Yu, D.I.; Jerng, D.W.; Kim, M.H.; Ahn, H.S. Review of boiling heat transfer enhancement on micro/nanostructured surfaces. Exp. Therm. Fluid Sci. 2015, 66, 173–196. [Google Scholar] [CrossRef]
- Attinger, D.; Frankiewicz, C.; Betz, A.R.; Schutzius, T.M.; Ganguly, R.; Das, A.; Kim, C.-J.; Megaridis, C.M. Surface engineering for phase change heat transfer: A review. MRS Energy Sustain. 2014, 1. [Google Scholar] [CrossRef] [Green Version]
- Honda, H.; Wei, J. Enhanced boiling heat transfer from electronic components by use of surface microstructures. Exp. Therm. Fluid Sci. 2004, 28, 159–169. [Google Scholar] [CrossRef]
- Lu, Y.-W.; Kandlikar, S.G. Nanoscale surface modification techniques for pool boiling enhancement—A critical review and future directions. Heat Transf. Eng. 2011, 32, 827–842. [Google Scholar] [CrossRef]
- Patil, C.M.; Kandlikar, S.G. Review of the manufacturing techniques for porous surfaces used in enhanced pool boiling. Heat Transf. Eng. 2014, 35, 887–902. [Google Scholar] [CrossRef]
- Mori, S.; Utaka, Y. Critical heat flux enhancement by surface modification in a saturated pool boiling: A review. Int. J. Heat Mass Transf. 2017, 108, 2534–2557. [Google Scholar] [CrossRef]
- Rioux, R.P.; Nolan, E.C.; Li, C.H. A systematic study of pool boiling heat transfer on structured porous surfaces: From nanoscale through microscale to macroscale. AIP Adv. 2014, 4, 117133. [Google Scholar] [CrossRef]
- Jo, B.; Jeon, P.; Yoo, J.; Kim, H. Wide range parametric study for the pool boiling of nano-fluids with a circular plate heater. Visualization 2009, 12, 37–46. [Google Scholar] [CrossRef]
- Patil, C.M.; Kandlikar, S.G. Pool boiling enhancement through microporous coatings selectively electrodeposited on fin tops of open microchannels. Int. J. Heat Mass Transf. 2014, 79, 816–828. [Google Scholar] [CrossRef]
- Jaikumar, A.; Kandlikar, S.G. Enhanced pool boiling heat transfer mechanisms for selectively sintered open microchannels. Int. J. Heat Mass Transf. 2015, 88, 652–661. [Google Scholar] [CrossRef]
- Sarafraz, M.; Hormozi, F.; Peyghambarzadeh, S. Pool boiling heat transfer to aqueous alumina nano-fluids on the plain and concentric circular micro-structured (CCM) surfaces. Exp. Therm. Fluid Sci. 2016, 72, 125–139. [Google Scholar] [CrossRef]
- Shahmoradi, Z.; Etesami, N.; Esfahany, M.N. Pool boiling characteristics of nanofluid on flat plate based on heater surface analysis. Int. Commun. Heat Mass Transf. 2013, 47, 113–120. [Google Scholar] [CrossRef]
- Cheng, L.; Mewes, D.; Luke, A. Boiling phenomena with surfactants and polymeric additives: A state-of-the-art review. Int. J. Heat Mass Transf. 2007, 50, 2744–2771. [Google Scholar] [CrossRef]
- Wasekar, V.M.; Manglik, R.M. A review of enhanced heat transfer in nucleate pool boiling of aqueous surfactant and polymeric solutions. Enhanc. Heat Transf. 1999, 6, 135–150. [Google Scholar] [CrossRef]
- Ciloglu, D.; Bolukbasi, A. A comprehensive review on pool boiling of nanofluids. Appl. Therm. Eng. 2015, 84, 45–63. [Google Scholar] [CrossRef]
- Yu, W.; France, D.M.; Routbort, J.L.; Choi, S.U. Review and comparison of nanofluid thermal conductivity and heat transfer enhancements. Heat Transf. Eng. 2008, 29, 432–460. [Google Scholar] [CrossRef]
- Li, Y.; Tung, S.; Schneider, E.; Xi, S. A review on development of nanofluid preparation and characterization. Powder Technol. 2009, 196, 89–101. [Google Scholar] [CrossRef]
- Özerinç, S.; Kakaç, S.; Yazıcıoğlu, A.G. Enhanced thermal conductivity of nanofluids: A state-of-the-art review. Microfluid. Nanofluid. 2010, 8, 145–170. [Google Scholar] [CrossRef]
- Kleinstreuer, C.; Feng, Y. Experimental and theoretical studies of nanofluid thermal conductivity enhancement: A review. Nanosc. Res. Lett. 2011, 6, 229. [Google Scholar] [CrossRef] [Green Version]
- Ghadimi, A.; Saidur, R.; Metselaar, H. A review of nanofluid stability properties and characterization in stationary conditions. Int. J. Heat Mass Transf. 2011, 54, 405–4068. [Google Scholar] [CrossRef]
- Saidur, R.; Leong, K.; Mohammad, H.A. A review on applications and challenges of nanofluids. Renew. Sustain. Energy Rev. 2011, 15, 1646–1668. [Google Scholar] [CrossRef]
- Ramesh, G.; Prabhu, N.K. Review of thermo-physical properties, wetting and heat transfer characteristics of nanofluids and their applicability in industrial quench heat treatment. Nanosc. Res. Lett. 2011, 6, 334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, W.; Xie, H. A review on nanofluids: Preparation, stability mechanisms, and applications. J. Nanomater. 2012, 2012, 1. [Google Scholar] [CrossRef] [Green Version]
- Mahian, O.; Kianifar, A.; Kalogirou, S.A.; Pop, I.; Wongwises, S. A review of the applications of nanofluids in solar energy. Int. J. Heat Mass Transf. 2013, 57, 582–594. [Google Scholar] [CrossRef]
- Sidik, N.A.C.; Mohammed, H.; Alawi, O.A.; Samion, H.; Transfer, M. A review on preparation methods and challenges of nanofluids. Int. Commun. Heat Mass Transf. 2014, 54, 115–125. [Google Scholar] [CrossRef]
- Haddad, Z.; Abid, C.; Oztop, H.F.; Mataoui, A. A review on how the researchers prepare their nanofluids. Int. J. Therm. Sci. 2014, 76, 168–189. [Google Scholar] [CrossRef]
- Shahrul, I.; Mahbubul, I.; Khaleduzzaman, S.; Saidur, R.; Sabri, M. A comparative review on the specific heat of nanofluids for energy perspective. Renew. Sustain. Energy Rev. 2014, 38, 88–98. [Google Scholar] [CrossRef]
- Kasaeian, A.; Eshghi, A.T.; Sameti, M. A review on the applications of nanofluids in solar energy systems. Renew. Sustain. Energy Rev. 2015, 43, 584–598. [Google Scholar] [CrossRef]
- Devendiran, D.K.; Amirtham, V.A. A review on preparation, characterization, properties and applications of nanofluids. Renew. Sustain. Energy Rev. 2016, 60, 21–40. [Google Scholar] [CrossRef]
- Linge, J.M.; Erikson, H.; Kozlova, J.; Sammelselg, V.; Tammeveski, K. Oxygen reduction reaction on electrochemically deposited silver nanoparticles from non-aqueous solution. Electroanal. Chem. 2018, 810, 129–134. [Google Scholar] [CrossRef]
- Qin, L.; Huang, D.; Xu, P.; Zeng, G.; Lai, C.; Fu, Y.; Yi, H.; Li, B.; Zhang, C.; Cheng, M. In-situ deposition of gold nanoparticles onto polydopamine-decorated g-C3N4 for highly efficient reduction of nitroaromatics in environmental water purification. Colloid Interface Sci. 2019, 534, 357–369. [Google Scholar] [CrossRef]
- Cieśliński, J.T.; Krygier, K. Augmentation of the critical heat flux in Water-Al2O3, Water-TiO2 and Water-Cu Nanofluids. In Proceedings of the MATEC Web of Conferences, Chengdu, China, 6–8 September 2014; EDP Sciences: Les Ulis, France, 2014. [Google Scholar]
- Truong, B.H. Determination of pool boiling critical heat flux enhancement in nanofluids. In Proceedings of the ASME 2007 International Mechanical Engineering Congress and Exposition, Seattle, WA, USA, 11–15 November 2007; American Society of Mechanical Engineers: New York, NY, USA, 2007. [Google Scholar]
- Liu, Y.; Williams, M.G.; Miller, T.J.; Teplyakov, A.V. Nanoparticle layer deposition for highly controlled multilayer formation based on high-coverage monolayers of nanoparticles. Thin Solid Films 2016, 598, 16–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- You, S.; Kim, J.; Kim, K. Effect of nanoparticles on critical heat flux of water in pool boiling heat transfer. Appl. Phys. Lett. 2003, 83, 3374–3376. [Google Scholar] [CrossRef]
- Vassallo, P.; Kumar, R.; D’Amico, S. Pool boiling heat transfer experiments in silica–water nano-fluids. Int. J. Heat Mass Transf. 2004, 47, 407–411. [Google Scholar] [CrossRef]
- Bang, I.C.; Chang, S.H. Boiling heat transfer performance and phenomena of Al2O3–water nano-fluids from a plain surface in a pool. Int. J. Heat Mass Transf. 2005, 48, 2407–2419. [Google Scholar] [CrossRef]
- Kim, H.-D.; Kim, J.-B.; Kim, M.-H. Experimental Study on CHF Characteristics of Water-Ti02 Nano-Fluids. Nucl. Eng. Technol. 2006, 38, 61–68. [Google Scholar]
- Kim, S.; Bang, I.C.; Buongiorno, J.; Hu, L. Effects of nanoparticle deposition on surface wettability influencing boiling heat transfer in nanofluids. Appl. Phys. Lett. 2006, 89, 153107. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.J.; Bang, I.C.; Buongiorno, J.; Hu, L. Surface wettability change during pool boiling of nanofluids and its effect on critical heat flux. Int. J. Heat Mass Transf. 2007, 50, 4105–4116. [Google Scholar] [CrossRef]
- Liu, Z.-H.; Xiong, J.-G.; Bao, R. Boiling heat transfer characteristics of nanofluids in a flat heat pipe evaporator with micro-grooved heating surface. Int. J. Multiph. Flow 2007, 33, 1284–1295. [Google Scholar] [CrossRef]
- Coursey, J.S.; Kim, J. Nanofluid boiling: The effect of surface wettability. Int. J. Heat Fluid Flow 2008, 29, 1577–1585. [Google Scholar] [CrossRef]
- Golubovic, M.N.; Hettiarachchi, H.M.; Worek, W.; Minkowycz, W. Nanofluids and critical heat flux, experimental and analytical study. Appl. Therm. Eng. 2009, 29, 1281–1288. [Google Scholar] [CrossRef]
- Kwark, S.M.; Kumar, R.; Moreno, G.; Yoo, J.; You, S.M. Pool boiling characteristics of low concentration nanofluids. Int. J. Heat Mass Transf. 2010, 53, 972–981. [Google Scholar] [CrossRef]
- Huang, C.-K.; Lee, C.-W.; Wang, C.-K. Boiling enhancement by TiO2 nanoparticle deposition. Int. J. Heat Mass Transf. 2011, 54, 4895–4903. [Google Scholar] [CrossRef]
- Sheikhbahai, M.; Esfahany, M.N.; Etesami, N. Experimental investigation of pool boiling of Fe3O4/ethylene glycol–water nanofluid in electric field. Int. J. Therm. Sci. 2012, 62, 149–153. [Google Scholar] [CrossRef]
- Hegde, R.N.; Rao, S.S.; Reddy, R. Experimental studies on CHF enhancement in pool boiling with CuO-water nanofluid. Heat Mass Transf. 2012, 48, 1031–1041. [Google Scholar] [CrossRef]
- Kole, M.; Dey, T. Investigations on the pool boiling heat transfer and critical heat flux of ZnO-ethylene glycol nanofluids. Appl. Therm. Eng. 2012, 37, 112–119. [Google Scholar] [CrossRef]
- Vazquez, D.M.; Kumar, R. Surface effects of ribbon heaters on critical heat flux in nanofluid pool boiling. Int. Commun. Heat Mass Transf. 2013, 41, 1–9. [Google Scholar] [CrossRef]
- Sharma, V.I.; Buongiorno, J.; McKrell, T.J.; Hu, L.W. Experimental investigation of transient critical heat flux of water-based zinc–oxide nanofluids. Int. J. Heat Mass Transf. 2013, 61, 425–431. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Kim, E.; Kim, M.H. Effect of nanoparticle deposit layer properties on pool boiling critical heat flux of water from a thin wire. Int. J. Heat Mass Transf. 2014, 69, 164–172. [Google Scholar] [CrossRef]
- Naphon, P.; Thongjing, C. Pool boiling heat transfer characteristics of refrigerant-nanoparticle mixtures. Int. Commun. Heat Mass Transf. 2014, 52, 84–89. [Google Scholar] [CrossRef]
- Sakashita, H. Pressure effect on CHF enhancement in pool boiling of nanofluids. Nucl. Sci. Technol. 2016, 53, 797–802. [Google Scholar] [CrossRef] [Green Version]
- Sarafraz, M.; Kiani, T.; Hormozi, F. Critical heat flux and pool boiling heat transfer analysis of synthesized zirconia aqueous nano-fluids. Int. Commun. Heat Mass Transf. 2016, 70, 75–83. [Google Scholar] [CrossRef]
- Ali, H.M.; Generous, M.M.; Ahmad, F.; Irfan, M. Experimental investigation of nucleate pool boiling heat transfer enhancement of TiO2-water based nanofluids. Appl. Therm. Eng. 2017, 113, 1146–1151. [Google Scholar] [CrossRef]
- Kshirsagar, J.M.; Shrivastava, R. Experimental investigation of nucleate pool boiling characteristics of high concentrated alumina/water nanofluids. Heat Mass Transf. 2018, 54, 1779–1790. [Google Scholar] [CrossRef]
- Kangude, P.; Srivastava, A. Performance of SiO2-water nanofluids for single bubble-based nucleate pool boiling heat transfer. Int. J. Therm. Sci. 2019, 138, 612–625. [Google Scholar] [CrossRef]
- Kathiravan, R.; Kumar, R.; Gupta, A.; Chandra, R. Characterization and pool boiling heat transfer studies of nanofluids. Heat Transf. 2009, 131, 081902. [Google Scholar] [CrossRef]
- Kathiravan, R.; Kumar, R.; Gupta, A.; Chandra, R. Preparation and pool boiling characteristics of copper nanofluids over a flat plate heater. Int. J. Heat Mass Transf. 2010, 53, 1673–1681. [Google Scholar] [CrossRef]
- Zhou, D. Heat transfer enhancement of copper nanofluid with acoustic cavitation. Int. Commun. Heat Mass Transf. 2004, 47, 3109–3117. [Google Scholar] [CrossRef]
- Krishna, K.H.; Ganapathy, H.; Sateesh, G.; Das, S.K. Pool boiling characteristics of metallic nanofluids. Heat Transf. 2011, 133, 111501. [Google Scholar] [CrossRef]
- Kole, M.; Dey, T. Pool boiling heat transfer and critical heat flux enhancement of copper nanoparticles dispersed in distilled water. Nanofluids 2014, 3, 85–96. [Google Scholar] [CrossRef]
- Shi, M.; Shuai, M.; Chen, Z.; Li, Q.; Xuan, Y.-M. Study on pool boiling heat transfer of nano-particle suspensions on plate surface. Enhanc. Heat Transf. 2007, 14, 223–231. [Google Scholar] [CrossRef]
- Cieslinski, J.T.; Kaczmarczyk, T.Z. Pool boiling of water-Al 2 O 3 and water-Cu nanofluids on horizontal smooth tubes. Nanosc. Res. Lett. 2011, 6, 220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldsmith, A.; Waterman, T.E. Thermophysical Properties of Solid Materials; Armour Research Foundation: Chicago, IL, USA, 1960. [Google Scholar]
- Touloukian, Y.S. Thermophysical Properties of High Temperature Solid Materials. Volume 2. Nonferrous Alloys. Part 1. Nonferrous Binary Alloys; Thermophysical and Electronic Properties Information Analysis Center: New York, NY, USA, 1966. [Google Scholar]
- Younglove, B.A. Thermophysical properties of fluids. Argon, I., ethylene, parahydrogen, nitrogen, nitrogen trifluoride, and oxygen. J. Phys. Chem. Ref. Data 1982, 11. Available online: https://srd.nist.gov/JPCRD/jpcrdS1Vol11.pdf (accessed on 30 July 2020). [CrossRef] [Green Version]
- Das, S.K.; Putra, N.; Roetzel, W. Pool boiling characteristics of nano-fluids. Int. Commun. Heat Mass Transf. 2003, 46, 851–862. [Google Scholar] [CrossRef]
- Wen, D.; Ding, Y. Experimental investigation into the pool boiling heat transfer of aqueous based γ-alumina nanofluids. Nanopart. Res. 2005, 7, 265–274. [Google Scholar] [CrossRef]
- Soltani, S.; Etemad, S.G.; Thibault, J. Pool boiling heat transfer performance of Newtonian nanofluids. Heat Mass Transf. 2009, 45, 1555–1560. [Google Scholar] [CrossRef]
- Lee, S.; Choi, S.-S.; Li, S.; Eastman, J. Measuring thermal conductivity of fluids containing oxide nanoparticles. Heat Transf. 1999, 121, 280–289. [Google Scholar] [CrossRef]
- Kwak, K.; Kim, C. Viscosity and thermal conductivity of copper oxide nanofluid dispersed in ethylene glycol. Korea Aust. Rheol. J. 2005, 17, 35–40. [Google Scholar]
- Suganthi, K.; Rajan, K. Temperature induced changes in ZnO–water nanofluid: Zeta potential, size distribution and viscosity profiles. Int. Commun. Heat Mass Transf. 2012, 55, 7969–7980. [Google Scholar] [CrossRef]
- Kutateladze, S.S. A hydrodynamic theory of changes in a boiling process under free convection. Izv. Akad. Nauk Otd. Tekhnicheski Nauk 1951, 4, 529–536. [Google Scholar]
- Hong, T.-K.; Yang, H.-S.; Choi, C. Study of the enhanced thermal conductivity of Fe nanofluids. Appl. Phys. Lett. 2005, 97, 064311. [Google Scholar] [CrossRef]
- Quan, X.; Wang, D.; Cheng, P. An experimental investigation on wettability effects of nanoparticles in pool boiling of a nanofluid. Int. J. Heat Mass Transf. 2017, 108, 32–40. [Google Scholar] [CrossRef]
- Bourdon, B.; Rioboo, R.; Marengo, M.; Gosselin, E.; De Coninck, J. Influence of the wettability on the boiling onset. Langmuir 2012, 28, 1618–1624. [Google Scholar] [CrossRef] [PubMed]
- Bourdon, B.; Di Marco, P.; Riobóo, R.; Marengo, M.; De Coninck, J. Enhancing the onset of pool boiling by wettability modification on nanometrically smooth surfaces. Int. Commun. Heat Mass Transf. 2013, 45, 11–15. [Google Scholar] [CrossRef]
- Bourdon, B.; Bertrand, E.; Di Marco, P.; Marengo, M.; Rioboo, R.; De Coninck, J. Wettability influence on the onset temperature of pool boiling: Experimental evidence onto ultra-smooth surfaces. Adv. Colloid Interface Sci. 2015, 221, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Jo, H.; Kim, S.; Kim, H.; Kim, J.; Kim, M.H. Nucleate boiling performance on nano/microstructures with different wetting surfaces. Nanosc. Res. Lett. 2012, 7, 242. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Kim, H.; Kim, H.D.; Ahn, H.S.; Kim, M.H.; Kim, J.; Park, G.-C. Experimental investigation of critical heat flux enhancement by micro/nanoscale surface modification in pool boiling. In Proceedings of the ASME 2008 6th International Conference on Nanochannels, Microchannels, and Minichannels, Darmstadt, Germany, 23–5 June 2008; American Society of Mechanical Engineers: New York, NY, USA, 2008. [Google Scholar]
- Sun, R.-D.; Nakajima, A.; Fujishima, A.; Watanabe, T.; Hashimoto, K. Photoinduced surface wettability conversion of ZnO and TiO2 thin films. Phys. Chem. B 2001, 105, 1984–1990. [Google Scholar] [CrossRef]
- Takata, Y.; Hidaka, S.; Masuda, M.; Ito, T. Pool boiling on a superhydrophilic surface. Int. J. Energy Res. 2003, 27, 111–119. [Google Scholar] [CrossRef]
- Takata, Y.; Hidaka, S.; Cao, J.; Nakamura, T.; Yamamoto, H.; Masuda, M.; Ito, T. Effect of surface wettability on boiling and evaporation. Energy 2005, 30, 209–220. [Google Scholar] [CrossRef]
- Liaw, S.-P.; Dhir, V. Void fraction measurements during saturated pool boiling of water on partially wetted vertical surfaces. Heat Transf. 1989, 111, 731–738. [Google Scholar] [CrossRef]
- Maeng, Y.H.; Song, S.L.; Lee, J.Y. Unaffectedness of improved wettability on critical heat flux enhancement with TiO2 sputtered surface. Appl. Phys. Lett. 2016, 108, 074101. [Google Scholar] [CrossRef]
- Kim, J.M.; Yu, D.I.; Park, H.S.; Moriyama, K.; Kim, M.H. Smart surface in pool boiling: Thermally-induced wetting transition. Int. Commun. Heat Mass Transf. 2017, 109, 231–241. [Google Scholar] [CrossRef]
- Kim, J.M.; Kim, T.; Yu, D.I.; Kim, M.H.; Moriyama, K.; Park, H.S. Time effect on wetting transition of smart surface and prediction of the wetting transition for critical heat flux in pool boiling. Int. Commun. Heat Mass Transf. 2017, 114, 735–742. [Google Scholar] [CrossRef]
- Kestin, J.; Wakeham, W. A contribution to the theory of the transient hot-wire technique for thermal conductivity measurements. Phys. A Stat. Mech. Appl. 1978, 92, 102–116. [Google Scholar] [CrossRef]
- Wang, X.; Xu, X.; Choi, S.S.U. Thermal conductivity of nanoparticle-fluid mixture. Thermophys. Heat Transf. 1999, 13, 474–480. [Google Scholar] [CrossRef]
- Das, S.K.; Putra, N.; Thiesen, P.; Roetzel, W. Temperature dependence of thermal conductivity enhancement for nanofluids. Heat Transf. 2003, 125, 567–574. [Google Scholar] [CrossRef]
- Xuan, Y.; Li, Q.; Hu, W. Aggregation structure and thermal conductivity of nanofluids. AIChE 2003, 49, 1038–1043. [Google Scholar] [CrossRef]
- Xue, Q.-Z. Model for effective thermal conductivity of nanofluids. Phys. Lett. A 2003, 307, 313–317. [Google Scholar] [CrossRef]
- Xie, H.; Fujii, M.; Zhang, X. Effect of interfacial nanolayer on the effective thermal conductivity of nanoparticle-fluid mixture. Int. J. Heat Mass Transf. 2005, 48, 2926–2932. [Google Scholar] [CrossRef]
- Xue, Q.; Xu, W.-M. A model of thermal conductivity of nanofluids with interfacial shells. Mater. Chem. Phys. 2005, 90, 298–301. [Google Scholar] [CrossRef]
- Hamilton, R.L.; Crosser, O. Thermal conductivity of heterogeneous two-component systems. Ind. Eng. Chem. Fundam. 1962, 1, 187–191. [Google Scholar] [CrossRef]
- Yu, W.; Choi, S. The role of interfacial layers in the enhanced thermal conductivity of nanofluids: A renovated Maxwell model. Nanopart. Res. 2003, 5, 167–171. [Google Scholar] [CrossRef]
- Maxwell, J.C. A Treatise on Electricity and Magnetism; Clarendon Press: Oxford, UK, 1881; Volume 1. [Google Scholar]
- Kakaç, S.; Pramuanjaroenkij, A. Review of convective heat transfer enhancement with nanofluids. Int. Commun. Heat Mass Transf. 2009, 52, 3187–3196. [Google Scholar] [CrossRef]
- Kamatchi, R.; Venkatachalapathy, S. Parametric study of pool boiling heat transfer with nanofluids for the enhancement of critical heat flux: A review. Int. J. Therm. Sci. 2015, 87, 228–240. [Google Scholar] [CrossRef]
- Kathiravan, R.; Kumar, R.; Gupta, A.; Chandra, R.; Jain, P. Pool boiling characteristics of multiwalled carbon nanotube (CNT) based nanofluids over a flat plate heater. Int. Commun. Heat Mass Transf. 2011, 54, 1289–1296. [Google Scholar] [CrossRef]
- Ganapathy, H.; Sajith, V. Semi-analytical model for pool boiling of nanofluids. Int. J. Heat Mass Transf. 2013, 57, 32–47. [Google Scholar] [CrossRef]
- Ahmed, O.; Hamed, M. Experimental investigation of the effect of particle deposition on pool boiling of nanofluids. Int. J. Heat Mass Transf. 2012, 55, 3423–3436. [Google Scholar] [CrossRef]
- Ahn, H.S.; Kim, J.M.; Kaviany, M.; Kim, M.H. Pool boiling experiments in reduced graphene oxide colloids. Part I–Boiling characteristics. Int. Commun. Heat Mass Transf. 2014, 74, 501–512. [Google Scholar] [CrossRef]
- Kim, H.-D.; Kim, M.-H. Critical heat flux behavior in pool boiling of water-TiO 2 nano-fluids. In Proceedings of the KSME Conference, Taejon, Korea, 3–5 November 2004. [Google Scholar]
- Pantzali, M.; Mouza, A.; Paras, S. Investigating the efficacy of nanofluids as coolants in plate heat exchangers (PHE). Chem. Eng. Sci. 2009, 64, 3290–3300. [Google Scholar] [CrossRef]
- Hwang, Y.; Ahn, Y.; Shin, H.; Lee, C.; Kim, G.; Park, H.; Lee, J. Investigation on characteristics of thermal conductivity enhancement of nanofluids. Curr. Appl. Phys. 2006, 6, 1068–1071. [Google Scholar] [CrossRef]
- Zhou, S.-Q.; Ni, R. Measurement of the specific heat capacity of water-based Al2O3 nanofluid. Appl. Phys. Lett. 2008, 92, 093123. [Google Scholar] [CrossRef]
- Brinkman, H. The viscosity of concentrated suspensions and solutions. Chem. Phys. 1952, 20, 571. [Google Scholar] [CrossRef]
- Anoop, K.; Kabelac, S.; Sundararajan, T.; Das, S.K. Rheological and flow characteristics of nanofluids: Influence of electroviscous effects and particle agglomeration. Appl. Phys. Lett. 2009, 106, 034909. [Google Scholar] [CrossRef]
- Namburu, P.K.; Kulkarni, D.P.; Misra, D.; Das, D.K. Viscosity of copper oxide nanoparticles dispersed in ethylene glycol and water mixture. Exp. Therm. Fluid Sci. 2007, 32, 397–402. [Google Scholar] [CrossRef]
- Nguyen, C.; Desgranges, F.; Galanis, N.; Roy, G.; Maré, T.; Boucher, S.; Mintsa, H.A. Viscosity data for Al2O3–water nanofluid—hysteresis: Is heat transfer enhancement using nanofluids reliable? Int. J. Therm. Sci. 2008, 47, 103–111. [Google Scholar] [CrossRef]
- Khanafer, K.; Vafai, K. A critical synthesis of thermophysical characteristics of nanofluids. Int. J. Heat Mass Transf. 2011, 54, 4410–4428. [Google Scholar] [CrossRef]
- Chang, T.-B.; Syu, S.-C.; Yang, Y.-K. Effects of particle volume fraction on spray heat transfer performance of Al2O3–water nanofluid. Int. J. Heat Mass Transf. 2012, 55, 1014–1021. [Google Scholar] [CrossRef]
- Utomo, A.T.; Poth, H.; Robbins, P.T.; Pacek, A.W. Experimental and theoretical studies of thermal conductivity, viscosity and heat transfer coefficient of titania and alumina nanofluids. Int. J. Heat Mass Transf. 2012, 55, 7772–7781. [Google Scholar] [CrossRef]
- Vafaei, S.; Purkayastha, A.; Jain, A.; Ramanath, G.; Borca-Tasciuc, T. The effect of nanoparticles on the liquid–gas surface tension of Bi2Te3 nanofluids. Nanotechnology 2009, 20, 185702. [Google Scholar] [CrossRef]
- Kumar, R.; Milanova, D. Effect of surface tension on nanotube nanofluids. Appl. Phys. Lett. 2009, 94, 073107. [Google Scholar] [CrossRef] [Green Version]
- Jeong, Y.H.; Chang, W.J.; Chang, S.H. Wettability of heated surfaces under pool boiling using surfactant solutions and nano-fluids. Int. J. Heat Mass Transf. 2008, 51, 3025–3031. [Google Scholar] [CrossRef]
- Dixon, A.G. Wall and particle-shape effects on heat transfer in packed beds. Chem. Eng. Commun. 1988, 71, 217–237. [Google Scholar] [CrossRef]
- Lin, Y.-J.; Hwang, K.-S. Effects of powder shape and processing parameters on heat dissipation of heat pipes with sintered porous wicks. Mater. Trans. 2009, 50, 2427–2434. [Google Scholar] [CrossRef] [Green Version]
- Fakour, M.; Rahbari, A.; Moghadasi, H.; Rahimipetroudi, I.; Domairry-Ganji, D.; Varmazyar, M. Analytical study of unsteady sedimentation analysis of spherical particle in Newtonian fluid media. Therm. Sci. 2018, 22, 847–855. [Google Scholar] [CrossRef]
- Deng, D.; Tang, Y.; Shao, H.; Zeng, J.; Zhou, W.; Liang, D. Effects of structural parameters on flow boiling performance of reentrant porous microchannels. Micromech. Microeng. 2014, 24, 065025. [Google Scholar] [CrossRef]
- Akbari, E.; Gheitaghy, A.M.; Saffari, H.; Hosseinalipour, S.M. Effect of silver nanoparticle deposition in re-entrant inclined minichannel on bubble dynamics for pool boiling enhancement. Exp. Therm. Fluid Sci. 2017, 82, 390–401. [Google Scholar] [CrossRef]
- Lu, W.-Q.; Fan, Q.-M. Study for the particle’s scale effect on some thermophysical properties of nanofluids by a simplified molecular dynamics method. Eng. Anal. Bound. Elem. 2008, 32, 282–289. [Google Scholar] [CrossRef]
- Murshed, S.; Leong, K.; Yang, C. Investigations of thermal conductivity and viscosity of nanofluids. Int. J. Therm. Sci. 2008, 47, 560–568. [Google Scholar] [CrossRef]
- Peng, H.; Ding, G.; Hu, H.; Jiang, W. Effect of nanoparticle size on nucleate pool boiling heat transfer of refrigerant/oil mixture with nanoparticles. Int. J. Heat Mass Transf. 2011, 54, 1839–1850. [Google Scholar] [CrossRef] [Green Version]
- Das, S.K.; Narayan, G.P.; Baby, A.K. Survey on nucleate pool boiling of nanofluids: The effect of particle size relative to roughness. Nanopart. Res. 2008, 10, 1099–1108. [Google Scholar] [CrossRef]
- Phan, H.T.; Caney, N.; Marty, P.; Colasson, S.; Gavillet, J. Surface wettability control by nanocoating: The effects on pool boiling heat transfer and nucleation mechanism. Int. J. Heat Mass Transf. 2009, 52, 5459–5471. [Google Scholar] [CrossRef]
- Hu, Y.; Li, H.; He, Y.; Liu, Z.; Zhao, Y. Effect of nanoparticle size and concentration on boiling performance of SiO2 nanofluid. Int. J. Heat Mass Transf. 2017, 107, 820–828. [Google Scholar] [CrossRef]
- Kim, H.; Kim, M. Experimental study of the characteristics and mechanism of pool boiling CHF enhancement using nanofluids. Heat Mass Transf. 2009, 45, 991–998. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, T.; Jeong, Y.H. Experimental study on the pool boiling CHF enhancement using magnetite-water nanofluids. Int. J. Heat Mass Transf. 2012, 55, 2656–2663. [Google Scholar] [CrossRef]
- Jung, J.-Y.; Kim, E.S.; Kang, Y.T. Stabilizer effect on CHF and boiling heat transfer coefficient of alumina/water nanofluids. Int. J. Heat Mass Transf. 2012, 55, 1941–1946. [Google Scholar] [CrossRef]
- Liu, Z.-H.; Yang, X.-F.; Xiong, J.-G. Boiling characteristics of carbon nanotube suspensions under sub-atmospheric pressures. Int. J. Therm. Sci. 2010, 49, 1156–1164. [Google Scholar] [CrossRef]
- Kim, J.; Kim, K.; You, S. Pool boiling heat transfer in saturated nanofluids. In Proceedings of the ASME 2004 International Mechanical Engineering Congress and Exposition, Anaheim, CA, USA, 13–19 November 2004; American Society of Mechanical Engineers: New York, NY, USA, 2004. [Google Scholar]
- Moreno, G.; Oldenburg, S.J.; You, S.M.; Kim, J.H. Pool boiling heat transfer of alumina-water, zinc oxide-water and alumina-water+ ethylene glycol nanofluids. In Proceedings of the ASME 2005 Summer Heat Transfer Conference collocated with the ASME 2005 Pacific Rim Technical Conference and Exhibition on Integration and Packaging of MEMS, NEMS, and Electronic Systems, San Francisco, CA, USA, 17–22 July 2005; American Society of Mechanical Engineers: New York, NY, USA, 2005. [Google Scholar]
- Ham, J.; Kim, H.; Shin, Y.; Cho, H. Experimental investigation of pool boiling characteristics in Al2O3 nanofluid according to surface roughness and concentration. Int. J. Therm. Sci. 2017, 114, 86–97. [Google Scholar] [CrossRef]
- Hiswankar, S.; Kshirsagar, J. Determination of critical heat flux in pool boiling using ZnO nanofluids. Int. J. Eng. Res. Technol. 2013, 2, 2091–2095. [Google Scholar]
- Kim, H.D.; Kim, J.; Kim, M.H. Experimental studies on CHF characteristics of nanofluids at pool boiling. Int. J. Multiph. Flow 2007, 33, 691–706. [Google Scholar] [CrossRef]
- Prakash, C.J.; Prasanth, R. Enhanced boiling heat transfer by nano structured surfaces and nanofluids. Renew. Sustain. Energy Rev. 2018, 82, 4028–4043. [Google Scholar] [CrossRef]
- Sulaiman, M.Z.; Matsuo, D.; Enoki, K.; Okawa, T. Systematic measurements of heat transfer characteristics in saturated pool boiling of water-based nanofluids. Int. J. Heat Mass Transf. 2016, 102, 264–276. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Kim, J.; Kim, M.H. Effect of nanoparticles on CHF enhancement in pool boiling of nano-fluids. Int. J. Heat Mass Transf. 2006, 49, 5070–5074. [Google Scholar] [CrossRef]
- Kim, H.D.; Kim, M.H. Effect of nanoparticle deposition on capillary wicking that influences the critical heat flux in nanofluids. Appl. Phys. Lett. 2007, 91, 014104. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Ahn, H.S.; Kim, M.H. On the mechanism of pool boiling critical heat flux enhancement in nanofluids. Heat Transf. 2010, 132, 061501. [Google Scholar] [CrossRef]
- Suriyawong, A.; Wongwises, S. Nucleate pool boiling heat transfer characteristics of TiO2–water nanofluids at very low concentrations. Exp. Therm. Fluid Sci. 2010, 34, 992–999. [Google Scholar] [CrossRef]
- Chopkar, M.; Das, A.; Manna, I.; Das, P. Pool boiling heat transfer characteristics of ZrO2–water nanofluids from a flat surface in a pool. Heat Mass Transf. 2008, 44, 999–1004. [Google Scholar] [CrossRef]
- Jung, J.-Y.; Kim, E.S.; Nam, Y.; Kang, Y.T. The study on the critical heat flux and pool boiling heat transfer coefficient of binary nanofluids (H2O/LiBr+ Al2O3). Int. J. Refrig. 2013, 36, 1056–1061. [Google Scholar] [CrossRef]
- Lotfi, H.; Shafii, M. Boiling heat transfer on a high temperature silver sphere in nanofluid. Int. J. Therm. Sci. 2009, 48, 2215–2220. [Google Scholar] [CrossRef]
- Mori, S.; Suazlan, M.; Okuyama, K. The CHF enhancement of a saturated pool boiling using nano-fluids and honeycomb porous plates. In Proceedings of the Annual Meeting of the Atomic Energy Society of Japan, Kyoto University, Kyoto, Japan, 30 May 2014. [Google Scholar]
- Mori, S.; Mt Aznam, S.; Yanagisawa, R.; Okuyama, K. CHF enhancement by honeycomb porous plate in saturated pool boiling of nanofluid. Nucl. Sci. Technol. 2016, 53, 1028–1035. [Google Scholar] [CrossRef]
- Park, K.-J.; Jung, D.; Shim, S.E. Nucleate boiling heat transfer in aqueous solutions with carbon nanotubes up to critical heat fluxes. Int. J. Multiph. Flow 2009, 35, 525–532. [Google Scholar] [CrossRef]
- Truong, B.; Hu, L.-W.; Buongiorno, J.; McKrell, T. Modification of sandblasted plate heaters using nanofluids to enhance pool boiling critical heat flux. Int. J. Heat Mass Transf. 2010, 53, 85–94. [Google Scholar] [CrossRef]
- Choi, S.U. Nanofluids: From vision to reality through research. Heat Transf. 2009, 131, 033106. [Google Scholar] [CrossRef]
- Milanova, D.; Kumar, R.; Kuchibhatla, S.; Seal, S. Heat transfer behavior of oxide nanoparticles in pool boiling experiment. In Proceedings of the ASME 4th International Conference on Nanochannels, Microchannels, and Minichannels, Limerick, Ireland, 19–21 June 2006; American Society of Mechanical Engineers: New York, NY, USA, 2006. [Google Scholar]
- Milanova, D.; Kumar, R. Heat transfer behavior of silica nanoparticles in pool boiling experiment. Heat Transf. 2008, 130, 042401. [Google Scholar] [CrossRef]
- Ahmed, O.; Hamed, M.S. Effects of acidity and method of preparation on nucleate pool boiling of nanofluids. Heat Transf. Eng. 2012, 33, 1148–1155. [Google Scholar] [CrossRef]
- Wu, W.; Yang, Y. Enhanced boiling heat transfer by surfactant additives. Pool Extern. Flow Boil. 1992, 1992, 361–366. [Google Scholar]
- Jeong, Y.H.; Chang, S.H.; Baek, W.-P. Critical heat flux experiments on the reactor vessel wall using 2-D slice test section. Nucl. Technol. 2005, 152, 162–169. [Google Scholar] [CrossRef]
- Jeong, J.; Kwon, Y. Effects of ultrasonic vibration on subcooled pool boiling critical heat flux. Heat Mass Transf. 2006, 42, 1155–1161. [Google Scholar] [CrossRef]
- Giancane, G.; Ruland, A.; Sgobba, V.; Manno, D.; Serra, A.; Farinola, G.M.; Omar, O.H.; Guldi, D.M.; Valli, L. Aligning single-walled carbon nanotubes by means of langmuir–blodgett film deposition: Optical, morphological, and photo-electrochemical studies. Adv. Funct. Mater. 2010, 20, 2481–2488. [Google Scholar] [CrossRef]
- Hotze, E.M.; Phenrat, T.; Lowry, G.V. Nanoparticle aggregation: Challenges to understanding transport and reactivity in the environment. Environ. Qual. 2010, 39, 1909–1924. [Google Scholar] [CrossRef] [Green Version]
- Lambert, K.; Capek, R.K.; Bodnarchuk, M.I.; Kovalenko, M.V.; Van Thourhout, D.; Heiss, W.; Hens, Z. Langmuir–Schaefer deposition of quantum dot multilayers. Langmuir 2010, 26, 7732–7736. [Google Scholar] [CrossRef]
- Kim, H. Enhancement of critical heat flux in nucleate boiling of nanofluids: A state-of-art review. Nanosc. Res. Lett. 2011, 6, 415. [Google Scholar] [CrossRef] [Green Version]
- Kwark, S.-M.; Amaya, M.; Moon, H.-J.; You, S.-M. Effect of soluble additives, boric acid (H3BO3) and salt (NaCl), in pool boiling heat transfer. Nucl. Eng. Technol. 2011, 43, 195–204. [Google Scholar] [CrossRef] [Green Version]
- Harish, G.; Emlin, V.; Sajith, V. Effect of surface particle interactions during pool boiling of nanofluids. Int. J. Therm. Sci. 2011, 50, 2318–2327. [Google Scholar] [CrossRef]
- Wen, D.; Corr, M.; Hu, X.; Lin, G. Boiling heat transfer of nanofluids: The effect of heating surface modification. Int. J. Therm. Sci. 2011, 50, 480–485. [Google Scholar] [CrossRef]
- Jung, J.-Y.; Kim, H.; Kim, M.H. Effect of ionic additive on pool boiling critical heat flux of titania/water nanofluids. Heat Mass Transf. 2013, 49, 1–10. [Google Scholar] [CrossRef]
- Saffari, H.; Moghadasi, H.; Malekian, N.; Hosseinalipour, S.M. Modeling heat transf. in nucleate pool boiling: Influence of nucleation sites density, contact angle and prandtl number. In Proceedings of the Third International Conference on Innovation and Research in Engineering Sciences, Tbilisi, Gerorgia, 21 July 2019. [Google Scholar]
- Kolev, N.I. How accurately can we predict nucleate boiling. In Multiphase Flow Dynamics; Springer: Berlin/Heidelberg, Germany, 2007; Volume 2, pp. 439–470. [Google Scholar]
- Gheitaghy, A.M.; Saffari, H.; Arshadi, S.S.; Tabatabaei, S.S. Prediction of pool boiling angle effect on isolated bubble. Heat. Trans. Res. 2018, 49, 423–435. [Google Scholar] [CrossRef]
- Barber, J.; Brutin, D.; Tadrist, L. A review on boiling heat transfer enhancement with nanofluids. Nanosc. Res. Lett. 2011, 6, 280. [Google Scholar] [CrossRef] [Green Version]
- Park, S.D.; Won Lee, S.; Kang, S.; Bang, I.C.; Kim, J.H.; Shin, H.S.; Lee, D.W.; Won Lee, D. Effects of nanofluids containing graphene/graphene-oxide nanosheets on critical heat flux. Appl. Phys. Lett. 2010, 97, 023103. [Google Scholar] [CrossRef] [Green Version]
- Ahn, H.S.; Kim, J.M.; Park, C.; Jang, J.-W.; Lee, J.S.; Kim, H.; Kaviany, M.; Kim, M.H. A novel role of three dimensional graphene foam to prevent heater failure during boiling. Sci. Rep. 2013, 3, 1960. [Google Scholar] [CrossRef] [Green Version]
- Ahn, H.S.; Kim, J.M.; Kim, M.H. Experimental study of the effect of a reduced graphene oxide coating on critical heat flux enhancement. Int. J. Heat Mass Transf. 2013, 60, 763–771. [Google Scholar] [CrossRef]
- Zuber, N. On the stability of boiling heat transfer. Trans. Am. Soc. Mech. Engrs. 1958, 80. Available online: https://www.osti.gov/biblio/4326542 (accessed on 30 July 2020).
- Haramura, Y.; Katto, Y. A new hydrodynamic model of critical heat flux, applicable widely to both pool and forced convection boiling on submerged bodies in saturated liquids. Int. J. Heat Mass Transf. 1983, 26, 389–399. [Google Scholar] [CrossRef]
- Rohsenow, W.M.; Griffith, P. Correlation of Maximum Heat Flux Data for Boiling of Saturated Liquids; Massachusetts Institute of Technology, Division of Industrial Cooperation: Cambridge, MA, USA, 1955. [Google Scholar]
- Yagov, V.V. Is a crisis in pool boiling actually a hydrodynamic phenomenon? Int. J. Heat Mass Transf. 2014, 73, 265–273. [Google Scholar] [CrossRef]
- Theofanous, T.; Dinh, T.-N.; Tu, J.; Dinh, A. The boiling crisis phenomenon: Part II: Dryout dynamics and burnout. Exp. Therm. Fluid Sci. 2002, 26, 793–810. [Google Scholar] [CrossRef]
- Zuber, N. Hydrodynamic Aspects of Heat Transfer; UCLA: Los Angeles, CA, USA, 1959. [Google Scholar]
- Lienhard, J.; Dhir, V. Hydrodynamic prediction of peak pool-boiling heat fluxes from finite bodies. Heat Transf. 1973, 95, 152–158. [Google Scholar] [CrossRef]
- Dinh, T.; Thu, J.; Theofanous, T. Burnout in high heat flux boiling: The hydrodynamic and physico-chemical factors. In Proceedings of the 42nd AIAA Aerospace Sciences Meeting and Exhibit, Reno, Nevada, 5–8 January 2004. [Google Scholar]
- Sadasivan, P.; Chappidi, P.; Unal, C.; Nelson, R. Possible mechanisms of macrolayer formation. Int. Commun. Heat Mass Transf. 1992, 19, 801–815. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Dhir, V. Effect of surface wettability on active nucleation site density during pool boiling of water on a vertical surface. Heat Transf. 1993, 115, 659–669. [Google Scholar] [CrossRef]
- Yagov, V. A physical model and calculation formula for critical heat fluxes with nucleate pool boiling of liquids. Therm. Eng. 1988, 35, 333–339. [Google Scholar]
- Ha, S.J.; No, H.C. A dry-spot model of critical heat flux in pool and forced convection boiling. Int. J. Heat Mass Transf. 1998, 41, 303–311. [Google Scholar] [CrossRef]
- Ha, S.J.; No, H.C. A dry-spot model of critical heat flux applicable to both pool boiling and subcooled forced convection boiling. Int. J. Heat Mass Transf. 2000, 43, 241–250. [Google Scholar] [CrossRef]
- Chung, H.J.; No, H.C. A nucleate boiling limitation model for the prediction of pool boiling CHF. Int. J. Heat Mass Transf. 2007, 50, 2944–2951. [Google Scholar] [CrossRef]
- Xiao, B.; Yu, B. A fractal model for critical heat flux in pool boiling. Int. J. Therm. Sci. 2007, 46, 426–433. [Google Scholar] [CrossRef]
- Van Ouwerkerk, H. Burnout in pool boiling the stability of boiling mechanisms. Int. J. Heat Mass Transf. 1972, 15, 25–34. [Google Scholar] [CrossRef]
- Liang, G.; Mudawar, I. Pool boiling critical heat flux (CHF)–Part 1: Review of mechanisms, models, and correlations. Int. J. Heat Mass Transf. 2018, 117, 1352–1367. [Google Scholar] [CrossRef]
- Kam, D.H.; Jeong, Y.H.; Cheon, H. A heat transfer model development for CHF prediction with consideration of dry patch characteristics. Int. J. Heat Mass Transf. 2020, 148, 25–34. [Google Scholar] [CrossRef]
- Theofanous, T.; Tu, J.; Dinh, A.; Dinh, T.-N. The boiling crisis phenomenon: Part I: Nucleation and nucleate boiling heat transfer. Exp. Therm. Fluid Sci. 2002, 26, 775–792. [Google Scholar] [CrossRef]
- Jung, J.; Kim, S.J.; Kim, J. Observations of the critical heat flux process during pool boiling of FC-72. Heat Transf. 2014, 136, 041501. [Google Scholar] [CrossRef]
- Theofanous, T.G.; Dinh, T.-N. High heat flux boiling and burnout as microphysical phenomena: Mounting evidence and opportunities. Multiph. Sci. Technol. 2006, 18. [Google Scholar] [CrossRef]
- Gupta, S. Capillary action in narrow and wide tubes—A unified approach. Metrologia 2004, 41, 361. [Google Scholar] [CrossRef]
- Chu, I.-C.; No, H.C.; Song, C.-H.; Euh, D.J. Observation of critical heat flux mechanism in horizontal pool boiling of saturated water. Nucl. Eng. Des. 2014, 279, 189–199. [Google Scholar] [CrossRef]
- Choi, J.Y.; No, H.C.; Kim, J. Development of a dry patch model for critical heat flux prediction. Int. J. Heat Mass Transf. 2016, 100, 386–395. [Google Scholar] [CrossRef]
- Kim, D.E.; Song, J.; Kim, H. Simultaneous observation of dynamics and thermal evolution of irreversible dry spot at critical heat flux in pool boiling. Int. J. Heat Mass Transf. 2016, 99, 409–424. [Google Scholar] [CrossRef]
- Chu, I.-C.; No, H.C.; Song, C.-H. Visualization of boiling structure and critical heat flux phenomenon for a narrow heating surface in a horizontal pool of saturated water. Int. J. Heat Mass Transf. 2013, 62, 142–152. [Google Scholar] [CrossRef]
- Galloway, J.; Mudawar, I. CHF mechanism in flow boiling from a short heated wall—I. Examination of near-wall conditions with the aid of photomicrography and high-speed video imaging. Int. J. Heat Mass Transf. 1993, 36, 2511–2526. [Google Scholar] [CrossRef]
- Galloway, J.; Mudawar, I. CHF mechanism in flow boiling from a short heated wall—II. Theoretical CHF model. Int. J. Heat Mass Transf. 1993, 36, 2527–2540. [Google Scholar] [CrossRef]
- Mudawar, I.; Howard, A.H.; Gersey, C.O. An analytical model for near-saturated pool boiling critical heat flux on vertical surfaces. Int. J. Heat Mass Transf. 1997, 40, 2327–2339. [Google Scholar] [CrossRef]
- Howard, A.H.; Mudawar, I. Orientation effects on pool boiling critical heat flux (CHF) and modeling of CHF for near-vertical surfaces. Int. J. Heat Mass Transf. 1999, 42, 1665–1688. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, S.; Kim, J.; Noh, S.; Suh, K.; Rempe, J.; Cheung, F.; Kim, S. Visualization of boiling phenomena in inclined rectangular gap. Int. J. Multiph. Flow 2005, 31, 618–642. [Google Scholar] [CrossRef]
- Zhong, D.; Meng, J.A.; Li, Z.; Guo, Z. Critical heat flux for downward-facing saturated pool boiling on pin fin surfaces. Int. J. Heat Mass Transf. 2015, 87, 201–211. [Google Scholar] [CrossRef]
- Zhang, H.; Mudawar, I.; Hasan, M.M. Experimental assessment of the effects of body force, surface tension force, and inertia on flow boiling CHF. Int. J. Heat Mass Transf. 2002, 45, 4079–4095. [Google Scholar] [CrossRef]
- Zhang, H.; Mudawar, I.; Hasan, M.M. Flow boiling CHF in microgravity. Int. J. Heat Mass Transf. 2005, 48, 3107–3118. [Google Scholar] [CrossRef]
- Konishi, C.; Mudawar, I. Review of flow boiling and critical heat flux in microgravity. Int. J. Heat Mass Transf. 2015, 80, 469–493. [Google Scholar] [CrossRef]
- Guan, C.-K.; Klausner, J.F.; Mei, R. A new mechanistic model for pool boiling CHF on horizontal surfaces. Int. J. Heat Mass Transf. 2011, 54, 3960–3969. [Google Scholar] [CrossRef]
- Rajvanshi, A.; Saini, J.; Prakash, R. Investigation of macrolayer thickness in nucleate pool boiling at high heat flux. Int. J. Heat Mass Transf. 1992, 35, 343–350. [Google Scholar] [CrossRef]
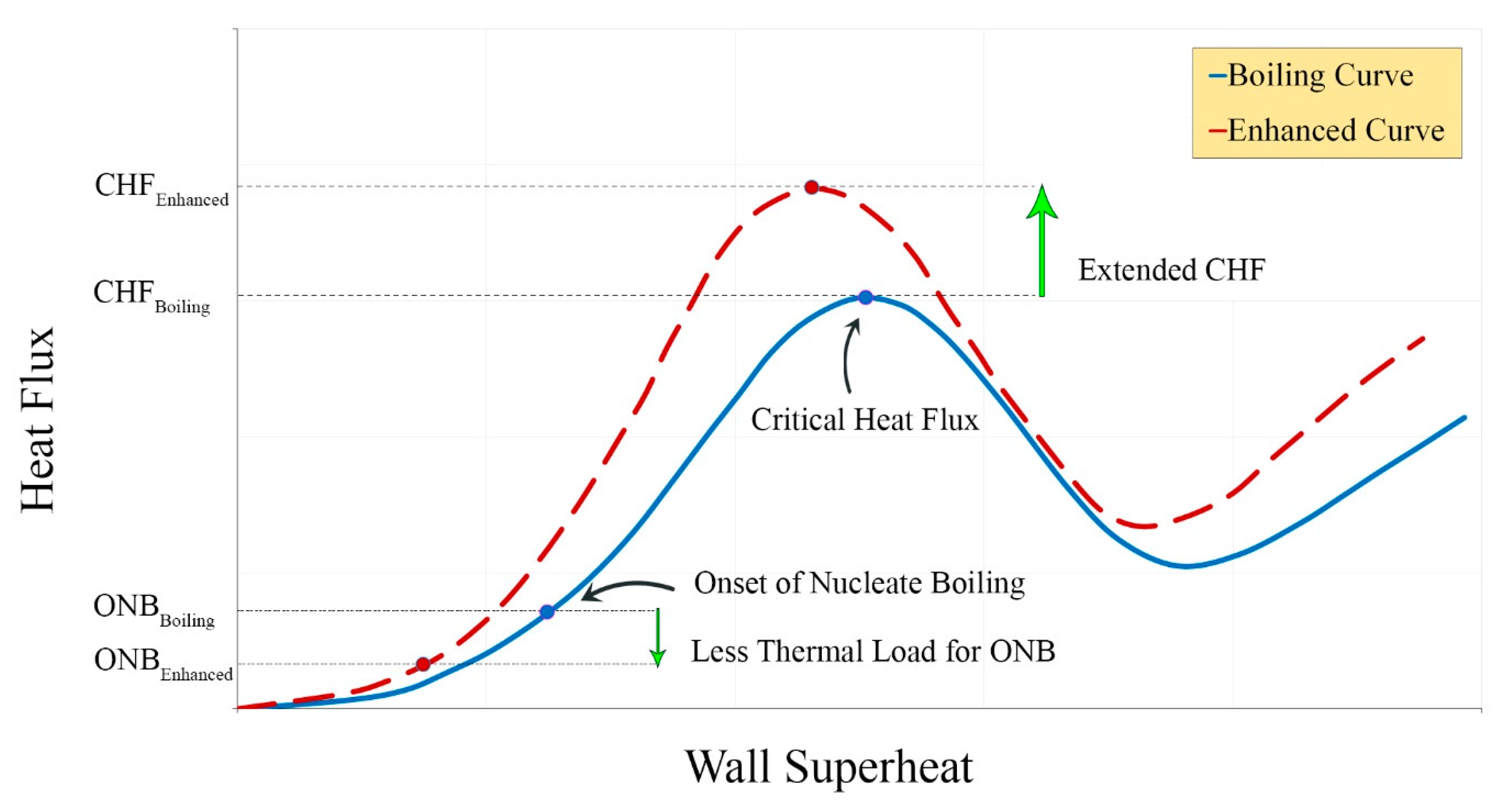
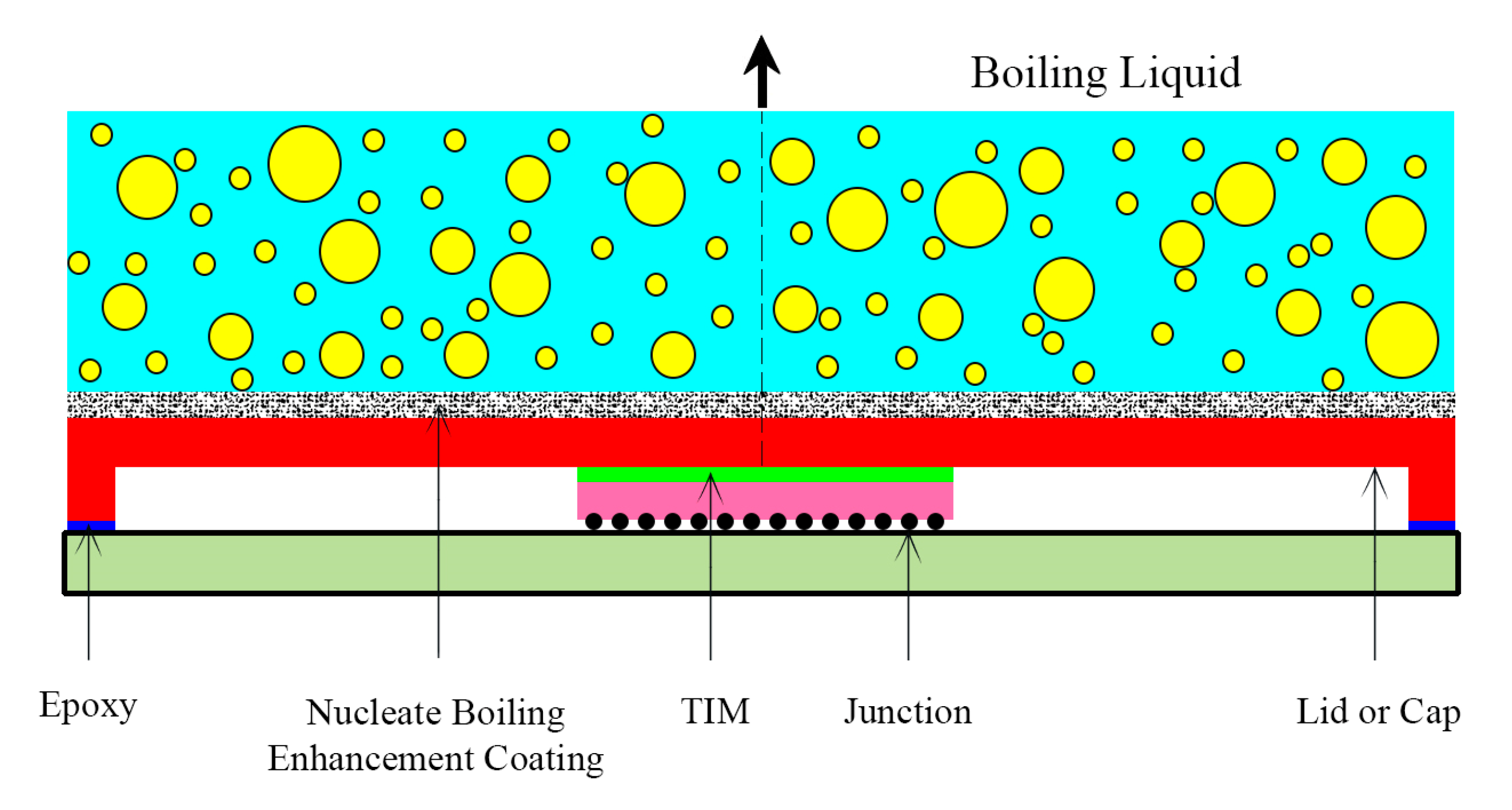

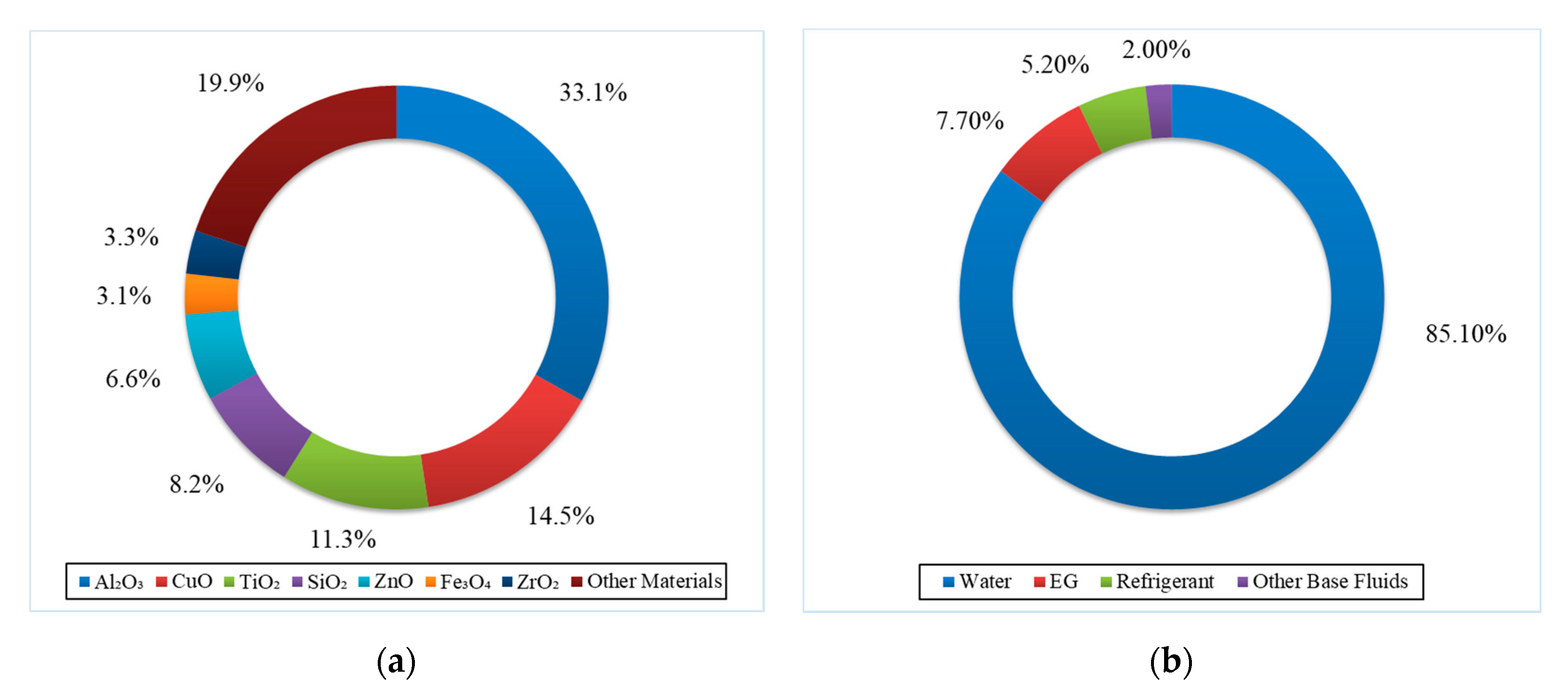



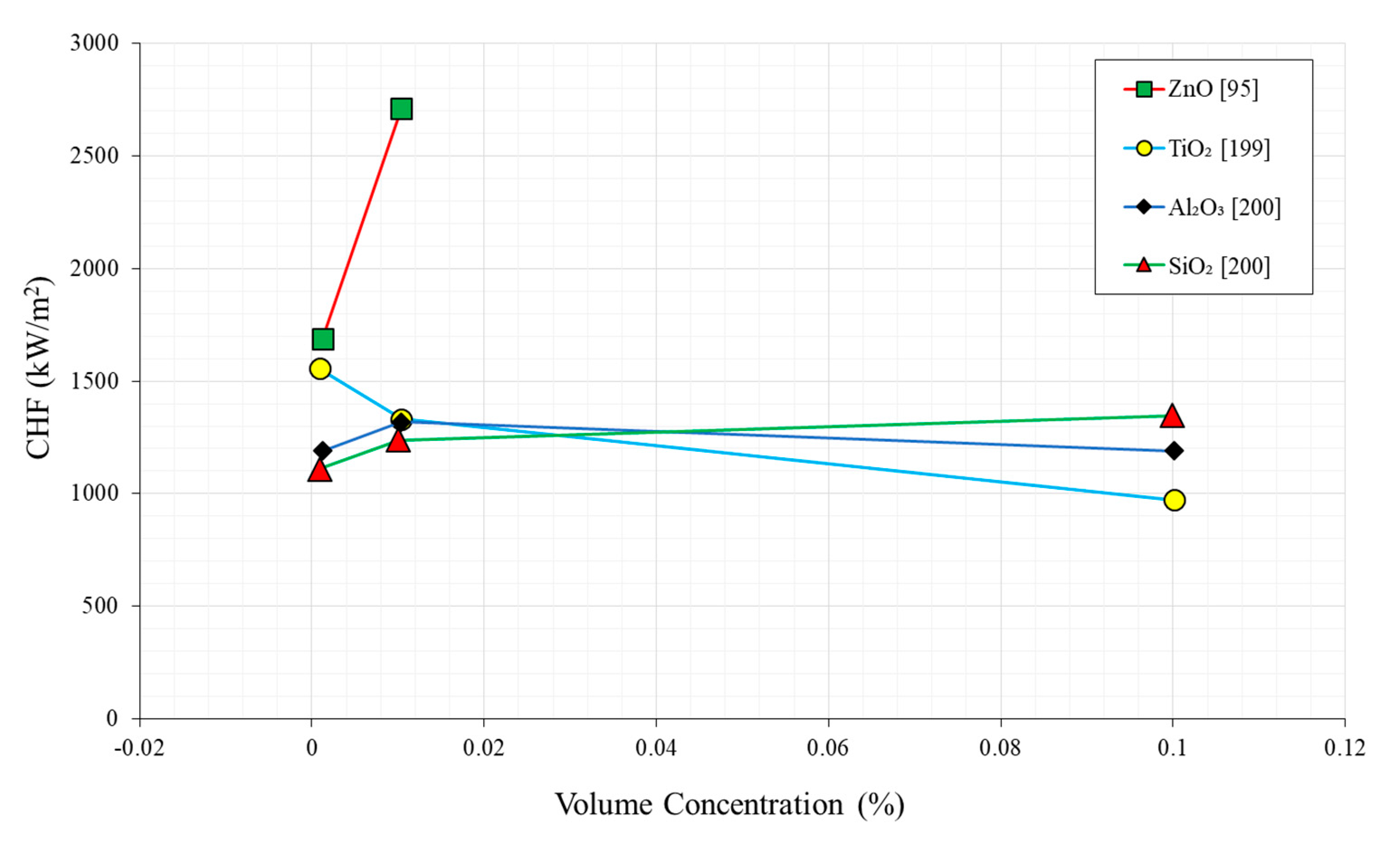
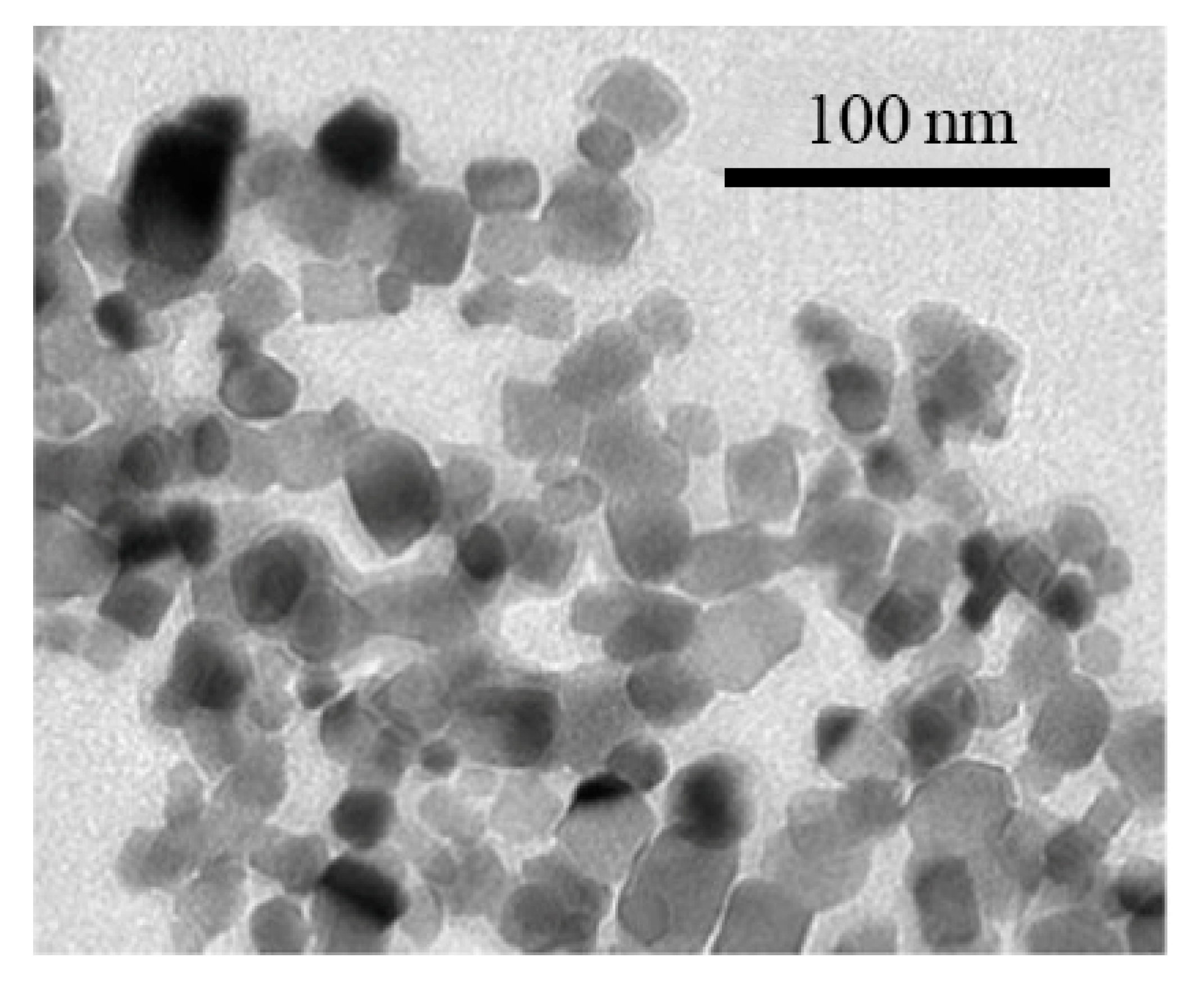
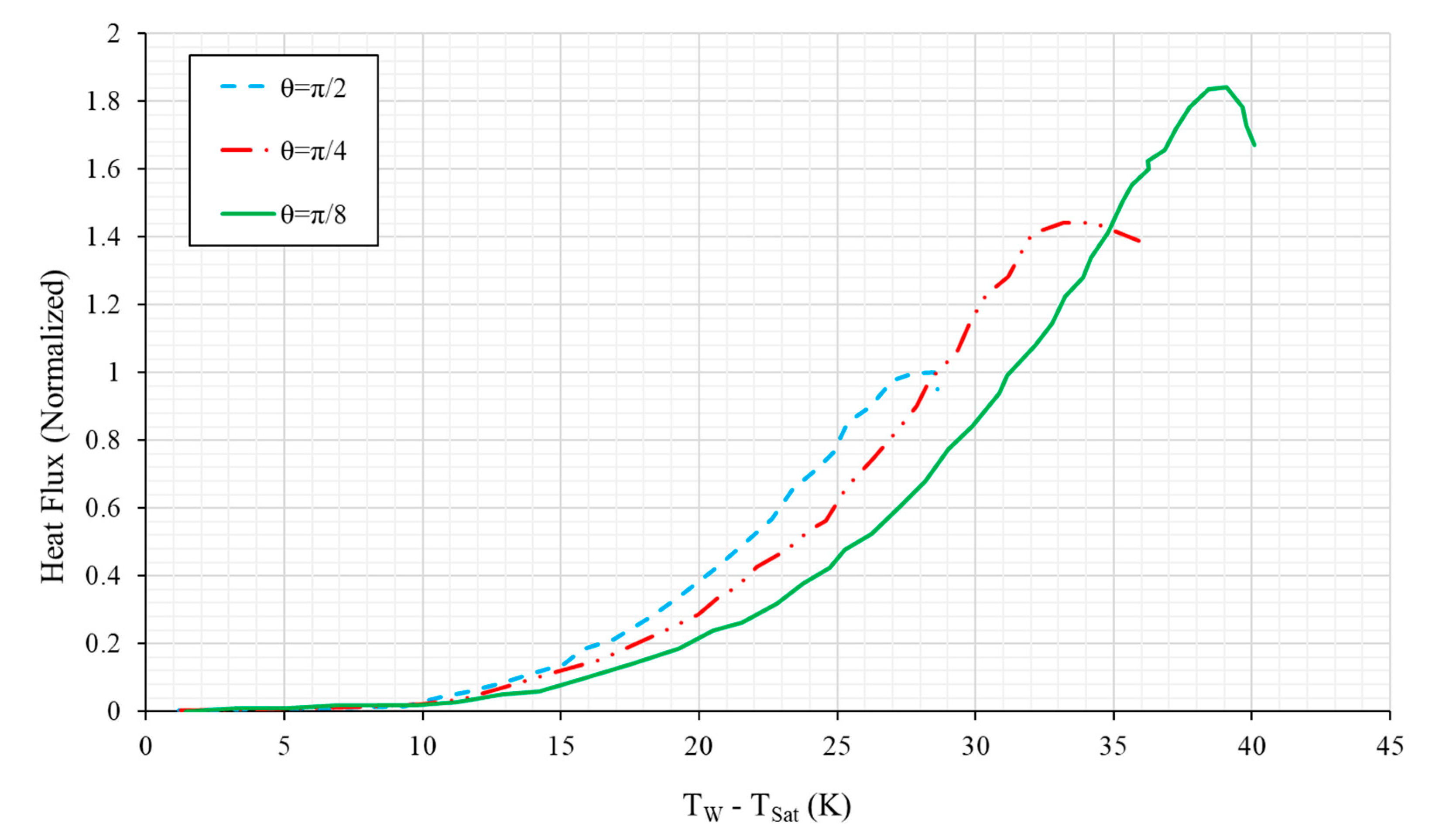
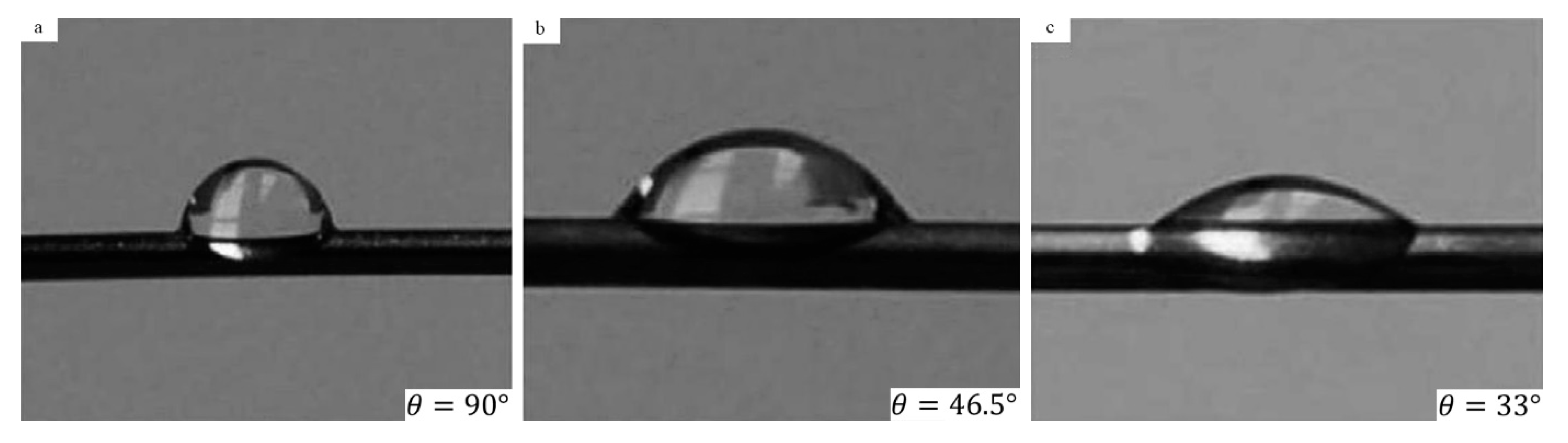

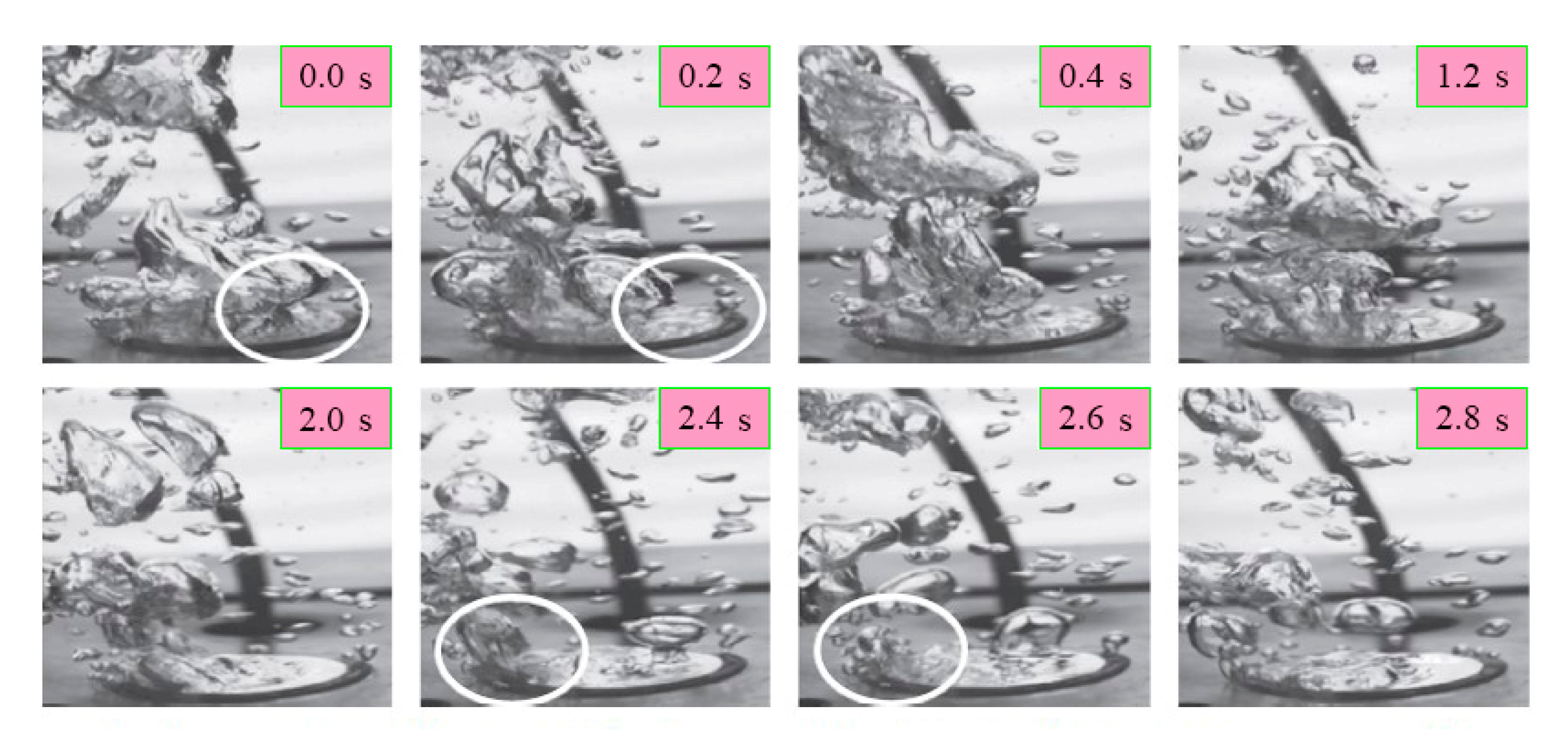
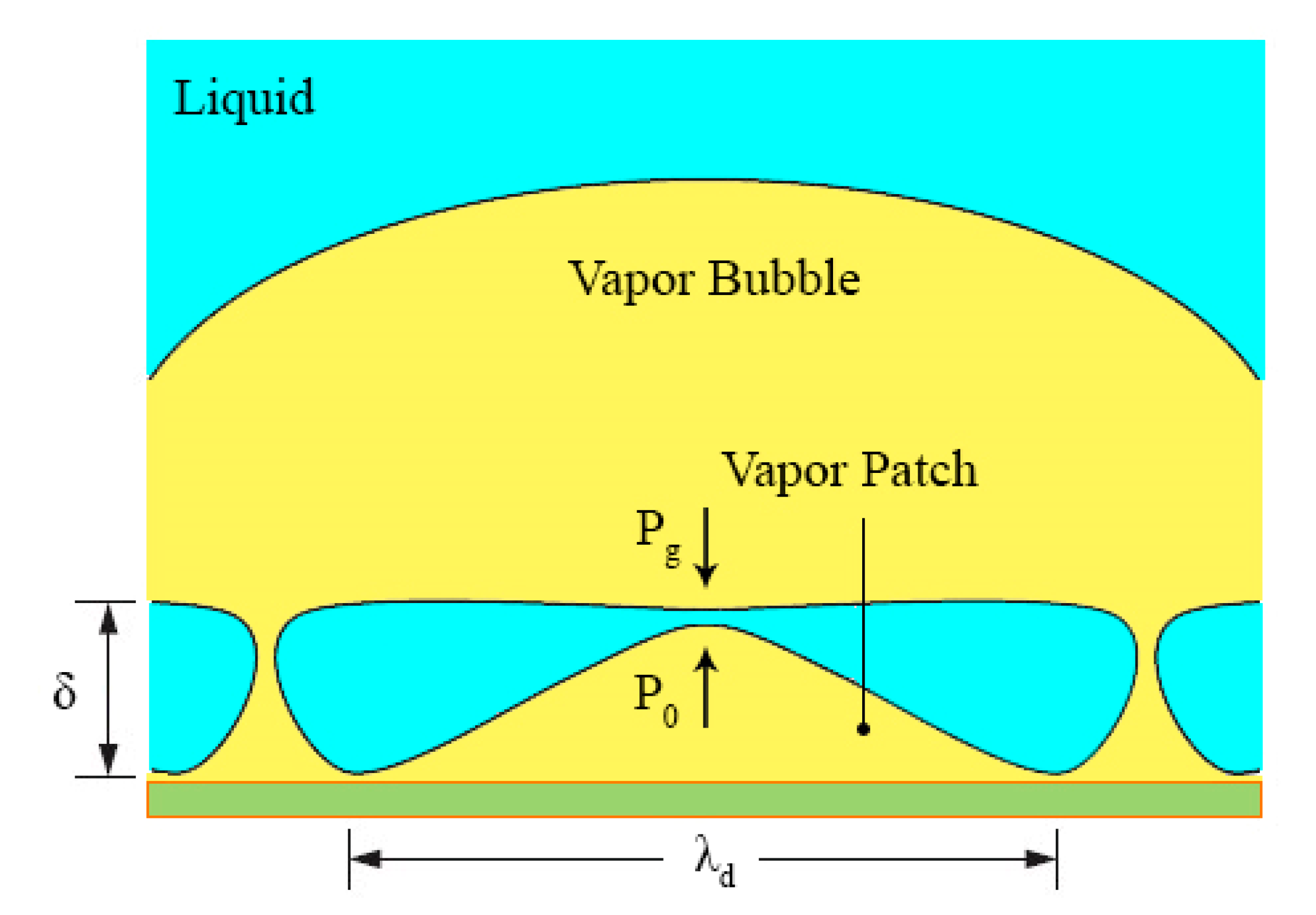
| No. | Authors | Year | Nanoparticle Material | Base Fluid | Particle Size (nm) | Concentration | Material/Type of Heater | CHF Enhancement (%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| 1 | You et al. | 2003 | Al2O3 | Water | - | 0.001–0.025 g/L | Copper block | 200 | [97] |
| 2 | Vassallo et al. | 2004 | SiO2 | Water | 15 and 20 | 0.5 vol.% | Ni-Cr Wire | 60 | [98] |
| 3 | Bang and Chang | 2005 | Al2O3 | Water | - | 0–4 vol.% | Plate heater | 51 | [99] |
| 5 | Kim et al. | 2006 | TiO2Al2O3 | Water | 85 47 | 10−5–0.1 vol.% | Ni-Cr heater | 200 176 | [100] |
| 6 | Kim et al. | 2006 | Al2O3SiO2ZrO2 | Water | 110–210 20–40 110–250 | 10−3–0.1 vol.% | SS Wire | 50 80 75 | [101] |
| 7 | Kim et al. | 2007 | Al2O3TiO2 | Water | 47 23 | 0.00001–0.1 vol.% | Ni-Cr Wire | 100 | [102] |
| 8 | Liu et al. | 2007 | CuO | Water | 30 | 0.1–2 wt% | Copper plate with grooves | 50 | [103] |
| 9 | Coursey and Kim | 2008 | Al2O3 | Water | 45 | 0.001–10 g/L | Polished Copper | 37 | [104] |
| 10 | Golubovic et al. | 2009 | Al2O3BiO2 | Water | 22.6–46 30 | 0–0.01 vol.% 0–0.01 vol.% | Ni-Cr Wire Copper | 50 33 | [105] |
| 11 | Kwark et al. | 2010 | Al2O3CuO | Water | 139 143 | 0.001–1 g/L | Copper block | 80 | [106] |
| 12 | Huang et al. | 2011 | TiO2 | Water | 110–220 | 0.01–1 wt% | Nickel tube | 82.7 | [107] |
| 13 | Sheikhbahai et al. | 2012 | Fe3O4 | EG-Water | 50 | 0–0.1 vol.% | Ni-Cr wire | 100 | [108] |
| 14 | Hegde et al. | 2012 | CuO | Water | 10–100 | 0.01–0.5 vol.% | Ni-Cr wire | 130 | [109] |
| 15 | Kole and Dey | 2012 | ZnO | EG | 30–50 | 0.5–3.75 vol.% | Copper block | 117 | [110] |
| 16 | Vazquez and Kumar | 2013 | SiO2 | Water | 10 | 0.1–2 vol.% | Ni-Cr wire | 270 | [111] |
| 17 | Sharma et al. | 2013 | ZnO | Water | 38–68 | 0.01 vol.% | Copper | 160 | [112] |
| 18 | Kim ei al. | 2014 | TiO2 | Water | 47 | 0.01 vol.% | Ni-Cr wire | 175 | [113] |
| 19 | Naphon and Thongjing | 2014 | TiO2 | R141b-Ethanol | 21 | 0.01–0.075 vol.% | Cylindrical heater | - | [114] |
| 20 | Sakashita | 2015 | TiO2 | Water | 25 | 0.002 wt% | Copper | 200 | [115] |
| 21 | Sarafraz et al. | 2016 | ZrO2 | EG-Water | 20–25 | 0.025–0.1 vol.% | Copper | - | [116] |
| 22 | Ali et al. | 2017 | TiO2 | Water | - | 12 wt%, 15wt% | Copper block | 122 | [117] |
| 23 | Kshirsagar and Shrivastava | 2018 | Al2O3 | Water | 30 | 0.3–1.5 wt% | Ni-Cr wire heate | 87 | [118] |
| 24 | Kangude and Srivastava | 2019 | SiO2 | Water | - | 0.005 and 0.01% (V/V) | - | - | [119] |
| Parameter Variations | CHF | Reason |
|---|---|---|
| Increase in the surface roughness | Increase | The CHF causes a higher surface superheat. |
| The efficient contact area increases. | ||
| Increase in the surface wettability | Increase | The liquid traps in the porous structure and the vapour layer formation is prevented. |
| The number of active nucleation sites decreases because of the low contact angle and the vapour layer formation is prevented. | ||
| Increase in the capillarity | Increase | The dry regions under the vapour bubbles are wetted by fresh liquid. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moghadasi, H.; Malekian, N.; Saffari, H.; Mirza Gheitaghy, A.; Zhang, G.Q. Recent Advances in the Critical Heat Flux Amelioration of Pool Boiling Surfaces Using Metal Oxide Nanoparticle Deposition. Energies 2020, 13, 4026. https://doi.org/10.3390/en13154026
Moghadasi H, Malekian N, Saffari H, Mirza Gheitaghy A, Zhang GQ. Recent Advances in the Critical Heat Flux Amelioration of Pool Boiling Surfaces Using Metal Oxide Nanoparticle Deposition. Energies. 2020; 13(15):4026. https://doi.org/10.3390/en13154026
Chicago/Turabian StyleMoghadasi, Hesam, Navid Malekian, Hamid Saffari, Amir Mirza Gheitaghy, and Guo Qi Zhang. 2020. "Recent Advances in the Critical Heat Flux Amelioration of Pool Boiling Surfaces Using Metal Oxide Nanoparticle Deposition" Energies 13, no. 15: 4026. https://doi.org/10.3390/en13154026
APA StyleMoghadasi, H., Malekian, N., Saffari, H., Mirza Gheitaghy, A., & Zhang, G. Q. (2020). Recent Advances in the Critical Heat Flux Amelioration of Pool Boiling Surfaces Using Metal Oxide Nanoparticle Deposition. Energies, 13(15), 4026. https://doi.org/10.3390/en13154026