The Nutritional and Health Effects of the COVID-19 Pandemic on Patients with Diabetes Mellitus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Questionnaire
2.3. Statistical Analysis
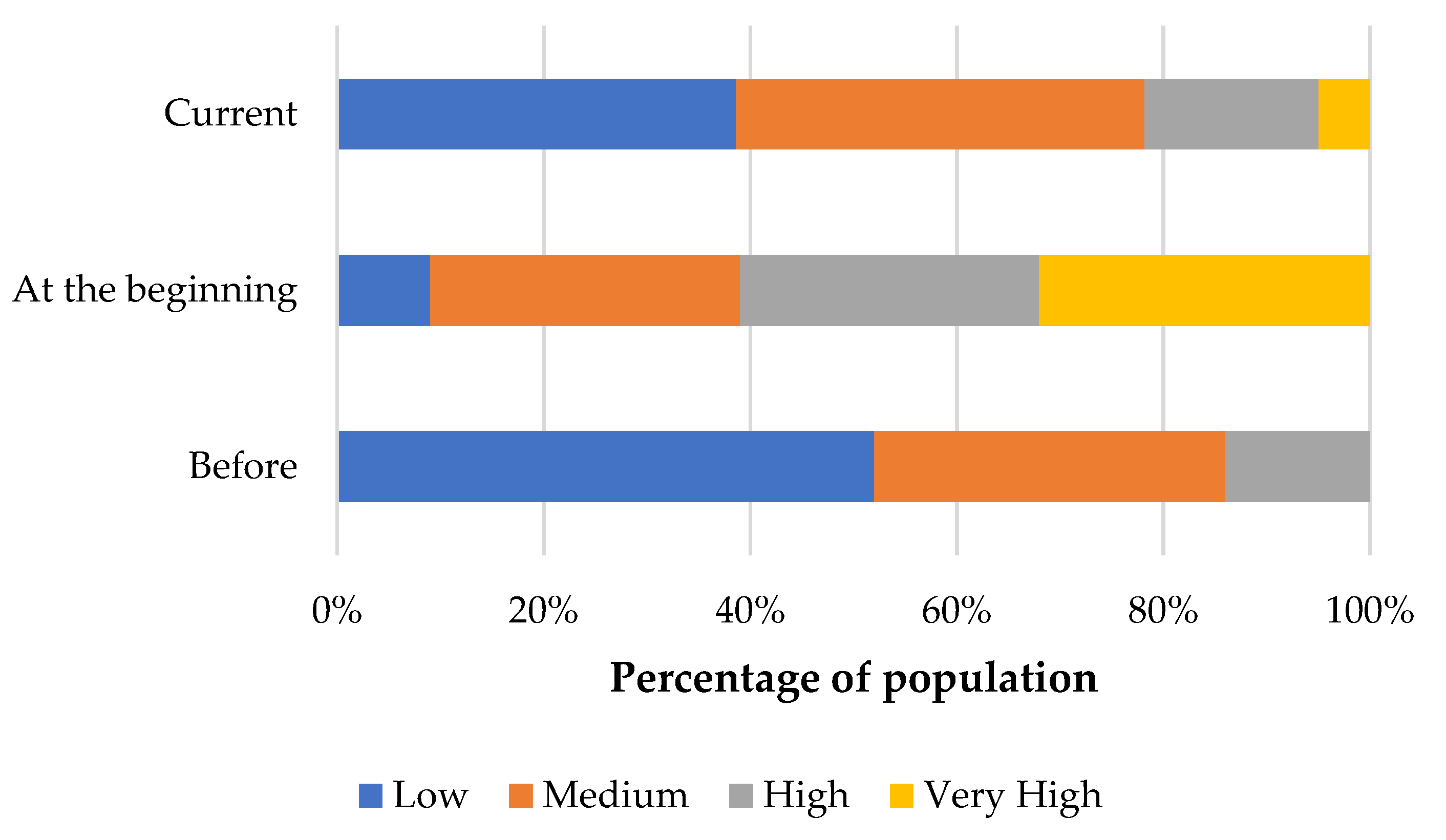
3. Results
3.1. Basic Information
3.2. Physical Activity
3.3. Eating Behaviours
3.4. Hygiene Behaviours
3.5. Lifestyle
3.6. Paediatric Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A. Questionnaire
- Fitness
- Gymnastics
- Gym
- Running
- Walking
- Swimming
- Cycling
- Dancing
- Salty snacks (crisps, crackers, bread sticks, etc.)
- Sweet snacks (cakes, cookies, chocolate bars)
- Delivery meals
- Fast Food
- Convenience food
- Red meat (beef, pork)
- White meat (chicken, turkey)
- Fresh fish
- Frozen fish
- Eggs
- Dairy products (milk, yoghurt, cottage cheese)
- Fresh bread
- Homemade bread
- Fresh fruit
- Fresh vegetables
- Grain products (rice, pasta etc.)
- Nuts
- Sweet beverages (except energy drinks)
- Energy drinks
- Coffee
- Water
- Snacks between main meals
- When you come home
- After using the toilet
- Before preparing food
- After contact with animals
- After leaving the public transport
- After leaving shops
References
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef] [Green Version]
- Polish Ministry of Health. Coronavirus Outbreak Map (SARS-CoV-2). Available online: https://www.gov.pl/web/koronawirus/wykaz-zarazen-koronawirusem-sars-cov-2 (accessed on 22 July 2020).
- Polish Ministry of Health. Current rules and Restrictions. Available online: https://www.gov.pl/web/koronawirus/aktualne-zasady-i-ograniczenia (accessed on 4 August 2020).
- Alberti, K.G.M.M.; Zimmet, P.Z. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus. Provisional report of a WHO Consultation. Diabet. Med. 1998, 15, 539–553. [Google Scholar] [CrossRef]
- Skyler, J.S.; Bakris, G.L.; Bonifacio, E.; Darsow, T.; Eckel, R.H.; Groop, L.; Groop, P.-H.; Handelsman, Y.; Insel, R.A.; Mathieu, C.; et al. Differentiation of Diabetes by Pathophysiology, Natural History, and Prognosis. Diabetes 2017, 66, 241–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, A.K.; Gupta, R.; Ghosh, A.; Misra, A. Diabetes in COVID-19: Prevalence, pathophysiology, prognosis and practical considerations. Diabetes Metab. Syndr. 2020, 14, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Colberg, S.R.; Sigal, R.J.; Yardley, J.E.; Riddell, M.C.; Dunstan, D.W.; Dempsey, P.C.; Horton, E.S.; Castorino, K.; Tate, D.F. Physical Activity/Exercise and Diabetes: A Position Statement of the American Diabetes Association. Diabetes Care 2016, 39, 2065–2079. [Google Scholar] [CrossRef] [Green Version]
- Di Renzo, L.; Gualtieri, P.; Pivari, F.; Soldati, L.; Attinà, A.; Cinelli, G.; Leggeri, C.; Caparello, G.; Barrea, L.; Scerbo, F.; et al. Eating habits and lifestyle changes during COVID-19 lockdown: an Italian survey. J. Transl. Med. 2020, 18, 229. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. People with Certain Medical Conditions. Available online: https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html (accessed on 5 August 2020).
- Visca, D.; Pignatti, P.; Spanevello, A.; Lucini, E.; La Rocca, E. Relationship between diabetes and respiratory diseases-Clinical and therapeutic aspects. Pharmacol Res 2018, 137, 230–235. [Google Scholar] [CrossRef]
- Valerius, N.H.; Eff, C.; Hansen, N.E.; Karle, H.; Nerup, J.; Søeberg, B.; Sørensen, S.F. Neutrophil and lymphocyte function in patients with diabetes mellitus. Acta Med Scand. 1982, 211, 463–467. [Google Scholar] [CrossRef]
- American Diabetes Association. How COVID-19 Impacts People with Diabetes. Available online: https://www.diabetes.org/coronavirus-covid-19/how-coronavirus-impacts-people-with-diabetes (accessed on 3 August 2020).
- Kulaga, Z.; Litwin, M.; Tkaczyk, M.; Rózdzyńska, A.; Barwicka, K.; Grajda, A.; Swiader, A.; Gurzkowska, B.; Napieralska, E.; Pan, H. The height-, weight-, and BMI-for-age of Polish school-aged children and adolescents relative to international and local growth references. BMC Public Health 2010, 10, 109. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Obesity: Preventing and Managing the Global Epidemic. Report of a WHO Consultation. Available online: https://www.who.int/nutrition/publications/obesity/WHO_TRS_894/en/ (accessed on 5 August 2020).
- Ammar, A.; Brach, M.; Trabelsi, K.; Chtourou, H.; Boukhris, O.; Masmoudi, L.; Bouaziz, B.; Bentlage, E.; How, D.; Ahmed, M.; et al. Effects of COVID-19 Home Confinement on Eating Behaviour and Physical Activity: Results of the ECLB-COVID19 International Online Survey. Nutrients 2020, 12, 1583. [Google Scholar] [CrossRef] [PubMed]
- Nachimuthu, S.; Vijayalakshmi, R.; Sudha, M.; Viswanathan, V. Coping with diabetes during the COVID - 19 lockdown in India: Results of an online pilot survey. Diabetes Metab. Syndr. 2020, 14, 579–582. [Google Scholar] [CrossRef]
- Scarmozzino, F.; Visioli, F. Covid-19 and the Subsequent Lockdown Modified Dietary Habits of Almost Half the Population in an Italian Sample. Foods 2020, 9, 675. [Google Scholar] [CrossRef] [PubMed]
- Sidor, A.; Rzymski, P. Dietary Choices and Habits during COVID-19 Lockdown: Experience from Poland. Nutrients 2020, 12, 1657. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Li, Y.; Wang, C.; Mao, Z.; Zhou, W.; Zhang, L.; Yang, X.; Cui, S.; Li, L. Dietary Protein Consumption and the Risk of Type 2 Diabetes: A Dose-Response Meta-Analysis of Prospective Studies. Nutrients 2019, 11, 2783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feskens, E.J.; Sluik, D.; van Woudenbergh, G.J. Meat consumption, diabetes, and its complications. Curr. Diab. Rep. 2013, 13, 298–306. [Google Scholar] [CrossRef]
- Duthie, S.J.; Duthie, G.G.; Russell, W.R.; Kyle, J.A.M.; Macdiarmid, J.I.; Rungapamestry, V.; Stephen, S.; Megias-Baeza, C.; Kaniewska, J.J.; Shaw, L.; et al. Effect of increasing fruit and vegetable intake by dietary intervention on nutritional biomarkers and attitudes to dietary change: a randomised trial. Eur. J. Nutr. 2018, 57, 1855–1872. [Google Scholar] [CrossRef] [Green Version]
- Cooper, A.J.; Forouhi, N.G.; Ye, Z.; Buijsse, B.; Arriola, L.; Balkau, B.; Barricarte, A.; Beulens, J.W.J.; Boeing, H.; Büchner, F.L.; et al. Fruit and vegetable intake and type 2 diabetes: EPIC-InterAct prospective study and meta-analysis. Eur. J. Clin. Nutr. 2012, 66, 1082–1092. [Google Scholar] [CrossRef] [Green Version]
- Gupta, L.; Khandelwal, D.; Dutta, D.; Kalra, S.; Lal, P.R.; Gupta, Y. The Twin White Herrings: Salt and Sugar. Indian J. Endocr. Metab. 2018, 22, 542–551. [Google Scholar] [CrossRef]
- Lamb, M.M.; Frederiksen, B.; Seifert, J.A.; Kroehl, M.; Rewers, M.; Norris, J.M. +Sugar intake is associated with progression from islet autoimmunity to type 1 diabetes: The Diabetes Autoimmunity Study in the Young. Diabetologia 2015, 58, 2027–2034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, T.F.; Lin, W.T.; Huang, H.L.; Lee, C.Y.; Wu, P.W.; Chiu, Y.W.; Huang, C.C.; Tsai, S.; Lin, C.L.; Lee, C.H. Consumption of sugar-sweetened beverages is associated with components of the metabolic syndrome in adolescents. Nutrients 2014, 6, 2088–2103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bleich, S.N.; Wang, Y.C. Consumption of sugar-sweetened beverages among adults with type 2 diabetes. Diabetes Care 2011, 34, 551–555. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Use of Glycated Haemoglobin (HbA1c) in the Diagnosis of Diabetes Mellitus: Abbreviated Report of a WHO Consultation. Available online: https://www.ncbi.nlm.nih.gov/books/NBK304267/ (accessed on 3 August 2020).
- Yannakoulia, M. Eating behavior among type 2 diabetic patients: a poorly recognized aspect in a poorly controlled disease. Rev. Diabet. Stud. 2006, 3, 11–16. [Google Scholar] [CrossRef] [Green Version]
- Savoca, M.R.; Miller, C.K.; Ludwig, D.A. Food habits are related to glycemic control among people with type 2 diabetes mellitus. J. Am. Diet. Assoc. 2004, 104, 560–566. [Google Scholar] [CrossRef]
- Falco, G.P.P.; Castellano, E.; Anfossi, M.; Borretta, G.; Gianotti, L. The Relationship between Stress and Diabetes Mellitus. J. Neurol. Psychol. 2015, 3, 1–7. [Google Scholar]
- Berlin, K.S.; Rabideau, E.M.; Hains, A.A. Empirically derived patterns of perceived stress among youth with type 1 diabetes and relationships to metabolic control. J. Pediatr. Psychol. 2012, 37, 990–998. [Google Scholar] [CrossRef] [Green Version]
- Pandit, A.U.; Bailey, S.C.; Curtis, L.M.; Seligman, H.K.; Davis, T.C.; Parker, R.M.; Schillinger, D.; DeWalt, D.; Fleming, D.; Mohr, D.C.; et al. Disease-related distress, self-care and clinical outcomes among low-income patients with diabetes. J. Epidemiol. Community Health 2014, 68, 557–564. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Baseline Characteristics of Study Groups | T1DM | T2DM | ||||
|---|---|---|---|---|---|---|
| Total (n = 90) | Women (n = 75) | Men (n = 15) | Total (n = 34) | Women (n = 28) | Men (n = 6) | |
| Age (years) | 20A** (17–28) | 20B** (17–27) | 19 (12–22) | 37.0A** (31–44) | 37.5B** (33–45) | 31.5 (20–35) |
| Body weight (kg) | 65A** (52–75) | 63B** (52–74) | 75 (55–78) | 75.0A** (66–90) | 73.5B** (63–90) | 78 (70–92) |
| Height (cm) | 168 (162–174) | 168 (160–171) | 180 (175–182) | 168 (160–175) | 165 (159–170) | 182.5 (179–186) |
| Body mass index (kg/m2) | 22.7A** (19.3–25.6) | 22.0B** (19.2–26.3) | 22.9 (21.9–23.6) | 26.1A** (22.4–30.9) | 27.0B** (23.5–31.2) | 23.3 (21.0–25.8) |
| Level of education | ||||||
| Kindergarten and primary school | 17% (15) | 14% (11) | 27% (4) | - | - | - |
| Secondary school | 34% (31) | 36% (27) | 27% (4) | 21% (7) | 10% (3) | 67% (4) |
| University | 49% (44) | 50% (37) | 46% (7) | 79% (27) | 89% (25) | 33% (2) |
| Place of residence | ||||||
| Village | 18% (16) | 16% (12) | 27% (4) | 24% (8) | 14% (4) | 67% (4) |
| City (≤150 k inhabitants) | 23% (21) | 28% (21) | - | 32% (11) | 39% (11) | - |
| City (150–250 k inhabitants) | 29% (26) | 25% (19) | 46% (7) | 20% (7) | 22% (6) | 17% (1) |
| City (≥250 k inhabitants) | 30% (27) | 31% (23) | 27% (4) | 24% (8) | 25% (7) | 17% (1) |
| Duration of diseaseC** | ||||||
| Up to 2 years | 15% (14) | 13% (10) | 27% (4) | 56% (19) | 53% (15) | 67% (4) |
| 2–5 years | 28% (25) | 28% (21) | 27% (4) | 29% (10) | 36% (10) | - |
| 5–10 years | 17% (15) | 17% (13) | 13% (2) | - | - | - |
| More than 10 years | 40% (36) | 42% (31) | 33% (5) | 15% (5) | 11% (3) | 33% (2) |
| HbA1c (%) D | 6.8 (6.0–7.6) | 6.9 (6.2–7.9) | 7.5 (6.8–7.9) | 6.3 (5.6–8.0) | 6.3 (5.7–8.1) | 7.5 (7.3–8.6) |
| Disease self-managementC* | ||||||
| No change | 17% (15) | 20% (15) | - | 6% (2) | 7% (2) | - |
| Deteriorated | 47% (42) | 40% (30) | 80% (12) | 44% (15) | 46.5% (13) | 33% (2) |
| Improved | 37% (33) | 40% (30) | 20% (3) | 50% (17) | 46.5% (13) | 66% (4) |
| Body mass changeC** | ||||||
| No change | 28% (25) | 20% (16) | 60% (9) | 41% (14) | 43% (11) | 33% (2) |
| Increased ≤5 kg | 31% (28) | 34% (26) | 13% (2) | 18% (6) | 21% (6) | - |
| Increased >5 kg | 11% (10) | 13% (10) | - | 12% (4) | 7% (2) | 33% (2) |
| Decreased ≤5 kg | 23% (21) | 26% (17) | 27% (4) | 9% (3) | 11% (3) | - |
| Decreased >5 kg | 7% (6) | 7% (6) | - | 20% (7) | 18% (5) | 33% (2) |
| Weekly Activity | Before/During the COVID-19 Pandemic | ||||||
|---|---|---|---|---|---|---|---|
| Total * (n = 124) | T1DM | T2DM | |||||
| Total * (n = 90) | Women * (n = 75) | Men (n = 15) | Total * (n = 34) | Women (n = 28) | Men (n = 6) | ||
| No activity | 21%/34% | 19%/33% | 22%/39% | -/7% | 26%/35% | 25%/35% | 33%/33% |
| 1–2 times/week | 36%/41% | 39%/42% | 40%/40% | 33%/53% | 26%/38% | 29%/39% | 17%/- |
| 3–4 times/week | 31%/19% | 30%/18% | 27%/17% | 47%/20% | 35%/24% | 39%/22% | 17%/33% |
| ≥5 times/week | 12%/6% | 12%/7% | 11%/4% | 20%/20% | 11%/3% | 7%/4% | 33%/33% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grabia, M.; Markiewicz-Żukowska, R.; Puścion-Jakubik, A.; Bielecka, J.; Nowakowski, P.; Gromkowska-Kępka, K.; Mielcarek, K.; Socha, K. The Nutritional and Health Effects of the COVID-19 Pandemic on Patients with Diabetes Mellitus. Nutrients 2020, 12, 3013. https://doi.org/10.3390/nu12103013
Grabia M, Markiewicz-Żukowska R, Puścion-Jakubik A, Bielecka J, Nowakowski P, Gromkowska-Kępka K, Mielcarek K, Socha K. The Nutritional and Health Effects of the COVID-19 Pandemic on Patients with Diabetes Mellitus. Nutrients. 2020; 12(10):3013. https://doi.org/10.3390/nu12103013
Chicago/Turabian StyleGrabia, Monika, Renata Markiewicz-Żukowska, Anna Puścion-Jakubik, Joanna Bielecka, Patryk Nowakowski, Krystyna Gromkowska-Kępka, Konrad Mielcarek, and Katarzyna Socha. 2020. "The Nutritional and Health Effects of the COVID-19 Pandemic on Patients with Diabetes Mellitus" Nutrients 12, no. 10: 3013. https://doi.org/10.3390/nu12103013
APA StyleGrabia, M., Markiewicz-Żukowska, R., Puścion-Jakubik, A., Bielecka, J., Nowakowski, P., Gromkowska-Kępka, K., Mielcarek, K., & Socha, K. (2020). The Nutritional and Health Effects of the COVID-19 Pandemic on Patients with Diabetes Mellitus. Nutrients, 12(10), 3013. https://doi.org/10.3390/nu12103013









