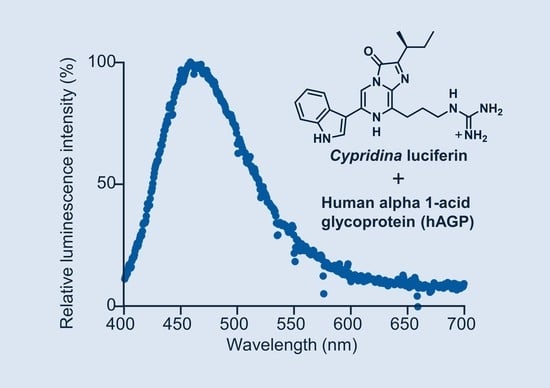
Luminescence of Cypridina Luciferin in the Presence of Human Plasma Alpha 1-Acid Glycoprotein
Abstract
:1. Introduction
2. Results
2.1. Luminescence of Cypridina Luciferin in the Presence of hAGP
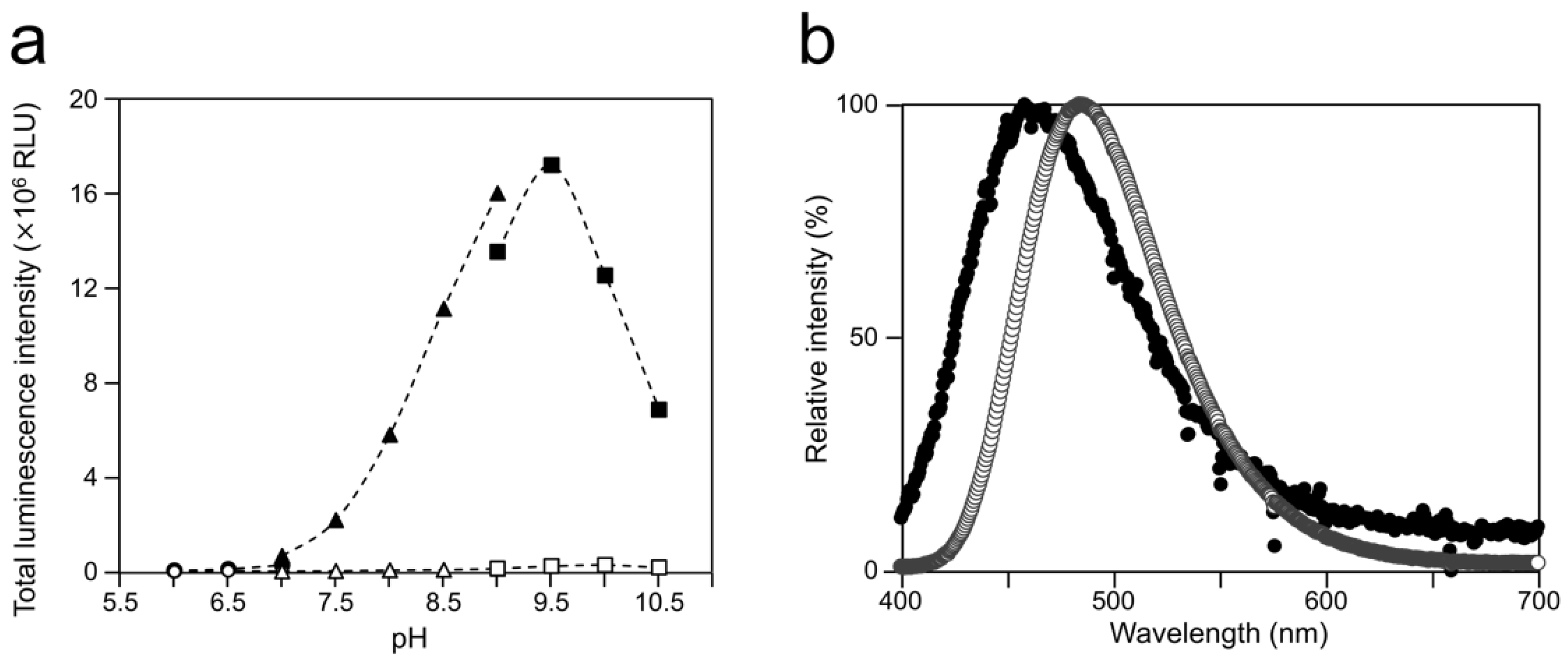
2.2. Luminescence Property of Cypridina Luciferin in the Presence of hAGP
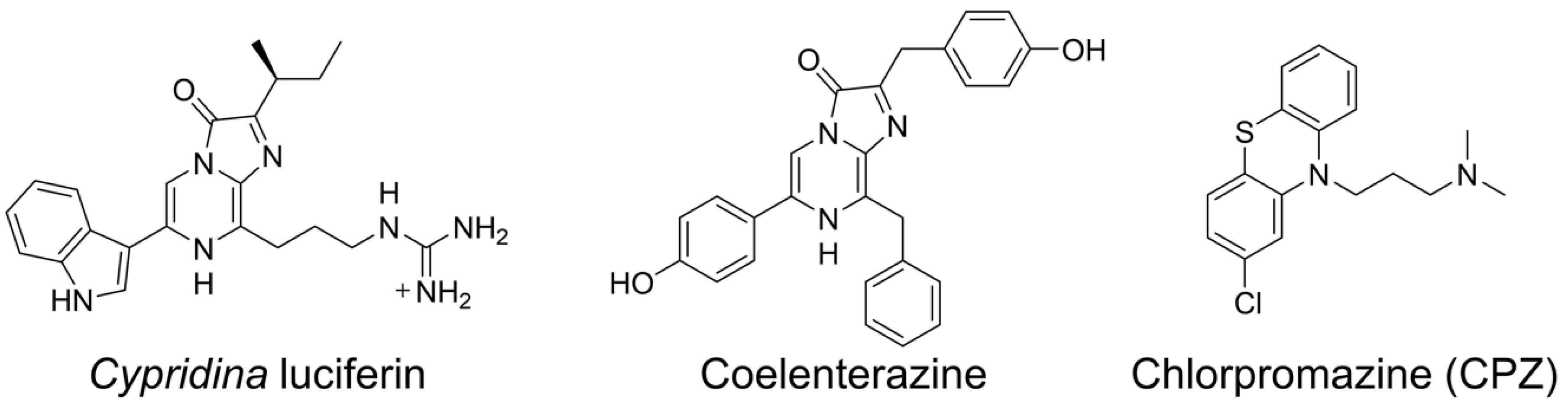
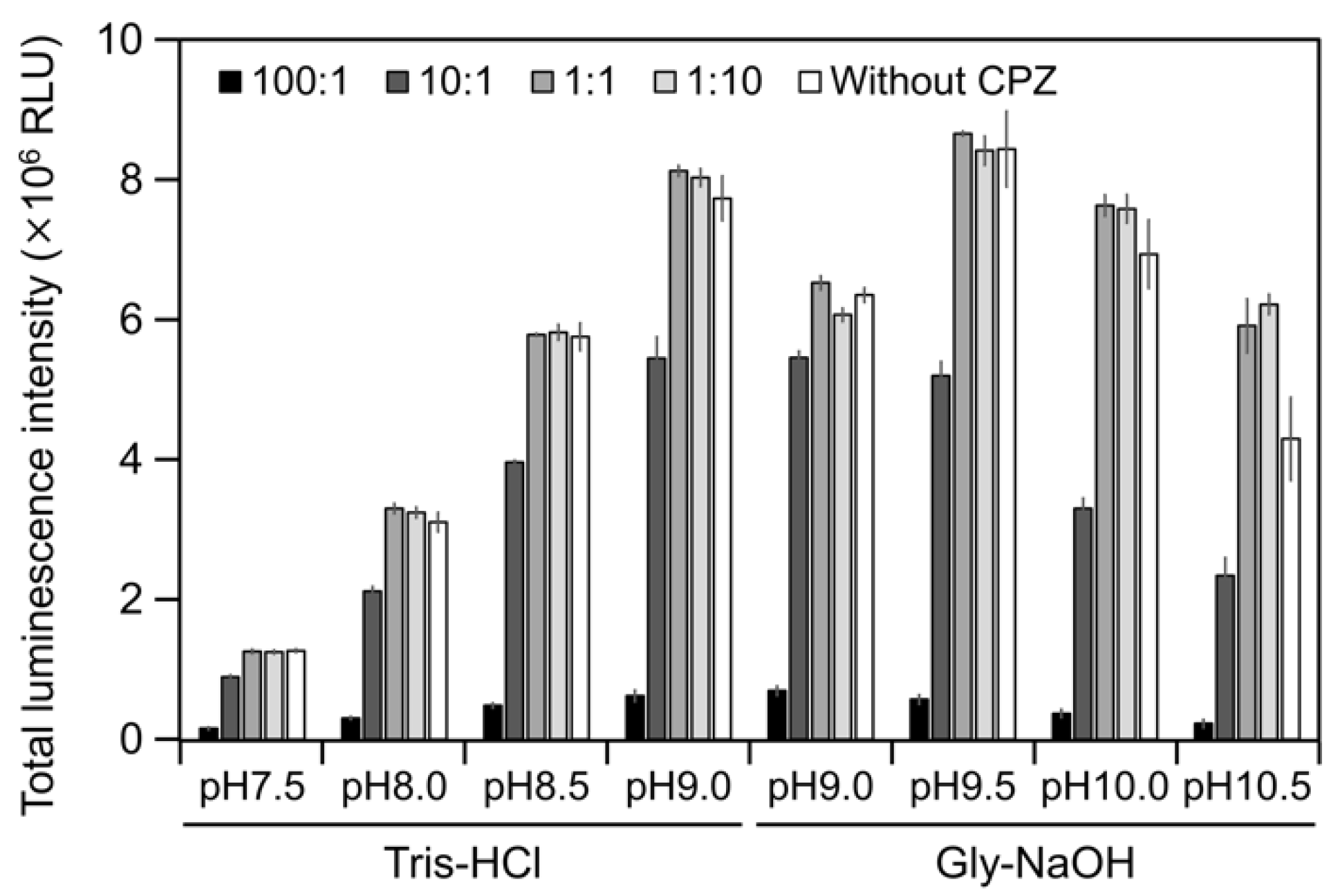
2.3. CPZ Effect on Luminescence of Cypridina Luciferin in the Presence of hAGP
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Measurement of Luminescence Intensity
4.3. Measurement of Luminescence Intensity of Cypridina Luciferin in the Presence of Proteins
4.4. Measurement of Luminescence Intensity of Cypridina Luciferin with hAGP under Various pH Conditions
4.5. Measurement of Luminescence Emission Spectrum
4.6. Measurement of Luminescence Intensity of Cypridina Luciferin with Heat-Treated hAGP
4.7. Measurement of Luminescence Intensity of Cypridina Luciferin and CPZ with hAGP or CLase
4.8. Linear Regression Analysis between Luminescence Intensity of Cypridina Luciferin and Amount of hAGP
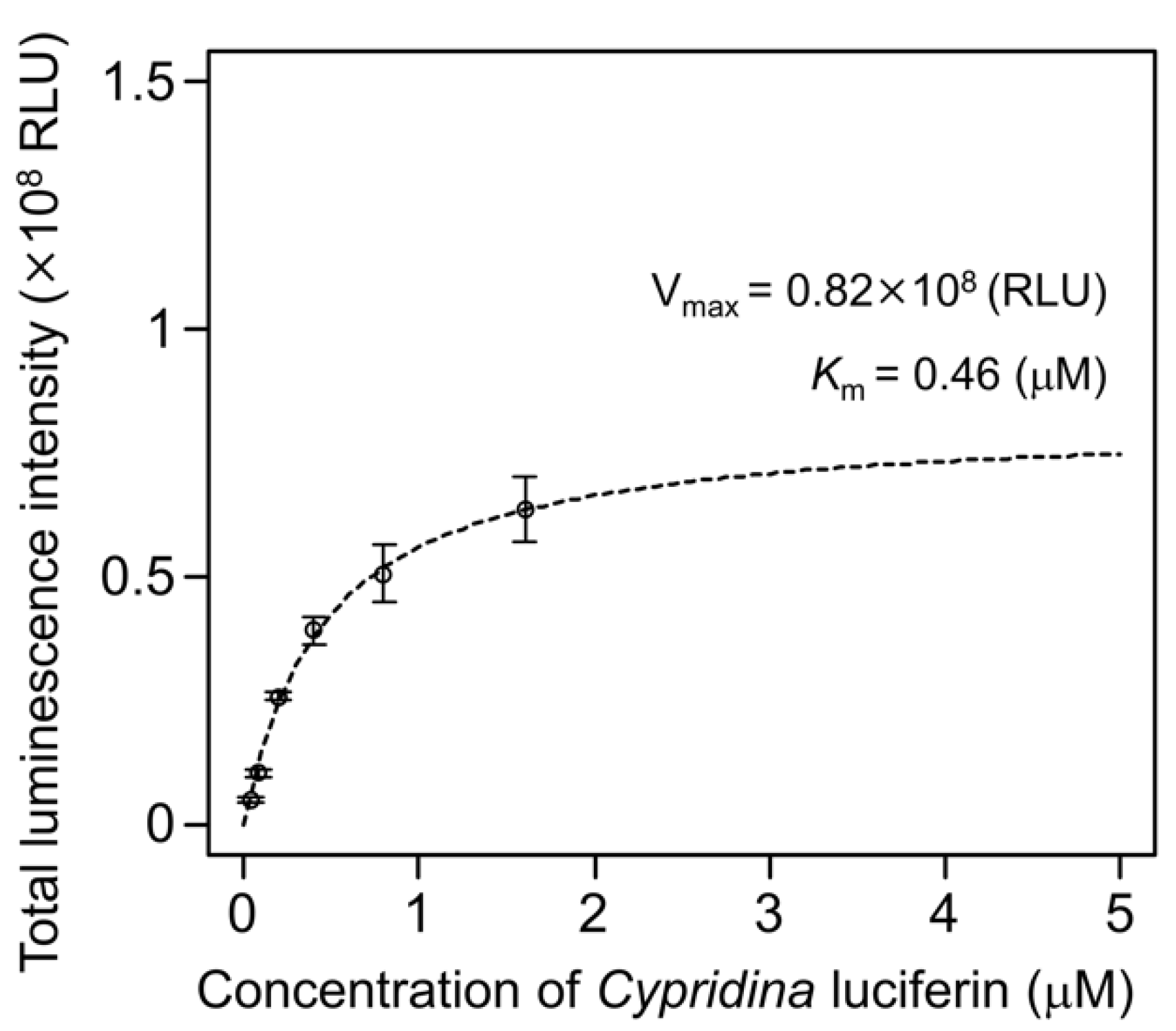
4.9. Kinetic Analysis of hAGP with Cypridina Luciferin
4.10. Comparison of Amino Acid Sequences between hAGP and CLases
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CLase | Cypridina luciferase |
| hAGP | Human alpha 1-acid glycoprotein |
| λmax | Maximum emission wavelength |
| ΦBL | Quantum yield of bioluminescence |
| DMSO | Dimethyl sulfoxide |
| OVA | Ovalbumin |
| BSA | Bovine serum albumin |
| HSA | Human serum albumin |
| Tris | Tris(hydroxymethyl)aminomethane |
| Gly | Glycine |
| CPZ | Chlorpromazine |
| BlastP | Protein-protein basic local alignment search tool |
| RLU | Relative light unit |
| E-value | Expect value |
| SD | Standard deviation |
| HCl | Hydrochloric acid |
| BisTris | Bis(2-hydroxyethyl)iminotris(hydroxymethyl)methane |
| NaOH | Sodium hydroxide |
| CCD | Charge coupled device |
Appendix A

| Protein | Buffer Conditions | λmax (nm) |
|---|---|---|
| hAGP | Tris-HCl buffer (pH 7.5) | 458 |
| Tris-HCl buffer (pH 8.5) | 457 | |
| Gly-NaOH buffer (pH 9.5) | 458 | |
| Gly-NaOH buffer (pH 10.5) | 458 | |
| CLase | Tris-HCl buffer (pH 7.5) | 478 |
| Tris-HCl buffer (pH 8.5) | 480 | |
| Gly-NaOH buffer (pH 9.5) | 484 | |
| Gly-NaOH buffer (pH 10.5) | 483 |



References
- Johnson, F.H.; Shimomura, O.; Saiga, Y.; Gershman, L.C.; Reynolds, G.T.; Waters, J.R. Quantum efficiency of Cypridina luminescence, with a note on that of Aequorea. J. Cell. Comp. Physiol. 1962, 60, 85–103. [Google Scholar] [CrossRef]
- Shimomura, O.; Johnson, F.H. Mechanisms in Quantum Yield of Cypridina Bioluminescence. Photochem. Photobiol. 1970, 12, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Shimomura, O.; Yampolsky, I.V. Bioluminescence: Chemical Principles and Methods, 3rd ed.; World Scientific: Hackensack, NJ, USA, 2019. [Google Scholar]
- Johnson, F.H.; Shimomura, O. Introduction to the Cypridina System. Methods Enzymol. 1978, 57, 331–364. [Google Scholar]
- Morin, J.G. Based on a review of the data, use of the term ‘cypridinid’ solves the Cypridina/Vargula dilemma for naming the constituents of the luminescent system of ostracods in the family Cypridinidae. Luminescence 2011, 26, 1–4. [Google Scholar] [CrossRef]
- Harvey, E.N. Bioluminescence; Academic Press: New York, NY, USA, 1952. [Google Scholar]
- Shimomura, O.; Goto, T.; Hirata, Y. Crystalline Cypridina Luciferin. Bull. Chem. Soc. Jpn. 1957, 30, 929–933. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, R.W. Classical Methods in Structure Elucidation of Natural Products; Wiley-VHCA: Zürich, Switzerland, 2018. [Google Scholar]
- Thompson, E.M.; Nagata, S.; Tsuji, F.I. Cloning and expression of cDNA for the luciferase from the marine ostracod Vargula hilgendorfii. Proc. Natl. Acad. Sci. USA 1989, 86, 6567–6571. [Google Scholar] [CrossRef] [Green Version]
- Nakajima, Y.; Kobayashi, K.; Yamagishi, K.; Enomoto, T.; Ohmiya, Y. cDNA cloning and characterization of a secreted luciferase from the luminous Japanese ostracod, Cypridina noctiluca. Biosci. Biotechnol. Biochem. 2004, 68, 565–570. [Google Scholar] [CrossRef] [Green Version]
- Hensley, N.M.; Ellis, E.A.; Leung, N.Y.; Coupart, J.; Mikhailovsky, A.; Taketa, D.A.; Tessler, M.; Gruber, D.F.; De Tomaso, A.W.; Mitani, Y.; et al. Molecular evolution of luciferase diversified bioluminescent signals in sea fireflies. bioRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Mitani, Y.; Oshima, Y.; Mitsuda, N.; Tomioka, A.; Sukegawa, M.; Fujita, M.; Kaji, H.; Ohmiya, Y. Efficient production of glycosylated Cypridina luciferase using plant cells. Protein Expr. Purif. 2017, 133, 102–109. [Google Scholar] [CrossRef]
- Kaskova, Z.M.; Tsarkova, A.S.; Yampolsky, I.V. 1001 lights: Luciferins, luciferases, their mechanisms of action and applications in chemical analysis, biology and medicine. Chem. Soc. Rev. 2016, 45, 6048–6077. [Google Scholar] [CrossRef]
- Takahashi, Y.; Kondo, H.; Maki, S.; Niwa, H.; Ikeda, H.; Hirano, T. Chemiluminescence of 6-aryl-2-methylimidazo 1,2-a pyrazin-3(7H)-ones in DMSO/TMG and in diglyme/acetate buffer: Support for the chemiexcitation process to generate the singlet-excited state of neutral oxyluciferin in a high quantum yield in the Cypridina (Vargula) bioluminescence mechanism. Tetrahedron Lett. 2006, 47, 6057–6061. [Google Scholar]
- Teranishi, K. Luminescence of imidazo 1,2-a pyrazin-3(7H)-one compounds. Bioorg. Chem. 2007, 35, 82–111. [Google Scholar] [CrossRef] [PubMed]
- Hirano, T.; Takahashi, Y.; Kondo, H.; Maki, S.; Kojima, S.; Ikeda, H.; Niwa, H. The reaction mechanism for the high quantum yield of Cypridina (Vargula) bioluminescence supported by the chemiluminescence of 6-aryl-2-methylimidazo 1,2-a pyrazin-3(7H)-ones (Cypridina luciferin analogues). Photochem. Photobiol. Sci. 2008, 7, 197–207. [Google Scholar] [PubMed]
- Naumov, P.; Wu, C.; Liu, Y.J.; Ohmiya, Y. Spectrochemistry and artificial color modulation of Cypridina luminescence: Indirect evidence for chemiexcitation of a neutral dioxetanone and emission from a neutral amide. Photochem. Photobiol. Sci. 2012, 11, 1151–1155. [Google Scholar] [CrossRef] [PubMed]
- Ding, B.W.; Naumov, P.; Liu, Y.J. Mechanistic Insight into Marine Bioluminescence: Photochemistry of the Chemiexcited Cypridina (Sea Firefly) Lumophore. J. Chem. Theory Comput. 2015, 11, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Magalhaes, C.M.; da Silva, J.; da Silva, L.P. Comparative study of the chemiluminescence of coelenterazine, coelenterazine-e and Cypridina luciferin with an experimental and theoretical approach. J. Photochem. Photobiol. B Biol. 2019, 190, 21–31. [Google Scholar]
- Min, C.G.; Liu, Q.B.; Leng, Y.; Magalhaes, C.M.; Huang, S.J.; Liu, C.X.; Yang, X.K.; da Silva, L.P. Mechanistic Insight into the Chemiluminescent Decomposition of Cypridina Dioxetanone and the Chemiluminescent, Fluorescent Properties of the Light Emitter of Cypridina Bioluminescence. J. Chem. Inf. Model. 2019, 59, 4393–4401. [Google Scholar]
- Johnson, F.H.; Stachel, H.D.; Taylor, E.C.; Shimomura, O. Chemiluminescence and Fluorescence of Cypridina Luciferin and of Some New Indole Compounds in Dimethylsulfoxide. In Bioluminescence in Progress; Johnson, F.H., Haneda, Y., Eds.; Princeton University Press: Princeton, NJ, USA, 1966; pp. 67–82. [Google Scholar]
- Goto, T. Chemistry of bioluminescence. Pure Appl. Chem. 1968, 17, 421–441. [Google Scholar]
- Goto, T.; Inoue, S.; Sugiura, S. Cypridina bioluminescence IV. Synthesis and chemiluminescence of 3,7-dihydroimidazo[1,2-a]pyrazin-3-one and its 2-methyl derivative. Tetrahedron Lett. 1968, 9, 3873–3876. [Google Scholar] [CrossRef]
- Goto, T.; Fukatsu, H. Cypridina bioluminescence VII. chemiluminescence in micelle solutions—A model system for cypridina bioluminescence. Tetrahedron Lett. 1969, 10, 4299–4302. [Google Scholar] [CrossRef]
- Shimomura, O.; Johnson, F.H.; Masugi, T. Cypridina Bioluminescence: Light-Emitting Oxyluciferin-Luciferase Complex. Science 1969, 164, 1299–1300. [Google Scholar] [CrossRef] [PubMed]
- Goto, T.; Kubota, I.; Suzuki, N.; Kishi, Y.; Inoue, S. Aspects of the Mechanism of Bioluminescence. In Chemiluminescence and Bioluminescence; Cormier, M.J., Hercules, D.M., Lee, J., Eds.; Springer US: Boston, MA, USA, 1973; pp. 325–335. [Google Scholar]
- Toya, Y. Chemistry of Vargula (formerly Cypridina) BIOLUMINESCENCE. Nippon Nogeikagaku Kaishi J. Jpn. Soc. Biosci. Biotechnol. Agrochem. 1992, 66, 742–747. (In Japanese) [Google Scholar]
- Mitani, M.; Sakaki, S.; Koinuma, Y.; Toya, Y.; Kosugi, M. Enhancement Effect of 2, 6-O-Dimethyl-β-cyclodextrin on the Chemiluminescent Detection of β-D-Galactosidase Using a Cypridina Luciferin Analog. Anal. Sci. 1995, 11, 1013–1015. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Xing, D.; He, Y.H.; Hu, X.J. Experimental study on photodynamic diagnosis of cancer mediated by chemiluminescence probe. FEBS Lett. 2002, 523, 128–132. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Zing, D.; Chen, Q. Enhancement of Fluoresceinyl Cypridina Luciferin Analog Chemiluminescence by Human Serum Albumin for Singlet Oxygen Detection. Photochem. Photobiol. 2006, 82, 1058–1064. [Google Scholar] [CrossRef] [Green Version]
- Xu, W.; Wei, Y.C.; Xing, D.; Chen, Q. A novel chemiluminescence technique for quantitative measurement of low concentration human serum albumin. Anal. Sci. 2008, 24, 115–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vassel, N.; Cox, C.D.; Naseem, R.; Morse, V.; Evans, R.T.; Power, R.L.; Brancale, A.; Wann, K.T.; Campbell, A.K. Enzymatic activity of albumin shown by coelenterazine chemiluminescence. Luminescence 2012, 27, 234–241. [Google Scholar] [CrossRef]
- Inouye, S.; Sahara-Miura, Y. A Novel Catalytic Function of Synthetic IgG-Binding Domain (Z Domain) from Staphylococcal Protein A: Light Emission with Coelenterazine. Photochem. Photobiol. 2014, 90, 137–144. [Google Scholar] [CrossRef]
- Bteich, M. An overview of albumin and alpha-1-acid glycoprotein main characteristics: Highlighting the roles of amino acids in binding kinetics and molecular interactions. Heliyon 2019, 5, e02879. [Google Scholar] [CrossRef] [Green Version]
- Nishi, K.; Ono, T.; Nakamura, T.; Fukunaga, N.; Izumi, M.; Watanabe, H.; Suenaga, A.; Maruyama, T.; Yamagata, Y.; Curry, S.; et al. Structural Insights into Differences in Drug-binding Selectivity between Two Forms of Human Alpha(1)-Acid Glycoprotein Genetic Variants, the A and F1*S Forms. J. Biol. Chem. 2011, 286, 14427–14434. [Google Scholar] [CrossRef] [Green Version]
- Smith, S.A.; Waters, N.J. Pharmacokinetic and Pharmacodynamic Considerations for Drugs Binding to Alpha-1-Acid Glycoprotein. Pharm. Res. 2019, 36, 30. [Google Scholar] [CrossRef]
- Matsumoto, K.; Sukimoto, K.; Nishi, K.; Maruyama, T.; Suenaga, A.; Otagiri, M. Characterization of Ligand Binding Sites on the α1-Acid Glycoprotein in Humans, Bovines and Dogs. Drug Metab. Pharmacokinet. 2002, 17, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Hunt, E.A.; Moutsiopoulou, A.; Broyles, D.; Head, T.; Dikici, E.; Daunert, S.; Deo, S.K. Expression of a soluble truncated Vargula luciferase in Escherichia coli. Protein Expr. Purif. 2017, 132, 68–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maeda, Y.; Ueda, H.; Kazami, J.; Kawano, G.; Suzuki, E.; Nagamune, T. Truncation of Vargula luciferase still results in retention of luminescence. J. Biochem. 1996, 119, 601–603. [Google Scholar] [CrossRef] [PubMed]
- Gannon, B.M.; Glesby, M.J.; Finkelstein, J.L.; Raj, T.; Erickson, D.; Mehta, S. A point-of-care assay for alpha-1-acid glycoprotein as a diagnostic tool for rapid, mobile-based determination of inflammation. Curr. Res. Biotechnol. 2019, 1, 41–48. [Google Scholar] [CrossRef]
- Mofford, D.M.; Reddy, G.R.; Miller, S.C. Latent luciferase activity in the fruit fly revealed by a synthetic luciferin. Proc. Natl. Acad. Sci. USA 2014, 111, 4443–4448. [Google Scholar] [CrossRef] [Green Version]
- Iwano, S.; Sugiyama, M.; Hama, H.; Watakabe, A.; Hasegawa, N.; Kuchimaru, T.; Tanaka, K.Z.; Takahashi, M.; Ishida, Y.; Hata, J.; et al. Single-cell bioluminescence imaging of deep tissue in freely moving animals. Science 2018, 359, 935–939. [Google Scholar] [CrossRef] [Green Version]
- Adams, S.T.; Miller, S.C. Enzymatic promiscuity and the evolution of bioluminescence. FEBS J. 2020, 287, 1369–1380. [Google Scholar] [CrossRef] [Green Version]
- Chaloupkova, R.; Liskova, V.; Toul, M.; Markova, K.; Sebestova, E.; Hernychova, L.; Marek, M.; Pinto, G.P.; Pluskal, D.; Waterman, J.; et al. Light-Emitting Dehalogenases: Reconstruction of Multifunctional Biocatalysts. ACS Catal. 2019, 9, 4810–4823. [Google Scholar] [CrossRef]
- Fallon, T.R.; Lower, S.E.; Chang, C.H.; Bessho-Uehara, M.; Martin, G.J.; Bewick, A.J.; Behringer, M.; Debat, H.J.; Wong, I.; Day, J.C.; et al. Firefly genomes illuminate parallel origins of bioluminescence in beetles. Elife 2018, 7, e36495. [Google Scholar] [CrossRef]
- Campbell, A.K. Darwin shines light on the evolution of bioluminescence. Luminescence 2012, 27, 447–449. [Google Scholar]
- Kishi, Y.; Goto, T.; Hirata, Y.; Shimomura, O.; Johnson, F.H. Cypridina bioluminescence I Sructure of Cypridina luciferin. Tetrahedron Lett. 1966, 7, 3427–3436. [Google Scholar]
- Peptide Property Calculator. Available online: http://biotools.nubic.northwestern.edu/proteincalc.html (accessed on 7 August 2020).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- BlastP. Available online: https://blast.ncbi.nlm.nih.gov/Blast.cgi?PAGE=Proteins (accessed on 7 August 2020).
- ClustalW. Available online: https://clustalw.ddbj.nig.ac.jp/ (accessed on 7 August 2020).


| Protein | Amount of Protein (μg) | Luminescence Intensity (RLU) | |||
|---|---|---|---|---|---|
| Maximum Intensity | Integration over 60 s | ||||
| None | 0 | 192 ± 94 | (0.04%) 1 | 48,651 ± 7566 | (0.02%) 1 |
| OVA | 2.5 | 330 ± 79 | (0.06%) 1 | 122,820 ± 13,239 | (0.04%) 1 |
| HSA | 2.5 | 319 ± 12 | (0.06%) 1 | 151,578 ± 8893 | (0.05%) 1 |
| BSA | 2.5 | 403 ± 14 | (0.08%) 1 | 193,741 ± 5228 | (0.07%) 1 |
| hAGP | 2.5 | 4426 ± 23 | (0.84%) 1 | 2,439,420 ± 20,104 | (0.82%) 1 |
| CLase | 0.25 (ng) | 524,803 ± 56,087 | (100%) 1 | 297,210,024 ± 31,692,238 | (100%) 1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanie, S.; Komatsu, M.; Mitani, Y. Luminescence of Cypridina Luciferin in the Presence of Human Plasma Alpha 1-Acid Glycoprotein. Int. J. Mol. Sci. 2020, 21, 7516. https://doi.org/10.3390/ijms21207516
Kanie S, Komatsu M, Mitani Y. Luminescence of Cypridina Luciferin in the Presence of Human Plasma Alpha 1-Acid Glycoprotein. International Journal of Molecular Sciences. 2020; 21(20):7516. https://doi.org/10.3390/ijms21207516
Chicago/Turabian StyleKanie, Shusei, Mami Komatsu, and Yasuo Mitani. 2020. "Luminescence of Cypridina Luciferin in the Presence of Human Plasma Alpha 1-Acid Glycoprotein" International Journal of Molecular Sciences 21, no. 20: 7516. https://doi.org/10.3390/ijms21207516
APA StyleKanie, S., Komatsu, M., & Mitani, Y. (2020). Luminescence of Cypridina Luciferin in the Presence of Human Plasma Alpha 1-Acid Glycoprotein. International Journal of Molecular Sciences, 21(20), 7516. https://doi.org/10.3390/ijms21207516