Dietary Advanced Glycation End-Products (dAGEs) Intake and Bone Health: A Cross-Sectional Analysis in the Rotterdam Study
Abstract
1. Introduction
2. Materials and Methods
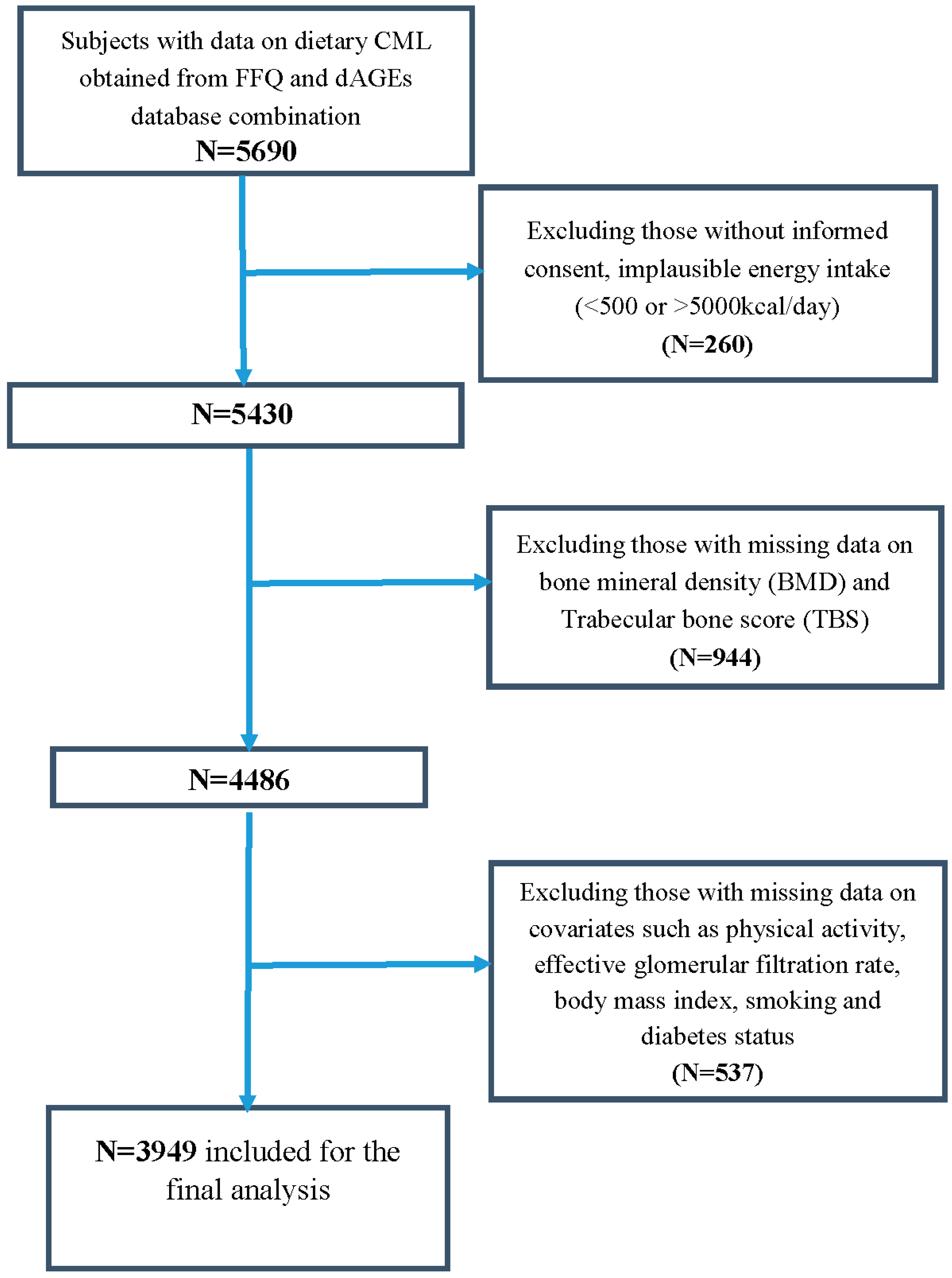
2.1. Study Population
2.2. Dietary Advanced Glycation End-Products (dAGEs) Assessment
Food Frequency Questionnaire
2.3. dAGEs Databases
2.4. Measurement of Bone Mineral Density (BMD) and Trabecular Bone Score (TBS)
2.5. Prevalent Major Osteoporotic Fractures (MOFs)
2.6. Prevalent Vertebral Fractures (VFs)
2.7. Assessment of Covariates
2.8. Statistical Methods
3. Results
3.1. Descriptives
3.2. Linear Regression Analysis of Energy-Adjusted CML Intake (CML) with Bone Mineral Density (BMD) and Trabecular Bone Score (TBS)
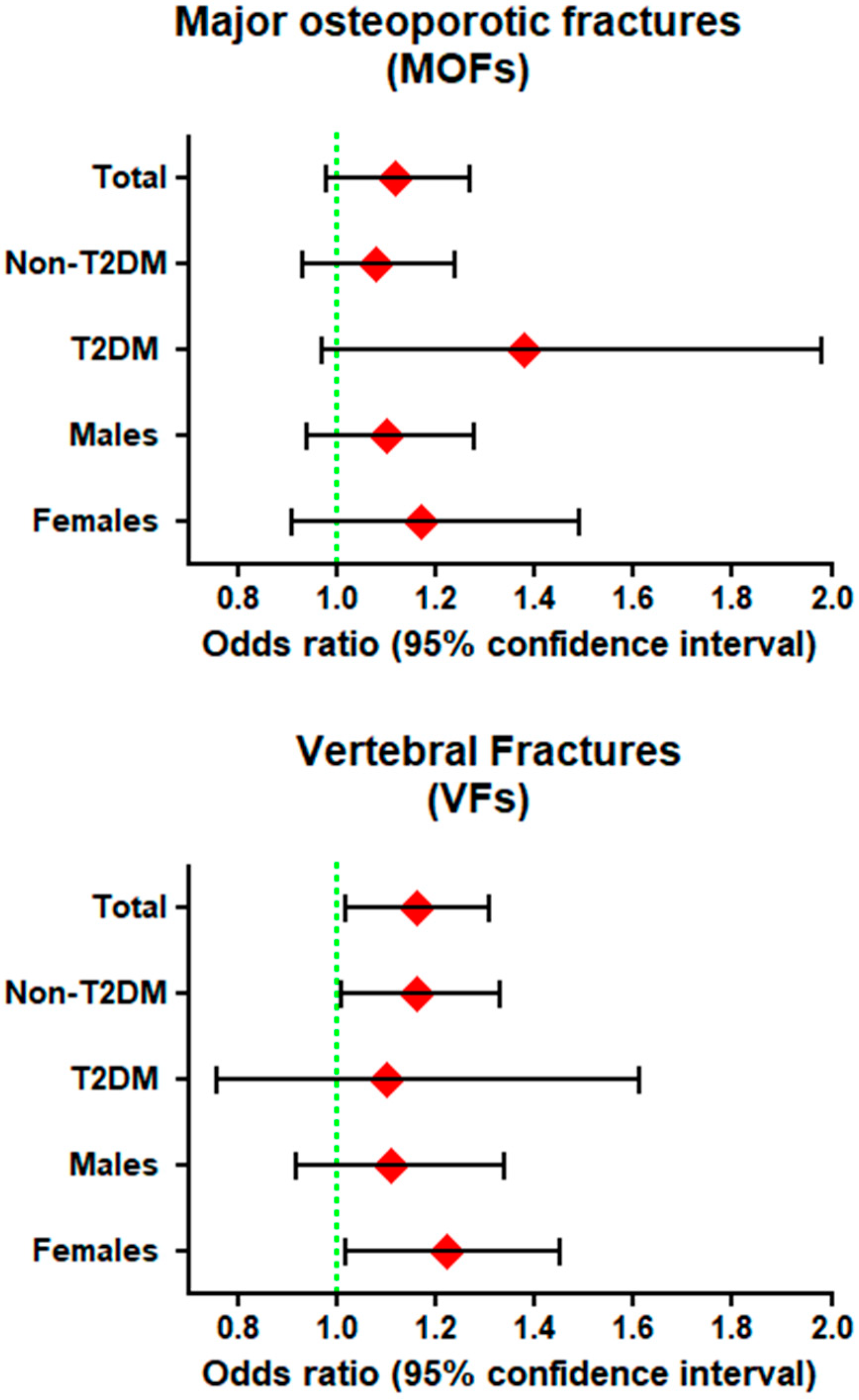
3.3. Logistic Regression Analysis for the Association between Energy-Adjusted CML Intake (CML) and Prevalence of Fractures
3.3.1. Major Osteoporotic Fractures (MOFs)
3.3.2. Vertebral Fractures (VFs)
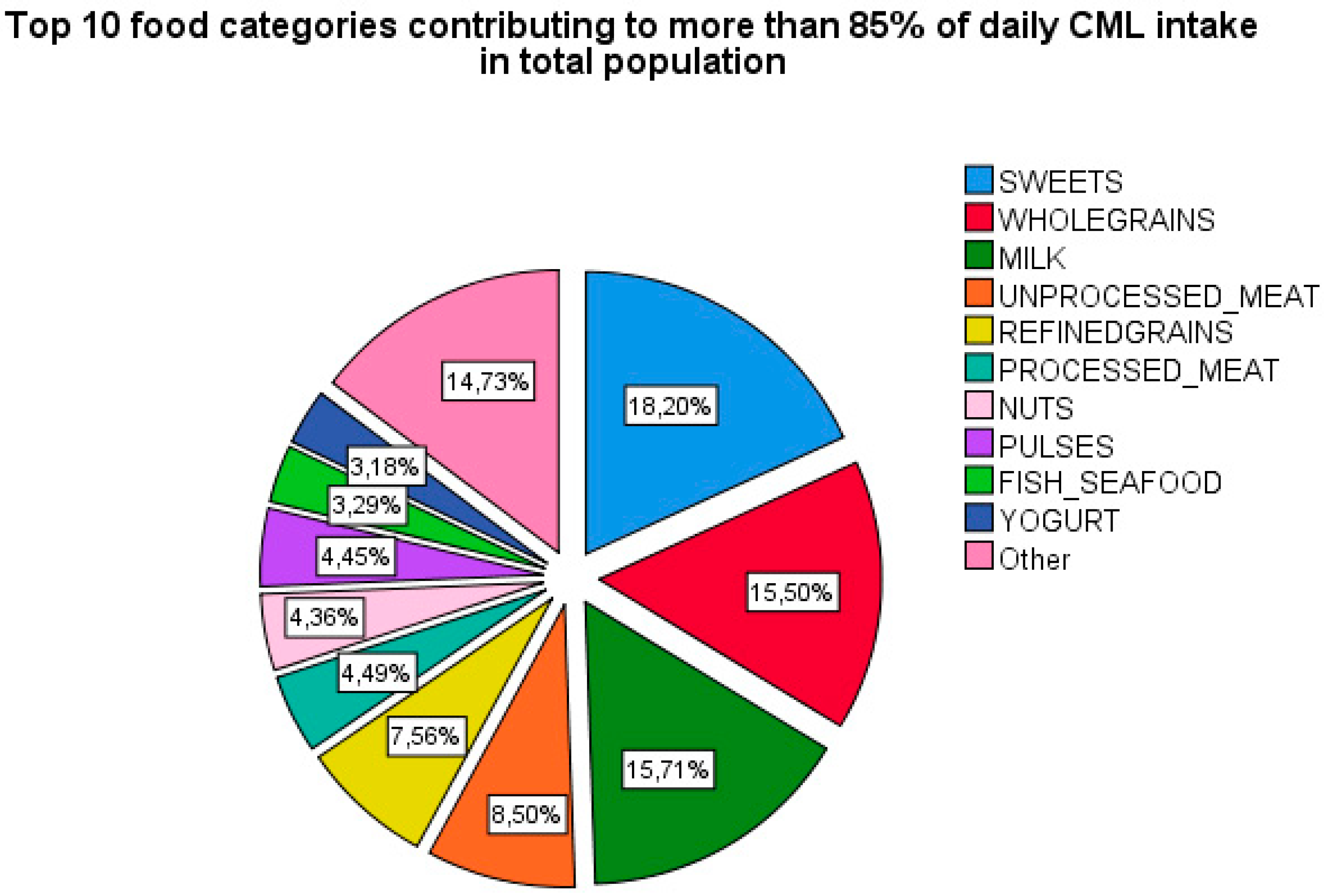
3.4. Logistic Regression Analysis for the Association between Top Food Categories Contributing to CML and Prevalence of Fractures
3.4.1. Major Osteoporotic Fractures (MOFs)
3.4.2. Vertebral Fractures (VFs)
3.5. Subgroup and Sensitivity Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CML | Carboxymethyllysine |
| CEL | Carboxyethyllysine |
| dAGEs | dietary advanced glycation end-products |
| MG-H1 | Methylglyoxal-derived hydroimidazolone |
| DQS | Dietary Quality Score |
| FFQ | Food frequency questionnaire |
| LS-BMD | Lumbar spine bone mineral density |
| FN-BMD | Femoral neck bone mineral density |
| TBS | Trabecular bone score |
| MOFs | prevalent major osteoporotic fractures |
| VFs | prevalent vertebral fractures |
| UPLC-MS/MS | Ultra performance liquid chromatography-Tandem Mass spectrometry |
| ELISA | Enzyme linked immunosorbent assay |
References
- Fabiani, R.; Naldini, G.; Chiavarini, M. Dietary Patterns in Relation to Low Bone Mineral Density and Fracture Risk: A Systematic Review and Meta-Analysis. Adv. Nutr. 2019, 10, 219–236. [Google Scholar] [CrossRef]
- Langsetmo, L.; Hanley, D.A.; Prior, J.C.; Barr, S.I.; Anastassiades, T.; Towheed, T.; Goltzman, D.; Morin, S.; Poliquin, S.; Kreiger, N.; et al. Dietary patterns and incident low-trauma fractures in postmenopausal women and men aged ≥ 50 y: A population-based cohort study. Am. J. Clin. Nutr. 2011, 93, 192–199. [Google Scholar] [CrossRef]
- McNaughton, S.A.; Wattanapenpaiboon, N.; Wark, J.D.; Nowson, C.A. An energy-dense, nutrient-poor dietary pattern is inversely associated with bone health in women. J. Nutr. 2011, 141, 1516–1523. [Google Scholar] [CrossRef] [PubMed]
- McTiernan, A.; Wactawski-Wende, J.; Wu, L.; Rodabough, R.J.; Watts, N.B.; Tylavsky, F.; Freeman, R.; Hendrix, S.; Jackson, R. Low-fat, increased fruit, vegetable, and grain dietary pattern, fractures, and bone mineral density: The Women’s Health Initiative Dietary Modification Trial. Am. J. Clin. Nutr. 2009, 89, 1864–1876. [Google Scholar] [PubMed]
- Ward, K.A.; Prentice, A.; Kuh, D.L.; Adams, J.E.; Ambrosini, G.L. Life Course Dietary Patterns and Bone Health in Later Life in a British Birth Cohort Study. J. Bone Miner. Res. 2016, 31, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Benetou, V.; Orfanos, P.; Pettersson-Kymmer, U.; Bergstrom, U.; Svensson, O.; Johansson, I.; Berrino, F.; Tumino, R.; Borch, K.B.; Lund, E.; et al. Mediterranean diet and incidence of hip fractures in a European cohort. Osteoporos. Int. 2013, 24, 1587–1598. [Google Scholar] [CrossRef] [PubMed]
- Hardcastle, A.C.; Aucott, L.; Fraser, W.D.; Reid, D.M.; Macdonald, H.M. Dietary patterns, bone resorption and bone mineral density in early post-menopausal Scottish women. Eur. J. Clin. Nutr. 2011, 65, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.J.; Roman, G.; Yeboah, F.; Konishi, Y. The road to advanced glycation end products: A mechanistic perspective. Curr. Med. Chem. 2007, 14, 1653–1671. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Barden, A.; Mori, T.; Beilin, L. Advanced glycation end-products: A review. Diabetologia 2001, 44, 129–146. [Google Scholar] [CrossRef]
- Friedman, E.A. Advanced glycosylated end products and hyperglycemia in the pathogenesis of diabetic complications. Diabetes Care 1999, 22, B65–B71. [Google Scholar]
- Witko-Sarsat, V.; Nguyen-Khoa, T.; Jungers, P.; Drueke, T.B.; Descamps-Latscha, B. Advanced oxidation protein products as a novel molecular basis of oxidative stress in uraemia. Nephrol. Dial. Transplant 1999, 14, 76–78. [Google Scholar] [CrossRef] [PubMed]
- Semba, R.D.; Nicklett, E.J.; Ferrucci, L. Does accumulation of advanced glycation end products contribute to the aging phenotype? J. Gerontol. A Biol. Sci. Med. Sci. 2010, 65, 963–975. [Google Scholar] [CrossRef] [PubMed]
- Cerami, C.; Founds, H.; Nicholl, I.; Mitsuhashi, T.; Giordano, D.; Vanpatten, S.; Lee, A.; Al-Abed, Y.; Vlassara, H.; Bucala, R.; et al. Tobacco smoke is a source of toxic reactive glycation products. Proc. Natl. Acad. Sci. USA 1997, 94, 13915–13920. [Google Scholar] [CrossRef] [PubMed]
- Uribarri, J.; Woodruff, S.; Goodman, S.; Cai, W.; Chen, X.; Pyzik, R.; Yong, A.; Striker, G.E.; Vlassara, H. Advanced glycation end products in foods and a practical guide to their reduction in the diet. J. Am. Diet Assoc. 2010, 110, 911–916.e12. [Google Scholar] [CrossRef]
- Snelson, M.; Coughlan, M.T. Dietary Advanced Glycation End Products: Digestion, Metabolism and Modulation of Gut Microbial Ecology. Nutrients 2019, 11, 215. [Google Scholar] [CrossRef]
- Poulsen, M.W.; Hedegaard, R.V.; Andersen, J.M.; de Courten, B.; Bugel, S.; Nielsen, J.; Skibsted, L.H.; Dragsted, L.O. Advanced glycation endproducts in food and their effects on health. Food Chem. Toxicol. 2013, 60, 10–37. [Google Scholar] [CrossRef]
- Goldberg, T.; Cai, W.; Peppa, M.; Dardaine, V.; Baliga, B.S.; Uribarri, J.; Vlassara, H. Advanced glycoxidation end products in commonly consumed foods. J. Am. Diet. Assoc. 2004, 104, 1287–1291. [Google Scholar] [CrossRef]
- Niu, L.; Sun, X.; Tang, J.; Wang, J.; Wang, J.; Rasco, B.A. Combination effects of salts and cold storage on the formation of protein-bound N (varepsilon)-(carboxymethyl) lysine and N (varepsilon)-(carboxyethyl) lysine in raw and subsequently commercially sterilized ground pork. Food Chem. 2018, 264, 455–461. [Google Scholar] [CrossRef]
- Liang, Z.; Chen, X.; Li, L.; Li, B.; Yang, Z. The fate of dietary advanced glycation end products in the body: From oral intake to excretion. Crit. Rev. Food Sci. Nutr. 2019, 59, 1–17. [Google Scholar] [CrossRef]
- Hellwig, M.; Geissler, S.; Matthes, R.; Peto, A.; Silow, C.; Brandsch, M.; Henle, T. Transport of Free and Peptide-Bound Glycated Amino Acids: Synthesis, Transepithelial Flux at Caco-2 Cell Monolayers, and Interaction with Apical Membrane Transport Proteins. ChemBioChem 2011, 12, 1270–1279. [Google Scholar] [CrossRef]
- Uribarri, J.; Cai, W.; Sandu, O.; Peppa, M.; Goldberg, T.; Vlassara, H. Diet-derived advanced glycation end products are major contributors to the body’s AGE pool and induce inflammation in healthy subjects. Ann. N. Y. Acad. Sci. 2005, 1043, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Avery, N.C.; Bailey, A.J. Enzymic and non-enzymic cross-linking mechanisms in relation to turnover of collagen: Relevance to aging and exercise. Scand. J. Med. Sci. Sports 2005, 15, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.; Fujii, K.; Soshi, S.; Tanaka, T. Reductions in degree of mineralization and enzymatic collagen cross-links and increases in glycation-induced pentosidine in the femoral neck cortex in cases of femoral neck fracture. Osteoporos. Int. 2006, 17, 986–995. [Google Scholar] [CrossRef] [PubMed]
- Kume, S.; Kato, S.; Yamagishi, S.-I.; Inagaki, Y.; Ueda, S.; Arima, N.; Okawa, T.; Kojiro, M.; Nagata, K. Advanced Glycation End-Products Attenuate Human Mesenchymal Stem Cells and Prevent Cognate Differentiation Into Adipose Tissue, Cartilage, and Bone. J. Bone Miner. Res. 2005, 20, 1647–1658. [Google Scholar] [CrossRef] [PubMed]
- Sanguineti, R.; Puddu, A.; Mach, F.; Montecucco, F.; Viviani, G.L. Advanced Glycation End Products Play Adverse Proinflammatory Activities in Osteoporosis. Mediat. Inflamm. 2014, 2014, 975872. [Google Scholar] [CrossRef]
- Yamamoto, M.; Sugimoto, T. Advanced Glycation End Products, Diabetes, and Bone Strength. Curr. Osteoporos. Rep. 2016, 14, 320–326. [Google Scholar] [CrossRef]
- Delgado-Andrade, C.; Roncero-Ramos, I.; Carballo, J.; Rufián-Henares, J.A.; Seiquer, I.; Navarro, M.P. Composition and functionality of bone affected by dietary glycated compounds. Food Funct. 2013, 4, 549–556. [Google Scholar] [CrossRef]
- Illien-Jünger, S.; Palacio-Mancheno, P.; Kindschuh, W.F.; Chen, X.; E Sroga, G.; Vashishth, D.; Iatridis, J.C. Dietary Advanced Glycation End Products Have Sex- and Age-Dependent Effects on Vertebral Bone Microstructure and Mechanical Function in Mice. J. Bone Miner. Res. 2017, 33, 437–448. [Google Scholar] [CrossRef]
- Carnovali, M.; Luzi, L.; Terruzzi, I.; Banfi, G.; Mariotti, M. Metabolic and bone effects of high-fat diet in adult zebrafish. Endocrine 2017, 61, 317–326. [Google Scholar] [CrossRef]
- Ganeko, K.; Masaki, C.; Shibata, Y.; Mukaibo, T.; Kondo, Y.; Nakamoto, T.; Miyazaki, T.; Hosokawa, R. Bone Aging by Advanced Glycation End Products. J. Dent. Res. 2015, 94, 1684–1690. [Google Scholar] [CrossRef]
- Scheijen, J.; Clevers, E.; Engelen, L.; Dagnelie, P.C.; Brouns, F.; Stehouwer, C.D.; Schalkwijk, C.G. Analysis of advanced glycation endproducts in selected food items by ultra-performance liquid chromatography tandem mass spectrometry: Presentation of a dietary AGE database. Food Chem. 2016, 190, 1145–1150. [Google Scholar] [CrossRef] [PubMed]
- Ikram, M.K.; Brusselle, G.; Ghanbari, M.; Goedegebure, A.; Kavousi, M.; Kieboom, B.C.T.; Klaver, C.C.W.; De Knegt, R.J.; Luik, A.I.; Nijsten, T.E.C.; et al. Objectives, design and main findings until 2020 from the Rotterdam Study. Eur. J. Epidemiol. 2020, 35, 483–517. [Google Scholar] [CrossRef] [PubMed]
- Voortman, T.; Jong, J.C.K.-D.; Ikram, M.A.; Stricker, B.H.; Van Rooij, F.J.A.; LaHousse, L.; Tiemeier, H.; Brusselle, G.G.; Franco, O.H.; Schoufour, J.D. Adherence to the 2015 Dutch dietary guidelines and risk of non-communicable diseases and mortality in the Rotterdam Study. Eur. J. Epidemiol. 2017, 32, 993–1005. [Google Scholar] [CrossRef] [PubMed]
- A Goldbohm, R.; Brandt, P.A.V.D.; A Brants, H.; Veer, P.V.; Al, M.; Sturmans, F.; Hermus, R.J. Validation of a dietary questionnaire used in a large-scale prospective cohort study on diet and cancer. Eur. J. Clin. Nutr. 1994, 48, 253–265. [Google Scholar]
- I Feunekes, G.; A Van Staveren, W.; De Vries, J.H.; Burema, J.; Hautvast, J.G. Relative and biomarker-based validity of a food-frequency questionnaire estimating intake of fats and cholesterol. Am. J. Clin. Nutr. 1993, 58, 489–496. [Google Scholar] [CrossRef]
- Hull, G.L.J.; WoodsidebJennifer, J.V.; Ames, J.M.; Cuskelly, G.J. Ne-(carboxymethyl) lysine content of foods commonly consumed in a Western style diet. Food Chem. 2012, 131, 170–174. [Google Scholar] [CrossRef]
- Chen, J.; Waqas, K.; Tan, R.C.; Voortman, T.; A Ikram, M.; Nijsten, T.; De Groot, L.C.P.G.M.; Uitterlinden, A.G.; Zillikens, M.C. The association between dietary and skin advanced glycation end products: The Rotterdam Study. Am. J. Clin. Nutr. 2020. [Google Scholar] [CrossRef]
- Hofman, A.; Brusselle, G.G.O.; Murad, S.D.; Van Duijn, C.M.; Franco, O.H.; Goedegebure, A.; Ikram, M.A.; Klaver, C.C.W.; Nijsten, T.E.C.; Peeters, R.P.; et al. The Rotterdam Study: 2016 objectives and design update. Eur. J. Epidemiol. 2015, 30, 661–708. [Google Scholar] [CrossRef]
- Looker, A.C.; Wahner, H.W.; Dunn, W.L.; Calvo, M.S.; Harris, T.B.; Heyse, S.P.; Jr, C.C.J.; Lindsay, R. Updated Data on Proximal Femur Bone Mineral Levels of US Adults. Osteoporos. Int. 1998, 8, 468–489. [Google Scholar] [CrossRef]
- Hans, D.B.; Goertzen, A.L.; Krieg, M.-A.; Leslie, W. Bone microarchitecture assessed by TBS predicts osteoporotic fractures independent of bone density: The manitoba study. J. Bone Miner. Res. 2011, 26, 2762–2769. [Google Scholar] [CrossRef]
- Kanis, J.; Johnell, O.; Oden, A.; Johansson, H.; McCloskey, E. FRAX™ and the assessment of fracture probability in men and women from the UK. Osteoporos. Int. 2008, 19, 385–397. [Google Scholar] [CrossRef] [PubMed]
- Oei, L.; Koromani, F.; Breda, S.J.; Schousboe, J.T.; Clark, E.M.; Van Meurs, J.B.; A Ikram, M.; Waarsing, J.H.; Van Rooij, F.J.; Zillikens, M.C.; et al. Osteoporotic Vertebral Fracture Prevalence Varies Widely Between Qualitative and Quantitative Radiological Assessment Methods: The Rotterdam Study. J. Bone Miner. Res. 2017, 33, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Genant, H.K.; Wu, C.Y.; Van Kuijk, C.; Nevitt, M.C. Vertebral fracture assessment using a semiquantitative technique. J. Bone Miner. Res. 2009, 8, 1137–1148. [Google Scholar] [CrossRef] [PubMed]
- Ferrar, L.; Jiang, G.; Adams, J.; Eastell, R. Identification of vertebral fractures: An update. Osteoporos. Int. 2005, 16, 717–728. [Google Scholar] [CrossRef] [PubMed]
- Stel, V.S.; Smit, J.H.; Pluijm, S.; Visser, M.; Deeg, D.J.; Lips, P. Comparison of the LASA Physical Activity Questionnaire with a 7-day diary and pedometer. J. Clin. Epidemiol. 2004, 57, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Caspersen, C.J.; Bloemberg, B.P.M.; Saris, W.H.M.; Merritt, R.K.; Kromhout, D. The Prevalence of Selected Physical Activities and Their Relation with Coronary Heart Disease Risk Factors in Elderly Men: The Zutphen Study, 1985. Am. J. Epidemiol. 1991, 133, 1078–1092. [Google Scholar] [CrossRef] [PubMed]
- Roncero-Ramos, I.; Delgado-Andrade, C.; Tessier, F.J.; Niquet-Leridon, C.; Strauch, C.; Monnier, V.M.; Navarro, M.P. Metabolic transit of N(epsilon)-carboxymethyl-lysine after consumption of AGEs from bread crust. Food Funct. 2013, 4, 1032–1039. [Google Scholar] [CrossRef]
- Roncero-Ramos, I.; Delgado-Andrade, C.; Rufián-Henares, J.A.; Carballo, J.; Navarro, M.P. Effects of model Maillard compounds on bone characteristics and functionality. J. Sci. Food Agric. 2013, 93, 2816–2821. [Google Scholar] [CrossRef]
- Karim, L.; Tang, S.G.; Sroga, G.E.; Vashishth, D. Differences in non-enzymatic glycation and collagen cross-links between human cortical and cancellous bone. Osteoporos. Int. 2013, 24, 2441–2447. [Google Scholar] [CrossRef]
- Pérez-Rey, J.; Roncero-Martin, R.; Rico-Martín, S.; Rey-Sanchez, P.; Pedrera-Zamorano, J.D.; Pedrera-Canal, M.; Lopez-Espuela, F.; Lavado-Garcia, J.M. Adherence to a Mediterranean Diet and Bone Mineral Density in Spanish Premenopausal Women. Nutrients 2019, 11, 555. [Google Scholar] [CrossRef]
- Palomeras-Vilches, A.; Viñals-Mayolas, E.; Bou-Mias, C.; Jordà-Castro, M.; Agüero-Martínez, M.; Busquets-Barceló, M.; Pujol-Busquets, G.; Carrion, C.; Bosque-Prous, M.; Serra-Majem, L.; et al. Adherence to the Mediterranean Diet and Bone Fracture Risk in Middle-Aged Women: A Case Control Study. Nutrients 2019, 11, 2508. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, T.R.; Martins, C.C.; Ferreira, L.L.; Spritzer, P.M. Mediterranean diet is associated with bone mineral density and muscle mass in postmenopausal women. Climacteric 2019, 22, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Julián, C.; Huybrechts, I.; Gracia-Marco, L.; González-Gil, E.M.; Gutierrez, A.; Gonzalez-Gross, M.; Marcos, A.; Widhalm, K.; Kafatos, A.; Vicente-Rodriguez, G.; et al. Mediterranean diet, diet quality, and bone mineral content in adolescents: The HELENA study. Osteoporos. Int. 2018, 29, 1329–1340. [Google Scholar] [CrossRef] [PubMed]
- Pérez, A.R.; Velasco, A.R. Adherence to Mediterranean diet and bone health. Nutr. Hospitalaria 2014, 29, 989–996. [Google Scholar]
- Trichopoulou, A.; Lagiou, P. Healthy traditional Mediterranean diet: An expression of culture, history, and lifestyle. Nutr. Rev. 1997, 55, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Benetou, V.; Orfanos, P.; Feskanich, D.; Michaëlsson, K.; Pettersson-Kymmer, U.; Byberg, L.; Eriksson, S.; Grodstein, F.; Wolk, A.; Jankovic, N.; et al. Mediterranean diet and hip fracture incidence among older adults: The CHANCES project. Osteoporos. Int. 2018, 29, 1591–1599. [Google Scholar] [CrossRef] [PubMed]
- Tosti, V.; Bertozzi, B.; Fontana, L. Health Benefits of the Mediterranean Diet: Metabolic and Molecular Mechanisms. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2017, 73, 318–326. [Google Scholar] [CrossRef]
- Sánchez, E.; The ILERVAS Project Investigators; Betriu, À.; Salas-Salvadó, J.; Pamplona, R.; Barbé, F.; Purroy, F.; Farràs, C.; Fernández, E.; López-Cano, C.; et al. Mediterranean diet, physical activity and subcutaneous advanced glycation end-products’ accumulation: A cross-sectional analysis in the ILERVAS project. Eur. J. Nutr. 2019, 59, 1233–1242. [Google Scholar]
- Huggins, C.E.; Coughlan, M.T.; Reid, C.M. Association between habitual dietary and lifestyle behaviours and skin autofluorescence (SAF), a marker of tissue accumulation of advanced glycation endproducts (AGEs), in healthy adults. Eur. J. Nutr. 2017, 57, 2209–2216. [Google Scholar]
- Bo, S.; Gambino, R.; Ponzo, V.; Cioffi, I.; Goitre, I.; Evangelista, A.; Ciccone, G.; Cassader, M.; Procopio, M. Effects of resveratrol on bone health in type 2 diabetic patients. A double-blind randomized-controlled trial. Nutr. Diabetes 2018, 8, 51. [Google Scholar] [CrossRef]
- Quezada-Fernández, P.; Trujillo-Quiros, J.; Pascoe-González, S.; Trujillo-Rangel, W.A.; Cardona-Müller, D.; Ramos-Becerra, C.G.; Barocio-Pantoja, M.; La Cerda, M.R.-D.; Sánchez-Rodríguez, E.N.; Cardona-Muñóz, E.G.; et al. Effect of green tea extract on arterial stiffness, lipid profile and sRAGE in patients with type 2 diabetes mellitus: A randomised, double-blind, placebo-controlled trial. Int. J. Food Sci. Nutr. 2019, 70, 977–985. [Google Scholar] [CrossRef] [PubMed]
- Tucker, K.; Chen, H.-L.; Hannan, M.T.; Cupples, L.A.; Wilson, P.W.F.; Felson, D.; Kiel, D. Bone mineral density and dietary patterns in older adults: The Framingham Osteoporosis Study. Am. J. Clin. Nutr. 2002, 76, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Michaëlsson, K.; Wolk, A.; Langenskiöld, S.; Basu, S.; Lemming, E.W.; Melhus, H.; Byberg, L. Milk intake and risk of mortality and fractures in women and men: Cohort studies. BMJ 2014, 349, g6015. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Bao, M.; Li, D.; Li, Y.M. Advanced glycation in d-galactose induced mouse aging model. Mech. Ageing Dev. 1999, 108, 239–251. [Google Scholar] [CrossRef]
- Mangano, K.M.; Sahni, S.; Kiel, D.P.; Tucker, K.L.; Dufour, A.; Hannan, M.T. Bone Mineral Density and Protein-Derived Food Clusters from the Framingham Offspring Study. J. Acad. Nutr. Diet. 2015, 115, 1605–1613.e1. [Google Scholar] [CrossRef]
- Delgado-Andrade, C. Carboxymethyl-lysine: Thirty years of investigation in the field of AGE formation. Food Funct. 2016, 7, 46–57. [Google Scholar] [CrossRef]
- Barzilay, J.I.; Buzkova, P.; Zieman, S.J.; Kizer, J.R.; Djoussé, L.; Ix, J.H.; Tracy, R.P.; Siscovick, D.; Cauley, J.A.; Mukamal, K.J. Circulating Levels of Carboxy-Methyl-Lysine (CML) Are Associated With Hip Fracture Risk: The Cardiovascular Health Study. J. Bone Miner. Res. 2014, 29, 1061–1066. [Google Scholar] [CrossRef]
- Lamb, L.S.; Alfonso, H.; Norman, P.E.; E Davis, T.M.; Forbes, J.M.; Müench, G.; Irrgang, F.; Almeida, O.P.; Golledge, J.; Hankey, G.J.; et al. Advanced Glycation End Products and esRAGE Are Associated With Bone Turnover and Incidence of Hip Fracture in Older Men. J. Clin. Endocrinol. Metab. 2018, 103, 4224–4231. [Google Scholar] [CrossRef]
| Total Participants (N = 3949) | T2DM (n = 473) 12% | Non-T2DM (n = 3476) 88% | |
|---|---|---|---|
| CML (mg/day, energy adjusted) | 2.42 ± 0.88 | 2.49 ± 0.93 | 2.41 ± 0.86 |
| MGH1 (mg/day, energy adjusted) | 28.4 ± 7.73 | 29.1 ±7.8 * | 28.3 ± 7.7 |
| CEL (mg/day, energy adjusted) | 2.42 ± 0.87 | 2.57 ± 0.97 * | 2.39 ± 0.86 |
| Age (years) | 66.7 ± 10.5 | 72.0 ± 9.2 * | 66.2 ± 10.6 |
| Males, n (%) | 1703 (43%) | 235 (49.7%) * | 1469 (42.2%) |
| BMI (kg/m2) | 27.4 ± 4.2 | 29.7 ± 4.8 * | 27.0 ± 3.97 |
| eGFR (mL/min per 1.73 m2) | 77.9 ± 14.9 | 77.6 (22.9) * | 79.5 (19.6) * |
| eGFR < 60, n (%) | 466 (12%) | 92 (19.5%) * | 378 (10.9%) |
| Never smokers, (%) | 32% | 27.7% | 33.1% |
| Ex-smokers, (%) | 52% | 59.0% | 50.8% |
| Current smokers, (%) | 16% | 13.3% | 16.1% |
| Total energy intake, kcal/day | 2154 ± 683 | 2045 ± 690 * | 2169 ± 680 |
| Fat intake, g/d | 77.9 ± 35.4 | 74.2 ± 35.1 | 77.9 ± 34.7 |
| carbohydrate intake, g/day | 243.0 ± 87.0 | 229.6 ± 87.8 * | 245.1 ± 85.3 |
| protein intake, g/day | 82.6 ± 26.2 | 81.6 ± 27.9 | 82.7 ± 25.5 |
| Physical activity (MET hours/week) | 41.5 (64.6) | 31.6 (54.9) * | 42.7 (65.8) |
| Major osteoporotic fractures, n (%) | 334 (8.5%) | 42 (9%) | 292 (8.4%) |
| Vertebral fractures, n (%) | 296 (7.5%) | 261 (7.5%) | 35 (7.4%) |
| Femoral neck BMD, g/cm2 | 0.901 ± 0.137 | 0.916 ± 0.142 * | 0.899 ±0.136 |
| Lumbar spine BMD, g/cm2 | 1.140 ± 0.217 | 1.194 ± 0.215 * | 1.132 ± 0.216 |
| TBS (unitless) | 1.311 ± 0.101 | 1.297 ± 0.107 | 1.313 ± 0.101 |
| Outcome | Standardized Coefficient β (p-Value) | ||
|---|---|---|---|
| Model 1 | Model 2 | Model 3 | |
| Femoral Neck BMD | 0.000 (0.97) | −0.001 (0.73) | −0.006 (0.70) |
| Lumbar spine BMD | −0.012 (0.41) | −0.012 (0.42) | −0.013 (0.38) |
| Trabecular bone score | 0.003 (0.89) | −0.008 (0.73) | −0.015 (0.48) |
| Major Osteoporotic Fractures (MOFs) | Vertebral Fractures (VFs) | |||
|---|---|---|---|---|
| ORs (95% CI) | p-Value | ORs (95% CI) | p-Value | |
| N (%) | 334 (8.5%) | 296 (7.5%) | ||
| Model 1 | 1.08 (0.95–1.24) | 0.23 | 1.13 (0.98–1.27) | 0.06 |
| Model 2 | 1.12 (0.98–1.28) | 0.10 | 1.15 (1.01–1.31) | 0.02 |
| Model 3 | 1.12 (0.98–1.27) | 0.11 | 1.16 (1.01–1.31) | 0.02 |
| Model 4 (BMD) | 1.11 (0.98–1.27) | 0.11 | 1.15 (1.01–1.31) | 0.025 |
| N = 3949 Fully Adjusted Models | Major Osteoporotic Fractures (MOFs) | Vertebral Fractures (VFs) | ||
|---|---|---|---|---|
| ORs (95% CI) | p-Value | ORs (95% CI) | p-Value | |
| Sweets | 1.04 (0.80–1.38) | 0.77 | 1.09 (0.83–1.43) | 0.52 |
| Whole grains | 1.02 (0.90–1.17) | 0.73 | 1.02 (0.90–1.15) | 0.78 |
| Milk | 1.06 (0.92–1.18) | 0.51 | 1.09 (0.97–1.22) | 0.15 |
| Unprocessed meat | 1.06 (0.95–1.18) | 0.33 | 1.11 (0.997–1.24) | 0.06 |
| Refined grains | 1.07 (0.94–1.21) | 0.30 | 1.05 (0.94–1.18) | 0.38 |
| Processed meat | 1.40 (0.62–3.12) | 0.42 | 1.74 (0.83–3.67) | 0.15 |
| Nuts | 1.03 (0.29–1.12) | 0.10 | 0.62 (0.32–1.20) | 0.15 |
| Pulses | 0.87 (0.49–1.55) | 0.63 | 1.05 (0.63–1.77) | 0.85 |
| Fish and Seafood | 0.40 (0.12–1.37) | 0.15 | 0.52 (0.16–1.71) | 0.28 |
| Yogurt | 1.21 (0.96–1.54) | 0.11 | 1.14 (0.88–1.48) | 0.33 |
| Top 6 combined | 1.09 (0.97–1.22) | 0.17 | 1.15 (1.03–1.29) | 0.02 |
| Top 10 combined | 1.11 (0.98–1.27) | 0.10 | 1.15 (1.01–1.30) | 0.03 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Waqas, K.; Chen, J.; van der Eerden, B.C.J.; Ikram, M.A.; Uitterlinden, A.G.; Voortman, T.; Zillikens, M.C. Dietary Advanced Glycation End-Products (dAGEs) Intake and Bone Health: A Cross-Sectional Analysis in the Rotterdam Study. Nutrients 2020, 12, 2377. https://doi.org/10.3390/nu12082377
Waqas K, Chen J, van der Eerden BCJ, Ikram MA, Uitterlinden AG, Voortman T, Zillikens MC. Dietary Advanced Glycation End-Products (dAGEs) Intake and Bone Health: A Cross-Sectional Analysis in the Rotterdam Study. Nutrients. 2020; 12(8):2377. https://doi.org/10.3390/nu12082377
Chicago/Turabian StyleWaqas, Komal, Jinluan Chen, Bram C. J. van der Eerden, M. Arfan Ikram, André G. Uitterlinden, Trudy Voortman, and M. Carola Zillikens. 2020. "Dietary Advanced Glycation End-Products (dAGEs) Intake and Bone Health: A Cross-Sectional Analysis in the Rotterdam Study" Nutrients 12, no. 8: 2377. https://doi.org/10.3390/nu12082377
APA StyleWaqas, K., Chen, J., van der Eerden, B. C. J., Ikram, M. A., Uitterlinden, A. G., Voortman, T., & Zillikens, M. C. (2020). Dietary Advanced Glycation End-Products (dAGEs) Intake and Bone Health: A Cross-Sectional Analysis in the Rotterdam Study. Nutrients, 12(8), 2377. https://doi.org/10.3390/nu12082377






