The Relative Content and Distribution of Absorbed Volatile Organic Compounds in Rats Administered Asari Radix et Rhizoma Are Different between Powder- and Decoction-Treated Groups
Abstract
:1. Introduction
2. Results and Discussion
2.1. Identification of ARR VOCs In Vivo in Rats
2.2. Distribution of ARR VOCs In Vivo in Rats
2.2.1. Distribution of VOCs in ARR Powder-Treated Group
2.2.2. Distribution of VOCs in Decoction-Treated Group
2.2.3. Distribution of Main Compounds Identified In Vivo in Rats
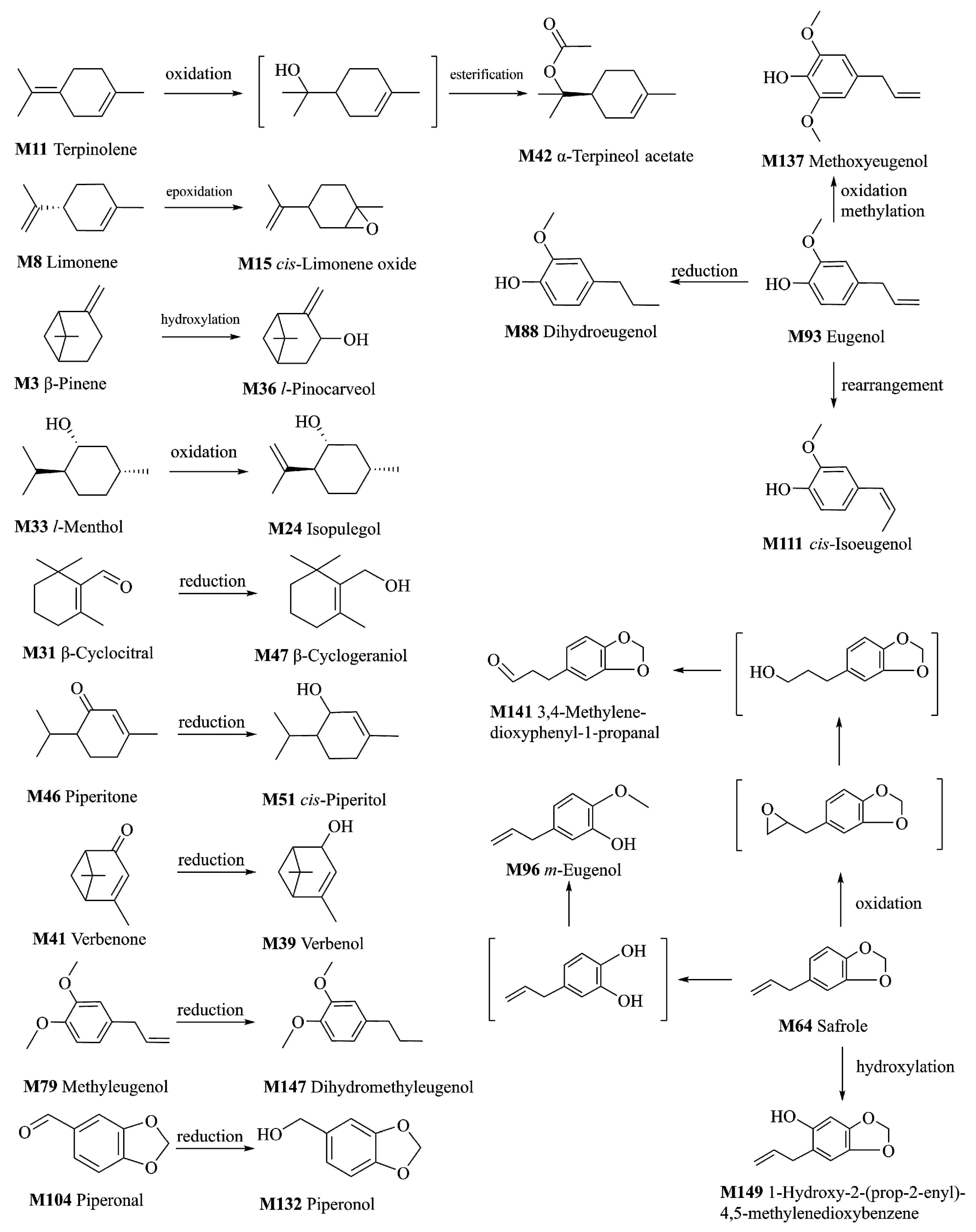
2.3. Metabolism of VOCs of ARR
2.3.1. Metabolites of the Oxidation Reaction
2.3.2. Metabolites of the Reduction Reaction
2.4. Review of the Bioactivity and Acute Toxicity of Main Absorbed Constituents
3. Experiment
3.1. Chemicals and Reagents
3.2. Plant Material
3.3. Sample Preparation
3.3.1. Dosage of Administration
3.3.2. Decoction of ARR
3.3.3. Suspension Solution of ARR Powder
3.4. HS-SPME-GC–MS Analysis
3.5. Animals and Drug Administration
3.6. Sample Collection and Preparation
3.7. Identification of Compounds
- Level 1:
- Compounds were identified by comparing their retention time and mass spectra with those of reference compounds.
- Level 2:
- Compounds were identified by comparing their RI and mass spectra with those of the literature.
- Level 3:
- Compounds were identified by searching mass spectra in NIST 11.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ARR | Asari Radix et Rhizoma |
| AHM | Asarum heterotropoides Fr. Schmidt var. mandshuricum (Maxim.) Kitag. |
| VOCs | Volatile organic compounds |
| RI | Retention index |
| LD50 | Median lethal dose |
| 3,5-DMT | 3,5-Dimethoxytoluene |
| 2,3,5-TMT | 2,3,5-Trimethoxytoluene |
| 3,4,5-TMT | 3,4,5-Trimethoxytoluene |
| GC–MS | Gas chromatography–mass spectrometry |
| HPLC | High–performance liquid chromatography |
| UPLC | Ultra–performance liquid chromatography |
| HS–SPME–GC–MS | Headspace solid–phase microextraction gas–chromatographymass spectrometry |
| HPLC–APCI–IT–TOF–MSn | High-performance liquid chromatography–atmospheric pressurechemical ionization–ion trap–time of flight–multistage mass spectrometry |
References
- Commission of Chinese Pharmacopoeia. Pharmacopoeia of the People’s Republic of China; China Medical Science Press: Beijing, China, 2015; Volume 1, pp. 230–231. [Google Scholar]
- Chang, H.M.; But, P.P.H. Pharmacology and Applications of Chinese Materia Medica; World Scientific: Hackensack, NJ, USA, 1987; pp. 807–811. [Google Scholar]
- Cai, S.Q.; Yu, J.; Wang, X.; Wang, R.Q.; Ran, F.X.; Shang, M.Y.; Cui, J.R.; Komatsu, K.; Namba, T. Cytotoxic activity of some Asarum plants. Fitoterapia 2008, 79, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Ramalingam, M.; Kim, S.J. Phytochemical, toxicological and pharmacological studies of asiasari radix et rhizoma: A review. Trop. J. Pharm. Res. 2015, 14, 545–554. [Google Scholar] [CrossRef] [Green Version]
- Jing, Y.; Zhang, Y.F.; Shang, M.Y.; Liu, G.X.; Li, Y.L.; Wang, X.; Cai, S.Q. Chemical constituents from the roots and rhizomes of Asarum heterotropoides var. mandshuricum and the in vitro anti-inflammatory activity. Molecules 2017, 22, 125. [Google Scholar] [CrossRef] [Green Version]
- Jing, Y.; Zhang, Y.F.; Shang, M.Y.; Yu, J.; Tang, J.W.; Liu, G.X.; Li, Y.L.; Li, X.M.; Wang, X.; Cai, S.Q.; et al. Phenanthrene derivatives from roots and rhizomes of Asarum heterotropoides var. mandshuricum. Fitoterapia 2017, 117, 101–108. [Google Scholar] [CrossRef]
- Li, C.; Xu, F.; Cao, C.; Shang, M.Y.; Zhang, C.Y.; Yu, J.; Liu, G.X.; Wang, X.; Cai, S.Q. Comparative analysis of two species of Asari Radix et Rhizoma by electronic nose, headspace GC-MS and chemometrics. J. Pharm. Biomed. Anal. 2013, 85, 231–238. [Google Scholar] [CrossRef]
- Cao, C.; Wang, J.; Wang, L.; Shang, M.; Liu, G.; Xu, F.; Wang, X.; Cai, S. Simultaneous determination of seven principal constituents in Asari Radix et Rhizoma by HPLC. J. Chin. Pharm. Sci. 2015, 24, 530–537. [Google Scholar] [CrossRef]
- Wen, H.; Gao, H.Y.; Qi, W.; Xiao, F.; Wang, L.L.; Wang, D.; Yuan, D. Simultaneous determination of twenty-two components in Asari Radix et Rhizoma by ultra-performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry. Planta Med. 2014, 80, 1753–1762. [Google Scholar] [CrossRef] [Green Version]
- Xie, D.M.; Liu, G.X.; Xu, F.; Shang, M.Y.; Zhang, Z.W.; Wang, X.; Cai, S.Q. Qualitative and quantitative analysis of dodecatetraenamides A, B in Asari Radix et Rhizoma. China J. Chin. Mater. Med. 2015, 40, 691–699. [Google Scholar] [CrossRef]
- Zhang, Z.W.; Liu, G.X.; Xie, D.M.; Tian, F.; Jia, Y.K.; Xu, F.; Shang, M.Y.; Wang, X.; Cai, S.Q. Quantitative determination of seven major absorbed volatile constituents in mice brain, liver and blood after intragastric administration of Asari Radix et Rhizoma suspension by headspace-solid phase microextraction-gas chromatography-mass spectrometry. China J. Chin. Mater. Med. 2016, 41, 285–293. [Google Scholar] [CrossRef]
- Li, C.; Xu, F.; Xie, D.M.; Jing, Y.; Shang, M.Y.; Liu, G.X.; Wang, X.; Cai, S.Q. Identification of absorbed constituents in the rabbit plasma and cerebrospinal fluid after intranasal administration of Asari Radix et Rhizoma by HS-SPME-GC-MS and HPLC-APCI-IT-TOF-MSn. Molecules 2014, 19, 4857–4879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Y.; Xu, K.; Han, Y.; Wang, S. Simultaneous determination of two epimeric furofuran lignans (sesamin and asarinin) of Asarum heterotropoides extract in rat plasma by LC/MS/MS: Application to pharmacokinetic study. J. Chromatogr. Sci. 2014, 52, 793–798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.; Spriano, D.; Lehmann, T.; Meier, B. Reduction of safrole and methyleugenol in Asari radix et rhizoma by decoction. Forsch Komplement. 2009, 16, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, L.; Goen, T. R-Limonene metabolism in humans and metabolite kinetics after oral administration. Arch. Toxicol. 2017, 91, 1175–1185. [Google Scholar] [CrossRef]
- Schmidt, L.; Goen, T. Human metabolism of alpha-pinene and metabolite kinetics after oral administration. Arch. Toxicol. 2017, 91, 677–687. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, M.S.; Malnoë, A.; Broillet, A.L. Absorption, metabolism and excretion of safrole in the rat and man. Toxicology 1977, 7, 69–83. [Google Scholar] [CrossRef]
- Klungsøyr, J.; Scheline, R.R. Metabolism of Safrole in the Rat. Acta Pharmacol. Toxicol. 1983, 52, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.H.; Zhang, L.; Zhi, D.X.; Liu, W.L.; Gao, X.; He, X. Identification and analysis of the reactive metabolites related to the hepatotoxicity of safrole. Xenobiotica 2018, 48, 1164–1172. [Google Scholar] [CrossRef]
- Klungsoyr, J.; Scheline, R.R. Metabolism of piperonal and piperonyl alcohol in the rat with special reference to the scission of the methylenedioxy group. Acta Pharm. Suec. 1984, 21, 67–72. [Google Scholar]
- Cartus, A.T.; Herrmann, K.; Weishaupt, L.W.; Merz, K.H.; Engst, W.; Glatt, H.; Schrenk, D. Metabolism of methyleugenol in liver microsomes and primary hepatocytes: Pattern of metabolites, cytotoxicity, and DNA-adduct formation. Toxicol. Sci. 2012, 129, 21–34. [Google Scholar] [CrossRef] [Green Version]
- Han, A.R.; Kim, H.J.; Shin, M.; Hong, M.; Kim, Y.S.; Bae, H. Constituents of Asarum sieboldii with Inhibitory Activity on Lipopolysaccharide (LPS)-Induced NO production in BV-2 microglial cells. Chem. Biodivers. 2008, 5, 346–351. [Google Scholar] [CrossRef]
- Zheng, Z.C.; Shu, Y.C.; Hua, M.C.; Lin, W.J. Quantitative determination of methyleugenol and safrole in Herba Asari by gas chromatography. Chin. Pharm. J. 1999, 34, 44–46. [Google Scholar]
- Choi, Y.K.; Cho, G.S.; Hwang, S.; Kim, B.W.; Lim, J.H.; Lee, J.C.; Kim, H.C.; Kim, W.K.; Kim, Y.S. Methyleugenol reduces cerebral ischemic injury by suppression of oxidative injury and inflammation. Free Radic. Res. 2010, 44, 925–935. [Google Scholar] [CrossRef] [PubMed]
- Yano, S.; Suzuki, Y.; Yuzurihara, M.; Kase, Y.; Takeda, S.; Watanabe, S.; Aburada, M.; Miyamoto, K. Antinociceptive effect of methyleugenol on formalin-induced hyperalgesia in mice. Eur. J. Pharmacol. 2006, 553, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Lima, C.C.; Criddle, D.N.; Coelho-de-Souza, A.N.; Monte, F.J.Q.; Jaffar, M.; Leal-Cardoso, J.H. Relaxant and Antispasmodic Actions of Methyleugenol on Guinea-Pig Isolated Ileum. Planta Med. 2000, 66, 408–411. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, K.; Yanagisawa, T.; Okui, Y.; Ikeya, Y.; Maruno, M.; Fujita, T. Studies on anti-allergic components in the roots of Asiasarum sieboldi. Planta Med. 1994, 60, 124–127. [Google Scholar] [CrossRef] [PubMed]
- Lahlou, S.; Figueiredo, A.F.; Magalhaes, P.J.; Leal-Cardoso, J.H.; Gloria, P.D. Cardiovascular effects of methyleugenol, a natural constituent of many plant essential oils, in normotensive rats. Life Sci. 2004, 74, 2401–2412. [Google Scholar] [CrossRef]
- Sayyah, M.; Valizadeh, J.; Kamalinejad, M. Anticonvulsant activity of the leaf essential oil of Laurus nobilis against pentylenetetrazole- and maximal electroshock-induced seizures. Phytomedicine 2002, 9, 212–216. [Google Scholar] [CrossRef]
- Lou, F.S.; Ping, L.L.; Jun, B.K. Determination of five active constituents of asiasarum essential oil with capillary gas chromatography by using double-internal standard method. Chin. J. Mod. Appl. Pharm. 2010, 27, 908–911. [Google Scholar] [CrossRef]
- Barboza, J.N.; da Silva Maia Bezerra Filho, C.; Silva, R.O.; Medeiros, J.V.R.; de Sousa, D.P. An overview on the anti-inflammatory potential and antioxidant profile of eugenol. Oxid. Med. Cell. Longev. 2018, 2018, 3957262. [Google Scholar] [CrossRef]
- Wei, X.Z.; Fu, Y.Q.; Wang, H.; Wang, Y.; Han, Y.; Sun, K.F. The actute toxicty appraises of the Asarum Heterotropoides fr. schmidt var. Mandshuricum (Maxim.) kitag, Asarum Sieboldii Miq. And Asarum Sieboldii Miq. var. seoulense nakai. Asia Pac. Tradit. Med. 2010, 6, 23–25. [Google Scholar]
- Lewis, R.J. Sax’s Dangerous Properties of Industrial Materials, 9th ed.; Van Nostrand Reinhold: New York, NY, USA, 1996; pp. 1–3. [Google Scholar]
- Bingham, E.; Cohrssen, B.; Powell, C.H. Patty’s Toxicology, 5th ed.; John Wiley & Sons: New York, NY, USA, 2001; pp. 1–9. [Google Scholar]
- Horikawa, E.; Okada, T. Experimental study on acute toxicity of phenol camphor. Shikwa Gakuho 1975, 75, 934–939. [Google Scholar] [PubMed]
- Jenner, P.M.; Hagan, E.C.; Taylor, J.M.; Cook, E.L.; Fitzhugh, O.G. Food flavourings and compounds of related structure I. Acute oral toxicity. Food Cosmet. Toxicol. 1964, 2, 327–343. [Google Scholar] [CrossRef]
- Taylor, J.M.; Jenner, P.M.; Jones, W.I. A comparison of the toxicity of some allyl, propenyl, and propyl compounds in the rat. Toxicol. Appl. Pharmacol. 1964, 6, 378–387. [Google Scholar] [CrossRef]
- Belova, L.F.; Alibekov, S.D.; Baginskaia, A.I.; Sokolov, S.; Pokrovskaia, G.V. Asarone and its biological properties. Farmakol. Toksikol. 1985, 48, 17–20. [Google Scholar] [PubMed]
- Daukshas, V.K.; Gaidyalis, P.G.; Pyatrauskas, O.Y.; Udrenaite, É.; Gasperavichene, G.A.; Raguotene, N.V. Synthesis and antiinflammatory activity of acylated benzoxa-and benzodioxaheterocycles and their acyclic analogs. Pharm. Chem. J. 1987, 21, 341–345. [Google Scholar] [CrossRef]
- Daukshas, V.K.; Gaidyalis, P.G.; Udrenaite, É.; Labanauskas, L.K.; Gasperavichene, G.A.; Gumbaragite, L.F.; Ramanauskas, D.V. Synthesis, anti-inflammatory activity and metabolism of alkyl aryl ketones and their derivatives. Pharm. Chem. J. 1989, 23, 990–995. [Google Scholar] [CrossRef]
Sample Availability: Samples of ARR and the compounds M3, M8, M12, M22, M37, M60, M64, M79, M82, M83, M93, M107, M112, M122 and M138 are available from the authors. Raw GC-MS data in CDF format are available from the MetaboLights repository, https://www.ebi.ac.uk/metabolights/ (Study Identifier: MTBLS2053). |
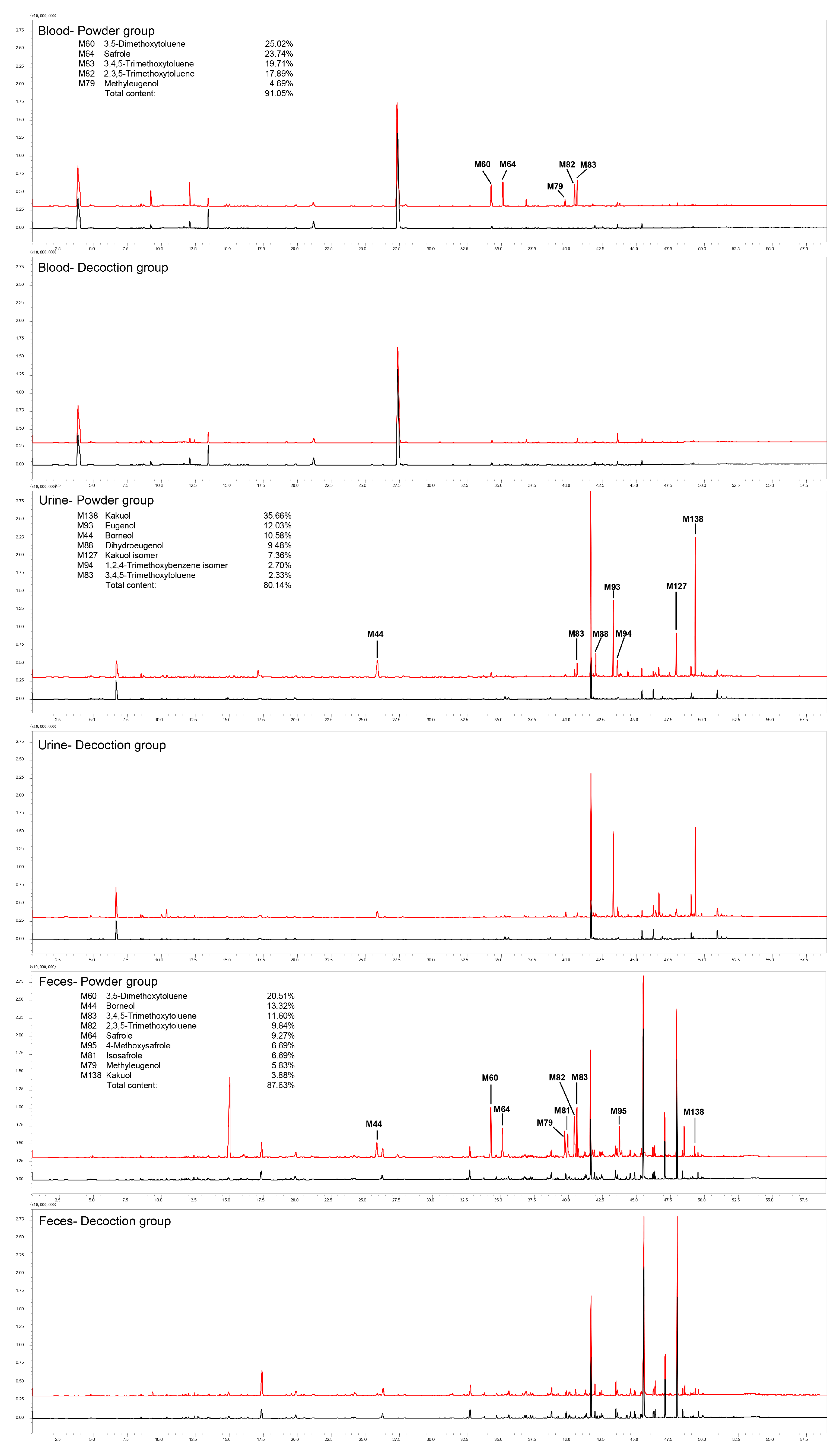
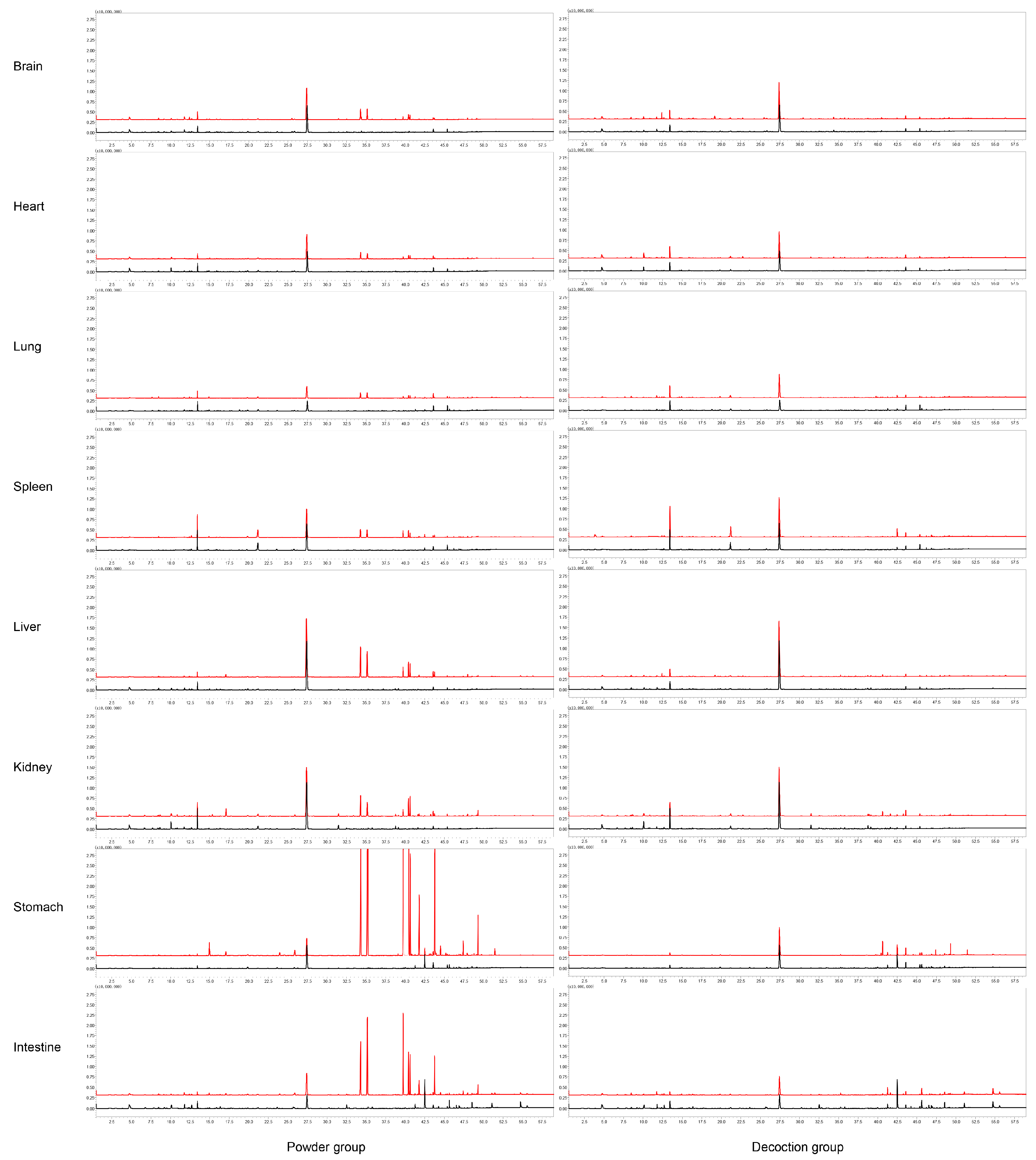
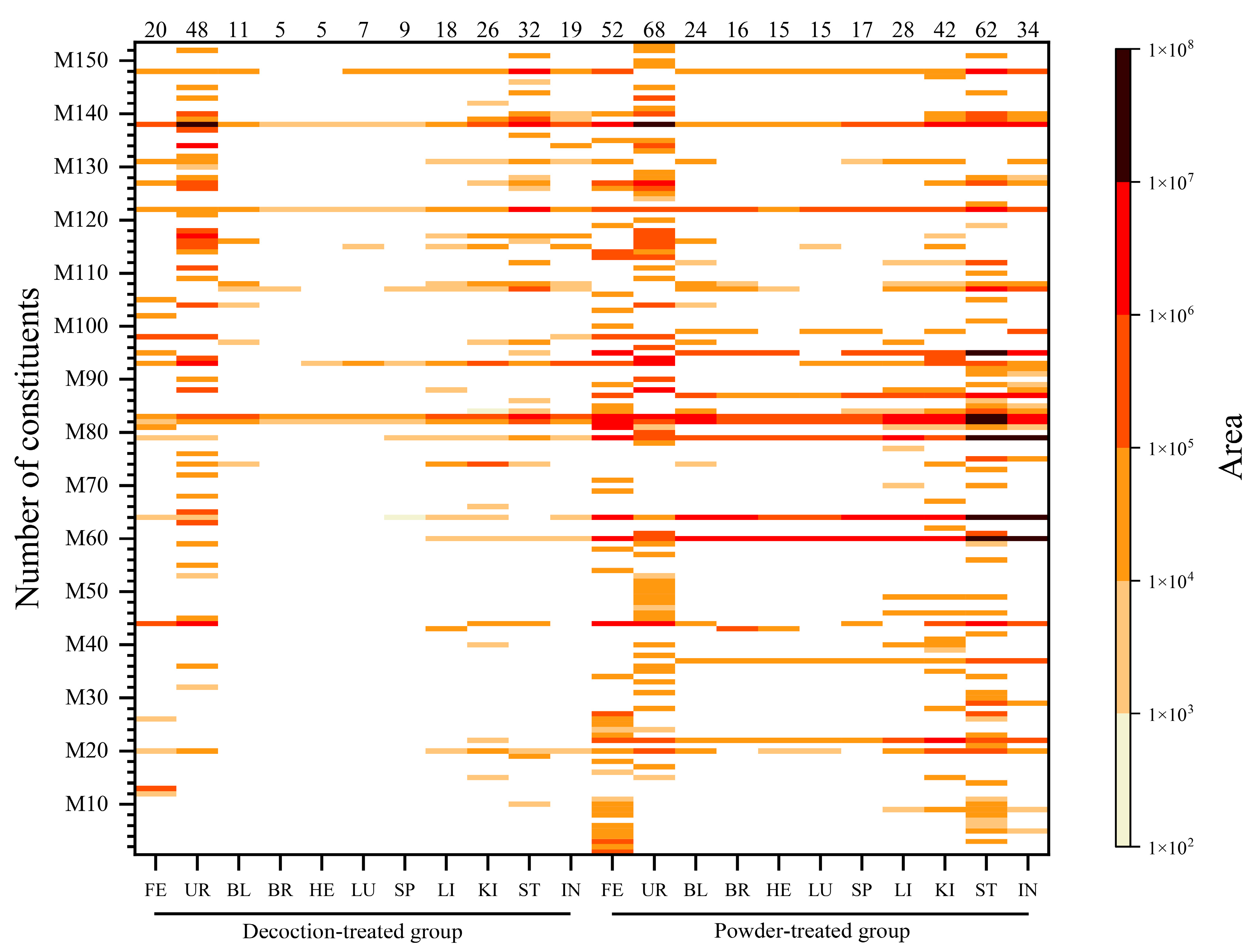
| No. a | Name of Compounds | CAS b | tR (min) | MW c | Formula | RI d | Identification e |
|---|---|---|---|---|---|---|---|
| M1 | α-Pinene | 80-56-8 | 3.785 | 136 | C10H16 | 1021 | MS, RI |
| M2 | Camphene | 79-92-5 | 4.375 | 136 | C10H16 | 1060 | MS, RI |
| M3 | β-Pinene | 127-91-3 | 4.955 | 136 | C10H16 | 1099 | MS, RI, REF |
| M4 | Sabinene | 3387-41-5 | 5.175 | 136 | C10H16 | 1110 | MS, RI |
| M5 | 3-Carene | 13466-78-9 | 5.700 | 136 | C10H16 | 1135 | MS, RI, |
| M6 | β-Myrcene | 123-35-3 | 6.080 | 136 | C10H16 | 1154 | MS, RI |
| M7 | d-4-Carene | 29050-33-7 | 6.405 | 136 | C10H16 | 1170 | MS, RI |
| M8 | Limonene | 138-86-3 | 6.830 | 136 | C10H16 | 1191 | MS, RI, REF |
| M9 | Eucalyptol | 470-82-6 | 7.095 | 154 | C10H18O | 1203 | MS, RI |
| M10 | o-Cymene | 527-84-4 | 8.630 | 134 | C10H14 | 1267 | MS, RI |
| M11 | Terpinolene | 586-62-9 | 8.895 | 136 | C10H16 | 1279 | MS, RI |
| M12 | Tridecane | 629-50-5 | 9.217 | 184 | C13H28 | 1293 | MS, RI, REF |
| M13 | Acetoin | 513-86-0 | 9.328 | 88 | C4H8O2 | 1298 | MS, RI |
| M14 | p-Cymenene | 1195-32-0 | 12.885 | 132 | C10H12 | 1433 | MS, RI |
| M15 | cis-Limonene oxide | 13837-75-7 | 13.135 | 152 | C10H16O | 1441 | MS, RI |
| M16 | cis-4-Thujanol | 15537-55-0 | 13.795 | 154 | C10H18O | 1462 | MS, RI |
| M17 | 2-Nonen-4-one | 32064-72-5 | 14.270 | 140 | C9H16O | 1477 | MS, RI |
| M18 | α-Copaene | 3856-25-5 | 14.485 | 204 | C15H24 | 1485 | MS, RI |
| M19 | Pentadecane | 629-62-9 | 14.953 | 212 | C15H32 | 1500 | MS, RI |
| M20 | dl-Camphor | 76-22-2 | 15.305 | 152 | C10H16O | 1507 | MS, RI |
| M21 | 1-Pentadecene | 13360-61-7 | 16.070 | 210 | C15H30 | 1523 | MS, RI |
| M22 | Eucarvone | 503-93-5 | 17.060 | 150 | C10H14O | 1543 | MS, REF |
| M23 | l-Aristolene | 6831-16-9 | 17.560 | 204 | C15H24 | 1553 | MS, RI |
| M24 | Isopulegol | 89-79-2 | 17.720 | 154 | C10H18O | 1556 | MS, RI |
| M25 | β-Copaene | 18252-44-3 | 18.115 | 204 | C15H24 | 1565 | MS, RI |
| M26 | Bornyl acetate | 76-49-3 | 18.305 | 196 | C12H20O2 | 1568 | MS, RI |
| M27 | 1(10)-Aristolene | 17334-55-3 | 18.760 | 204 | C15H24 | 1577 | MS, RI |
| M28 | Fenchol | 1632-73-1 | 18.920 | 154 | C10H18O | 1581 | MS, RI |
| M29 | Thymol methyl ether | 1076-56-8 | 19.390 | 164 | C11H16O | 1590 | MS, RI |
| M30 | l-Aristolene isomer | 19.650 | 204 | C15H24 | 1596 | MS | |
| M31 | β-Cyclocitral | 432-25-7 | 20.510 | 152 | C10H16O | 1610 | MS, RI |
| M32 | Methyl benzoate | 93-58-3 | 20.816 | 136 | C8H8O2 | 1615 | MS, RI |
| M33 | l-Menthol | 2216-51-5 | 21.220 | 156 | C10H20O | 1621 | MS, RI |
| M34 | δ-Guaiene | 3691-11-0 | 21.535 | 204 | C15H24 | 1626 | MS, RI |
| M35 | Umbellulone | 24545-81-1 | 21.815 | 150 | C10H14O | 1630 | MS, RI |
| M36 | l-Pinocarveol | 547-61-5 | 23.120 | 152 | C10H16O | 1651 | MS, RI |
| M37 | Estragole | 140-67-0 | 23.905 | 148 | C10H12O | 1663 | MS, RI, REF |
| M38 | Isomenthol | 490-99-3 | 24.295 | 156 | C10H20O | 1670 | MS, RI |
| M39 | Verbenol | 473-67-6 | 24.570 | 152 | C10H16O | 1674 | MS, RI |
| M40 | Eucarvone isomer | 24.990 | 150 | C10H14O | 1680 | MS | |
| M41 | Verbenone | 80-57-9 | 25.240 | 150 | C10H14O | 1684 | MS, RI |
| M42 | α-Terpineol acetate | 80-26-2 | 25.300 | 196 | C12H20O2 | 1685 | MS, RI |
| M43 | 4-Ethylbenzaldehyde | 4748-78-1 | 25.830 | 134 | C9H10O | 1693 | MS, RI |
| M44 | Borneol | 507-70-0 | 25.875 | 154 | C10H18O | 1692 | MS, RI |
| M45 | Phellandral | 21391-98-0 | 26.415 | 154 | C10H18O | 1703 | MS, RI |
| M46 | Piperitone | 89-81-6 | 26.610 | 152 | C10H16O | 1706 | MS, RI |
| M47 | β-Cyclogeraniol | 472-20-8 | 26.960 | 154 | C10H18O | 1711 | MS |
| M48 | l-Carvone | 2244-16-8 | 27.270 | 150 | C10H14O | 1716 | MS, RI |
| M49 | Berbenone | 80-57-9 | 28.190 | 150 | C10H14O | 1730 | MS, RI |
| M50 | trans-Piperitol | 16721-39-4 | 29.165 | 154 | C10H18O | 1745 | MS, RI |
| M51 | cis-Piperitol | 16721-38-3 | 29.485 | 154 | C10H18O | 1750 | MS, RI |
| M52 | Methyl benzeneacetate | 101-41-7 | 29.955 | 150 | C9H10O2 | 1758 | MS, RI |
| M53 | Myrtenol | 515-00-4 | 31.910 | 152 | C10H16O | 1788 | MS, RI |
| M54 | 6-Methyl-2-hepten-4-one | 49852-35-9 | 32.060 | 126 | C8H14O | 1790 | MS |
| M55 | 3,4-Dimethylbenzaldehyde | 5973-71-7 | 32.061 | 134 | C9H10O | 1790 | MS, RI |
| M56 | Cuparene | 16982-00-6 | 32.520 | 202 | C15H22 | 1797 | MS, RI |
| M57 | cis-Sabinol | 3310-02-9 | 32.645 | 152 | C10H16O | 1799 | MS, RI |
| M58 | Anethol | 104-46-1 | 33.265 | 148 | C10H12O | 1815 | MS, RI |
| M59 | cis-Carveol | 1197-06-4 | 34.050 | 152 | C10H16O | 1835 | MS, RI |
| M60 | 3,5-Dimethoxytoluene | 4179-19-5 | 34.260 | 152 | C9H12O2 | 1840 | MS, RI, REF |
| M61 | p-Cymen-8-ol | 1197-01-9 | 34.660 | 150 | C10H14O | 1851 | MS, RI |
| M62 | 2,3-Dimethyldecahydronaphthalene | 1008-80-6 | 34.995 | 166 | C12H22 | 1859 | MS |
| M63 | Guaiacol | 90-05-1 | 35.000 | 124 | C7H8O2 | 1860 | MS, RI |
| M64 | Safrole | 94-59-7 | 35.108 | 162 | C10H10O2 | 1862 | MS, RI, REF |
| M65 | Benzyl alcohol | 100-51-6 | 35.662 | 108 | C7H8O | 1877 | MS, RI |
| M66 | Verbenone isomer | 36.215 | 150 | C10H14O | 1891 | MS | |
| M67 | Verbenone isomer | 36.360 | 150 | C10H14O | 1895 | MS | |
| M68 | 2-Phenylethanol | 60-12-8 | 36.725 | 122 | C8H10O | 1906 | MS, RI |
| M69 | Agarospirol | 1460-73-7 | 37.365 | 222 | C15H26O | 1928 | MS |
| M70 | 3,4-Methylenedioxyanisole | 7228-35-5 | 37.450 | 152 | C8H8O3 | 1931 | MS |
| M71 | Isosafrole isomer | 37.575 | 162 | C10H10O2 | 1936 | MS | |
| M72 | Creosol | 93-51-6 | 38.108 | 138 | C8H10O2 | 1954 | MS, RI |
| M73 | 2-Allyl-1,4-dimethoxybenzene | 19754-22-4 | 38.535 | 178 | C11H14O2 | 1968 | MS |
| M74 | Thymoquinone | 490-91-5 | 38.880 | 164 | C10H12O2 | 1980 | MS |
| M75 | Methyleugenol isomer | 39.055 | 178 | C11H14O2 | 1986 | MS | |
| M76 | 6-Allyl-o-cresol | 3354-58-3 | 39.435 | 148 | C10H12O | 2000 | MS |
| M77 | 2,4-Dimethylanisole | 6738-23-4 | 39.485 | 136 | C9H12O | 2002 | MS |
| M78 | p-Methoxybenzaldehyde | 123-11-5 | 39.630 | 136 | C8H8O2 | 2008 | MS, RI |
| M79 | Methyleugenol | 93-15-2 | 39.715 | 178 | C11H14O2 | 2011 | MS, RI, REF |
| M80 | o-Methylphenol | 95-48-7 | 39.730 | 108 | C7H8O | 2012 | MS, RI |
| M81 | Isosafrole | 120-58-1 | 39.925 | 162 | C10H10O2 | 2020 | MS, RI |
| M82 | 2,3,5-Trimethoxytoluene | 38790-14-6 | 40.415 | 182 | C10H14O3 | 2040 | REF |
| M83 | 3,4,5-Trimethoxytoluene | 6443-69-2 | 40.610 | 182 | C10H14O3 | 2048 | MS, RI, REF |
| M84 | 3,4,5-Trimethoxybenzoic acid | 118-41-2 | 40.780 | 212 | C10H12O5 | 2055 | MS |
| M85 | Globulol | 51371-47-2 | 40.910 | 222 | C15H26O | 2061 | MS, RI |
| M86 | 1,2,4-Trimethoxybenzene | 135-77-3 | 41.430 | 168 | C9H12O3 | 2083 | MS, RI |
| M87 | E-Isocroweacin | 194609-21-7 | 41.755 | 192 | C11H12O3 | 2096 | MS |
| M88 | Dihydroeugenol | 2785-87-7 | 41.970 | 166 | C10H14O2 | 2106 | MS, RI |
| M89 | Spathulenol | 6750-60-3 | 42.110 | 220 | C15H24O | 2112 | MS, RI |
| M90 | Dihydroeugenol isomer | 42.190 | 166 | C10H14O2 | 2116 | MS | |
| M91 | Viridiflorol | 552-02-3 | 42.310 | 222 | C15H26O | 2122 | MS, RI |
| M92 | Patchoulol | 5986-55-0 | 43.065 | 222 | C15H26O | 2157 | MS, RI |
| M93 | Eugenol | 97-53-0 | 43.250 | 164 | C10H12O2 | 2166 | MS, RI, REF |
| M94 | 1,2,4-Trimethoxybenzene isomer | 43.565 | 168 | C9H12O3 | 2181 | MS | |
| M95 | 4-Methoxysafrole | 18607-93-7 | 43.745 | 192 | C11H12O3 | 2189 | MS, RI |
| M96 | m-Eugenol | 501-19-9 | 43.780 | 164 | C10H12O2 | 2191 | MS, RI |
| M97 | 2,4,5-Trimethoxybenzoic acid | 490-64-2 | 43.815 | 212 | C10H12O5 | 2192 | MS |
| M98 | Thymol | 89-83-8 | 43.850 | 150 | C10H14O | 2194 | MS, RI |
| M99 | p-Methoxypropiophenone | 121-97-1 | 43.910 | 164 | C10H12O2 | 2197 | MS |
| M100 | Bulnesol | 22451-73-6 | 44.010 | 222 | C15H26O | 2202 | MS, RI |
| M101 | α-Bisabolol | 515-69-5 | 44.200 | 222 | C15H26O | 2212 | MS, RI |
| M102 | 2-Aminoacetophenone | 551-93-9 | 44.232 | 135 | C8H9NO | 2214 | MS, RI |
| M103 | α-Eudesmol | 473-16-5 | 44.325 | 222 | C15H26O | 2219 | MS, RI |
| M104 | Piperonal | 120-57-0 | 44.340 | 150 | C8H6O3 | 2219 | MS, RI |
| M105 | Isothymol | 499-75-2 | 44.390 | 150 | C10H14O | 2222 | MS, RI |
| M106 | α-Cadinol | 481-34-5 | 44.435 | 222 | C15H26O | 2225 | MS, RI |
| M107 | Elemicin | 487-11-6 | 44.505 | 208 | C12H16O3 | 2228 | MS, RI, REF |
| M108 | 3,4,5-Trimethoxytoluene isomer | 44.765 | 182 | C10H14O3 | 2242 | MS | |
| M109 | Methoxyeugenol isomer | 44.870 | 194 | C11H14O3 | 2248 | MS | |
| M110 | Isospathulenol | 88395-46-4 | 44.935 | 220 | C15H24O | 2251 | MS, RI |
| M111 | cis-Isoeugenol | 5912-86-7 | 45.080 | 164 | C10H12O2 | 2258 | MS, RI |
| M112 | β-Asarone | 5273-86-9 | 45.180 | 208 | C12H16O3 | 2264 | MS, RI, REF |
| M113 | 1,2,4-Trimethoxybenzene isomer | 45.360 | 168 | C9H12O3 | 2273 | MS | |
| M114 | 1,2,3,4-Tetramethoxybenzene | 21450-56-6 | 45.590 | 198 | C10H14O4 | 2286 | MS, RI |
| M115 | 1,2,4-Trimethoxybenzene isomer | 46.360 | 168 | C9H12O3 | 2334 | MS | |
| M116 | 3,4-Methylenedioxyacetophenone | 3162-29-6 | 46.400 | 164 | C9H8O3 | 2337 | MS |
| M117 | Chavicol | 501-92-8 | 46.605 | 134 | C9H10O | 2350 | MS, RI |
| M118 | 1,2,4-Trimethoxybenzene isomer | 46.670 | 168 | C9H12O3 | 2355 | MS | |
| M119 | Kaurene | 34424-57-2 | 46.785 | 272 | C20H32 | 2362 | MS, RI |
| M120 | Methylvanillin | 120-14-9 | 47.240 | 166 | C9H10O3 | 2394 | MS, RI |
| M121 | Methoxyeugenol isomer | 47.304 | 194 | C11H14O3 | 2398 | MS | |
| M122 | 3,4-Methylenedioxypropiophenone | 28281-49-4 | 47.395 | 178 | C10H10O3 | 2405 | MS, REF |
| M123 | Apiol | 523-80-8 | 47.510 | 222 | C12H14O4 | 2415 | MS, RI |
| M124 | Amilfenol | 80-46-6 | 47.560 | 164 | C11H16O | 2418 | MS |
| M125 | 6-Allyl-2-methylphenol | 3354-58-3 | 47.755 | 148 | C10H12O | 2435 | MS |
| M126 | Methoxyeugenol isomer | 47.835 | 194 | C11H14O3 | 2441 | MS | |
| M127 | Kakuol isomer | 47.895 | 194 | C10H10O4 | 2446 | MS | |
| M128 | Mellein | 1200-93-7 | 48.255 | 178 | C10H10O3 | 2475 | MS, RI |
| M129 | 2,4-Dimethoxyacetophenone | 829-20-9 | 48.345 | 180 | C10H12O3 | 2483 | MS |
| M130 | 2,6-Dimethoxyacetophenone | 2040-04-2 | 48.585 | 180 | C10H12O3 | 2503 | MS |
| M131 | 1-(3,4-Methylenedioxyphenyl)-propane-1-ol | 6890-30-8 | 48.777 | 180 | C10H12O3 | 2521 | MS |
| M132 | Piperonol | 495-76-1 | 48.928 | 152 | C8H8O3 | 2534 | MS |
| M133 | Methoxyeugenol isomer | 48.935 | 194 | C11H14O3 | 2534 | MS | |
| M134 | 3-Methoxy-5-methylphenol | 3209-13-0 | 48.985 | 138 | C8H10O2 | 2538 | MS, RI |
| M135 | 2′,4′-Dimethoxypropiophenone | 831-00-5 | 49.045 | 194 | C11H14O3 | 2544 | MS |
| M136 | 4,6-Dimethoxy-phthalide | 58545-97-4 | 49.065 | 194 | C10H10O4 | 2546 | MS |
| M137 | Methoxyeugenol | 6627-88-9 | 49.069 | 194 | C11H14O3 | 2547 | MS, RI |
| M138 | Kakuol | 18607-90-4 | 49.295 | 194 | C10H10O4 | 2567 | MS, REF |
| M139 | 2′,4′-Dimethoxy-3′-methylpropiophenone isomer | 49.690 | 208 | C12H16O3 | 2602 | MS | |
| M140 | Xanthoxylin | 90-24-4 | 49.760 | 196 | C10H10O4 | 2608 | MS |
| M141 | 3,4-Methylenedioxyphenyl-1-propanal | 30830-55-8 | 49.915 | 178 | C10H10O3 | 2620 | MS |
| M142 | 2′,4′-Dimethoxy-3′-methylpropiophenone isomer | 50.226 | 208 | C12H16O3 | 2645 | MS | |
| M143 | 3,4,5-Trimethoxyphenyl-2-propanone | 16603-18-2 | 50.270 | 224 | C12H16O4 | 2648 | MS |
| M144 | 4-(Dimethoxymethyl)-1,2-dimethoxybenzene | 59276-33-4 | 50.615 | 212 | C11H16O4 | 2676 | MS |
| M145 | Propioveratrone | 1835-04-7 | 50.740 | 194 | C11H14O3 | 2686 | MS |
| M146 | 2′,4′-Dimethoxypropiophenone | 831-00-5 | 50.760 | 194 | C11H14O3 | 2687 | MS |
| M147 | Dihydromethyleugenol | 5888-52-8 | 51.135 | 180 | C11H16O2 | 2714 | MS |
| M148 | 2′,4′-Dimethoxy-3′-methylpropiophenone | 77942-13-3 | 51.435 | 208 | C12H16O3 | 2734 | MS |
| M149 | 1-Hydroxy-2-(prop-2-enyl)-4,5- methylenedioxybenzene | 19202-23-4 | 51.670 | 178 | C10H10O3 | 2750 | MS |
| M150 | 3-(2,4,6-Trimethoxyphenyl)-2-butanone | 26537-69-9 | 51.850 | 238 | C13H18O4 | 2761 | MS |
| M151 | 1,2-Dimethoxy-4-(1,2-dimethox yethyl)-benzene | 477884-01-8 | 52.195 | 226 | C12H18O4 | 2785 | MS |
| M152 | 1-Hydroxy-2-(prop-2-enyl)-4,5- methylenedioxybenzene isomer | 52.665 | 178 | C10H10O3 | 2814 | MS | |
| M153 | Syringic acid | 530-57-4 | 55.055 | 198 | C9H10O5 | 2934 | MS |
| No. | Constituents | RI | Powder-Treated Group | Decoction-Treated Group | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FE | UR | BL | BR | HE | LU | SP | LI | KI | ST | IN | FE | UR | BL | BR | HE | LU | SP | LI | KI | ST | IN | |||
| M1 | α-Pinene | 1021 | ● | |||||||||||||||||||||
| M2 | Camphene | 1060 | ● | |||||||||||||||||||||
| M3 | β-Pinene | 1099 | ● | ● | ||||||||||||||||||||
| M4 | Sabinene | 1110 | ● | |||||||||||||||||||||
| M5 | 3-Carene | 1135 | ● | ● | ● | |||||||||||||||||||
| M6 | β-Myrcene | 1154 | ● | ● | ||||||||||||||||||||
| M7 | d-4-Carene | 1170 | ● | |||||||||||||||||||||
| M8 | Limonene | 1191 | ● | ● | ||||||||||||||||||||
| M9 | Eucalyptol | 1203 | ● | ● | ● | ● | ● | |||||||||||||||||
| M10 | o-Cymene | 1267 | ● | ● | ● | |||||||||||||||||||
| M11 | Terpinolene | 1279 | ● | ● | ||||||||||||||||||||
| M12 | Tridecane | 1293 | ● | |||||||||||||||||||||
| M13 | Acetoin | 1298 | ● | |||||||||||||||||||||
| M14 | p-Cymenene | 1433 | ● | |||||||||||||||||||||
| M16 | cis-4-Thujanol | 1462 | ● | |||||||||||||||||||||
| M18 | α-Copaene | 1485 | ● | |||||||||||||||||||||
| M19 | Pentadecane | 1500 | ● | |||||||||||||||||||||
| M20 | dl-Camphor | 1507 | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | |||||||
| M21 | 1-Pentadecene | 1523 | ● | |||||||||||||||||||||
| M22 | Eucarvone | 1543 | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ||||||||||
| M23 | l-Aristolene | 1553 | ● | ● | ||||||||||||||||||||
| M25 | β-Copaene | 1565 | ● | |||||||||||||||||||||
| M26 | Bornyl acetate | 1568 | ● | ● | ● | |||||||||||||||||||
| M27 | 1(10)-Aristolene | 1577 | ● | ● | ||||||||||||||||||||
| M28 | Fenchol | 1581 | ● | ● | ||||||||||||||||||||
| M29 | Thymol methyl ether | 1590 | ● | ● | ||||||||||||||||||||
| M30 | l-Aristolene isomer | 1596 | ● | |||||||||||||||||||||
| M31 | β-Cyclocitral | 1610 | ● | ● | ||||||||||||||||||||
| M33 | l-Menthol | 1621 | ● | |||||||||||||||||||||
| M34 | δ-Guaiene | 1626 | ● | ● | ||||||||||||||||||||
| M35 | Umbellulone | 1630 | ● | ● | ||||||||||||||||||||
| M37 | Estragole | 1663 | ● | ● | ● | ● | ● | ● | ● | ● | ● | |||||||||||||
| M38 | Isomenthol | 1670 | ● | |||||||||||||||||||||
| M40 | Eucarvone isomer | 1680 | ● | ● | ● | ● | ||||||||||||||||||
| M41 | Verbenone | 1684 | ● | |||||||||||||||||||||
| M44 | Borneol | 1692 | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | |||||||||||
| M45 | Phellandral | 1703 | ● | ● | ||||||||||||||||||||
| M46 | Piperitone | 1706 | ● | ● | ● | ● | ||||||||||||||||||
| M48 | l-Carvone | 1716 | ● | |||||||||||||||||||||
| M49 | Berbenone | 1730 | ● | ● | ● | ● | ||||||||||||||||||
| M50 | trans-Piperitol | 1745 | ● | |||||||||||||||||||||
| M53 | Myrtenol | 1788 | ● | ● | ||||||||||||||||||||
| M56 | Cuparene | 1797 | ● | |||||||||||||||||||||
| M57 | cis-Sabinol | 1799 | ● | |||||||||||||||||||||
| M59 | cis-Carveol | 1835 | ● | ● | ● | |||||||||||||||||||
| M60 | 3,5-Dimethoxytoluene | 1840 | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | |||||||
| M61 | p-Cymen-8-ol | 1851 | ● | ● | ||||||||||||||||||||
| M62 | 2,3-Dimethyldecahydronaphthalene | 1859 | ● | |||||||||||||||||||||
| M64 | Safrole | 1862 | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | |||||
| M67 | Verbenone isomer | 1895 | ● | |||||||||||||||||||||
| M69 | Agarospirol | 1928 | ● | |||||||||||||||||||||
| M70 | 3,4-Methylenedioxyanisole | 1931 | ● | ● | ||||||||||||||||||||
| M71 | Isosafrole isomer | 1936 | ● | |||||||||||||||||||||
| M73 | 2-Allyl-1,4-dimethoxybenzene | 1968 | ● | |||||||||||||||||||||
| M74 | Thymoquinone | 1980 | ● | ● | ● | ● | ● | ● | ● | |||||||||||||||
| M75 | Methyleugenol isomer | 1986 | ● | ● | ||||||||||||||||||||
| M77 | 2,4-Dimethylanisole | 2002 | ● | |||||||||||||||||||||
| M79 | Methyleugenol | 2011 | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ||||
| M81 | Isosafrole | 2020 | ● | ● | ● | ● | ● | ● | ● | |||||||||||||||
| M82 | 2,3,5-Trimethoxytoluene | 2040 | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● |
| M83 | 3,4,5-Trimethoxytoluene | 2048 | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● |
| M84 | 3,4,5-Trimethoxybenzoic acid | 2055 | ● | ● | ● | ● | ● | ● | ● | ● | ● | |||||||||||||
| M85 | Globulol | 2061 | ● | ● | ● | |||||||||||||||||||
| M86 | 1,2,4-Trimethoxybenzene | 2083 | ● | ● | ||||||||||||||||||||
| M87 | E-Isocroweacin | 2096 | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ||||||||||||
| M89 | Spathulenol | 2112 | ● | ● | ● | |||||||||||||||||||
| M91 | Viridiflorol | 2122 | ● | ● | ||||||||||||||||||||
| M92 | Patchoulol | 2157 | ● | ● | ||||||||||||||||||||
| M93 | Eugenol | 2166 | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | |||||
| M95 | 4-Methoxysafrole | 2189 | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | |||||||||||
| M97 | 2,4,5-Trimethoxybenzoic acid | 2192 | ● | ● | ● | ● | ● | |||||||||||||||||
| M98 | Thymol | 2194 | ● | ● | ● | ● | ● | |||||||||||||||||
| M99 | p-Methoxypropiophenone | 2197 | ● | ● | ● | ● | ● | ● | ||||||||||||||||
| M100 | Bulnesol | 2202 | ● | |||||||||||||||||||||
| M101 | α-Bisabolol | 2212 | ● | |||||||||||||||||||||
| M103 | α-Eudesmol | 2219 | ● | |||||||||||||||||||||
| M104 | Piperonal | 2219 | ● | ● | ● | ● | ||||||||||||||||||
| M105 | Isothymol | 2222 | ● | ● | ||||||||||||||||||||
| M106 | α-Cadinol | 2225 | ● | |||||||||||||||||||||
| M107 | Elemicin | 2228 | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ||||||||
| M108 | 3,4,5-Trimethoxytoluene isomer | 2242 | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | |||||||||||
| M109 | Methoxyeugenol isomer | 2248 | ● | ● | ||||||||||||||||||||
| M110 | Isospathulenol | 2251 | ● | |||||||||||||||||||||
| M112 | β-Asarone | 2264 | ● | ● | ● | ● | ● | |||||||||||||||||
| M113 | 1,2,4-Trimethoxybenzene isomer | 2273 | ● | ● | ||||||||||||||||||||
| M114 | 1,2,3,4-Tetramethoxybenzene | 2286 | ● | ● | ● | |||||||||||||||||||
| M116 | 3,4-Methylenedioxyacetophenone | 2337 | ● | ● | ● | ● | ● | |||||||||||||||||
| M117 | Chavicol | 2350 | ● | ● | ● | ● | ● | ● | ● | |||||||||||||||
| M119 | Kaurene | 2362 | ● | ● | ||||||||||||||||||||
| M122 | 3,4-Methylenedioxypropiophenone | 2405 | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● |
| M123 | Apiol | 2415 | ● | |||||||||||||||||||||
| M126 | Methoxyeugenol isomer | 2441 | ● | ● | ● | ● | ||||||||||||||||||
| M127 | Kakuol isomer | 2446 | ● | ● | ● | ● | ● | ● | ● | ● | ● | |||||||||||||
| M128 | Mellein | 2475 | ● | ● | ● | ● | ● | |||||||||||||||||
| M131 | 1-(3,4-Methylenedioxyphenyl)-propane-1-ol | 2520 | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ||||||||||
| M135 | 2′,4′-Dimethoxypropiophenone | 2544 | ● | ● | ||||||||||||||||||||
| M138 | Kakuol | 2567 | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● |
| M139 | 2′,4′-Dimethoxy-3′-methylpropiophenone isomer | 2602 | ● | ● | ● | ● | ● | ● | ● | |||||||||||||||
| M140 | Xanthoxylin | 2608 | ● | ● | ● | ● | ● | ● | ● | ● | ||||||||||||||
| M148 | 2′,4′-Dimethoxy-3′-methylpropiophenone | 2734 | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | |||
| M153 | Syringic acid | 2934 | ● | |||||||||||||||||||||
| Total: | 49 | 40 | 24 | 15 | 14 | 14 | 17 | 27 | 36 | 59 | 33 | 19 | 26 | 11 | 5 | 5 | 6 | 9 | 15 | 22 | 28 | 17 | ||
| Total 98 original constituents were identified in the powder group. | Total 43 original constituents were identified in the decoction group. | |||||||||||||||||||||||
| No. | Metabolites | RI | Powder-Treated Group | Decoction-Treated Group | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FE | UR | BL | BR | HE | LU | SP | LI | KI | ST | IN | FE | UR | BL | BR | HE | LU | SP | LI | KI | ST | IN | |||
| M15 | cis-Limonene oxide | 1441 | ● | ● | ● | |||||||||||||||||||
| M24 | Isopulegol | 1556 | ● | ● | ||||||||||||||||||||
| M36 | l-Pinocarveol | 1651 | ● | ● | ||||||||||||||||||||
| M39 | Verbenol | 1674 | ● | |||||||||||||||||||||
| M42 | α-Terpineol acetate | 1685 | ● | |||||||||||||||||||||
| M47 | β-Cyclogeraniol | 1711 | ● | |||||||||||||||||||||
| M51 | cis-Piperitol | 1750 | ● | |||||||||||||||||||||
| M88 | Dihydroeugenol | 2106 | ● | ● | ● | ● | ● | ● | ||||||||||||||||
| M96 | m-Eugenol | 2191 | ● | |||||||||||||||||||||
| M111 | cis-Isoeugenol | 2258 | ● | |||||||||||||||||||||
| M132 | Piperonol | 2534 | ● | |||||||||||||||||||||
| M137 | Methoxyeugenol | 2547 | ● | |||||||||||||||||||||
| M141 | 3,4-Methylenedioxyphenyl-1-propanal | 2620 | ● | |||||||||||||||||||||
| M147 | Dihydromethyleugenol | 2714 | ● | |||||||||||||||||||||
| M149 | 1-Hydroxy-2-(prop-2-enyl)-4,5-methylenedioxybenzene | 2750 | ● | |||||||||||||||||||||
| Total: | 1 | 9 | 0 | 0 | 0 | 0 | 0 | 1 | 4 | 1 | 1 | 0 | 5 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | ||
| Total 12 metabolites were identified in the powder group. | Total 6 metabolites were identified in the decoction group. | |||||||||||||||||||||||
| 3,5-Dimethoxytoluene (M60) | Safrole (M64) | Methyleugenol (M79) | 2,3,5-Trimethoxytoluene (M82) | 3,4,5-Trimethoxytoluene (M83) | Eugenol (M93) | Kakuol (M138) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P | D | Ratio | P | D | Ratio | P | D | Ratio | P | D | Ratio | P | D | Ratio | P | D | Ratio | P | D | Ratio | |
| FE | 7.19 | n.d. | / | 3.25 | 0.0058 | 560.3 | 2.04 | 0.0052 | 392.3 | 3.45 | 0.005 | 690.0 | 4.07 | 0.01 | 407.0 | 0.10 | 0.02 | 5.4 | 1.36 | 0.70 | 1.9 |
| UR | 0.54 | n.d. | / | 0.04 | 0.0038 | 10.5 | 0.12 | 0.0064 | 18.8 | 0.65 | 0.030 | 21.7 | 1.10 | 0.32 | 3.4 | 5.66 | 6.58 | 0.9 | 16.79 | 10.51 | 1.6 |
| BL | 2.68 | n.d. | / | 2.54 | n.d. | / | 0.5 | n.d. | / | 1.91 | 0.032 | 59.7 | 2.11 | 0.31 | 6.8 | n.d. | n.d. | / | 0.10 | 0.02 | 6.4 |
| BR | 2.48 | n.d. | / | 1.94 | n.d. | / | 0.4 | n.d. | / | 0.75 | 0.007 | 107.1 | 0.64 | 0.06 | 10.7 | n.d. | n.d. | / | 0.07 | 0.01 | 13.0 |
| HE | 1.45 | n.d. | / | 0.98 | n.d. | / | 0.26 | n.d. | / | 0.54 | 0.007 | 77.1 | 0.46 | 0.05 | 9.2 | n.d. | 0.004 | / | 0.06 | 0.005 | 12.5 |
| LU | 1.27 | n.d. | / | 0.98 | n.d. | / | 0.24 | n.d. | / | 0.39 | 0.008 | 48.8 | 0.33 | 0.05 | 6.6 | 0.05 | 0.01 | 5.2 | 0.09 | 0.01 | 10.9 |
| SP | 1.86 | n.d. | / | 1.43 | 0.0004 | 3575.0 | 0.9 | 0.0016 | 562.5 | 0.97 | 0.009 | 107.8 | 0.59 | 0.06 | 9.8 | 0.02 | 0.01 | 3.0 | 0.16 | 0.01 | 21.7 |
| LI | 7.09 | 0.0017 | 4170.6 | 5.03 | 0.0012 | 4191.7 | 1.29 | 0.0032 | 403.1 | 2.26 | 0.031 | 72.9 | 1.89 | 0.24 | 7.9 | 0.05 | 0.02 | 2.6 | 0.05 | 0.04 | 1.2 |
| KI | 4.63 | 0.0019 | 2436.8 | 2.48 | 0.0018 | 1377.8 | 0.88 | 0.0051 | 172.5 | 2.58 | 0.056 | 46.1 | 2.78 | 0.56 | 5.0 | 0.22 | 0.23 | 0.9 | 1.13 | 0.31 | 3.6 |
| ST | 35.16 | 0.0025 | 14,064.0 | 46.54 | n.d. | / | 34.99 | 0.0801 | 436.8 | 18.84 | 0.293 | 64.3 | 17.07 | 1.90 | 9.0 | 0.20 | 0.05 | 4.1 | 8.58 | 2.36 | 3.6 |
| IN | 13.06 | 0.0021 | 6219.0 | 16.02 | 0.0025 | 6408.0 | 12.38 | 0.0069 | 1794.2 | 6.56 | 0.026 | 252.3 | 5.83 | 0.13 | 44.8 | 0.06 | 0.12 | 0.5 | 2.01 | 0.22 | 9.2 |
| No. | Compounds | LD50 (Oral) a | Distribution b | Content c |
|---|---|---|---|---|
| M20 | dl-Camphor | mouse, 1310 mg/kg [35] | P (FE, UR, BL, HE, LU, LI, KI, ST, IN) D (FE, UR, LI, KI, ST, IN) | 0.11% |
| M37 | Estragole | mouse, 1250 mg/kg [36] | P (BL, BR, HE, LU, SP, LI, KI, ST, IN) D (n.d.) | 0.28% |
| M44 | Borneol | mouse, 1059 mg/kg [35] | P (FE, UR, BL, SP, KI, ST, IN) D (FE, UR, KI, ST) | 0.12% |
| M64 | safrole | mouse, 2350 mg/kg [33,36] | P (FE, UR, BL, BR, HE, LU, SP, LI, KI, ST, IN) D (FE, UR, SP, LI, KI, IN) | 23.74% |
| M79 | Methyleugenol | rat, 1179 mg/kg [33] | P (FE, UR, BL, BR, HE, LU, SP, LI, KI, ST, IN) D (FE, UR, SP, LI, KI, ST, IN) | 4.69% |
| M104 | Piperonal | rat, 2700 mg/kg [37] | P (UR, BL) D (UR, BL) | 0.07% |
| M112 | β-Asarone | mouse, 418 mg/kg [38] | P (BL, LI, KI, ST) D (ST) | 0.04% |
| M122 | 3,4-Methylenedioxypropiophenone | mouse, 2150 mg/kg [39] | P (FE, UR, BL, BR, HE, LU, SP, LI, KI, ST, IN) D (FE, UR, BL, BR, HE, LU, SP, LI, KI, ST, IN) | 1.58% |
| M131 | 1-(3,4-Methylenedioxyphenyl)-propane-1-ol | mouse, 720 mg/kg [40] | P (FE, BL, SP, LI, KI, IN) D (FE, UR, LI, KI, ST, IN) | 0.15% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, G.-X.; Xu, F.; Shang, M.-Y.; Wang, X.; Cai, S.-Q. The Relative Content and Distribution of Absorbed Volatile Organic Compounds in Rats Administered Asari Radix et Rhizoma Are Different between Powder- and Decoction-Treated Groups. Molecules 2020, 25, 4441. https://doi.org/10.3390/molecules25194441
Liu G-X, Xu F, Shang M-Y, Wang X, Cai S-Q. The Relative Content and Distribution of Absorbed Volatile Organic Compounds in Rats Administered Asari Radix et Rhizoma Are Different between Powder- and Decoction-Treated Groups. Molecules. 2020; 25(19):4441. https://doi.org/10.3390/molecules25194441
Chicago/Turabian StyleLiu, Guang-Xue, Feng Xu, Ming-Ying Shang, Xuan Wang, and Shao-Qing Cai. 2020. "The Relative Content and Distribution of Absorbed Volatile Organic Compounds in Rats Administered Asari Radix et Rhizoma Are Different between Powder- and Decoction-Treated Groups" Molecules 25, no. 19: 4441. https://doi.org/10.3390/molecules25194441
APA StyleLiu, G.-X., Xu, F., Shang, M.-Y., Wang, X., & Cai, S.-Q. (2020). The Relative Content and Distribution of Absorbed Volatile Organic Compounds in Rats Administered Asari Radix et Rhizoma Are Different between Powder- and Decoction-Treated Groups. Molecules, 25(19), 4441. https://doi.org/10.3390/molecules25194441





