Lactoferrin Functionalized Biomaterials: Tools for Prevention of Implant-Associated Infections
Abstract
:1. Introduction
2. Implant-Associated Infections
3. Surface Functionalization with Antimicrobial Peptides
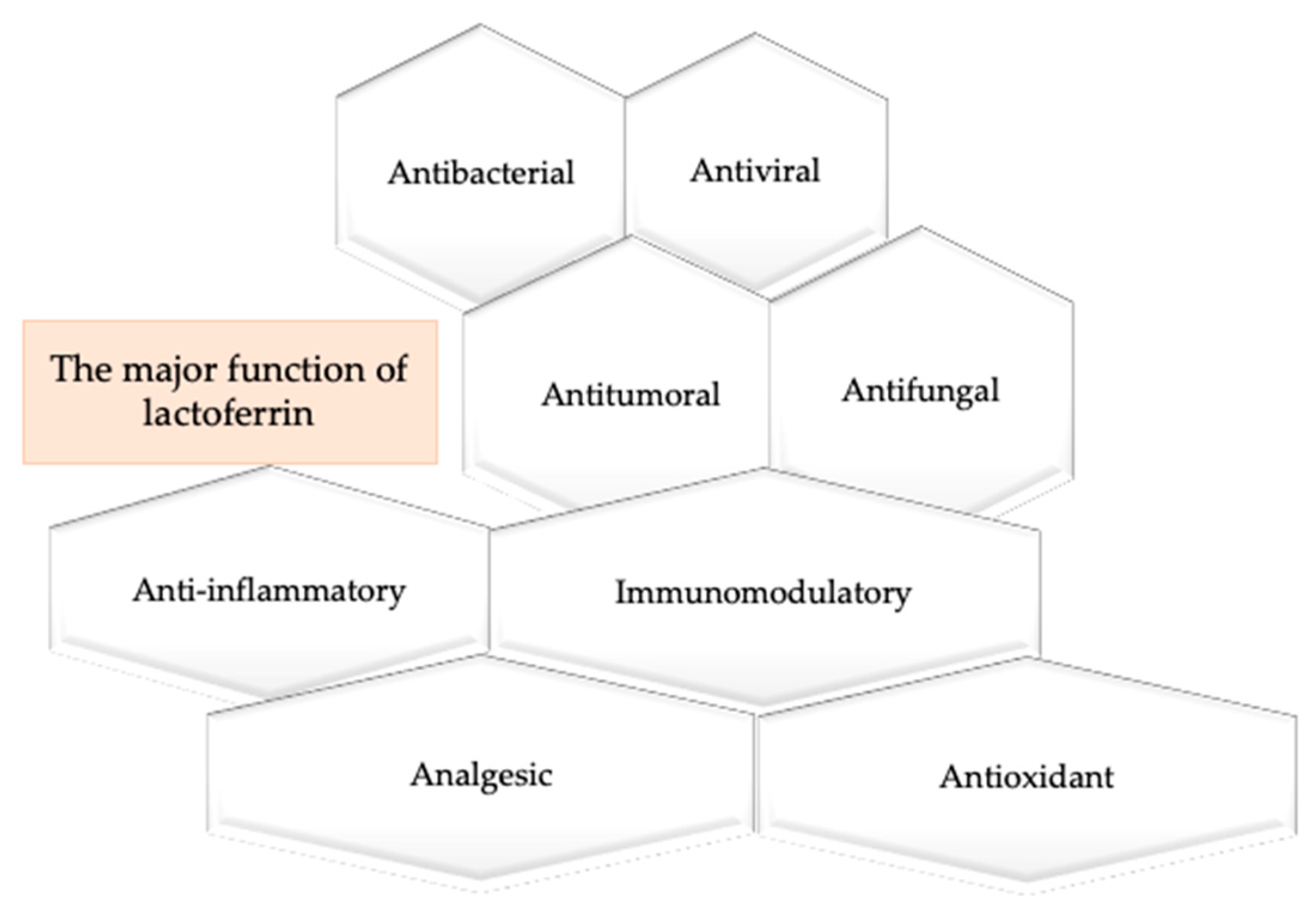
4. The Multiple Properties of Lactoferrin
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Navarro, M.; Michiardi, A.; Castaño, O.; Planell, J.A. Biomaterials in orthopaedics. J. R. Soc. Interface 2008, 5, 1137–1158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribeiro, M.; Monteiro, F.J.; Ferraz, M.P. Infection of orthopedic implants with emphasis on bacterial adhesion process and techniques used in studying bacterial-material interactions. Biomatter 2012, 2, 176–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, D.F. The Williams Dictionary of Biomaterials; Liverpool University Press: Liverpool, UK, 1999; pp. 340–357. [Google Scholar]
- Nair, L.S.; Cato, T.L. Biodegradable polymers as biomaterials. Prog. Polym. Sci. 2007, 32, 762–798. [Google Scholar] [CrossRef]
- Hench, L.L.; Polak, J.M. Third-generation biomedical materials. Science 2002, 295, 1014–1017. [Google Scholar] [CrossRef] [Green Version]
- Davis, J.R. Handbook of Materials for Medical Devices; ASM International: Materials Park, OH, USA, 2003; pp. 179–194. [Google Scholar]
- Chen, Q.; Thouas, G. Biomaterials: A Basic Introduction; CRC Press: Boca Raton, FL, USA, 2014; pp. 63–84. [Google Scholar]
- Narayan, R. Encyclopedia of Biomedical Engineering; Elsevier: Amsterdam, The Netherlands, 2019; pp. 425–439. [Google Scholar]
- Campoccia, D.; Montanaro, L.; Speziale, P.; Arciola, C.R. Antibiotic-loaded biomaterials and the risks for the spread of antibiotic resistance following their prophylactic and therapeutic clinical use. Biomaterials 2010, 31, 6363–6377. [Google Scholar] [CrossRef]
- Paital, S.R.; Dahotre, N.B. Calcium phosphate coatings for bio-implant applications: Materials, performance factors, and methodologies. Mater. Sci. Eng. Rep. 2009, 66, 1–70. [Google Scholar] [CrossRef]
- Rehman, M.; Madni, A.; Webster, T.J. The era of biofunctional biomaterials in orthopedics: What does the future hold? Expert Rev. Med. Devices 2018, 15, 193–204. [Google Scholar] [CrossRef]
- Wang, J.L.; Xu, J.K.; Hopkins, C.; Chow, D.H.; Qin, L. Biodegradable Magnesium-Based Implants in Orthopedics-A General Review and Perspectives. Adv. Sci. 2020, 28, 1902443. [Google Scholar] [CrossRef]
- Klimek, K.; Ginalska, G. Proteins and Peptides as Important Modifiers of the Polymer Scaffolds for Tissue Engineering Applications-A Review. Polymers 2020, 12, 844. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Webster, T.J. Nanotechnology and nanomaterials: Promises for improved tissue regeneration. Nanotoday 2009, 4, 66–80. [Google Scholar] [CrossRef]
- Lloyd, A.W. Interfacial bioengineering to enhance surface biocompatibility. Med. Device Technol. 2002, 13, 18–21. [Google Scholar] [PubMed]
- Song, R.; Murphy, M.; Li, C.; Ting, K.; Soo, C.; Zheng, Z. Current development of biodegradable polymeric materials for biomedical applications. Drug Des. Dev. Ther. 2018, 12, 3117–3145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kohane, D.; Langer, R. Polymeric Biomaterials in Tissue Engineering. Pediatr. Res. 2008, 63, 487–491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loureiro dos Santos, L.A. Natural Polymeric Biomaterials: Processing and Properties. Reference Module in Materials Science and Materials Engineering. In Reference Module in Materials Science and Materials Engineering; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1–5. [Google Scholar]
- Freyman, T.M.; Yannas, I.V.; Yokoo, R.; Gibson, L.J. Fibroblast contraction of a collagen—GAG matrix. Biomaterials 2001, 22, 2883–2891. [Google Scholar] [CrossRef]
- Park, H.; Choi, B.; Hu, J.; Lee, M. Injectable chitosan hyaluronic acid hydrogels for cartilage tissue engineering. Acta Biomater. 2013, 9, 4779–4786. [Google Scholar] [CrossRef]
- Kato, K.; Eika, Y.; Ikada, Y. Deposition of a hydroxyapatite thin layer onto a polymer surface carrying grafted phosphate polymer chains. J. Biomed. Mater. Res. 1996, 32, 687–691. [Google Scholar] [CrossRef]
- Ma, Z.; Gao, C.; Gong, Y.; Ji, J.; Shen, J. Immobilization of natural macromolecules on poly-L-lactic acid membrane surface in order to improve its cytocompatibility. J. Biomed. Mater. Res. 2002, 63, 838–847. [Google Scholar] [CrossRef]
- Hutmacher, D.; Hürzeler, M.B.; Schliephake, H. A review of material properties of biodegradable and bioresorbable polymer for GTR and GBR. Int. J. Oral Maxillofac. Implants 2000, 11, 667–678. [Google Scholar]
- Riool, M.; de Breij, A.; Drijfhout, J.W.; Nibbering, P.H.; Zaat, S.A.J. Antimicrobial Peptides in Biomedical Device Manufacturing. Front. Chem. 2017, 5, 63. [Google Scholar] [CrossRef]
- Diefenbeck, M.; Mückley, T.; Gunther, O.; Hofmann, G. Prophylaxis and treatment of implant-related infections by local application of antibiotics. Injury 2006, 32, S95–S104. [Google Scholar] [CrossRef]
- Kazemzadeh-Narbat, M.; Kindrachuk, J.; Duan, K.; Jenssen, H.; Hancock, R.E.; Wang, R. Antimicrobial peptides on calcium phosphate-coated titanium for the prevention of implant-associated infections. Biomaterials 2010, 31, 9519–9526. [Google Scholar] [CrossRef] [PubMed]
- Dhammi, I.K.; Ul Haq, R.; Kumar, S. Prophylactic antibiotics in orthopedic surgery: Controversial issues in its use. Indian J. Orthop. 2015, 49, 373–376. [Google Scholar] [CrossRef] [PubMed]
- Boxma, H.; Broekhuizen, T.; Patka, P.; Oosting, H. Randomised controlled trial of single-dose antibiotic prophylaxis in surgical treatment of closed fractures: The Dutch Trauma trial. Lancet 1996, 347, 1133–1137. [Google Scholar] [CrossRef]
- Ewald, A.; Glückermann, S.K.; Thull, R.; Gbureck, U. Antimicrobial titanium/silver PVD coatings on titanium. Biomed. Eng. Online 2006, 5, 22. [Google Scholar] [CrossRef] [Green Version]
- Romanò, C.L.; Scarponi, S.; Gallazzi, E.; Romanò, D.; Drago, L. Antibacterial coating of implants in orthopaedics and trauma: A classification proposal in an evolving panorama. J. Orthop. Surg. Res. 2015, 10, 157. [Google Scholar] [CrossRef] [Green Version]
- Arciola, C.R.; Campoccia, D.; Montanaro, L. Implant infections: Adhesion, biofilm formation and immune evasion. Nat Rev Microbiol 2018, 16, 397–409. [Google Scholar] [CrossRef]
- Trampuz, A.; Osmon, D.R.; Hanssen, A.D.; Steckelberg, J.M.; Patel, R. Molecular and antibiofilm approaches to prosthetic joint infection. Clin. Orthop. Relat. Res. 2003, 414, 69–88. [Google Scholar] [CrossRef]
- Bormann, N.; Koliszak, A.; Kasper, S.; Schoen, L.; Hilpert, K.; Volkmer, R.; Kikhney, J.; Wildemann, B. A short artificial antimicrobial peptide shows potential to prevent or treat bone infections. Sci. Rep. 2017, 7, 1506. [Google Scholar] [CrossRef] [Green Version]
- Zilberman, M.; Elsner, J.J. Antibiotic-eluting medical devices for various applications. J. Control. Release 2008, 130, 202–215. [Google Scholar] [CrossRef]
- Nandakumar, V.; Chittaranjan, S.; Kurian, V.; Doble, M. Characteristics of bacterial biofilm associated with implant material in clinical practice. Polym. J. 2013, 45, 137–152. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, W.; Zhai, Z.; Gao, C. Adaptive antibacterial biomaterial surfaces and their applications. Mater. Today Bio. 2019, 2, 100017. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Yu, Q.; Sun, H. Novel strategies for the prevention and treatment of biofilm related infections. Int. J. Mol. Sci. 2013, 14, 18488–18501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khatoon, Z.; McTiernan, C.D.; Suuronen, E.J.; Mah, T.F.; Alarcon, E.I. Bacterial biofilm formation on implantable devices and approaches to its treatment and prevention. Heliyon 2018, 4, e01067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benito, N.; Franco, M.; Ribera, A.; Soriano, A.; Rodriguez-Pardo, D.; Sorlí, L.; Fresco, G.; Fernández-Sampedro, M.; Dolores Del Toro, M.; Guío, L.; et al. Time trends in the aetiology of prosthetic joint infections: A multicentre cohort study. Clin. Microbiol. Infect. 2016, 22, 732.e1–732.e8. [Google Scholar] [CrossRef] [Green Version]
- Pfang, B.G.; García-Cañete, J.; García-Lasheras, J.; Blanco, A.; Auñón, Á.; Parron-Cambero, R.; Macías-Valcayo, A.; Esteban, J. Orthopedic Implant-Associated Infection by Multidrug Resistant Enterobact. J. Clin. Med. 2019, 8, 220. [Google Scholar] [CrossRef] [Green Version]
- Osmon, D.R.; Berbari, E.F.; Berendt, A.R.; Lew, D.; Zimmerli, W.; Steckelberg, J.M.; Rao, N.; Hanssen, A.; Wilson, W.R. Diagnosis and Management of Prosthetic Joint Infection: Clinical Practice Guidelines by the Infectious Diseases Society of America. Clin. Infect. Dis. 2013, 56, e1–e25. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Barrena, E.; Esteban, J.; Medel, F.; Molina-Manso, D.; Ortiz-Pérez, A.; Cordero-Ampuero, J.; Puértolas, J.A. Bacterial adherence to separated modular components in joint prosthesis: A clinical study. J. Orthop. Res. 2012, 30, 1634–1639. [Google Scholar] [CrossRef] [Green Version]
- Ariza, J.; Cobo, J.; Baraia-Etxaburu, J.; Benito, N.; Bori, G.; Cabo, J.; Corona, P.; Esteban, J.; Horcajada, J.P.; Lora-Tamayo, J.; et al. Executive summary of management of prosthetic joint infections. Clinical practice guidelines by the Spanish Society of Infectious Diseases and Clinical Microbiology (SEIMC). Enferm. Infecc. Microbiol. Clínica 2017, 35, 189–195. [Google Scholar] [CrossRef]
- Li, B.; Webster, T.J. Bacteria antibiotic resistance: New challenges and opportunities for implant-associated orthopedic infections. J. Orthop. Res. 2018, 36, 22–32. [Google Scholar] [CrossRef] [Green Version]
- Turner, I.G.; Pilliar, R.M.; Srichana, T.; Domb, A.J.; Lacroix, D.; Planell, J.A. Sterility and Infection. In Biomedical Materials; Narayan, R., Ed.; Springer Science: New York, NY, USA, 2009; pp. 239–258. [Google Scholar]
- Magiorakos, A.-P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [Green Version]
- Ventola, C.L. The antibiotic resistance crisis: Part 1: Causes and threats. Pharm. Ther. 2015, 40, 277–283. [Google Scholar]
- Pfalzgraff, A.; Brandenburg, K.; Weindl, G. Antimicrobial Peptides and Their Therapeutic Potential for Bacterial Skin Infections and Wounds. Front. Pharmacol. 2018, 9, 281. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.; Sun, L.; Huang, S.; Zhu, C.; Li, P.; He, J.; Mackey, V.; Coy, D.H.; He, Q. The antimicrobial peptides and their potential clinical applications. Am. J. Transl. Res. 2019, 11, 3919–3931. [Google Scholar] [PubMed]
- Darouiche, R.O. Antimicrobial coating of devices for prevention of infection: Principles and protection. Int. J. Artif. Organs 2007, 30, 820–827. [Google Scholar] [CrossRef]
- Knetsch, M.L.; Koole, L.H. New strategies in the development of antimicrobial coatings: The example of increasing usage of silver and silver nanoparticles. Polymers 2011, 3, 340–366. [Google Scholar] [CrossRef]
- Siedenbiedel, F.; Tiller, J.C. Antimicrobial polymers in solution and on surfaces: Overview and functional principles. Polymers 2012, 4, 46–71. [Google Scholar] [CrossRef] [Green Version]
- Chen, R.; Willcox, M.D.; Ho, K.K.; Smyth, D.; Kumar, N. Antimicrobial peptide melimine coating for titanium and its in vivo antibacterial activity in rodent subcutaneous infection models. Biomaterials. 2016, 85, 142–151. [Google Scholar] [CrossRef]
- Khurshid, Z.; Zafar, M.S.; Najeeb, S.; Nejatian, T.; Sefat, F. Introduction to dental biomaterials and their advances. In Advanced Dental Biomaterials; Woodhead Publishing: Amsterdam, The Netherlands, 2019; pp. 1–5. [Google Scholar]
- Kamaly, N.; Yameen, B.; Wu, J.; Farokhzad, O.C. Degradable Controlled-Release Polymers and Polymeric Nanoparticles: Mechanisms of Controlling Drug Release. Chem. Rev. 2016, 116, 2602–2663. [Google Scholar] [CrossRef] [Green Version]
- Gould, I.M.; Bal, A.M. New antibiotic agents in the pipeline and how they can help overcome microbial resistance. Virulence 2013, 4, 185–191. [Google Scholar] [CrossRef] [Green Version]
- Nocerino, N.; Fulgione, A.; Iannaccone, M.; Tomasetta, L.; Ianniello, F.; Martora, F.; Lelli, M.; Roveri, N.; Capuano, F.; Capparelli, R. Biological activity of lactoferrin-functionalized biomimetic hydroxyapatite nanocrystals. Int. J. Nanomed. 2014, 9, 1175–1184. [Google Scholar]
- World Health Organization. Antibiotic Resistance. 5 February 2018. Available online: https://www.who.int/news-room/fact-sheets/detail/antibiotic-resistance (accessed on 7 May 2020).
- Centers for Disease Control and Prevention. Office of Infectious Disease Antibiotic Resistance Threats in the United States. Available online: http://www.cdc.gov/drugresistance/threat-report-2013 (accessed on 7 May 2020).
- Aslam, B.; Wang, W.; Arshad, M.I.; Khurshid, M.; Muzammil, S.; Rasool, M.H.; Nisar, M.A.; Alvi, R.F.; Aslam, M.A.; Qamar, M.U.; et al. Antibiotic resistance: A rundown of a global crisis. Infect. Drug Resist. 2018, 11, 1645–1658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yazici, H.; O’Neill, M.B.; Kacar, T.; Wilson, B.R.; Oren, E.E.; Sarikaya, M.; Tamerler, C. Engineered Chimeric Peptides as Antimicrobial Surface Coating Agents toward Infection-Free Implants. ACS Appl Mater. Interfaces. 2016, 8, 5070–5081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wimley, W.C.; Hristova, K. Antimicrobial peptides: Successes, challenges and unanswered questions. J. Membr. Biol. 2011, 239, 27–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Townsend, L.; Williams, R.L.; Anuforom, O.; Berwick, M.R.; Halstead, F.; Hughes, E.; Stamboulis, A.; Oppenheim, B.; Gough, J.; Grover, L.; et al. Antimicrobial peptide coatings for hydroxyapatite: Electrostatic and covalent attachment of antimicrobial peptides to surfaces. J. R. Soc. Interface. 2017, 14, 20160657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corrales-Ureña, Y.R.; Souza-Schiaber, Z.; Lisboa-Filho, P.N.; Marquenet, F.; Noeske, P.M.; Gätjen, L.; Rischka, K. Functionalization of hydrophobic surfaces with antimicrobial peptides immobilized on a biointerfactant layer. RSC Adv. 2020, 10, 376. [Google Scholar] [CrossRef] [Green Version]
- Jenssen, H.; Hamill, P.; Hancock, R.E. Peptide antimicrobial agents. Clin. Microbiol. Rev. 2006, 19, 491–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahman, A.; Choudhary, M.I. Applications of NMR Spectroscopy; Betham eBooks: Bussum, The Netherlands, 2016; eISSN 2405-4682. [Google Scholar]
- Teixeira, V.; Feio, M.J.; Bastos, M. Role of lipids in the interaction of antimicrobial peptides with membranes. Prog. Lipid Res. 2012, 51, 149–177. [Google Scholar] [CrossRef]
- Chai, H.; Allen, W.E.; Hicks, R.P. Synthetic Antimicrobial Peptides Exhibit Two Different Binding Mechanisms to the Lipopolysaccharides Isolated from Pseudomonas aeruginosa and Klebsiella pneumoniae. Int. J. Med. Chem. 2014, 2014, 809283. [Google Scholar]
- Gao, W.; Xing, L.; Qu, P.; Tan, T.; Yang, N.; Li, D.; Chen, H.; Feng, X. Identification of a novel cathelicidin antimicrobial peptide from ducks and determination of its functional activity and antibacterial mechanism. Sci. Rep. 2015, 5, 17260. [Google Scholar] [CrossRef] [Green Version]
- Kang, H.K.; Kim, C.; Seo, C.H.; Park, Y. The therapeutic applications of antimicrobial peptides (AMPs): A patent review. J. Microbiol. 2017, 55, 1–12. [Google Scholar] [CrossRef]
- Goytia, M.; Kandler, J.L.; Shafer, W.M. Mechanisms and significance of bacterial resistance to human cationic antimicrobial peptides. In Antimicrobial Peptides and Innate Immunity; Hiemstra, P., Zaat, S., Eds.; Springer: Basel, Switzerland, 2013; pp. 219–254. [Google Scholar]
- Ernst, C.M.; Kuhn, S.; Slavetinsky, C.J.; Krismer, B.; Heilbronner, S.; Gekeler, C.; Kraus, D.; Wagner, S.; Peschel, A. The lipid-modifying multiple peptide resistance factor is an oligomer consisting of distinct interacting synthase and flippase subunits. MBio 2015, 6, e02340-14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joo, H.S.; Fu, C.I.; Otto, M. Bacterial strategies of resistance to antimicrobial peptides. Philos Trans. R. Soc. B Biol. Sci. 2016, 371, 20150292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bahar, A.A.; Ren, D. Antimicrobial peptides. Pharmaceuticals 2013, 28, 1543–1575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohammad, H.; Thangamani, S.; Seleem, M.N. Antimicrobial peptides and peptidomimetics—Potent therapeutic allies for staphylococcal infections. Curr. Pharm. Des. 2015, 21, 2073–2088. [Google Scholar] [CrossRef]
- Mohamed, M.; Abdelkhalek, A.; Seleem, M. Evaluation of short synthetic antimicrobial peptides for treatment of drug-resistant and intracellular Staphylococcus aureus. Sci. Rep. 2016, 6, 29707. [Google Scholar] [CrossRef]
- Kumar, P.; Kizhakkedathu, J.N.; Straus, S.K. Antimicrobial Peptides: Diversity, Mechanism of Action and Strategies to Improve the Activity and Biocompatibility In Vivo. Biomolecules 2018, 8, 4. [Google Scholar] [CrossRef] [Green Version]
- Ebner, P.; Reichert, S.; Luqman, A.; Krismer, B.; Popella, P.; Götz, F. Lantibiotic production is a burden for the producing staphylococci. Sci. Rep. 2018, 8, 7471. [Google Scholar] [CrossRef]
- Umu, Ö.C.; Bäuerl, C.; Oostindjer, M.; Pope, P.B.; Hernández, P.E.; Pérez-Martínez, G.; Diep, D.B. The Potential of Class II Bacteriocins to Modify Gut Microbiota to Improve Host Health. PLoS ONE 2016, 11, e0164036. [Google Scholar] [CrossRef] [Green Version]
- Kościuczuk, E.M.; Lisowski, P.; Jarczak, J.; Strzałkowska, N.; Jóźwik, A.; Horbańczuk, J.; Krzyżewski, J.; Zwierzchowski, L.; Bagnicka, E. Cathelicidins: Family of antimicrobial peptides. A review. Mol. Biol. Rep. 2012, 39, 10957–10970. [Google Scholar] [CrossRef] [Green Version]
- Elsbach, P. What is the real role of antimicrobial polypeptides that can mediate several other inflammatory responses? J. Clin. Investig. 2003, 111, 1643–1645. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.H.; Lu, T.K. Development and Challenges of Antimicrobial Peptides for Therapeutic Applications. Antibiotics 2020, 9, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zanetti, M. The role of cathelicidins in the innate host defenses of mammals. Curr. Issues Mol. Biol. 2005, 7, 179–196. [Google Scholar] [PubMed]
- Selsted, M.E.; Ouellette, A.J. Mammalian defensins in the antimicrobial immune response. Nat. Immunol. 2005, 6, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Chairatana, P.; Chu, H.; Castillo, P.A.; Shen, B.; Bevins, C.L.; Nolan, E.M. Proteolysis Triggers Self-Assembly and Unmasks Innate Immune Function of a Human α-Defensin Peptide. Chem. Sci. 2016, 7, 1738–1752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Overhage, J.; Campisano, A.; Bains, M.; Torfs, E.C.W.; Rehm, B.H.A.; Hancock, R.E.W. Human host defense peptide LL-37 prevents bacterial biofilm formation. Infect. Immun. 2008, 76, 4176–4182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melicherčík, P.; Nešuta, O.; Čeřovský, V. Antimicrobial Peptides for Topical Treatment of Osteomyelitis and Implant-Related Infections: Study in the Spongy Bone. Pharmaceuticals 2018, 11, 20. [Google Scholar]
- Sakellariou, V.I.; Savvidou, O.; Markopoulos, C.; Drakou, A.; Mavrogenis, A.F.; Papagelopoulos, P.J. Combination of Calcium Hydroxyapatite Antibiotic Carrier with Cement Spacers in Peri-Prosthetic Knee Infections. Surg. Infect. 2015, 16, 748–754. [Google Scholar] [CrossRef]
- Qiu, Y.; Zhang, N.; An, Y.H.; Wen, X. Biomaterial strategies to reduce implant-associated infections. Int. J. Artif. Organs 2007, 30, 828–841. [Google Scholar] [CrossRef]
- García-Montoya, I.A.; Cendón, T.S.; Arévalo-Gallegos, S.; Rascón-Cruz, Q. Lactoferrin a multiple bioactive protein: An overview. Biochim. Biophys. Acta. 2012, 1820, 226–236. [Google Scholar] [CrossRef]
- Sinha, M.; Sanket, K.; Punit, K.; Sharma, S.; Singh, T.P. Antimicrobial Lactoferrin Peptides: The Hidden Players in the Protective Function of a Multifunctional Protein. Int. J. Pept. 2013, 390230, 12. [Google Scholar] [CrossRef] [Green Version]
- Amini, A.A.; Nair, L.S. Recombinant human lactoferrin as a biomaterial for bone tissue engineering: Mechanism of antiapoptotic and osteogenic activity. Adv. Healthc Mater. 2014, 3, 897–905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naot, D.; Grey, A.; Reid, I.R.; Cornish, J. Lactoferrin—A novel bone growth factor. Clin. Med. Res. 2005, 3, 93–101. [Google Scholar] [CrossRef] [Green Version]
- Vandrovcova, M.; Douglas, T.E.; Heinemann, S.; Scharnweber, D.; Dubruel, P.; Bacakova, L. Collagen-lactoferrin fibrillar coatings enhance osteoblast proliferation and differentiation. J. Biomed. Mater. Res. Part A 2015, 103, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Kruzel, M.L.; Zimecki, M.; Actor, J.K. Lactoferrin in a Context of Inflammation-Induced Pathology. Front. Immunol. 2017, 8, 1438. [Google Scholar] [CrossRef] [PubMed]
- Görmez, U.; Kürkcü, M.E.; Benlidayi, M.; Ulubayram, K.; Sertdemir, Y.; Dağlioğlu, K. Effects of bovine lactoferrin in surgically created bone defects on bone regeneration around implants. J. Oral Sci. 2015, 57, 7–15. [Google Scholar] [CrossRef]
- Kilic, E.; Novoselova, M.V.; Lim, S.H.; Pyataev, N.; Pinyaev, M.V.; Kulikov, O.A.; Sindeeva, O.A.; Mayorova, O.A.; Murney, R.; Antipina, M.A.; et al. Formulation for Oral Delivery of Lactoferrin Based on Bovine Serum Albumin and Tannic Acid Multilayer Microcapsules. Sci. Rep. 2017, 7, 44159. [Google Scholar] [CrossRef] [Green Version]
- Hao, L.; Shan, Q.; Wei, J.; Ma, F.; Sun, P. Lactoferrin: Major Physiological Functions and Applications. Curr. Protein Pept. Sci. 2019, 20, 139–144. [Google Scholar] [CrossRef]
- Jenssen, H.; Hancock, R.E. Antimicrobial properties of lactoferrin. Biochimie 2009, 91, 19–29. [Google Scholar] [CrossRef]
- Giansanti, F.; Panella, G.; Leboffe, L.; Antonini, G. Lactoferrin from Milk: Nutraceutical and Pharmacological Properties. Pharmaceuticals 2016, 9, 61. [Google Scholar] [CrossRef] [Green Version]
- Moreno-Expósito, L.; Illescas-Montes, R.; Melguizo-Rodríguez, L.; Ruiz, C.; Ramos-Torrecillas, J.; de Luna-Bertos, E. Multifunctional capacity and therapeutic potential of lactoferrin. Life Sci. 2018, 195, 61–64. [Google Scholar] [CrossRef]
- Embleton, N.D.; Berrington, J.E.; McGuire, W.; Stewart, C.J.; Cummings, S.P. Lactoferrin: Antimicrobial activity and therapeutic potential. In Semin in Fetal and Neonatal Medicine; WB Saunders: Philadelphia, PA, USA, 2013; Volume 18, pp. 143–149. [Google Scholar]
- Miyazawa, K.; Mantel, C.; Lu, L.; Morrison, D.C.; Broxmeyer, H.E. Lactoferrin-lipopolysaccharide interactions. Effect on lactoferrin binding to monocyte/macrophage-differentiated HL-60 cells. J. Immunol. 1991, 146, 723–729. [Google Scholar] [PubMed]
- González-Chávez, S.A.; Arévalo-Gallegos, S.; Rascón-Cruz, Q. Lactoferrin: Structure, function and applications, Int. J. Antimicrob. Agents 2009, 33, e1–e8. [Google Scholar] [CrossRef] [PubMed]
- Yen, C.C.; Shen, C.J.; Hsu, W.H.; Chang, Y.H.; Lin, H.T.; Chen, H.L.; Chen, C.M. Lactoferrin: An iron-binding antimicrobial protein against Escherichia coli infection. Biometals 2011, 24, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Ammons, M.C.; Copié, V. Mini-review: Lactoferrin: A bioinspired, anti-biofilm therapeutic. Biofouling 2013, 29, 443–455. [Google Scholar] [CrossRef] [Green Version]
- Icriverzi, M.; Dinca, V.; Moisei, M.; Evans, R.W.; Trif, M.; Roseanu, A. Lactoferrin in Bone Tissue Regeneration. Curr. Med. Chem. 2020, 27, 838–853. [Google Scholar] [CrossRef]
- Owen, R.; Reill, G.C. In vitro Models of Bone Remodelling and Associated Disorders. Front. Bioeng. Biotech. 2018, 6, 134. [Google Scholar] [CrossRef]
- Bastos, A.R.; da Silva, L.P.; Maia, F.R.; Pina, S.; Rodrigues, T.; Sousa, F.; Oliveira, J.M.; Cornish, J.; Correlo, V.M.; Reis, R.L. Lactoferrin-Hydroxyapatite Containing Spongy-Like Hydrogels for Bone Tissue Engineering. Materials 2019, 12, 2074. [Google Scholar] [CrossRef] [Green Version]
- Icriverzi, M.; Bonciu, A.; Rusen, L.; Sima, L.E.; Brajnicov, S.; Cimpean, A.; Evans, R.W.; Dinca, V.; Roseanu, A. Human Mesenchymal Stem Cell Response to Lactoferrin-based Composite Coatings. Materials 2019, 12, 3414. [Google Scholar] [CrossRef] [Green Version]
- Ying, X.; Cheng, S.; Wang, W.; Lin, Z.; Chen, Q.; Zhang, W.; Kou, D.; Shen, Y.; Cheng, X.; Peng, L.; et al. Effect of lactoferrin on osteogenic differentiation of human adipose stem cells. Int. Orthop. 2012, 36, 647–653. [Google Scholar] [CrossRef] [Green Version]
- Cornish, J.; Callon, K.E.; Naot, D.; Palmano, K.P.; Banovic, T.; Bava, U.; Watson, M.; Lin, J.-M.; Tong, P.C.; Chen, Q.; et al. Lactoferrin is a potent regulator of bone cell activity and increases bone formation in vivo. Endocrinology 2004, 145, 4366–4374. [Google Scholar] [CrossRef] [Green Version]
- Takaoka, R.; Hikasa, Y.; Hayashi, K.; Tabata, Y. Bone regeneration by lactoferrin released from a gelatin hydrogel. J. Biomater Sci. Polym. Ed. 2011, 22, 1581–1589. [Google Scholar] [CrossRef] [Green Version]
- Montesi, M.; Panseri, S.; Iafisco, M.; Adamiano, A.; Tampieri, A. Coupling Hydroxyapatite Nanocrystals with Lactoferrin as a Promising Strategy to Fine Regulate Bone Homeostasis. PLoS ONE 2015, 10, e0132633. [Google Scholar] [CrossRef] [PubMed]
- Onishi, H.; Machida, Y.; Koyama, K. Preparation and in vitro characteristics of lactoferrin-loaded chitosan microparticles. Drug Dev. Ind. Pharm. 2007, 33, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Shi, P.; Wang, Q.; Yu, C.; Fan, F.; Liu, M.; Tu, M.; Lu, W.; Du, M. Hydroxyapatite nanorod and microsphere functionalized with bioactive lactoferrin as a new biomaterial for enhancement bone regeneration. Colloids Surf. B 2017, 155, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Fox, K.; Tran, P.A.; Tran, N. Recent advances in research applications of nanophase hydroxyapatite. Chemphyschem 2012, 13, 2495–2506. [Google Scholar] [CrossRef]
- James, E.N.; Nair, L.S. Development and characterization of lactoferrin loaded poly (epsilon-caprolactone) nanofibers. J. Biomed. Nanotechnol. 2014, 10, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Bolscher, J.; Nazmi, K.; van Marle, J.; van’t Hof, W.; Veerman, E. Chimerization of lactoferricin and lactoferrampin peptides strongly potentiates the killing activity against. Biochem. Cell Biol. 2012, 90, 378–388. [Google Scholar] [CrossRef]
- Singh, P.; Parsek, M.; Greenberg, E.; Welsh, J.M. A component of innate immunity prevents bacterial biofilm development. Nature 2002, 417, 552–555. [Google Scholar] [CrossRef]
- Chen, R.; Cole, N.; Dutta, D.; Kumar, N.; Willcox, M.D.P. Antimicrobial activity of immobilized lactoferrin and lactoferricin. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 2612–2617. [Google Scholar] [CrossRef]
- Fulgione, A.; Nocerino, N.; Iannaccone, M.; Roperto, S.; Capuano, F.; Roveri, N.; Lelli, M.; Crasto, A.; Calogero, A.; Pilloni, A.P.; et al. Lactoferrin Adsorbed onto Biomimetic Hydroxyapatite Nanocrystals Controlling-In Vivo-the Helicobacter pylori Infection. PLoS ONE 2016, 11, e0158646. [Google Scholar] [CrossRef] [Green Version]
- Stoleru, E.; Zaharescu, T.; Hitruc, G.E.; Vesel, A.; Ioanid, E.G.; Coroaba, A.; Safrany, A.; Pricope, G.; Lungu, M.; Schick, C.; et al. Lactoferrin-Immobilized Surfaces onto Functionalized PLA Assisted by the Gamma-Rays and Nitrogen Plasma to Create Materials with Multifunctional Properties. ACS Appl. Mater. Interfaces 2016, 8, 31902–31915. [Google Scholar] [CrossRef] [PubMed]
- Jinkyu, L.; Jinki, L.; Sangmin, L.; Taufiq, A.; Sajeesh, K.M.P.; Eun, M.K.; Sang, W.L.; Heungsoo, S. Bioactive Membrane Immobilized with Lactoferrin for Modulation of Bone Regeneration and Inflammation. Tissue Eng. Part. A 2020, 1–15. [Google Scholar]
- Godoy-Gallardo, M.; Mas-Moruno, C.; Yu, K.; Manero, J.M.; Gil, F.J.; Kizhakkedathu, J.N.; Rodriguez, D. Antibacterial properties of hLf1-11 peptide onto titanium surfaces: A comparison study between silanization and surface initiated polymerization. Biomacromolecules 2015, 16, 483–496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brouwer, C.P.; Rahman, M.; Welling, M.M. Discovery and development of a synthetic peptide derived from lactoferrin for clinical use. Peptides 2011, 32, 1953–1963. [Google Scholar] [CrossRef]
- Costa, F.; Maia, S.; Gomes, J.; Gomes, P.; Martins, M.C. Characterization of hLF1-11 immobilization onto chitosan ultrathin films, and its effects on antimicrobial activity. Acta Biomater. 2014, 10, 3513–3521. [Google Scholar] [CrossRef] [Green Version]
- Nagano-Takebe, F.; Miyakawa, H.; Nakazawa, F.; Endo, K. Inhibition of initial bacterial adhesion on titanium surfaces by lactoferrin coating. Biointerphases 2014, 9, 029006. [Google Scholar] [CrossRef]
- Yoshinari, M.; Kato, T.; Matsuzaka, K.; Hayakawa, T.; Shiba, K. Prevention of biofilm formation on titanium surfaces modified with conjugated molecules comprised of antimicrobial and titanium-binding peptides. Biofouling 2010, 26, 103–110. [Google Scholar] [CrossRef] [Green Version]

| Material Description | Biologic Activity | Reference |
|---|---|---|
| Hydroxyapatite loaded PEG–PCL and lactoferrin | Osteoconductive and osteoinductive properties | [101] |
| Collagen membranes treated with Lf | Osteogenic lineage differentiation cells proliferation and differentiation | [94,91] |
| Gelatin hydrogel treated with Lf | Bone regeneration | [102] |
| AMSCs treatment with Lf | Osteogenic differentiation-related marker genes | [91] |
| Inorganic bovine bone and lactoferrin | Bone regeneration | [96] |
| rhLF on MC3T3 pre-osteoblast cells | Anti-apoptotic effect, support cell viability, proliferation and differentiation | [92] |
| Chitosan–alginate–Ca microparticles with Lf | Anti-inflammatory properties | [105,117] |
| Multilayer microcapsules with bovine serum albumin, tannic acid and Lf | Anti-inflammatory properties | [96] |
| Hydroxyapatites conjugated with lactoferrin | Antimicrobial property | [105] |
| Glass surfaces covalently bound with lactoferrin | Antimicrobial and antibiofilm property | [111] |
| Biopolymer loaded with hLf1-11 | Antimicrobial and antibiofilm property | [112] |
| Chitosan ultrathin films with hLF1–11 peptide | Antimicrobial property | [113,114] |
| Human Lf on titanium-based biomaterial | Antimicrobial property | [115,116] |
| Biomimetic hydroxyapatite | Antimicrobial property | [122] |
| Functionalized the poly (lactic acid) substrate anchoring with lactoferrin | Antioxidant, antimicrobial and cell-proliferation activity | [123] |
| Electrospun nanofibers immobilized with lactoferrin | Anti-inflammatory and bone regeneration | [124] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pall, E.; Roman, A. Lactoferrin Functionalized Biomaterials: Tools for Prevention of Implant-Associated Infections. Antibiotics 2020, 9, 522. https://doi.org/10.3390/antibiotics9080522
Pall E, Roman A. Lactoferrin Functionalized Biomaterials: Tools for Prevention of Implant-Associated Infections. Antibiotics. 2020; 9(8):522. https://doi.org/10.3390/antibiotics9080522
Chicago/Turabian StylePall, Emoke, and Alexandra Roman. 2020. "Lactoferrin Functionalized Biomaterials: Tools for Prevention of Implant-Associated Infections" Antibiotics 9, no. 8: 522. https://doi.org/10.3390/antibiotics9080522
APA StylePall, E., & Roman, A. (2020). Lactoferrin Functionalized Biomaterials: Tools for Prevention of Implant-Associated Infections. Antibiotics, 9(8), 522. https://doi.org/10.3390/antibiotics9080522






