Chrysanthemum morifolium Flower Extract Inhibits Adipogenesis of 3T3-L1 Cells via AMPK/SIRT1 Pathway Activation
Abstract
:1. Introduction
2. Materials and Methods
2.1. HCF Preparation
2.2. Ultra-Performance Liquid Chromatography (UPLC) Analysis
2.3. Cell Culture
2.4. Cytotoxicity Assay
2.5. Intracellular Lipid and Triglyceride (TG) Assay
2.6. Glycerol-3-Phosphate Dehydrogenase (GPDH) Activity
2.7. Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR)
2.8. AMPK and SIRT1 Activity
2.9. Statistical Analysis
3. Results
3.1. Content of Polyphenolic Compounds in HCF
3.2. Effect of HCF on 3T3-L1 Cell Viability
3.3. Effects of HCF on Lipid and TG Content
3.4. Effect of HCF on GPDH Activity
3.5. Effects of HCF on Adipogenesis/Lipogenesis-Related Gene Expression
3.6. Effects of HCF on AMPK and SIRT1 Activity
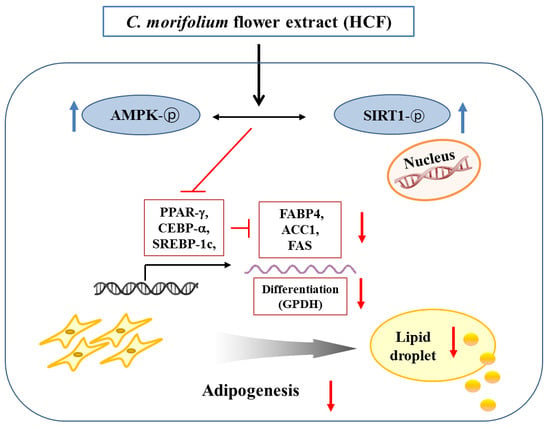
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Friedman, J.M. Obesity in the new millennium. Nature 2000, 404, 632–634. [Google Scholar] [CrossRef] [PubMed]
- Spiegelman, B.M.; Flier, J.S. Adipogenesis and obesity: Rounding out the big picture. Cell 1996, 87, 377–389. [Google Scholar] [CrossRef] [Green Version]
- O’Shea Alvarez, M.S. 3T3 cells in adipocytic conversion. Arch. Invest. Med. 1991, 22, 235–244. [Google Scholar]
- Ntambi, J.M.; Young-Cheul, K. Adipocyte differentiation and gene expression. J. Nutr. 2000, 130, 3122S–3126S. [Google Scholar] [CrossRef]
- Fajas, L.; Schoonjans, K.; Gelman, L.; Kim, J.B.; Najib, J.; Martin, G.; Fruchart, J.C.; Briggs, M.; Spiegelman, B.M.; Auwerx, J. Regulation of peroxisome proliferator-activated receptor gamma expression by adipocyte differentiation and determination factor 1/sterol regulatory element binding protein 1: Implications for adipocyte differentiation and metabolism. Mol. Cell Biol. 1999, 19, 5495–5503. [Google Scholar] [CrossRef] [Green Version]
- Spiegelman, B.M.; Choy, L.; Hotamisligil, G.S.; Graves, R.A.; Tontonoz, P. Regulation of adipocyte gene expression in differentiation and syndromes of obesity/diabetes. J. Biol. Chem. 1993, 268, 6823–6826. [Google Scholar]
- Hardie, D.G.; Ross, F.A.; Hawley, S.A. AMPK: A nutrient and energy sensor that maintains energy homeostasis. Nat. Rev. Mol. Cell Biol. 2012, 13, 251–262. [Google Scholar] [CrossRef] [Green Version]
- Ruderman, N.B.; Xu, X.J.; Nelson, L.; Cacicedo, J.M.; Saha, A.K.; Lan, F.; Ido, Y. AMPK and SIRT1: A long-standing partnership? Am. J. Physiol. Endocrinol. Metab. 2010, 298, E751–E760. [Google Scholar] [CrossRef]
- Villena, J.A.; Viollet, B.; Andreelli, F.; Kahn, A.; Vaulont, S.; Sul, H.S. Induced adiposity and adipocyte hypertrophy in mice lacking the AMP-activated protein kinase-alpha2 subunit. Diabetes 2004, 53, 2242–2249. [Google Scholar] [CrossRef] [Green Version]
- Stefanowicz, M.; Nikołajuk, A.; Matulewicz, N.; Karczewska-Kupczewska, M. Adipose tissue, but not skeletal muscle, sirtuin 1 expression is decreased in obesity and related to insulin sensitivity. Endocrine 2018, 60, 263–271. [Google Scholar] [CrossRef] [Green Version]
- Cui, Y.; Wang, X.; Xue, J.; Liu, J.; Xie, M. Chrysanthemum morifolium extract attenuates high-fat milk-induced fatty liver through peroxisome proliferator-activated receptor α-mediated mechanism in mice. Nutr. Res. 2014, 34, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Fu, X.; Lan, N.; Li, S.; Zhang, J.; Wang, S.; Li, C.; Shang, Y.; Huang, T.; Zhang, L. Luteolin protects against high fat diet-induced cognitive deficits in obesity mice. Behav. Brain Res. 2014, 267, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.S.; Qu, W. Dietary Apigenin promotes lipid catabolism, thermogenesis, and browning in adipose tissues of HFD-Fed mice. Food Chem. Toxicol. 2019, 133, 110780. [Google Scholar] [CrossRef] [PubMed]
- Cho, A.S.; Jeon, S.M.; Kim, M.J.; Yeo, J.; Seo, K.I.; Choi, M.S.; Lee, M.K. Chlorogenic acid exhibits anti-obesity property and improves lipid metabolism in high-fat diet-induced-obese mice. Food Chem. Toxicol. 2010, 48, 937–943. [Google Scholar] [CrossRef]
- Lin, G.H.; Lin, L.; Liang, H.W.; Ma, X.; Wang, J.Y.; Wu, L.P.; Jiang, H.D.; Bruce, I.C.; Xia, Q. Antioxidant action of a Chrysanthemum morifolium extract protects rat brain against ischemia and reperfusion injury. J. Med. Food 2010, 13, 306–311. [Google Scholar] [CrossRef]
- Tian, Z.; Jia, H.; Jin, Y.; Wang, M.; Kou, J.; Wang, C.; Rong, X.; Xie, X.; Han, G.; Pang, X. Chrysanthemum extract attenuates hepatotoxicity via inhibiting oxidative stress in vivo and in vitro. Food Nutr. Res. 2019, 63. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, J.; Tadaishi, M.; Yamane, T.; Oishi, Y.; Shimizu, M.; Kobayashi-Hattori, K. Hot water extracts of edible Chrysanthemum morifolium Ramat. exert antidiabetic effects in obese diabetic KK-Ay mice. Biosci. Biotechnol. Biochem. 2015, 79, 1147–1154. [Google Scholar] [CrossRef] [Green Version]
- Commission, C.P. Chinese Pharmacopoeia 2015 (English Edition); China Medical Science and Technology Press: Beijing, China, 2015; p. 110. [Google Scholar]
- Lee, M.S.; Kim, Y. Effects of Isorhamnetin on Adipocyte Mitochondrial Biogenesis and AMPK Activation. Molecules 2018, 23. [Google Scholar] [CrossRef] [Green Version]
- Wise, L.S.; Green, H. Participation of one isozyme of cytosolic glycerophosphate dehydrogenase in the adipose conversion of 3T3 cells. J. Biol. Chem. 1979, 254, 273–275. [Google Scholar]
- Campitelli, M.; Desiderio, A.; Cacace, G.; Nigro, C.; Prevenzano, I.; Leone, A.; de Simone, S.; Campiglia, P.; Formisano, P.; Raciti, G.A.; et al. Citrus aurantium L. Dry Extracts Ameliorate Adipocyte Differentiation of 3T3-L1 Cells Exposed to TNFα by Down-Regulating miR-155 Expression. Nutrients 2020, 12. [Google Scholar] [CrossRef]
- Chayaratanasin, P.; Caobi, A.; Suparpprom, C.; Saenset, S.; Pasukamonset, P.; Suanpairintr, N.; Barbieri, M.A.; Adisakwattana, S. Clitoria ternatea Flower Petal Extract Inhibits Adipogenesis and Lipid Accumulation in 3T3-L1 Preadipocytes by Downregulating Adipogenic Gene Expression. Molecules 2019, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, E.; Lee, C.G.; Kim, J.; Yeo, S.; Kim, J.A.; Choi, C.W.; Jeong, S.Y. Antiobesity Effects of Gentiana lutea Extract on 3T3-L1 Preadipocytes and a High-Fat Diet-Induced Mouse Model. Molecules 2020, 25. [Google Scholar] [CrossRef]
- Kim, H.-Y.; Kim, J.H.; Zuo, G.; Lim, S.S. Anti-obesity effect of Melandrium firmum Rohrbach extract in 3T3-L1 cells and high-fat diet-induced obese C57BL/6N mice. Food Sci. Nutr. 2020, 8, 2251–2261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nepali, S.; Cha, J.Y.; Ki, H.H.; Lee, H.Y.; Kim, Y.H.; Kim, D.K.; Song, B.J.; Lee, Y.M. Chrysanthemum indicum Inhibits Adipogenesis and Activates the AMPK Pathway in High-Fat-Diet-Induced Obese Mice. Am. J. Chin. Med. 2018, 46, 119–136. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.Y.; Nepali, S.; Lee, H.Y.; Hwang, S.W.; Choi, S.Y.; Yeon, J.M.; Song, B.J.; Kim, D.K.; Lee, Y.M. Chrysanthemum indicum L. ethanol extract reduces high-fat diet-induced obesity in mice. Exp. Ther. Med. 2018, 15, 5070–5076. [Google Scholar] [CrossRef] [PubMed]
- Park, M.J.; Song, J.H.; Shon, M.S.; Kim, H.O.; Kwon, O.J.; Roh, S.S.; Kim, C.Y.; Kim, G.N. Anti-Adipogenic Effects of Ethanol Extracts Prepared from Selected Medicinal Herbs in 3T3-L1 Cells. Prev. Nutr. Food Sci. 2016, 21, 227–235. [Google Scholar] [CrossRef]
- Park, J.A.; Jin, K.-S.; Kwon, H.J.; Kim, B.W. Antiobesity activity of Chrysanthemum zawadskii methanol extract. J. Life Sci. 2015, 25, 299–306. [Google Scholar] [CrossRef] [Green Version]
- Han, A.-R.; Kim, H.Y.; So, Y.; Nam, B.; Lee, I.; Nam, J.-W.; Jo, Y.D.; Kim, S.H.; Kim, J.-B.; Kang, S.-Y.; et al. Quantification of Antioxidant Phenolic Compounds in a New Chrysanthemum Cultivar by High-Performance Liquid Chromatography with Diode Array Detection and Electrospray Ionization Mass Spectrometry. Int. J. Anal. Chem. 2017, 2017, 1254721. [Google Scholar] [CrossRef]
- Mosqueda-Solis, A.; Lasa, A.; Gomez-Zorita, S.; Eseberri, I.; Pico, C.; Portillo, M.P. Screening of potential anti-adipogenic effects of phenolic compounds showing different chemical structure in 3T3-L1 preadipocytes. Food Funct. 2017, 8, 3576–3586. [Google Scholar] [CrossRef]
- Guo, X.; Liu, J.; Cai, S.; Wang, O.; Ji, B. Synergistic interactions of apigenin, naringin, quercetin and emodin on inhibition of 3T3-L1 preadipocyte differentiation and pancreas lipase activity. Obes. Res. Clin. Pract. 2016, 10, 327–339. [Google Scholar] [CrossRef]
- Duangjai, A.; Nuengchamnong, N.; Suphrom, N.; Trisat, K.; Limpeanchob, N.; Saokaew, S. Potential of Coffee Fruit Extract and Quinic Acid on Adipogenesis and Lipolysis in 3T3-L1 Adipocytes. Kobe J. Med. Sci. 2018, 64, E84. [Google Scholar] [PubMed]
- Cantó, C.; Gerhart-Hines, Z.; Feige, J.N.; Lagouge, M.; Noriega, L.; Milne, J.C.; Elliott, P.J.; Puigserver, P.; Auwerx, J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 2009, 458, 1056–1060. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.B.; Zhou, G.; Li, C. AMPK: An emerging drug target for diabetes and the metabolic syndrome. Cell Metab. 2009, 9, 407–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chalkiadaki, A.; Guarente, L. High-fat diet triggers inflammation-induced cleavage of SIRT1 in adipose tissue to promote metabolic dysfunction. Cell Metab. 2012, 16, 180–188. [Google Scholar] [CrossRef] [Green Version]
- Price, N.L.; Gomes, A.P.; Ling, A.J.; Duarte, F.V.; Martin-Montalvo, A.; North, B.J.; Agarwal, B.; Ye, L.; Ramadori, G.; Teodoro, J.S.; et al. SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab. 2012, 15, 675–690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.; Kang, R.; Bae, S.; Yoon, Y. AICAR, an activator of AMPK, inhibits adipogenesis via the WNT/β-catenin pathway in 3T3-L1 adipocytes. Int. J. Mol. Med. 2011, 28, 65–71. [Google Scholar] [CrossRef]
- Picard, F.; Kurtev, M.; Chung, N.; Topark-Ngarm, A.; Senawong, T.; Machado de Oliveira, R.; Leid, M.; McBurney, M.W.; Guarente, L. Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-gamma. Nature 2004, 429, 771–776. [Google Scholar] [CrossRef]







| Name | GenBank No. | Primer Sequence (5′-3′) |
|---|---|---|
| ACC1 | AY451393 | F: CAAGTGCTCAAGTTTGGCGC |
| R: CAAGAACCACCCCGAAGCTC | ||
| β-actin | NM_007393 | F: GGACCTGACAGACTACCTCA |
| R: GTTGCCAATAGTGATGACCT | ||
| CEBP-α | NM_007678 | F: ATAGACATCAGCGCCTACAT |
| R: TCCCGGGTAGTCAAAGTCAC | ||
| FABP4 | NM_024406 | F: CGACAGGAAGGTGAAGAGCA |
| R: ATTCCACCACCAGCTTGTCA | ||
| FAS | AF127033 | F: CTGGCATTCGTGATGGAGTC |
| R: TGTTTCCCCTGAGCCATGTA | ||
| PPAR-γ | NM_011146 | F: TTGATTTCTCCAGCATTTCT |
| R: TGTTGTAGAGCTGGGTCTTT | ||
| SREBP-1c | AF509567 | F: GGCTGTTGTCTACCATAAGC |
| R: AGGAAGAAACGTGTCAAGAA |
| Polyphenolic Compound | Content (mg/100 g) |
|---|---|
| Luteolin-7-O-glucoside | 567.40 ± 3.65 |
| Luteolin-7-O-glucuronide | 533.73 ± 3.38 |
| Apigenin-7-O-glucoside | 671.76 ± 3.58 |
| Chlorogenic acid | 329.41 ± 2.20 |
| 1,5-Dicaffeoylquinic acid | 101.12 ± 0.69 |
| 3,5-Dicaffeoylquinic acid | 427.21 ± 2.28 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, M.-S.; Kim, Y. Chrysanthemum morifolium Flower Extract Inhibits Adipogenesis of 3T3-L1 Cells via AMPK/SIRT1 Pathway Activation. Nutrients 2020, 12, 2726. https://doi.org/10.3390/nu12092726
Lee M-S, Kim Y. Chrysanthemum morifolium Flower Extract Inhibits Adipogenesis of 3T3-L1 Cells via AMPK/SIRT1 Pathway Activation. Nutrients. 2020; 12(9):2726. https://doi.org/10.3390/nu12092726
Chicago/Turabian StyleLee, Mak-Soon, and Yangha Kim. 2020. "Chrysanthemum morifolium Flower Extract Inhibits Adipogenesis of 3T3-L1 Cells via AMPK/SIRT1 Pathway Activation" Nutrients 12, no. 9: 2726. https://doi.org/10.3390/nu12092726
APA StyleLee, M.-S., & Kim, Y. (2020). Chrysanthemum morifolium Flower Extract Inhibits Adipogenesis of 3T3-L1 Cells via AMPK/SIRT1 Pathway Activation. Nutrients, 12(9), 2726. https://doi.org/10.3390/nu12092726