Mediterranean Diet Pyramid: A Proposal for Italian People
Abstract
:1. Introduction
2. Cereal Foods in the Traditional Mediterranean Diet of the Early 1960s
3. Traditional Mediterranean Diet of the Early 1960s as a Low GI and GL Diet
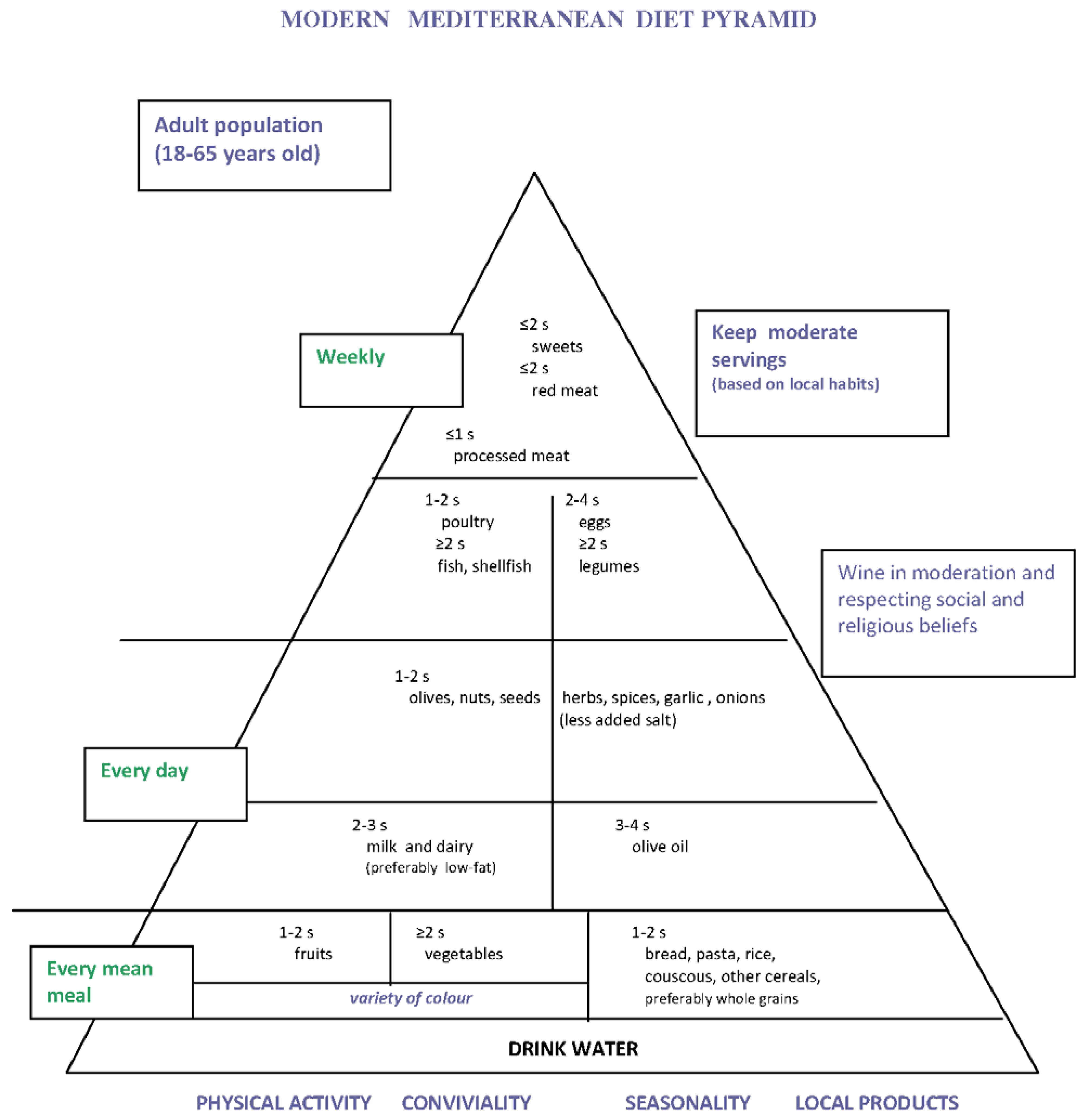
4. Proposal of Mediterranean Diet Pyramid for Italian People

5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dietary Guidelines for Americans. Available online: http://www.health.gov/dietaryguidelines/dga95/9DIETGUI.HTM (accessed on 28 March 2014).
- Willett, W.C.; Sacks, F.; Trichopoulou, A.; Drescher, G.; Ferro-Luzzi, A.; Helsing, E.; Trichopoulos, D. Mediterranean diet pyramid: A cultural model for healthy eating. Am. J. Clin. Nutr. 1995, 61, 1402–1406. [Google Scholar]
- The Oldways Mediterranean Diet Pyramid. Available online: http://www.oldwayspt.org (accessed on 28 March 2014).
- Supreme Scientific Health Council; Ministry of Health and Welfare. Dietary guidelines for adults in Greece. Arch. Hellenic Med. 1999, 16, 516–524. [Google Scholar]
- Aranceta, J.; Serra-Majem, L.; Working Party for the Development of Food-Based Dietary Guidelines for the Spanish Population. Dietary guidelines for the Spanish population. Public Health Nutr. 2001, 4, 1403–1408. [Google Scholar] [PubMed]
- Istituto Nazionale di Ricerca per gli alimenti e la Nutrizione. Piramide della Dieta Mediterranea Moderna. Available online: http://www.inran.it (accessed on 9 July 2013).
- Bach-Faig, A.; Berry, E.M.; Lairon, D.; Reguant, J.; Trichopoulou, A.; Dernini, S.; Medina, F.X.; Battino, M.; Belahsen, R.; Miranda, G.; et al. Mediterranean diet pyramid today. Science and cultural updates. Public Health Nutr. 2011, 14, 2274–2284. [Google Scholar] [CrossRef] [PubMed]
- Menotti, A.; Kromhout, D.; Blackburn, H.; Fidanza, F.; Buzina, R.; Nissinen, A. Food intake patterns and 25-year mortality from coronary heart disease: Cross-cultural correlations in the Seven Countries Study. The Seven Countries Study Research Group. Eur. J. Epidemiol. 1999, 15, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Kromhout, D.; Keys, A.; Aravanis, C.; Buzina, R.; Fidanza, F.; Giampaoli, S.; Jansen, A.; Menotti, A.; Nedeljkovic, S.; Pekkarinen, M.; et al. Food consumption patterns in the 1960s in seven countries. Am. J. Clin. Nutr. 1989, 49, 889–894. [Google Scholar] [PubMed]
- Keys, A.; Aravanis, C.; Sdrin, H. The diets of middle-aged men in two rural areas of Greece. In Dietary Studies and Epidemiology of Heart Diseases; Den Hartog, C., Buzina, K., Fidanza, F., Keys, A., Roine, P., Eds.; Stichting tot wetenschappelijke Voorlichting op Voedingsgebied: The Hague, The Netherlands, 1968; pp. 57–68. [Google Scholar]
- Alberti-Fidanza, A.; Fidanza, F.; Chiuchiù, M.P.; Verducci, G.; Fruttini, D. Dietary studies on two rural italian population groups of the Seven Countries Study. 3. Trend of food and nutrient intake from 1960 to 1991. Eur. J. Clin. Nutr. 1999, 53, 854–860. [Google Scholar] [CrossRef] [PubMed]
- Fidanza, F. La dieta di Nicotera nel 1960: Dieta Mediterranea Italiana di riferimento. In Dieta Mediterranea Italiana di Riferimento; E.M.S.I.: Roma, Italy, 2006; pp. 25–32. [Google Scholar]
- Istituto Nazionale per la Dieta Mediterranea e la Nutrigenomica (I.N.Di.M.); Barbalace, P. Pietanze di un Tempo. Saperi e Sapori Della Cucina Nicoterese. Available online: http://www.indim.it/Documenti/Capitolo-I-II.pdf (accessed on 11 February 2013).
- De Vuyst, L.; Neysens, P. The sourdough microflora: Biodiversity and metabolic interactions. Trends Food Sci. Tech. 2005, 16, 43–56. [Google Scholar] [CrossRef]
- Corsetti, A.; Settanni, L. Lactobacilli in sourdough fermentation. Food Res. Int. 2007, 40, 539–558. [Google Scholar] [CrossRef]
- D’Alessandro, A. La Dieta Mediterranea. In Le Evidenze Scientifiche del suo Ruolo Protettivo nei Confronti dell’Aterosclerosi Coronarica e Delle Malattie Dismetaboliche; Cacucci: Bari, Italy, 2013; pp. 1–237. [Google Scholar]
- Simopoulos, A.P. The Mediterranean diets: What is so special about the diet of Greece? The scientific evidence. J. Nutr. 2001, 131, 3065–3073. [Google Scholar]
- Gikas, G.; Hyz, A.; Vasileiou, K.; Georgakopoulos, G.; Sotiropoulos, I. Urban and rural dietary patterns in Greece in the years 1957–2008; an economic analysis. Sci. J. 2012, 12, pp. 5–14. Available online: http://www.wne.sggw.pl/czasopisma/pdf/PRS_2012_T12(27)_z3.pdf (accessed on 9 March 2013).
- Sotiropoulos, I.; Georgakopoulos, G.; Salavrakos, I.D. Alimentary expenditure of the different socio-vocational classes of the population in Greece (1957–2005): A description of the dietary models. Int. Bus. Res. 2009, 2, 17. [Google Scholar] [CrossRef]
- Catzeddu, P. Sourdough breads. In Flour and Breads and Their Fortification in Health and Disease Prevention; Preedy, V.R., Watson, R.R., Patel, V.B., Eds.; Academic Press Elsevier: London, UK, 2011; pp. 37–46. [Google Scholar]
- Minervini, F.; Di Cagno, R.; Lattanzi, A.; De Angelis, M.; Antonielli, L.; Cardinali, G.; Cappelle, S.; Gobbetti, M. Lactic acid bacterium and yeast microbiotas of 19 sourdoughs used for traditional/typical italian breads: Interactions between ingredients and microbial species diversity. Appl. Environ. Microbiol. 2012, 78, 1251–1264. [Google Scholar] [CrossRef] [PubMed]
- Björck, I.; Elmståhl, H.L. The glycaemic index: Importance of dietary fibre and other food properties. Proc. Nutr. Soc. 2003, 62, 201–206. [Google Scholar] [CrossRef]
- Poutanen, K.; Flander, L.; Katina, K. Sourdough and cereal fermentation in a nutritional perspective. Food Microbiol. 2009, 26, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Liljeberg, H.G.; Lönner, C.H.; Björck, I. Sourdough fermentation or addition of organic acids or corresponding salts to bread improves nutritional properties of starch in healthy humans. J. Nutr. 1995, 125, 1503–1511. [Google Scholar] [PubMed]
- Östman, E.M.; Nilsson, M.; Liljeberg Elmståhl, H.; Molin, G.; Björck, I. On the effect of lactic acid on blood glucose and insulin responses to cereal products: Mechanistic studies in healthy subjects and in vitro. J. Cereal Sci. 2002, 36, 339–346. [Google Scholar] [CrossRef]
- Liljeberg, H.; Björck, I. Delayed gastric emptying rate may explain improved glycaemia in healthy subjects to a starchy meal with added vinegar. Eur. J. Clin. Nutr. 1998, 52, 368–371. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, F.S.; Foster-Powell, K.; Brand-Miller, J.C. International tables of glycemic index and glycemic load values: 2008. Diabetes Care 2008, 31, 2281–2283. [Google Scholar] [CrossRef] [PubMed]
- Harvard Health Publications. Available online: http//www.health.harvard.edu (accessed on 23 August 2013).
- Mesci, B.; Oguz, A.; Sagun, H.G.; Uzunlulu, M.; Keskin, E.B.; Coksert, D. Dietary breads: Myth or reality? Diabetes Res. Clin. Pract. 2008, 81, 68–71. [Google Scholar] [CrossRef]
- Breen, C.; Ryan, M.; Gibney, M.J.; Corrigan, M.; O’Shea, D. Glycemic, insulinemic, and appetite responses of patients with type 2 diabetes to commonly consumed breads. Diabetes Educ. 2013, 39, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D.J.; Wesson, V.; Wolever, T.M.; Jenkins, A.L.; Kalmusky, J.; Guidici, S.; Csima, A.; Josse, R.G.; Wong, G.S. Wholemeal versus wholegrain breads: Proportion of whole or cracked grain and the glycaemic response. BMJ 1988, 297, 958–960. [Google Scholar] [CrossRef] [PubMed]
- Holt, S.H.; Miller, J.B. Particle size, satiety and the glycaemic response. Eur. J. Clin. Nutr. 1994, 48, 496–502. [Google Scholar] [PubMed]
- Liljeberg, H.; Granfeldt, Y.; Björck, I. Metabolic responses to starch in bread containing intact kernels versus milled flour. Eur. J. Clin. Nutr. 1992, 46, 561–575. [Google Scholar] [PubMed]
- Snow, P.; O’Dea, K. Factors affecting the rate of hydrolysis of starch in food. Am. J. Clin. Nutr. 1981, 34, 2721–2727. [Google Scholar] [PubMed]
- Scazzina, F.; Del Rio, D.; Pellegrini, N.; Brighenti, F. Sourdough bread: Starch digestibility and postprandial glycemic response. J. Cereal Sci. 2009, 49, 419–421. [Google Scholar] [CrossRef]
- Maioli, M.; Pes, G.M.; Sanna, M.; Cherchi, S.; Dettori, M.; Manca, E.; Farris, G.A. Sourdough leavened bread improves postprandial glucose and insulin plasma levels in subjects with impaired glucose tolerance. Acta Diabetol. 2008, 45, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Lappi, J.; Selinheimo, E.; Schwab, U.; Katina, K.; Lehtinen, P.; Mykkänen, H.; Kolehmainen, M.; Poutanen, K. Sourdough fermentation of wholemeal wheat bread increases solubility of arabinoxylan and protein and decreases postprandial glucose and insulin responses. J. Cereal Sci. 2010, 51, 152–158. [Google Scholar] [CrossRef]
- Lopez, H.W.; Krespine, V.; Guy, C.; Messager, A.; Demigne, C.; Remesy, C. Prolonged fermentation of whole wheat sourdough reduces phytate level and increases soluble magnesium. J. Agric. Food Chem. 2001, 49, 2657–2662. [Google Scholar] [CrossRef] [PubMed]
- Reale, A.; Konietzny, U.; Coppola, R.; Sorrentino, E.; Greiner, R. The importance of lactic acid bacteria for phytate degradation during cereal dough fermentation. J. Agric. Food Chem. 2007, 55, 2993–2997. [Google Scholar] [CrossRef] [PubMed]
- Schlemmer, U.; Frølich, W.; Prieto, R.M.; Grases, F. Phytate in foods and significance for humans: Food sources, intake, processing, bioavailability, protective role and analysis. Mol. Nutr. Food Res. 2009, 53, 330–375. [Google Scholar] [CrossRef]
- Bohn, L.; Meyer, A.S.; Rasmussen, S.K. Phytate: Impact on environment and human nutrition. A challenge for molecular breeding. J. Zhejiang Univ. Sci. B 2008, 9, 165–191. [Google Scholar] [CrossRef] [PubMed]
- Katina, K.; Arendt, E.; Liukkonen, K-H.; Autio, K.; Flander, L.; Poutanen, K. Potential of sourdough for healthier cereal products. Trends Food Sci. Tech. 2005, 16, 104–112. [Google Scholar] [CrossRef]
- Kirpitch, A.R.; Maryniuk, M.D. The 3 R’s of glycemic index: Recommendations, research, and the real world. Clin. Diabetes 2011, 29, 155–159. [Google Scholar] [CrossRef]
- Lennerz, B.S.; Alsop, D.C.; Holsen, L.M.; Stern, E.; Rojas, R.; Ebbeling, C.B.; Goldstein, J.M.; Ludwig, D.S. Effects of dietary glycemic index on brain regions related to reward and craving in men. Am. J. Clin. Nutr. 2013, 98, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Page, K.A.; Seo, D.; Belfort-DeAguiar, R.; Lacadie, C.; Dzuira, J.; Naik, S.; Amarnath, S.; Constable, R.T.; Sherwin, R.S.; Sinha, R. Circulating glucose levels modulate neural control of desire for high-calorie foods in humans. J. Clin. Investig. 2011, 121, 4161–4169. [Google Scholar] [CrossRef] [PubMed]
- Livesey, G.; Taylor, R.; Hulshof, T.; Howlett, J. Glycemic response and health—A systematic review and meta-analysis: Relations between dietary glycemic properties and health outcomes. Am. J. Clin. Nutr. 2008, 87, 258–268. [Google Scholar]
- Lau, C.; Toft, U.; Tetens, I.; Richelsen, B.; Jørgensen, T.; Borch-Johnsen, K.; Glümer, C. Association between dietary glycemic index, glycemic load, and body mass index in the Inter99 study: Is underreporting a problem? Am. J. Clin. Nutr. 2006, 84, 641–645. [Google Scholar]
- Murakami, K.; McCaffrey, T.A.; Livingstone, M.B. Associations of dietary glycaemic index and glycaemic load with food and nutrient intake and general and central obesity in British adults. Br. J. Nutr. 2013, 9, 1–11. [Google Scholar]
- Rossi, M.; Bosetti, C.; Talamini, R.; Lagiou, P.; Negri, E.; Franceschi, S.; La Vecchia, C. Glycemic index and glycemic load in relation to body mass index and waist to hip ratio. Eur. J. Nutr. 2010, 49, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Mendez, M.A.; Covas, M.I.; Marrugat, J.; Vila, J.; Schröder, H. Glycemic load, glycemic index, and body mass index in Spanish adults. Am. J. Clin. Nutr. 2009, 89, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Ford, E.S.; Liu, S. Glycemic index and serum high-density lipoprotein cholesterol concentration among US adults. Arch. Intern. Med. 2001, 161, 572–576. [Google Scholar] [CrossRef] [PubMed]
- Denova-Gutiérrez, E.; Huitrón-Bravo, G.; Talavera, J.O.; Castañón, S.; Gallegos-Carrillo, K.; Flores, Y.; Salmerón, J. Dietary glycemic index, dietary glycemic load, blood lipids, and coronary heart disease. J. Nutr. Metab. 2010, 2010. [Google Scholar] [CrossRef]
- Levitan, E.B.; Cook, N.R.; Stampfer, M.J.; Ridker, P.M.; Rexrode, K.M.; Buring, J.E.; Manson, J.E.; Liu, S. Dietary glycemic index, dietary glycemic load, blood lipids, and C-reactive protein. Metabolism 2008, 57, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Manson, J.E.; Stampfer, M.J.; Holmes, M.D.; Hu, F.B.; Hankinson, S.E.; Willett, W.C. Dietary glycemic load assessed by food-frequency questionnaire in relation to plasma high-density lipoprotein cholesterol and fasting plasma triacylglycerols in postmenopausal women. Am. J. Clin. Nutr. 2001, 73, 560–566. [Google Scholar] [PubMed]
- Amano, Y.; Kawakubo, K.; Lee, J.S.; Tang, A.C.; Sugiyama, M.; Mori, K. Correlation between dietary glycemic index and cardiovascular disease risk factors among Japanese women. Eur. J. Clin. Nutr. 2004, 58, 1472–1478. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, D.S. The glycemic index: Physiological mechanisms relating to obesity, diabetes, and cardiovascular disease. JAMA 2002, 287, 2414–2423. [Google Scholar] [CrossRef] [PubMed]
- Livesey, G.; Taylor, R.; Livesey, H.; Liu, S. Is there a dose-response relation of dietary glycemic load to risk of type 2 diabetes? Meta-analysis of prospective cohort studies. Am. J. Clin. Nutr. 2013, 97, 584–596. [Google Scholar] [CrossRef] [PubMed]
- Willett, W.; Manson, J.; Liu, S. Glycemic index, glycemic load, and risk of type 2 diabetes. Am. J. Clin. Nutr. 2002, 76, 274–280. [Google Scholar]
- Dong, J.Y.; Zhang, Y.H.; Wang, P.; Qin, L.Q. Meta-analysis of dietary glycemic load and glycemic index in relation to risk of coronary heart disease. Am. J. Cardiol. 2012, 109, 1608–1613. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Song, Y.; Wang, Y.; Hui, R.; Zhang, W. Dietary glycemic index, glycemic load, and risk of coronary heart disease, stroke, and stroke mortality: A systematic review with meta-analysis. PLoS One 2012, 7, e52182. [Google Scholar] [CrossRef] [PubMed]
- Mirrahimi, A.; de Souza, R.J.; Chiavaroli, L.; Sievenpiper, J.L.; Beyene, J.; Hanley, A.J.; Augustin, L.S.; Kendall, C.W.; Jenkins, D.J. Associations of glycemic index and load with coronary heart disease events: A systematic review and meta-analysis of prospective cohorts. J. Am. Heart Assoc. 2012, 1, e000752. [Google Scholar] [CrossRef] [PubMed]
- Mirrahimi, A.; Chiavaroli, L.; Srichaikul, K.; Augustin, L.S.; Sievenpiper, J.L.; Kendall, C.W.; Jenkins, D.J. The role of glycemic index and glycemic load in cardiovascular disease and its risk factors: A review of the recent literature. Curr. Atheroscler. Rep. 2014, 16, 1–10. [Google Scholar]
- Sieri, S.; Krogh, V.; Berrino, F.; Evangelista, A.; Agnoli, C.; Brighenti, F.; Pellegrini, N.; Palli, D.; Masala, G.; Sacerdote, C.; et al. Dietary glycemic load and index and risk of coronary heart disease in a large italian cohort: The EPICOR study. Arch. Intern. Med. 2010, 170, 640–647. [Google Scholar] [CrossRef] [PubMed]
- Knopp, R.H.; Paramsothy, P.; Retzlaff, B.M.; Fish, B.; Walden, C.; Dowdy, A.; Tsunehara, C.; Aikawa, K.; Cheung, M.C. Gender differences in lipoprotein metabolism and dietary response: Basis in hormonal differences and implications for cardiovascular disease. Curr. Atheroscler. Rep. 2005, 7, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Sarwar, N.; Gao, P.; Seshasai, S.R.; Gobin, R.; Kaptoge, S.; di Angelantonio, E.; Ingelsson, E.; Lawlor, D.A.; Selvin, E.; Stampfer, M.; et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: A collaborative meta-analysis of 102 prospective studies. The Emerging Risk Factors Collaboration. Lancet 2010, 375, 2215–2222. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Block, G.; Norkus, E.P.; Morrow, J.D.; Dietrich, M.; Hudes, M. Relations of glycemic index and glycemic load with plasma oxidative stress markers. Am. J. Clin. Nutr. 2006, 84, 70–76. [Google Scholar] [PubMed]
- De Pergola, G; Silvestris, F. Obesity as a major risk factor for cancer. J. Obes. 2013, 2013. [Google Scholar] [CrossRef]
- Arcidiacono, B.; Iiritano, S.; Nocera, A.; Possidente, K.; Nevolo, M.T.; Ventura, V.; Foti, D.; Chiefari, E.; Brunetti, A. Insulin resistance and cancer risk: An overview of the pathogenetic mechanisms. Exp. Diabetes Res. 2012, 2012. [Google Scholar] [CrossRef]
- Gnagnarella, P.; Gandini, S.; la Vecchia, C.; Maisonneuve, P. Glycemic index, glycemic load, and cancer risk: A meta-analysis. Am. J. Clin. Nutr. 2008, 87, 1793–1801. [Google Scholar] [PubMed]
- Mulholland, H.G.; Murray, L.J.; Cardwell, C.R.; Cantwell, M.M. Dietary glycaemic index, glycaemic load and endometrial and ovarian cancer risk: A systematic review and meta-analysis. Br. J. Cancer 2008, 99, 434–441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mulholland, H.G.; Murray, L.J.; Cardwell, C.R.; Cantwell, M.M. Glycemic index, glycemic load, and risk of digestive tract neoplasms: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2009, 89, 568–576. [Google Scholar] [PubMed]
- Aune, D.; Chan, D.S.; Greenwood, D.C.; Vieira, A.R.; Rosenblatt, D.A.; Vieira, R.; Norat, T. Dietary fiber and breast cancer risk: A systematic review and meta-analysis of prospective studies. Ann. Oncol. 2012, 23, 1394–1402. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.Y.; Qin, L.Q. Dietary glycemic index, glycemic load, and risk of breast cancer: Meta-analysis of prospettive cohort studies. Breast Cancer Res. Treat. 2011, 126, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; la Vecchia, C.; Augustin, L.S.; Negri, E.; de Groh, M.; Morrison, H.; Mery, L.; Canadian Cancer Registries Epidemiology Research Group. Glycemic index, glycemic load and cancer risk. Ann. Oncol. 2013, 24, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.A.; O’Reilly, E.; Augustsson, K.; Fraser, G.E.; Goldbourt, U.; Heitmann, B.L.; Hallmans, G.; Knekt, P.; Liu, S.; Pietinen, P.; et al. Dietary fiber and risk of coronary heart disease: A pooled analysis of cohort studies. Arch. Intern. Med. 2004, 164, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Schulze, M.B.; Schulz, M.; Heidemann, C.; Schienkiewitz, A.; Hoffmann, K.; Boeing, H. Fiber and magnesium intake and incidence of type 2 diabetes: A prospective study and meta-analysis. Arch. Intern. Med. 2007, 167, 956–965. [Google Scholar] [CrossRef] [PubMed]
- Koh-Banerjee, P.; Franz, M.; Sampson, L.; Liu, S.; Jacobs Jr, D.R.; Spiegelman, D.; Willett, W.; Rimm, E. Changes in whole-grain, bran, and cereal fiber consumption in relation to 8-y weight gain among men. Am. J. Clin. Nutr. 2004, 80, 1237–1245. [Google Scholar] [PubMed]
- Aune, D.; Chan, D.S.; Lau, R.; Vieira, R.; Greenwood, D.C.; Kampman, E.; Norat, T. Dietary fibre, whole grains, and risk of colorectal cancer: Systematic review and dose-response meta-analysis of prospective studies. BMJ 2011, 343. [Google Scholar] [CrossRef]
- Esposito, K.; Nappo, F.; Giugliano, F.; di Palo, C.; Ciotola, M.; Barbieri, M.; Paolisso, G.; Giugliano, D. Meal modulation of circulating interleukin 18 and adiponectin concentrations in healthy subjects and in patients with type 2 diabetes mellitus. Am. J. Clin. Nutr. 2003, 78, 1135–1140. [Google Scholar] [PubMed]
- Chuang, S.C.; Vermeulen, R.; Sharabiani, M.T.; Sacerdote, C.; Fatemeh, S.H.; Berrino, F.; Krogh, V.; Palli, D.; Panico, S.; Tumino, R.; et al. The intake of grain fibers modulates cytokine levels in blood. Biomarkers 2011, 16, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Weickert, M.O.; Möhlig, M.; Schöfl, C.; Arafat, A.M.; Otto, B.; Viehoff, H.; Koebnick, C.; Kohl, A.; Spranger, J.; Pfeiffer, A.F. Cereal fiber improves whole-body insulin sensitivity in overweight and obese women. Diabetes Care 2006, 29, 775–780. [Google Scholar] [CrossRef] [PubMed]
- Gil, A.; Ortega, R.M.; Maldonado, J. Wholegrain cereals and bread: A duet of the Mediterranean diet for the prevention of chronic diseases. Public Health Nutr. 2011, 14, 2316–2322. [Google Scholar] [CrossRef] [PubMed]
- Aune, D.; Norat, T.; Romundstad, P.; Vatten, L.J. Whole grain and refined grain consumption and the risk of type 2 diabetes: A systematic review and dose-response meta-analysis of cohort studies. Eur. J. Epidemiol. 2013, 28, 845–858. [Google Scholar] [CrossRef] [PubMed]
- Fardet, A. New hypotheses for the health-protective mechanisms of whole-grain cereals: What is beyond fibre? Nutr. Res. Rev. 2010, 23, 65–134. [Google Scholar] [CrossRef]
- Costabile, A.; Klinder, A.; Fava, F.; Napolitano, A.; Fogliano, V.; Leonard, C.; Gibson, G.R.; Tuohy, K.M. Whole-grain wheat breakfast cereal has a prebiotic effect on the human gut microbiota: A double-blind, placebo-controlled, crossover study. Br. J. Nutr. 2008, 99, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Slavin, J. Fiber and prebiotics: Mechanisms and health benefits. Nutrients 2013, 5, 1417–1435. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.K.; Omaye, S.T. Metabolic diseases and pro- and prebiotics: Mechanistic insights. Nutr. Metab. 2012, 9, 60. [Google Scholar] [CrossRef]
- Istituto Nazionale di Sociologia Rurale (I.N.S.O.R.). Atlante dei Prodotti Tipici: Il Pane, 2nd ed.; Agra-Rai Eri: Roma, Italy, 2000; pp. 1–302. [Google Scholar]
- Pérez Rodrigo, C.; Ruiz Vadillo, V. Wheat, bread and pasta in Mediterranean diets. Arch. Latinoam. Nutr. 2004, 54, 52–58. [Google Scholar]
- Wirfält, E.; McTaggart, A.; Pala, V.; Gullberg, B.; Frasca, G.; Panico, S.; Bueno-de-Mesquita, H.B.; Peeters, P.H.; Engeset, D.; Skeie, G.; et al. Food sources of carbohydrates in a European cohort of adults. Public Health Nutr. 2002, 5, 1197–1215. [Google Scholar] [CrossRef] [PubMed]
- Leclercq, C.; Arcella, D.; Piccinelli, R.; Sette, S.; Le Donne, C.; Turrini, A. The Italian National Food Consumption Survey INRAN-SCAI 2005–06: Main results in terms of food consumption. Public Health Nutr. 2009, 12, 2504–2532. [Google Scholar] [CrossRef] [PubMed]
- Vareiro, D.; Bach-Faig, A.; Raidó Quintana, B.; Bertomeu, I.; Buckland, G.; Vaz de Almeida, M.D.; Serra-Majem, L. Availability of Mediterranean and non-Mediterranean foods during the last four decades: Comparison of several geographical areas. Public Health Nutr. 2009, 12, 1667–1675. [Google Scholar] [CrossRef] [PubMed]
- Geoffrey Rose e la Strategia della Medicina Preventiva, 2nd ed.; Il Pensiero Scientifico Editore: Roma, Italy, 2012; pp. 12–33.
- Capone, R.; ElBilali, H.; Debs, P.; Cardone, G.; Driouech, N. Mediterranean food consumption patterns sustainability: Setting up a common ground for future research and action. Am. J. Nutr. Food Sci. 2014, 1, 37–52. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
D'Alessandro, A.; De Pergola, G. Mediterranean Diet Pyramid: A Proposal for Italian People. Nutrients 2014, 6, 4302-4316. https://doi.org/10.3390/nu6104302
D'Alessandro A, De Pergola G. Mediterranean Diet Pyramid: A Proposal for Italian People. Nutrients. 2014; 6(10):4302-4316. https://doi.org/10.3390/nu6104302
Chicago/Turabian StyleD'Alessandro, Annunziata, and Giovanni De Pergola. 2014. "Mediterranean Diet Pyramid: A Proposal for Italian People" Nutrients 6, no. 10: 4302-4316. https://doi.org/10.3390/nu6104302
APA StyleD'Alessandro, A., & De Pergola, G. (2014). Mediterranean Diet Pyramid: A Proposal for Italian People. Nutrients, 6(10), 4302-4316. https://doi.org/10.3390/nu6104302




