The Evolving Applications of Creatine Supplementation: Could Creatine Improve Vascular Health?
Abstract
:1. Introduction
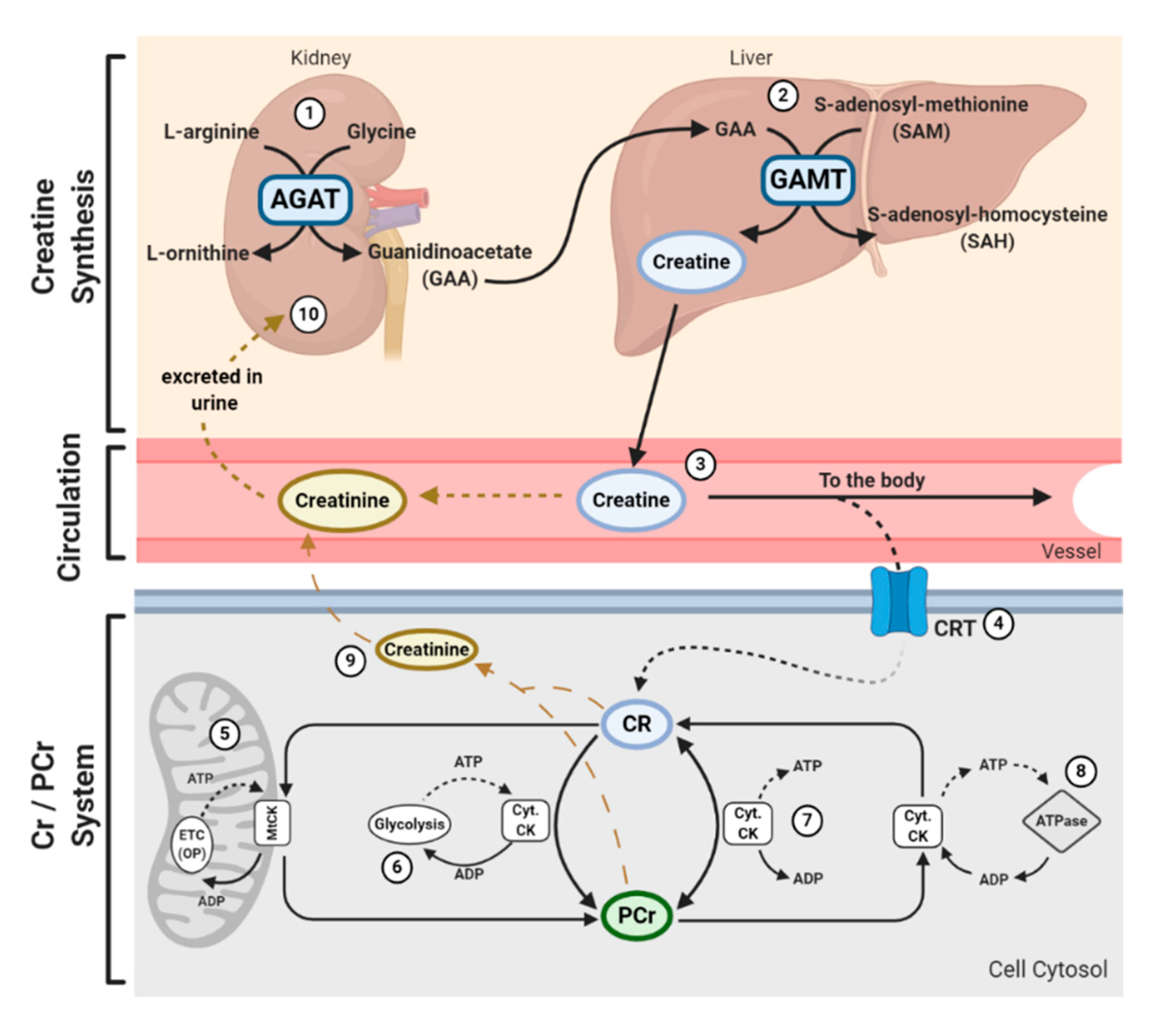
2. Brief Overview of Creatine Metabolism and the Cellular Actions of Creatine
3. Pleiotropic Application of Creatine
4. Current Clinical Trials of Creatine Supplementation and Vascular Health
5. Potential Mechanisms of How Creatine May Improve Vascular Health
5.1. Creatine, Oxidative Stress, and the Antioxidant System
5.2. Creatine and Homocysteine
5.3. Creatine and Inflammation
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chanutin, A. The fate of creatine when administered to man. J. Biol. Chem. 1926, 67, 29–41. [Google Scholar]
- Walker, J.B. Creatine: Biosynthesis, regulation, and function. Adv. Enzym. Relat. Areas Mol. Biol. 1979, 50, 177–242. [Google Scholar]
- Harris, R.C.; Soderlund, K.; Hultman, E. Elevation of creatine in resting and exercised muscle of normal subjects by creatine supplementation. Clin. Sci. 1992, 83, 367–374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gualano, B.; Artioli, G.G.; Poortmans, J.R.; Lancha Junior, A.H. Exploring the therapeutic role of creatine supplementation. Amino Acids 2010, 38, 31–44. [Google Scholar] [CrossRef]
- Kreider, R.B.; Kalman, D.S.; Antonio, J.; Ziegenfuss, T.N.; Wildman, R.; Collins, R.; Candow, D.G.; Kleiner, S.M.; Almada, A.L.; Lopez, H.L. International Society of Sports Nutrition position stand: Safety and efficacy of creatine supplementation in exercise, sport, and medicine. J. Int. Soc. Sports Nutr. 2017, 14, 18. [Google Scholar] [CrossRef]
- Wallimann, T.; Harris, R. Creatine: A miserable life without it. Amino Acids 2016, 48, 1739–1750. [Google Scholar] [CrossRef] [PubMed]
- Momaya, A.; Fawal, M.; Estes, R. Performance-enhancing substances in sports: A review of the literature. Sports Med. 2015, 45, 517–531. [Google Scholar] [CrossRef]
- Butts, J.; Jacobs, B.; Silvis, M. Creatine Use in Sports. Sports Health 2018, 10, 31–34. [Google Scholar] [CrossRef]
- Cooper, R.; Naclerio, F.; Allgrove, J.; Jimenez, A. Creatine supplementation with specific view to exercise/sports performance: An update. J. Int. Soc. Sports Nutr. 2012, 9, 33. [Google Scholar] [CrossRef] [Green Version]
- Lawler, J.M.; Barnes, W.S.; Wu, G.; Song, W.; Demaree, S. Direct antioxidant properties of creatine. Biochem. Biophys. Res. Commun. 2002, 290, 47–52. [Google Scholar] [CrossRef]
- Sestili, P.; Martinelli, C.; Colombo, E.; Barbieri, E.; Potenza, L.; Sartini, S.; Fimognari, C. Creatine as an antioxidant. Amino Acids 2011, 40, 1385–1396. [Google Scholar] [CrossRef] [PubMed]
- Van Bavel, D.; de Moraes, R.; Tibirica, E. Effects of dietary supplementation with creatine on homocysteinemia and systemic microvascular endothelial function in individuals adhering to vegan diets. Fundam. Clin. Pharm. 2018. [Google Scholar] [CrossRef]
- Korzun, W.J. Oral creatine supplements lower plasma homocysteine concentrations in humans. Clin. Lab. Sci. 2004, 17, 102–106. [Google Scholar]
- Bereket-Yucel, S. Creatine supplementation alters homocysteine level in resistance trained men. J. Sports Med. Phys. Fit. 2015, 55, 313–319. [Google Scholar]
- Candow, D.G.; Forbes, S.C.; Chilibeck, P.D.; Cornish, S.M.; Antonio, J.; Kreider, R.B. Effectiveness of Creatine Supplementation on Aging Muscle and Bone: Focus on Falls Prevention and Inflammation. J. Clin. Med. 2019, 8, 488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bassit, R.A.; Curi, R.; Costa Rosa, L.F. Creatine supplementation reduces plasma levels of pro-inflammatory cytokines and PGE2 after a half-ironman competition. Amino Acids 2008, 35, 425–431. [Google Scholar] [CrossRef]
- Nomura, A.; Zhang, M.; Sakamoto, T.; Ishii, Y.; Morishima, Y.; Mochizuki, M.; Kimura, T.; Uchida, Y.; Sekizawa, K. Anti-inflammatory activity of creatine supplementation in endothelial cells in vitro. Br. J. Pharm. 2003, 139, 715–720. [Google Scholar] [CrossRef] [Green Version]
- Rahimi, R. Creatine supplementation decreases oxidative DNA damage and lipid peroxidation induced by a single bout of resistance exercise. J. Strength Cond. Res. 2011, 25, 3448–3455. [Google Scholar] [CrossRef]
- Wald, D.S.; Law, M.; Morris, J.K. Homocysteine and cardiovascular disease: Evidence on causality from a meta-analysis. BMJ 2002, 325, 1202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willerson, J.T.; Ridker, P.M. Inflammation as a cardiovascular risk factor. Circulation 2004, 109, II-2–II-10. [Google Scholar] [CrossRef] [Green Version]
- Dhalla, N.S.; Temsah, R.M.; Netticadan, T. Role of oxidative stress in cardiovascular diseases. J. Hypertens. 2000, 18, 655–673. [Google Scholar] [CrossRef] [PubMed]
- Snow, R.J.; Murphy, R.M. Creatine and the creatine transporter: A review. Mol. Cell. BioChem. 2001, 224, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Brosnan, J.T.; Brosnan, M.E. Creatine: Endogenous metabolite, dietary, and therapeutic supplement. Annu. Rev. Nutr. 2007, 27, 241–261. [Google Scholar] [CrossRef] [Green Version]
- Kan, H.E.; van der Graaf, M.; Klomp, D.W.; Vlak, M.H.; Padberg, G.W.; Heerschap, A. Intake of 13C-4 creatine enables simultaneous assessment of creatine and phosphocreatine pools in human skeletal muscle by 13C MR spectroscopy. Magn. Reson. Med. 2006, 56, 953–957. [Google Scholar] [CrossRef]
- Cockcroft, D.W.; Gault, M.H. Prediction of creatinine clearance from serum creatinine. Nephron 1976, 16, 31–41. [Google Scholar] [CrossRef]
- Wallimann, T.; Wyss, M.; Brdiczka, D.; Nicolay, K.; Eppenberger, H.M. Intracellular compartmentation, structure and function of creatine kinase isoenzymes in tissues with high and fluctuating energy demands: The ‘phosphocreatine circuit’ for cellular energy homeostasis. Biochem. J. 1992, 281, 21–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Decking, U.K.; Alves, C.; Wallimann, T.; Wyss, M.; Schrader, J. Functional aspects of creatine kinase isoenzymes in endothelial cells. Am. J. Physiol. Cell Physiol. 2001, 281, C320–C328. [Google Scholar] [CrossRef]
- Schlattner, U.; Tokarska-Schlattner, M.; Wallimann, T. Mitochondrial creatine kinase in human health and disease. Biochim. Biophys. Acta 2006, 1762, 164–180. [Google Scholar] [CrossRef]
- Wyss, M.; Kaddurah-Daouk, R. Creatine and creatinine metabolism. Physiol. Rev. 2000, 80, 1107–1213. [Google Scholar] [CrossRef]
- Wallimann, T.; Hemmer, W. Creatine kinase in non-muscle tissues and cells. Mol. Cell. Biochem. 1994, 133, 193–220. [Google Scholar] [CrossRef]
- Greenhaff, P.L. The creatine-phosphocreatine system: There’s more than one song in its repertoire. J. Physiol. 2001, 537, 657. [Google Scholar] [CrossRef]
- Meyer, L.E.; Machado, L.B.; Santiago, A.P.; da-Silva, W.S.; De Felice, F.G.; Holub, O.; Oliveira, M.F.; Galina, A. Mitochondrial creatine kinase activity prevents reactive oxygen species generation: Antioxidant role of mitochondrial kinase-dependent ADP re-cycling activity. J. Biol. Chem. 2006, 281, 37361–37371. [Google Scholar] [CrossRef] [Green Version]
- Wallimann, T.; Dolder, M.; Schlattner, U.; Eder, M.; Hornemann, T.; O’Gorman, E.; Rück, A.; Brdiczka, D. Some new aspects of creatine kinase (CK): Compartmentation, structure, function and regulation for cellular and mitochondrial bioenergetics and physiology. Biofactors 1998, 8, 229–234. [Google Scholar] [CrossRef]
- Persky, A.M.; Brazeau, G.A. Clinical pharmacology of the dietary supplement creatine monohydrate. Pharm. Rev. 2001, 53, 161–176. [Google Scholar]
- Kreider, R.B.; Ferreira, M.; Wilson, M.; Grindstaff, P.; Plisk, S.; Reinardy, J.; Cantler, E.; Almada, A.L. Effects of creatine supplementation on body composition, strength, and sprint performance. Med. Sci. Sports Exerc. 1998, 30, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Volek, J.S.; Ratamess, N.A.; Rubin, M.R.; Gómez, A.L.; French, D.N.; McGuigan, M.M.; Scheett, T.P.; Sharman, M.J.; Häkkinen, K.; Kraemer, W.J. The effects of creatine supplementation on muscular performance and body composition responses to short-term resistance training overreaching. Eur. J. Appl. Physiol. 2004, 91, 628–637. [Google Scholar] [CrossRef]
- Aguiar, A.F.; Januário, R.S.; Junior, R.P.; Gerage, A.M.; Pina, F.L.; do Nascimento, M.A.; Padovani, C.R.; Cyrino, E.S. Long-term creatine supplementation improves muscular performance during resistance training in older women. Eur. J. Appl. Physiol. 2013, 113, 987–996. [Google Scholar] [CrossRef]
- Tarnopolsky, M.A. Potential benefits of creatine monohydrate supplementation in the elderly. Curr. Opin. Clin. Nutr. Metab. Care 2000, 3, 497–502. [Google Scholar] [CrossRef]
- Cooke, M.B.; Rybalka, E.; Williams, A.D.; Cribb, P.J.; Hayes, A. Creatine supplementation enhances muscle force recovery after eccentrically-induced muscle damage in healthy individuals. J. Int. Soc. Sports Nutr. 2009, 6, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, S.A.; Montain, S.J.; Matott, R.P.; Zientara, G.P.; Jolesz, F.A.; Fielding, R.A. Creatine supplementation and age influence muscle metabolism during exercise. J. Appl. Physiol. 1998, 85, 1349–1356. [Google Scholar] [CrossRef]
- Urbanski, R.L.; Vincent, W.J.; Yaspelkis, B.B., 3rd. Creatine supplementation differentially affects maximal isometric strength and time to fatigue in large and small muscle groups. Int. J. Sport Nutr. 1999, 9, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Rawson, E.S.; Stec, M.J.; Frederickson, S.J.; Miles, M.P. Low-dose creatine supplementation enhances fatigue resistance in the absence of weight gain. Nutrition 2011, 27, 451–455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stout, J.R.; Sue Graves, B.; Cramer, J.T.; Goldstein, E.R.; Costa, P.B.; Smith, A.E.; Walter, A.A. Effects of creatine supplementation on the onset of neuromuscular fatigue threshold and muscle strength in elderly men and women (64–86 years). J. Nutr. Health Aging 2007, 11, 459–464. [Google Scholar]
- Chilibeck, P.D.; Kaviani, M.; Candow, D.G.; Zello, G.A. Effect of creatine supplementation during resistance training on lean tissue mass and muscular strength in older adults: A meta-analysis. Open Access J. Sports Med. 2017, 8, 213–226. [Google Scholar] [CrossRef] [Green Version]
- Tarnopolsky, M.A.; MacLennan, D.P. Creatine monohydrate supplementation enhances high-intensity exercise performance in males and females. Int. J. Sport Nutr. Exerc. Metab. 2000, 10, 452–463. [Google Scholar] [CrossRef] [PubMed]
- Mihic, S.; MacDonald, J.R.; McKenzie, S.; Tarnopolsky, M.A. Acute creatine loading increases fat-free mass, but does not affect blood pressure, plasma creatinine, or CK activity in men and women. Med. Sci. Sports Exerc. 2000, 32, 291–296. [Google Scholar] [CrossRef]
- Pulido, S.M.; Passaquin, A.C.; Leijendekker, W.J.; Challet, C.; Wallimann, T.; Rüegg, U.T. Creatine supplementation improves intracellular Ca2+ handling and survival in mdx skeletal muscle cells. FEBS Lett. 1998, 439, 357–362. [Google Scholar] [CrossRef] [Green Version]
- Farshidfar, F.; Pinder, M.A.; Myrie, S.B. Creatine Supplementation and Skeletal Muscle Metabolism for Building Muscle Mass-Review of the Potential Mechanisms of Action. Curr. Protein Pept. Sci. 2017, 18, 1273–1287. [Google Scholar] [CrossRef]
- Parise, G.; Mihic, S.; MacLennan, D.; Yarasheski, K.E.; Tarnopolsky, M.A. Effects of acute creatine monohydrate supplementation on leucine kinetics and mixed-muscle protein synthesis. J. Appl. Physiol. 2001, 91, 1041–1047. [Google Scholar] [CrossRef] [Green Version]
- Olsen, S.; Aagaard, P.; Kadi, F.; Tufekovic, G.; Verney, J.; Olesen, J.L.; Suetta, C.; Kjaer, M. Creatine supplementation augments the increase in satellite cell and myonuclei number in human skeletal muscle induced by strength training. J. Physiol. 2006, 573, 525–534. [Google Scholar] [CrossRef]
- Tarnopolsky, M.A.; Parise, G. Direct measurement of high-energy phosphate compounds in patients with neuromuscular disease. Muscle Nerve 1999, 22, 1228–1233. [Google Scholar] [CrossRef]
- Tarnopolsky, M.; Martin, J. Creatine monohydrate increases strength in patients with neuromuscular disease. Neurology 1999, 52, 854–857. [Google Scholar] [CrossRef]
- Tarnopolsky, M.A.; Mahoney, D.J.; Vajsar, J.; Rodriguez, C.; Doherty, T.J.; Roy, B.D.; Biggar, D. Creatine monohydrate enhances strength and body composition in Duchenne muscular dystrophy. Neurology 2004, 62, 1771–1777. [Google Scholar] [CrossRef] [Green Version]
- Walter, M.C.; Lochmuller, H.; Reilich, P.; Klopstock, T.; Huber, R.; Hartard, M.; Hennig, M.; Pongratz, D.; Muller-Felber, W. Creatine monohydrate in muscular dystrophies: A double-blind, placebo-controlled clinical study. Neurology 2000, 54, 1848–1850. [Google Scholar] [CrossRef] [PubMed]
- Louis, M.; Lebacq, J.; Poortmans, J.R.; Belpaire-Dethiou, M.C.; Devogelaer, J.P.; Van Hecke, P.; Goubel, F.; Francaux, M. Beneficial effects of creatine supplementation in dystrophic patients. Muscle Nerve 2003, 27, 604–610. [Google Scholar] [CrossRef]
- Hyder, F.; Rothman, D.L.; Bennett, M.R. Cortical energy demands of signaling and nonsignaling components in brain are conserved across mammalian species and activity levels. Proc. Natl. Acad. Sci. USA 2013, 110, 3549–3554. [Google Scholar] [CrossRef] [Green Version]
- Watts, M.E.; Pocock, R.; Claudianos, C. Brain Energy and Oxygen Metabolism: Emerging Role in Normal Function and Disease. Front. Mol. Neurosci. 2018, 11, 216. [Google Scholar] [CrossRef] [PubMed]
- Hemmer, W.; Wallimann, T. Functional aspects of creatine kinase in brain. Dev. Neurosci. 1993, 15, 249–260. [Google Scholar] [CrossRef]
- Béard, E.; Braissant, O. Synthesis and transport of creatine in the CNS: Importance for cerebral functions. J. Neurochem. 2010, 115, 297–313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- National Organization of Rare Disorders Creatine Transporter Deficiency. Available online: https://rarediseases.org/rare-diseases/creatine-transporter-deficiency/ (accessed on 27 August 2020).
- Rae, C.; Digney, A.L.; McEwan, S.R.; Bates, T.C. Oral creatine monohydrate supplementation improves brain performance: A double-blind, placebo-controlled, cross-over trial. Proc. Biol. Sci. 2003, 270, 2147–2150. [Google Scholar] [CrossRef] [Green Version]
- McMorris, T.; Harris, R.C.; Swain, J.; Corbett, J.; Collard, K.; Dyson, R.J.; Dye, L.; Hodgson, C.; Draper, N. Effect of creatine supplementation and sleep deprivation, with mild exercise, on cognitive and psychomotor performance, mood state, and plasma concentrations of catecholamines and cortisol. Psychopharmacology 2006, 185, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Rawson, E.S.; Venezia, A.C. Use of creatine in the elderly and evidence for effects on cognitive function in young and old. Amino Acids 2011, 40, 1349–1362. [Google Scholar] [CrossRef]
- Pazini, F.L.; Cunha, M.P.; Rodrigues, A.L.S. The possible beneficial effects of creatine for the management of depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 2019, 89, 193–206. [Google Scholar] [CrossRef]
- Matthews, R.T.; Ferrante, R.J.; Klivenyi, P.; Yang, L.; Klein, A.M.; Mueller, G.; Kaddurah-Daouk, R.; Beal, M.F. Creatine and cyclocreatine attenuate MPTP neurotoxicity. Exp. Neurol. 1999, 157, 142–149. [Google Scholar] [CrossRef]
- Andres, R.H.; Ducray, A.D.; Perez-Bouza, A.; Schlattner, U.; Huber, A.W.; Krebs, S.H.; Seiler, R.W.; Wallimann, T.; Widmer, H.R. Creatine supplementation improves dopaminergic cell survival and protects against MPP+ toxicity in an organotypic tissue culture system. Cell Transpl. 2005, 14, 537–550. [Google Scholar] [CrossRef] [Green Version]
- Brewer, G.J.; Wallimann, T.W. Protective effect of the energy precursor creatine against toxicity of glutamate and beta-amyloid in rat hippocampal neurons. J. Neurochem. 2000, 74, 1968–1978. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, P.; Yu, Z.; Cong, Y.; Sun, H.; Zhang, J.; Zhang, J.; Sun, C.; Zhang, Y.; Ju, X. The effect of creatine and coenzyme q10 combination therapy on mild cognitive impairment in Parkinson′s disease. Eur. Neurol. 2015, 73, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Bender, A.; Koch, W.; Elstner, M.; Schombacher, Y.; Bender, J.; Moeschl, M.; Gekeler, F.; Müller-Myhsok, B.; Gasser, T.; Tatsch, K.; et al. Creatine supplementation in Parkinson disease: A placebo-controlled randomized pilot trial. Neurology 2006, 67, 1262–1264. [Google Scholar] [CrossRef]
- Hersch, S.; Gevorkian, S.; Marder, K.; Moskowitz, C.; Feigin, A.; Cox, M.; Como, P.; Zimmerman, C.; Lin, M.; Zhang, L. Creatine in Huntington disease is safe, tolerable, bioavailable in brain and reduces serum 8OH2′ dG. Neurology 2006, 66, 250–252. [Google Scholar] [CrossRef]
- Investigators, N.N.-P. A randomized, double-blind, futility clinical trial of creatine and minocycline in early Parkinson disease. Neurology 2006, 66, 664–671. [Google Scholar] [CrossRef]
- Kieburtz, K.; Tilley, B.C.; Elm, J.J.; Babcock, D.; Hauser, R.; Ross, G.W.; Augustine, A.H.; Augustine, E.U.; Aminoff, M.J.; Bodis-Wollner, I.G.; et al. Effect of creatine monohydrate on clinical progression in patients with Parkinson disease: A randomized clinical trial. JAMA 2015, 313, 584–593. [Google Scholar] [CrossRef] [PubMed]
- Verbessem, P.; Lemiere, J.; Eijnde, B.O.; Swinnen, S.; Vanhees, L.; Van Leemputte, M.; Hespel, P.; Dom, R. Creatine supplementation in Huntington′s disease: A placebo-controlled pilot trial. Neurology 2003, 61, 925–930. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, R.J.; Andreassen, O.A.; Jenkins, B.G.; Dedeoglu, A.; Kuemmerle, S.; Kubilus, J.K.; Kaddurah-Daouk, R.; Hersch, S.M.; Beal, M.F. Neuroprotective effects of creatine in a transgenic mouse model of Huntington’s disease. J. Neurosci. 2000, 20, 4389–4397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gualano, B.; de Salles Painelli, V.; Roschel, H.; Lugaresi, R.; Dorea, E.; Artioli, G.G.; Lima, F.R.; da Silva, M.E.; Cunha, M.R.; Seguro, A.C.; et al. Creatine supplementation does not impair kidney function in type 2 diabetic patients: A randomized, double-blind, placebo-controlled, clinical trial. Eur. J. Appl. Physiol. 2011, 111, 749–756. [Google Scholar] [CrossRef]
- Alves, C.R.; Santiago, B.M.; Lima, F.R.; Otaduy, M.C.; Calich, A.L.; Tritto, A.C.; de Sa Pinto, A.L.; Roschel, H.; Leite, C.C.; Benatti, F.B.; et al. Creatine supplementation in fibromyalgia: A randomized, double-blind, placebo-controlled trial. Arthritis Care Res. 2013, 65, 1449–1459. [Google Scholar] [CrossRef] [Green Version]
- Lenz, H.; Schmidt, M.; Welge, V.; Schlattner, U.; Wallimann, T.; Elsasser, H.P.; Wittern, K.P.; Wenck, H.; Stab, F.; Blatt, T. The creatine kinase system in human skin: Protective effects of creatine against oxidative and UV damage in vitro and in vivo. J. Investig. Derm. 2005, 124, 443–452. [Google Scholar] [CrossRef] [Green Version]
- Chilibeck, P.D.; Candow, D.G.; Landeryou, T.; Kaviani, M.; Paus-Jenssen, L. Effects of Creatine and Resistance Training on Bone Health in Postmenopausal Women. Med. Sci. Sports Exerc. 2015, 47, 1587–1595. [Google Scholar] [CrossRef]
- Da Silva, R.P.; Kelly, K.B.; Leonard, K.A.; Jacobs, R.L. Creatine reduces hepatic TG accumulation in hepatocytes by stimulating fatty acid oxidation. Biochim. Biophys. Acta 2014, 1841, 1639–1646. [Google Scholar] [CrossRef]
- Guidi, C.; Potenza, L.; Sestili, P.; Martinelli, C.; Guescini, M.; Stocchi, L.; Zeppa, S.; Polidori, E.; Annibalini, G.; Stocchi, V. Differential effect of creatine on oxidatively-injured mitochondrial and nuclear DNA. Biochim. Biophys. Acta 2008, 1780, 16–26. [Google Scholar] [CrossRef]
- Murphy, S.L.; Xu, J.; Kochanek, K.D.; Arias, E. Mortality in the United States, 2017. NCHS Data Br. 2018, 328, 1–8. [Google Scholar]
- Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; Das, S.R.; de Ferranti, S.; Despres, J.P.; Fullerton, H.J.; et al. Heart Disease and Stroke Statistics-2016 Update: A Report from the American Heart Association. Circulation 2016, 133, e38–e360. [Google Scholar] [CrossRef] [PubMed]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seals, D.R.; Jablonski, K.L.; Donato, A.J. Aging and vascular endothelial function in humans. Clin. Sci. 2011, 120, 357–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Widmer, R.J.; Lerman, A. Endothelial dysfunction and cardiovascular disease. Glob. Cardiol. Sci. Pr. 2014, 2014, 291–308. [Google Scholar] [CrossRef]
- Kohn, J.C.; Lampi, M.C.; Reinhart-King, C.A. Age-related vascular stiffening: Causes and consequences. Front. Genet. 2015, 6, 112. [Google Scholar] [CrossRef] [Green Version]
- Henderson, K.K.; Byron, K.L. Vasopressin-induced vasoconstriction: Two concentration-dependent signaling pathways. J. Appl. Physiol. 2007, 102, 1402–1409. [Google Scholar] [CrossRef] [Green Version]
- Ye, G.J.; Nesmith, A.P.; Parker, K.K. The role of mechanotransduction on vascular smooth muscle myocytes’ [corrected] cytoskeleton and contractile function. Anat. Rec. 2014, 297, 1758–1769. [Google Scholar] [CrossRef] [Green Version]
- Thomas, G.D. Neural control of the circulation. Adv. Physiol. Educ. 2011, 35, 28–32. [Google Scholar] [CrossRef] [Green Version]
- Rajendran, P.; Rengarajan, T.; Thangavel, J.; Nishigaki, Y.; Sakthisekaran, D.; Sethi, G.; Nishigaki, I. The vascular endothelium and human diseases. Int. J. Biol. Sci. 2013, 9, 1057–1069. [Google Scholar] [CrossRef] [Green Version]
- Pugsley, M.K.; Tabrizchi, R. The vascular system. An overview of structure and function. J. Pharm. Toxicol. Methods 2000, 44, 333–340. [Google Scholar] [CrossRef]
- Incalza, M.A.; D′Oria, R.; Natalicchio, A.; Perrini, S.; Laviola, L.; Giorgino, F. Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vasc. Pharm. 2018, 100, 1–19. [Google Scholar] [CrossRef]
- Castellon, X.; Bogdanova, V. Chronic Inflammatory Diseases and Endothelial Dysfunction. Aging Dis. 2016, 7, 81–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Popov, D. Endothelial cell dysfunction in hyperglycemia: Phenotypic change, intracellular signaling modification, ultrastructural alteration, and potential clinical outcomes. Int. J. Diabetes Mellit. 2010, 2, 189–195. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.A.; Montagnani, M.; Chandrasekran, S.; Quon, M.J. Role of lipotoxicity in endothelial dysfunction. Heart Fail. Clin. 2012, 8, 589–607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lobato, N.S.; Filgueira, F.P.; Akamine, E.H.; Tostes, R.C.; Carvalho, M.H.; Fortes, Z.B. Mechanisms of endothelial dysfunction in obesity-associated hypertension. Braz. J. Med. Biol. Res. 2012, 45, 392–400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michael Pittilo, R. Cigarette smoking, endothelial injury and cardiovascular disease. Int. J. Exp. Pathol. 2000, 81, 219–230. [Google Scholar] [CrossRef]
- Tanaka, A.; Cui, R.; Kitamura, A.; Liu, K.; Imano, H.; Yamagishi, K.; Kiyama, M.; Okada, T.; Iso, H. Heavy Alcohol Consumption is Associated with Impaired Endothelial Function. J. Atheroscler. Thromb. 2016, 23, 1047–1054. [Google Scholar] [CrossRef] [Green Version]
- Fleg, J.L.; Strait, J. Age-associated changes in cardiovascular structure and function: A fertile milieu for future disease. Heart Fail. Rev. 2012, 17, 545–554. [Google Scholar] [CrossRef] [Green Version]
- Hadi, H.A.; Carr, C.S.; Al Suwaidi, J. Endothelial dysfunction: Cardiovascular risk factors, therapy, and outcome. Vasc. Health Risk Manag. 2005, 1, 183–198. [Google Scholar]
- Kawashima, S.; Yokoyama, M. Dysfunction of Endothelial Nitric Oxide Synthase and Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 998–1005. [Google Scholar] [CrossRef]
- Widlansky, M.E.; Gokce, N.; Keaney, J.F., Jr.; Vita, J.A. The clinical implications of endothelial dysfunction. J. Am. Coll. Cardiol. 2003, 42, 1149–1160. [Google Scholar] [CrossRef] [Green Version]
- Kaiser Family Foundation. Data Note Prescription Drugs and Older Adults. Available online: https://www.kff.org/health-reform/issue-brief/data-note-prescription-drugs-and-older-adults/ (accessed on 27 August 2020).
- Council for Responsible Nutrition. 2018 Consumer Survey on Dietary Supplements. Available online: https://www.crnusa.org/sites/default/files/images/2018-survey/CRN-ConsumerSurvey-Infographic-2019f.pdf (accessed on 27 August 2020).
- Arciero, P.J.; Hannibal, N.S., 3rd; Nindl, B.C.; Gentile, C.L.; Hamed, J.; Vukovich, M.D. Comparison of creatine ingestion and resistance training on energy expenditure and limb blood flow. Metabolism 2001, 50, 1429–1434. [Google Scholar] [CrossRef] [PubMed]
- Englund, D.A.; Kirn, D.R.; Koochek, A.; Zhu, H.; Travison, T.G.; Reid, K.F.; von Berens, Å.; Melin, M.; Cederholm, T.; Gustafsson, T.; et al. Nutritional Supplementation with Physical Activity Improves Muscle Composition in Mobility-Limited Older Adults, the VIVE2 Study: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 73, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Beaudart, C.; Dawson, A.; Shaw, S.C.; Harvey, N.C.; Kanis, J.A.; Binkley, N.; Reginster, J.Y.; Chapurlat, R.; Chan, D.C.; Bruyère, O.; et al. Nutrition and physical activity in the prevention and treatment of sarcopenia: Systematic review. Osteoporos. Int. 2017, 28, 1817–1833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchez-Gonzalez, M.A.; Wieder, R.; Kim, J.S.; Vicil, F.; Figueroa, A. Creatine supplementation attenuates hemodynamic and arterial stiffness responses following an acute bout of isokinetic exercise. Eur. J. Appl. Physiol. 2011, 111, 1965–1971. [Google Scholar] [CrossRef]
- Cui, J.; Mascarenhas, V.; Moradkhan, R.; Blaha, C.; Sinoway, L.I. Effects of muscle metabolites on responses of muscle sympathetic nerve activity to mechanoreceptor(s) stimulation in healthy humans. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 294, R458–R466. [Google Scholar] [CrossRef] [Green Version]
- Jadhav, U.M.; Kadam, N.N. Non-invasive assessment of arterial stiffness by pulse-wave velocity correlates with endothelial dysfunction. Indian Heart J. 2005, 57, 226–232. [Google Scholar]
- Cole, C.R.; Blackstone, E.H.; Pashkow, F.J.; Snader, C.E.; Lauer, M.S. Heart-rate recovery immediately after exercise as a predictor of mortality. N. Engl. J. Med. 1999, 341, 1351–1357. [Google Scholar] [CrossRef]
- Moraes, R.; Van Bavel, D.; Moraes, B.S.; Tibirica, E. Effects of dietary creatine supplementation on systemic microvascular density and reactivity in healthy young adults. Nutr. J. 2014, 13, 115. [Google Scholar] [CrossRef] [Green Version]
- Raddino, R.; Caretta, G.; Teli, M.; Bonadei, I.; Robba, D.; Zanini, G.; Madureri, A.; Nodari, S.; Dei Cas, L. Nitric oxide and cardiovascular risk factors. Heart Int. 2007, 3, 18. [Google Scholar] [CrossRef]
- Sverdlov, A.L.; Ngo, D.T.; Chan, W.P.; Chirkov, Y.Y.; Horowitz, J.D. Aging of the nitric oxide system: Are we as old as our NO? J. Am. Heart Assoc. 2014, 3, e000973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lorenzo, S.; Minson, C.T. Human cutaneous reactive hyperaemia: Role of BKCa channels and sensory nerves. J. Physiol. 2007, 585, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Kaviani, M.; Shaw, K.; Chilibeck, P.D. Benefits of Creatine Supplementation for Vegetarians Compared to Omnivorous Athletes: A Systematic Review. Int. J. Env. Res. Public Health 2020, 17, 3041. [Google Scholar] [CrossRef]
- Dhingra, R.; Vasan, R.S. Age as a risk factor. Med. Clin. N. Am. 2012, 96, 87–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taddei, S.; Virdis, A.; Ghiadoni, L.; Salvetti, G.; Bernini, G.; Magagna, A.; Salvetti, A. Age-related reduction of NO availability and oxidative stress in humans. Hypertension 2001, 38, 274–279. [Google Scholar] [CrossRef]
- Yavuz, B.B.; Yavuz, B.; Sener, D.D.; Cankurtaran, M.; Halil, M.; Ulger, Z.; Nazli, N.; Kabakci, G.; Aytemir, K.; Tokgozoglu, L.; et al. Advanced age is associated with endothelial dysfunction in healthy elderly subjects. Gerontology 2008, 54, 153–156. [Google Scholar] [CrossRef]
- Chung, H.Y.; Kim, D.H.; Lee, E.K.; Chung, K.W.; Chung, S.; Lee, B.; Seo, A.Y.; Chung, J.H.; Jung, Y.S.; Im, E.; et al. Redefining Chronic Inflammation in Aging and Age-Related Diseases: Proposal of the Senoinflammation Concept. Aging Dis. 2019, 10, 367–382. [Google Scholar] [CrossRef] [Green Version]
- Clarke, H.; Kim, D.-H.; Meza, C.; Hickner, R. Pilot Study: The Effect of Acute 5-Day Creatine Supplementation on Macrovascular Endothelial Function in Older Adults. Curr. Dev. Nutr. 2020, 4, 15. [Google Scholar] [CrossRef]
- Rawlings, R.; Nohria, A.; Liu, P.-Y.; Donnelly, J.; Creager, M.A.; Ganz, P.; Selwyn, A.; Liao, J.K. Comparison of effects of rosuvastatin (10 mg) versus atorvastatin (40 mg) on rho kinase activity in caucasian men with a previous atherosclerotic event. Am. J. Cardiol. 2009, 103, 437–441. [Google Scholar] [CrossRef] [Green Version]
- Eskurza, I.; Myerburgh, L.A.; Kahn, Z.D.; Seals, D.R. Tetrahydrobiopterin augments endothelium-dependent dilatation in sedentary but not in habitually exercising older adults. J. Physiol. 2005, 568, 1057–1065. [Google Scholar] [CrossRef]
- Pierce, G.L.; Eskurza, I.; Walker, A.E.; Fay, T.N.; Seals, D.R. Sex-specific effects of habitual aerobic exercise on brachial artery flow-mediated dilation in middle-aged and older adults. Clin. Sci. 2011, 120, 13–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Betteridge, D.J. What is oxidative stress? Metabolism 2000, 49, 3–8. [Google Scholar] [CrossRef]
- Burton, G.J.; Jauniaux, E. Oxidative stress. Best Pr. Res. Clin. Obs. Gynaecol. 2011, 25, 287–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Meo, S.; Reed, T.T.; Venditti, P.; Victor, V.M. Harmful and Beneficial Role of ROS. Oxid. Med. Cell. Longev. 2016, 2016, 7909186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pham-Huy, L.A.; He, H.; Pham-Huy, C. Free radicals, antioxidants in disease and health. Int. J. Biomed. Sci. 2008, 4, 89–96. [Google Scholar]
- Dinkova-Kostova, A.T.; Talalay, P. Direct and indirect antioxidant properties of inducers of cytoprotective proteins. Mol. Nutr. Food Res. 2008, 52, S128–S138. [Google Scholar] [CrossRef]
- Tan, B.L.; Norhaizan, M.E.; Liew, W.P.; Sulaiman Rahman, H. Antioxidant and Oxidative Stress: A Mutual Interplay in Age-Related Diseases. Front. Pharm. 2018, 9, 1162. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Ren, Z.; Zhang, J.; Chuang, C.C.; Kandaswamy, E.; Zhou, T.; Zuo, L. Role of ROS and Nutritional Antioxidants in Human Diseases. Front. Physiol. 2018, 9, 477. [Google Scholar] [CrossRef] [Green Version]
- Simioni, C.; Zauli, G.; Martelli, A.M.; Vitale, M.; Sacchetti, G.; Gonelli, A.; Neri, L.M. Oxidative stress: Role of physical exercise and antioxidant nutraceuticals in adulthood and aging. Oncotarget 2018, 9, 17181–17198. [Google Scholar] [CrossRef] [Green Version]
- Fusco, D.; Colloca, G.; Lo Monaco, M.R.; Cesari, M. Effects of antioxidant supplementation on the aging process. Clin. Interv. Aging 2007, 2, 377–387. [Google Scholar]
- Senoner, T.; Dichtl, W. Oxidative Stress in Cardiovascular Diseases: Still a Therapeutic Target? Nutrients 2019, 11, 2090. [Google Scholar] [CrossRef] [Green Version]
- Wallimann, T.; Tokarska-Schlattner, M.; Schlattner, U. The creatine kinase system and pleiotropic effects of creatine. Amino Acids 2011, 40, 1271–1296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madamanchi, N.R.; Vendrov, A.; Runge, M.S. Oxidative stress and vascular disease. Arter. Thromb. Vasc. Biol. 2005, 25, 29–38. [Google Scholar] [CrossRef] [Green Version]
- Matthews, R.T.; Yang, L.; Jenkins, B.G.; Ferrante, R.J.; Rosen, B.R.; Kaddurah-Daouk, R.; Beal, M.F. Neuroprotective effects of creatine and cyclocreatine in animal models of Huntington′s disease. J. Neurosci. 1998, 18, 156–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sestili, P.; Martinelli, C.; Bravi, G.; Piccoli, G.; Curci, R.; Battistelli, M.; Falcieri, E.; Agostini, D.; Gioacchini, A.M.; Stocchi, V. Creatine supplementation affords cytoprotection in oxidatively injured cultured mammalian cells via direct antioxidant activity. Free Radic. Biol. Med. 2006, 40, 837–849. [Google Scholar] [CrossRef]
- Bray, A.W.; Ballinger, S.W. Mitochondrial DNA mutations and cardiovascular disease. Curr. Opin. Cardiol. 2017, 32, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Fimognari, C.; Sestili, P.; Lenzi, M.; Cantelli-Forti, G.; Hrelia, P. Protective effect of creatine against RNA damage. Mutat. Res. 2009, 670, 59–67. [Google Scholar] [CrossRef]
- Rambo, L.M.; Ribeiro, L.R.; Oliveira, M.S.; Furian, A.F.; Lima, F.D.; Souza, M.A.; Silva, L.F.; Retamoso, L.T.; Corte, C.L.; Puntel, G.O.; et al. Additive anticonvulsant effects of creatine supplementation and physical exercise against pentylenetetrazol-induced seizures. Neurochem. Int. 2009, 55, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Sestili, P.; Barbieri, E.; Martinelli, C.; Battistelli, M.; Guescini, M.; Vallorani, L.; Casadei, L.; D’Emilio, A.; Falcieri, E.; Piccoli, G.; et al. Creatine supplementation prevents the inhibition of myogenic differentiation in oxidatively injured C2C12 murine myoblasts. Mol. Nutr. Food Res. 2009, 53, 1187–1204. [Google Scholar] [CrossRef]
- Hosamani, R.; Ramesh, S.R. Attenuation of rotenone-induced mitochondrial oxidative damage and neurotoxicty in Drosophila melanogaster supplemented with creatine. Neurochem. Res. 2010, 35, 1402–1412. [Google Scholar] [CrossRef]
- Peng, C.; Wang, X.; Chen, J.; Jiao, R.; Wang, L.; Li, Y.M.; Zuo, Y.; Liu, Y.; Lei, L.; Ma, K.Y.; et al. Biology of Ageing and Role of Dietary Antioxidants. Biomed. Res. Int. 2014, 2014, 831841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tribble, D.L. Antioxidant Consumption and Risk of Coronary Heart Disease: Emphasis on Vitamin C, Vitamin E, and β-carotene: A statement for healthcare professionals from the American Heart Association. Circulation 1999, 99, 591–595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goszcz, K.; Deakin, S.J.; Duthie, G.G.; Stewart, D.; Leslie, S.J.; Megson, I.L. Antioxidants in Cardiovascular Therapy: Panacea or False Hope? Front. Cardiovasc. Med. 2015, 2, 29. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.A.; Figueroa, A.; Navaei, N.; Wong, A.; Kalfon, R.; Ormsbee, L.T.; Feresin, R.G.; Elam, M.L.; Hooshmand, S.; Payton, M.E.; et al. Daily Blueberry Consumption Improves Blood Pressure and Arterial Stiffness in Postmenopausal Women with Pre- and Stage 1-Hypertension: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. J. Acad. Nutr. Diet. 2015, 115, 369–377. [Google Scholar] [CrossRef]
- Alizadeh, M.; Kheirouri, S. Curcumin reduces malondialdehyde and improves antioxidants in humans with diseased conditions: A comprehensive meta-analysis of randomized controlled trials. Biomedicine 2019, 9, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, A.; Palfrey, H.A.; Pathak, R.; Kadowitz, P.J.; Gettys, T.W.; Murthy, S.N. The metabolism and significance of homocysteine in nutrition and health. Nutr. Metab. 2017, 14, 78. [Google Scholar] [CrossRef] [Green Version]
- Wyss, M.; Schulze, A. Health implications of creatine: Can oral creatine supplementation protect against neurological and atherosclerotic disease? Neuroscience 2002, 112, 243–260. [Google Scholar] [CrossRef]
- Tyagi, N.; Sedoris, K.C.; Steed, M.; Ovechkin, A.V.; Moshal, K.S.; Tyagi, S.C. Mechanisms of homocysteine-induced oxidative stress. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, H2649–H2656. [Google Scholar] [CrossRef] [Green Version]
- Brosnan, J.T.; da Silva, R.P.; Brosnan, M.E. The metabolic burden of creatine synthesis. Amino Acids 2011, 40, 1325–1331. [Google Scholar] [CrossRef]
- Mudd, S.H.; Ebert, M.H.; Scriver, C.R. Labile methyl group balances in the human: The role of sarcosine. Metabolism 1980, 29, 707–720. [Google Scholar] [CrossRef]
- McCarty, M.F. Supplemental creatine may decrease serum homocysteine and abolish the homocysteine ‘gender gap’ by suppressing endogenous creatine synthesis. Med. Hypotheses 2001, 56, 5–7. [Google Scholar] [CrossRef] [PubMed]
- Marcus, J.; Sarnak, M.J.; Menon, V. Homocysteine lowering and cardiovascular disease risk: Lost in translation. Can J Cardiol. 2007, 23, 707–710. [Google Scholar] [CrossRef] [Green Version]
- Stead, L.M.; Au, K.P.; Jacobs, R.L.; Brosnan, M.E.; Brosnan, J.T. Methylation demand and homocysteine metabolism: Effects of dietary provision of creatine and guanidinoacetate. Am. J. Physiol. Endocrinol. Metab. 2001, 281, E1095–E1100. [Google Scholar] [CrossRef]
- Steenge, G.R.; Verhoef, P.; Greenhaff, P.L. The Effect of Creatine and Resistance Training on Plasma Homocysteine Concentration in Healthy Volunteers. Arch. Intern. Med. 2001, 161, 1455–1457. [Google Scholar] [CrossRef]
- Taes, Y.E.; Delanghe, J.R.; De Vriese, A.S.; Rombaut, R.; Van Camp, J.; Lameire, N.H. Creatine supplementation decreases homocysteine in an animal model of uremia. Kidney Int. 2003, 64, 1331–1337. [Google Scholar] [CrossRef] [Green Version]
- Deminice, R.; Cella, P.S.; Padilha, C.S.; Borges, F.H.; da Silva, L.E.; Campos-Ferraz, P.L.; Jordao, A.A.; Robinson, J.L.; Bertolo, R.F.; Cecchini, R.; et al. Creatine supplementation prevents hyperhomocysteinemia, oxidative stress and cancer-induced cachexia progression in Walker-256 tumor-bearing rats. Amino Acids 2016, 48, 2015–2024. [Google Scholar] [CrossRef]
- Deminice, R.; Portari, G.V.; Vannucchi, H.; Jordao, A.A. Effects of creatine supplementation on homocysteine levels and lipid peroxidation in rats. Br. J. Nutr. 2009, 102, 110–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peters, B.A.; Hall, M.N.; Liu, X.; Parvez, F.; Siddique, A.B.; Shahriar, H.; Uddin, M.N.; Islam, T.; Ilievski, V.; Graziano, J.H.; et al. Low-Dose Creatine Supplementation Lowers Plasma Guanidinoacetate, but Not Plasma Homocysteine, in a Double-Blind, Randomized, Placebo-Controlled Trial. J. Nutr. 2015, 145, 2245–2252. [Google Scholar] [CrossRef] [Green Version]
- Deminice, R.; Rosa, F.T.; Franco, G.S.; da Cunha, S.F.; de Freitas, E.C.; Jordao, A.A. Short-term creatine supplementation does not reduce increased homocysteine concentration induced by acute exercise in humans. Eur. J. Nutr. 2014, 53, 1355–1361. [Google Scholar] [CrossRef]
- Taes, Y.E.; Delanghe, J.R.; De Bacquer, D.; Langlois, M.; Stevens, L.; Geerolf, I.; Lameire, N.H.; De Vriese, A.S. Creatine supplementation does not decrease total plasma homocysteine in chronic hemodialysis patients. Kidney Int. 2004, 66, 2422–2428. [Google Scholar] [CrossRef] [Green Version]
- Jahangir, E.; Vita, J.A.; Handy, D.; Holbrook, M.; Palmisano, J.; Beal, R.; Loscalzo, J.; Eberhardt, R.T. The effect of L-arginine and creatine on vascular function and homocysteine metabolism. Vasc. Med. 2009, 14, 239–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shelmadine, B.; Hudson, G.M.; Buford, T.W.; Grothe, A.; Moreillon, J.J.; Gutierrez, J.L.; Bowden, R.G.; Wilson, R.L.; Willoughby, D.W. The effects of supplementation of creatine on total homocysteine. J. Ren. Nurs. 2012, 4, 278–283. [Google Scholar] [CrossRef]
- Brattström, L.; Lindgren, A.; Israelsson, B.; Andersson, A.; Hultberg, B. Homocysteine and cysteine: Determinants of plasma levels in middle-aged and elderly subjects. J. Intern. Med. 1994, 236, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Strassburg, A.; Krems, C.; Lührmann, P.M.; Hartmann, B.; Neuhäuser-Berthold, M. Effect of age on plasma homocysteine concentrations in young and elderly subjects considering serum vitamin concentrations and different lifestyle factors. Int. J. Vitam. Nutr. Res. 2004, 74, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Kuo, H.-K.; Sorond, F.A.; Chen, J.-H.; Hashmi, A.; Milberg, W.P.; Lipsitz, L.A. The Role of Homocysteine in Multisystem Age-Related Problems: A Systematic Review. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2005, 60, 1190–1201. [Google Scholar] [CrossRef]
- Moore, K.J. Targeting inflammation in CVD: Advances and challenges. Nat. Rev. Cardiol. 2019, 16, 74–75. [Google Scholar] [CrossRef]
- Winter, C.A.; Risley, E.A.; Nuss, G.W. Carrageenin-Induced Edema in Hind Paw of the Rat as an Assay for Antiinflammatory Drugs. Proc. Soc. Exp. Biol. Med. 1962, 111, 544–547. [Google Scholar] [CrossRef]
- Madan, B.; Khanna, N. Effect of aminoacids on the carrageenan induced paw oedema in rats: A preliminary report. Indian J. Pharmacol. 1976, 8, 227–229. [Google Scholar]
- Khanna, N.K.; Madan, B.R. Studies on the anti-inflammatory activity of creatine. Arch. Int. Pharm. 1978, 231, 340–350. [Google Scholar]
- Madan, B.R.; Khanna, N.K. Effect of creatinine on various experimentally induced inflammatory models. Indian J. Physiol. Pharm. 1979, 23, 1–7. [Google Scholar]
- Luc, G.; Arveiler, D.; Evans, A.; Amouyel, P.; Ferrieres, J.; Bard, J.M.; Elkhalil, L.; Fruchart, J.C.; Ducimetiere, P. Circulating soluble adhesion molecules ICAM-1 and VCAM-1 and incident coronary heart disease: The PRIME Study. Atherosclerosis 2003, 170, 169–176. [Google Scholar] [CrossRef] [Green Version]
- Roldan, V.; Marin, F.; Lip, G.Y.; Blann, A.D. Soluble E-selectin in cardiovascular disease and its risk factors. A review of the literature. Thromb. Haemost. 2003, 90, 1007–1020. [Google Scholar] [CrossRef] [PubMed]
- Chistiakov, D.A.; Orekhov, A.N.; Bobryshev, Y.V. Endothelial Barrier and Its Abnormalities in Cardiovascular Disease. Front. Physiol. 2015, 6, 365. [Google Scholar] [CrossRef] [PubMed]
- Mundi, S.; Massaro, M.; Scoditti, E.; Carluccio, M.A.; van Hinsbergh, V.W.M.; Iruela-Arispe, M.L.; De Caterina, R. Endothelial permeability, LDL deposition, and cardiovascular risk factors-a review. Cardiovasc. Res. 2018, 114, 35–52. [Google Scholar] [CrossRef] [PubMed]
- Santos, R.V.; Bassit, R.A.; Caperuto, E.C.; Costa Rosa, L.F. The effect of creatine supplementation upon inflammatory and muscle soreness markers after a 30km race. Life Sci. 2004, 75, 1917–1924. [Google Scholar] [CrossRef] [PubMed]
- Deminice, R.; Rosa, F.T.; Franco, G.S.; Jordao, A.A.; de Freitas, E.C. Effects of creatine supplementation on oxidative stress and inflammatory markers after repeated-sprint exercise in humans. Nutrition 2013, 29, 1127–1132. [Google Scholar] [CrossRef] [Green Version]
- Silva, L.A.; Tromm, C.B.; Da Rosa, G.; Bom, K.; Luciano, T.F.; Tuon, T.; De Souza, C.T.; Pinho, R.A. Creatine supplementation does not decrease oxidative stress and inflammation in skeletal muscle after eccentric exercise. J. Sports Sci. 2013, 31, 1164–1176. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, J.; Kim, S.; Yoon, D.; Kim, J.; Sung, D.J. Role of creatine supplementation in exercise-induced muscle damage: A mini review. J. Exerc. Rehabil. 2015, 11, 244–250. [Google Scholar] [CrossRef] [Green Version]
- Hansson, G.K.; Robertson, A.K.; Söderberg-Nauclér, C. Inflammation and atherosclerosis. Annu. Rev. Pathol. 2006, 1, 297–329. [Google Scholar] [CrossRef]
- Anderson, O. Creatine propels British athletes to Olympic gold medals: Is creatine the one true ergogenic aid. Run. Res. News 1993, 9, 1–5. [Google Scholar]
- Bird, S.P. Creatine supplementation and exercise performance: A brief review. J. Sports Sci. Med. 2003, 2, 123–132. [Google Scholar]
- Persky, A.M.; Rawson, E.S. Safety of creatine supplementation. Subcell Biochem. 2007, 46, 275–289. [Google Scholar]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clarke, H.; Kim, D.-H.; Meza, C.A.; Ormsbee, M.J.; Hickner, R.C. The Evolving Applications of Creatine Supplementation: Could Creatine Improve Vascular Health? Nutrients 2020, 12, 2834. https://doi.org/10.3390/nu12092834
Clarke H, Kim D-H, Meza CA, Ormsbee MJ, Hickner RC. The Evolving Applications of Creatine Supplementation: Could Creatine Improve Vascular Health? Nutrients. 2020; 12(9):2834. https://doi.org/10.3390/nu12092834
Chicago/Turabian StyleClarke, Holly, Do-Houn Kim, Cesar A. Meza, Michael J. Ormsbee, and Robert C. Hickner. 2020. "The Evolving Applications of Creatine Supplementation: Could Creatine Improve Vascular Health?" Nutrients 12, no. 9: 2834. https://doi.org/10.3390/nu12092834
APA StyleClarke, H., Kim, D. -H., Meza, C. A., Ormsbee, M. J., & Hickner, R. C. (2020). The Evolving Applications of Creatine Supplementation: Could Creatine Improve Vascular Health? Nutrients, 12(9), 2834. https://doi.org/10.3390/nu12092834





