The Role of Piper sarmentosum Aqueous Extract as a Bone Protective Agent, a Histomorphometric Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Aqueous Piper sarmentosum Leaf Extract
2.2. Animals and Treatment
2.3. Bone Histomorphometric Analysis
2.3.1. Bone Microstructure Histomorphometric Analysis
2.3.2. Bone Static Histomorphometric Analysis
2.3.3. Bone Dynamic Histomorphometric Analysis
2.4. Statistical Analysis
3. Results
3.1. Structural Histomorphometry Parameters
3.2. Static Histomorphometry Parameters
3.3. Dynamic Histomorphometry Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chapurlat, R.D.; Genant, H.K. Osteoporosis. In Endocrinology: Adult and Pediatric, 7th ed.; Jameson, J.L., De Groot, L.J., de Kretser, D.M., Giudice, L.C., Grossman, A.B., Melmed, S., Potts, J.T., Weir, G.C., Eds.; W.B. Saunders: Philadelphia, PA, USA, 2016; pp. 1184–1213. ISBN 978-0-323-18907-1. [Google Scholar] [CrossRef]
- Weinstein, R.S. Glucocorticoid-induced bone disease. N. Engl. J. Med. 2011, 365, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Orcel, P. Prevention and treatment of glucocorticoid-induced osteoporosis in 2005. Joint Bone Spine 2005, 72, 461–465. [Google Scholar] [CrossRef] [PubMed]
- Canalis, E.; Bilezikian, J.P.; Angeli, A.; Giustina, A. Perspectives on glucocorticoid-induced osteoporosis. Bone 2004, 34, 593–598. [Google Scholar] [CrossRef]
- Van Staa, T.P.; Leufkens, H.G.M.; Abenhaim, L.; Zhang, B.; Cooper, C. Use of oral corticosteroids and risk of fractures. J. Bone Miner. Res. 2000, 15, 993–1000. [Google Scholar] [CrossRef] [Green Version]
- O’Brien, C.A.; Jia, D.; Plotkin, L.I.; Bellido, T.; Powers, C.C.; Stewart, S.A.; Manolagas, S.C.; Weinstein, R.S. Glucocorticoids act directly on osteoblasts and osteocytes to induce their apoptosis and reduce bone formation and strength. Endocrinology 2004, 145, 1835–1841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beavan, S.; Horner, A.; Bord, S.; Ireland, D.; Compston, J. Colocalization of glucocorticoid and mineralcorticoid receptors in human bone. J. Bone Miner. Res. 2001, 16, 1496–1504. [Google Scholar] [CrossRef]
- Stewart, P.M.; Krozowski, Z.S. 11β-hydroxysteroid dehydrogenase. Vitam. Horm. 1997, 57, 249–324. [Google Scholar] [CrossRef]
- Draper, N.; Stewart, P.M. 11β-Hydroxysteroid dehydrogenase and the pre-receptor regulation of corticosteroid hormone action. J. Endocrinol. 2005, 186, 251–271. [Google Scholar] [CrossRef] [Green Version]
- Hewison, M. Ligand regulation and nuclear receptor action. In Nuclear Receptors. Proteins and Cell Regulation; Bunce, C.M., Campbell, M.J., Eds.; Springer: Dordrecht, The Netherlands, 2010; Volume 8, pp. 381–417. ISBN 978-90-481-3303-1. [Google Scholar]
- Eijken, M.; Hewison, M.; Cooper, M.S.; de Jong, F.H.; Chiba, H.; Stewart, P.M.; Uitterlinden, A.G.; Pols, H.A.P.; van Leeuwen, J.P.T.M. 11β-hydroxysteroid dehydrogenase expression and glucocorticoid synthesis are directed by a molecular switch during osteoblast differentiation. Mol. Endocrinol. 2005, 19, 621–631. [Google Scholar] [CrossRef] [Green Version]
- Isbrucker, R.A.; Burdock, G.A. Risk and safety assessment on the consumption of Licorice root (Glycyrrhiza sp.), its extract and powder as a food ingredient, with emphasis on the pharmacology and toxicology of glycyrrhizin. Regul. Toxicol. Pharmacol. 2006, 46, 167–192. [Google Scholar] [CrossRef]
- Størmer, F.C.; Reistad, R.; Alexander, J. Glycyrrhizic acid in liquorice—Evaluation of health hazard. Food Chem. Toxicol. 1993, 31, 303–312. [Google Scholar] [CrossRef]
- Kao, T.C.; Wu, C.H.; Yen, G.C. Glycyrrhizic acid and 18β-glycyrrhetinic acid recover glucocorticoid resistance via PI3K-induced AP1, CRE and NFAT activation. Phytomedicine 2013, 20, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Livingstone, D.E.W.; Walker, B.R. Is 11β-hydroxysteroid dehydrogenase type 1 a therapeutic target? Effects of carbenoxolone in lean and obese Zucker rats. J. Pharmacol. Exp. Ther. 2003, 305, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Cooper, M.S.; Rabbitt, E.H.; Goddard, P.E.; Bartlett, W.A.; Hewison, M.; Stewart, P.M. Osteoblastic 11β-hydroxysteroid dehydrogenase type 1 activity increases with age and glucocorticoid exposure. J. Bone Miner. Res. 2002, 17, 979–986. [Google Scholar] [CrossRef]
- Ramli, E.S.M.; Suhaimi, F.; Asri, S.F.M.; Ahmad, F.; Soelaiman, I.N. Glycyrrhizic acid (GCA) as 11β-hydroxysteroid dehydrogenase inhibitor exerts protective effect against glucocorticoid-induced osteoporosis. J. Bone Miner. Metab. 2013, 31, 262–273. [Google Scholar] [CrossRef]
- Roxburgh, W.; Wallich, N. Flora Indica, or, Descriptions of Indian Plants; Mission Press: Serampore, India, 1820; pp. 162–163. [Google Scholar]
- Mohamad, S.; Zin, N.M.; Wahab, H.A.; Ibrahim, P.; Sulaiman, S.F.; Zahariluddin, A.S.M.; Noor, S.S.M. Antituberculosis potential of some ethnobotanically selected Malaysian plants. J. Ethnopharmacol. 2011, 133, 1021–1026. [Google Scholar] [CrossRef]
- Rahman, S.F.S.A.; Sijam, K.; Omar, D. Piper sarmentosum Roxb.: A mini review of ethnobotany, phytochemistry and pharmacology. J. Anal. Pharm. Res. 2016, 2, 00031. [Google Scholar] [CrossRef]
- Parmar, V.S.; Jain, S.C.; Bisht, K.S.; Jain, R.; Taneja, P.; Jha, A.; Tyagi, O.D.; Prasad, A.K.; Wengel, J.; Olsen, C.E.; et al. Phytochemistry of the genus Piper. Phytochemistry 1997, 46, 597–673. [Google Scholar] [CrossRef]
- Rukachaisirikul, T.; Siriwattanakit, P.; Sukcharoenphol, K.; Wongvein, C.; Ruttanaweang, P.; Wongwattanavuch, P.; Suksamrarn, A. Chemical constituents and bioactivity of Piper sarmentosum. J. Ethnopharmacol. 2004, 93, 173–176. [Google Scholar] [CrossRef]
- Ismail, M.; Chua, K.; Aminuddin, A.; Ugusman, A. Piper sarmentosum as an antioxidant: A systematic review. Sains Malays. 2018, 47, 2359–2368. [Google Scholar] [CrossRef]
- Hussain, K.; Ismail, Z.; Sadikun, A.; Ibrahim, P. Analysis of proteins, polysaccharides, glycosaponins contents of Piper sarmentosum Roxb. and anti-TB evaluation for bio-enhancing/interaction effects of leaf extracts with Isoniazid. Nat. Prod. Radiance 2008, 7, 402–408. [Google Scholar]
- Ariffin, S.H.Z.; Omar, W.H.H.W.; Ariffin, Z.Z.; Safian, M.F.; Senafi, S.; Wahab, R.M.A. Intrinsic anticarcinogenic effects of Piper sarmentosum ethanolic extract on a human hepatoma cell line. Cancer Cell Int. 2009, 9, 6. [Google Scholar] [CrossRef] [Green Version]
- Peungvicha, P.; Thirawarapan, S.S.; Temsiririrkkul, R.; Watanabe, H.; Prasain, J.K.; Kadota, S. Hypoglycemic effect of the water extract of Piper sarmentosum in rats. J. Ethnopharmacol. 1998, 60, 27–32. [Google Scholar] [CrossRef]
- Azlina, A.A.; Farihah, H.S.; Qodriyah, H.M.S.; Nur Azlina, M.F. Effects of Piper sarmentosum water extract on 11-β hydroxysteroid dehydrogenase type 1 bioactivity in ovariectomy-induced obese rats. Int. J. Pharmacol. 2009, 5, 362–369. [Google Scholar] [CrossRef] [Green Version]
- Rahman, N.N.N.A.; Furuta, T.; Kojima, S.; Takane, K.; Mohd, M.A. Antimalarial activity of extracts of Malaysian medicinal plants. J. Ethnopharmacol. 1999, 64, 249–254. [Google Scholar] [CrossRef]
- Subramaniam, V.; Adenan, M.I.; Ahmad, A.R.; Sahdan, R. Natural antioxidants: Piper sarmentosum (Kadok) and Morinda elliptica (Mengkudu). Malays. J. Nutr. 2003, 9, 41–51. [Google Scholar]
- Ridtitid, W.; Rattanaprom, W.; Thaina, P.; Chittrakarn, S.; Sunbhanich, M. Neuromuscular blocking activity of methanolic extract of Piper sarmentosum leaves in the rat phrenic nerve-hemidiaphragm preparation. J. Ethnopharmacol. 1998, 61, 135–142. [Google Scholar] [CrossRef]
- Amran, A.A.; Zakaria, Z.; Othman, F.; Das, S.; Raj, S.; Nordin, N.A.M. Aqueous extract of Piper sarmentosum decreases atherosclerotic lesions in high cholesterolemic experimental rabbits. Lipids Health Dis. 2010, 9, 44. [Google Scholar] [CrossRef] [Green Version]
- Zakaria, Z.A.; Patahuddin, H.; Mohamad, A.S.; Israf, D.A.; Sulaiman, M.R. In vivo anti-nociceptive and anti-inflammatory activities of the aqueous extract of the leaves of Piper sarmentosum. J. Ethnopharmacol. 2010, 128, 42–48. [Google Scholar] [CrossRef]
- Estai, M.A.; Suhaimi, F.H.; Das, S.; Fadzilah, F.M.; Alhabshi, S.M.I.; Shuid, A.N.; Soelaiman, I.N. Piper sarmentosum enhances fracture healing in ovariectomized osteoporotic rats: A radiological study. Clinics 2011, 66, 865–872. [Google Scholar] [CrossRef] [Green Version]
- Ramli, E.S.M.; Soelaiman, I.N.; Othman, F.; Ahmad, F.; Shuib, A.N.; Mohamed, N.; Muhammad, N.; Suhaimi, F.H. The effects of Piper sarmentosum water extract on the expression and activity of 11β-hydroxysteroid dehydrogenase type 1 in the bones with excessive glucocorticoids. Iran. J. Med. Sci. 2012, 37, 39–46. [Google Scholar]
- Li, J.; Zhang, N.; Huang, X.; Xu, J.; Fernandes, J.C.; Dai, K.; Zhang, X. Dexamethasone shifts bone marrow stromal cells from osteoblasts to adipocytes by C/EBPalpha promoter methylation. Cell Death Dis. 2013, 4, e832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takuma, A.; Kaneda, T.; Sato, T.; Ninomiya, S.; Kumegawa, M.; Hakeda, Y. Dexamethasone enhances osteoclast formation synergistically with transforming growth factor-beta by stimulating the priming of osteoclast progenitors for differentiation into osteoclast. J. Biol. Chem. 2003, 278, 44667–44674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klein, G.L. The effect of glucocorticoids on bone and muscle. Osteoporos. Sarcopenia 2015, 1, 39–45. [Google Scholar] [CrossRef] [Green Version]
- Ott, S.M. Histomorphometric measurements of bone turnover, mineralization, and volume. Clin. J. Am. Soc. Nephrol. 2008, 3, S151–S156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dalle Carbonare, L.; Bertoldo, F.; Valenti, M.T.; Zenari, S.; Zanatta, M.; Sella, S.; Giannini, S.; Lo Cascio, V. Histomorphometric analysis of glucocorticoid-induced osteoporosis. Micron 2005, 36, 645–652. [Google Scholar] [CrossRef]
- Kulak, C.A.M.; Dempster, D.W. Bone histomorphometry: A concise review for endocrinologists and clinicians. Arq. Bras. Endocrinol. Metabol. 2010, 54, 87–98. [Google Scholar] [CrossRef] [Green Version]
- Ima Nirwana, S.; Fakhrurazi, H. Palm Vitamin E protects bone against Dexamethasone-induced osteoporosis in male rats. Med. J. Malays. 2002, 57, 136–144. [Google Scholar]
- Lucinda, L.M.F.; Vieira, B.J.; Oliveira, T.T.; Sá, R.C.S.; Peters, V.M.; Reis, J.E.P.; Guerra, M.O. Evidences of osteoporosis improvement in Wistar rats treated with Ginkgo biloba extract: A histomorphometric study of mandible and femur. Fitoterapia 2010, 81, 982–987. [Google Scholar] [CrossRef]
- Ima-Nirwana, S.; Norazlina, M.; Khalid, B.A.K. Pattern of bone mineral density in growing male and female rats after gonadectomy. J. ASEAN Fed. Endocr. Soc. 1998, 16, 21–36. [Google Scholar]
- Elvy Suhana, M.R.; Farihah, H.S.; Faizah, O.; Nazrun, A.S.; Norazlina, M.; Norliza, M.; Ima-Nirwana, S. Effect of 11β-HSD1 dehydrogenase activity on bone histomorphometry of glucocorticoid-induced osteoporotic male Sprague-Dawley rats. Singap. Med. J. 2011, 52, 786–793. [Google Scholar]
- Baldock, P.A.; Morris, H.A.; Moore, R.J.; Need, A.G.; Durbridge, T.C. Prepubertal oophorectomy limits the accumulation of cancellous bone in the femur of growing rats with long-term effects on metaphyseal bone architecture. Calcif. Tissue Int. 1998, 62, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Ogoshi, T.; Hagino, H.; Fukata, S.; Tanishima, S.; Okano, T.; Teshima, R. Influence of glucocorticoid on bone in 3-, 6-, and 12-month-old rats as determined by bone mass and histomorphometry. Mod. Rheumatol. 2008, 18, 552–561. [Google Scholar] [CrossRef] [PubMed]
- Dempster, D.W.; Compston, J.E.; Drezner, M.K.; Glorieux, F.H.; Kanis, J.A.; Malluche, H.; Meunier, P.J.; Ott, S.M.; Recker, R.R.; Parfitt, A.M. Standardized nomenclature, symbols, and units for bone histomorphometry: A 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J. Bone Miner. Res. 2013, 28, 2–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freere, R.H.; Weibel, E.R. Stereologic techniques in microscopy. J. Microsc. 1967, 87, 25–34. [Google Scholar] [CrossRef]
- Ima-Nirwana, S.; Suhaniza, S. Effects of tocopherols and tocotrienols on body composition and bone calcium content in adrenalectomized rats replaced with dexamethasone. J. Med. Food 2004, 7, 45–51. [Google Scholar] [CrossRef]
- Liu, Y.; Cui, Y.; Chen, Y.; Gao, X.; Su, Y.; Cui, L. Effects of dexamethasone, celecoxib, and methotrexate on the histology and metabolism of bone tissue in healthy Sprague Dawley rats. Clin. Interv. Aging 2015, 10, 1245–1253. [Google Scholar] [CrossRef] [Green Version]
- Rauch, A.; Seitz, S.; Baschant, U.; Schilling, A.F.; Illing, A.; Stride, B.; Kirilov, M.; Mandic, V.; Takacz, A.; Schmidt-Ullrich, R.; et al. Glucocorticoids suppress bone formation by attenuating osteoblast differentiation via the monomeric glucocorticoid receptor. Cell Metab. 2010, 11, 517–531. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.; Qi, H.; Zhong, Y.; Lv, S.; Yu, J.; Liu, J.; Wang, L.; Bi, J.; Kong, X.; Di, W.; et al. 11β-Hydroxysteroid dehydrogenase type 1 selective inhibitor BVT.2733 protects osteoblasts against endogenous glucocorticoid induced dysfunction. Endocr. J. 2013, 60, 1047–1058. [Google Scholar] [CrossRef] [Green Version]
- Weinstein, R.S. Glucocorticoid-induced osteoporosis and osteonecrosis. Endocrinol. Metab. Clin. N. Am. 2012, 41, 595–611. [Google Scholar] [CrossRef] [Green Version]
- Hussain, K.; Hashmi, F.K.; Latif, A.; Ismail, Z.; Sadikun, A. A review of the literature and latest advances in research of Piper sarmentosum. Pharm. Biol. 2012, 50, 1045–1052. [Google Scholar] [CrossRef] [PubMed]
- Horcajada, M.N.; Habauzit, V.; Trzeciakiewicz, A.; Morand, C.; Gil-Izquierdo, A.; Mardon, J.; Lebecque, P.; Davicco, M.J.; Chee, W.S.S.; Coxam, V.; et al. Hesperidin inhibits ovariectomized-induced osteopenia and shows differential effects on bone mass and strength in young and adult intact rats. J. Appl. Physiol. 2008, 104, 648–654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ima-Nirwana, S.; Elvy-Suhana, M.R.; Faizah, O.; Farihah, S. Effects of Piper sarmentosum on bone resorption and its relationship to plasma cortisol in rats. Bone 2009, 44, S79–S80. [Google Scholar] [CrossRef]
- Azri, M.; Shuid, A.; Muhammad, N. Role of medicinal plants and natural products on osteoporotic fracture healing. Evid. Based Complement. Altern. Med. 2012, 2012, 714512. [Google Scholar] [CrossRef]
- Sumazian, Y.; Syahida, A.; Hakiman, M.; Maziah, M. Antioxidant activities, flavonoids, ascorbic acid and phenolic content of Malaysian vegetables. J. Med. Plants Res. 2010, 4, 881–890. [Google Scholar]
- Ugusman, A.; Zakaria, Z.; Hui, C.K.; Nordin, N.A.M.M.; Mahdy, Z.A. Flavonoids of Piper sarmentosum and its cytoprotective effects against oxidative stress. EXCLI J. 2012, 11, 705–714. [Google Scholar]
- Srivastava, S.; Bankar, R.; Roy, P. Assessment of the role of flavonoids for inducing osteoblast differentiation in isolated mouse bone marrow derived mesenchymal stem cells. Phytomedicine 2013, 20, 683–690. [Google Scholar] [CrossRef]
- An, J.; Yang, H.; Zhang, Q.; Liu, C.; Zhao, J.; Zhang, L.; Chen, B. Natural products for treatment of osteoporosis: The effects and mechanisms on promoting osteoblast-mediated bone formation. Life Sci. 2016, 147, 46–58. [Google Scholar] [CrossRef]
- Fiore, C.; Eisenhut, M.; Ragazzi, E.; Zanchin, G.; Armanini, D. A history of the therapeutic use of liquorice in Europe. J. Ethnopharmacol. 2005, 99, 317–324. [Google Scholar] [CrossRef]
- Ploeger, B.; Mensinga, T.; Sips, A.; Seinen, W.; Meulenbelt, J.; DeJongh, J. The pharmacokinetics of glycyrrhizic acid evaluated by physiologically based pharmacokinetic modeling. Drug Metab. Rev. 2001, 33, 125–147. [Google Scholar] [CrossRef]
- Mohamad Asri, S.F.; Mohd Ramli, E.S.; Soelaiman, I.N.; Mat Noh, M.A.; Abdul Rashid, A.H.; Suhaimi, F. Piper sarmentosum effects on 11β-hydroxysteroid dehydrogenase type 1 enzyme in serum and bone in rat model of glucocorticoid-induced osteoporosis. Molecules 2016, 21, 1523. [Google Scholar] [CrossRef] [Green Version]
- Nirwana, S.I.; Suhana, M.R.; Fadziyah, M.A.; Farihah, H.S.; Fairus, A.; Alfakri, M.N. Piper sarmentosum improves bone structure and biomechanical strength of rats given excess glucocorticoid. J. Pharm. Res. Int. 2012, 2, 168–187. [Google Scholar] [CrossRef]
- Estai, M.A.; Suhaimi, F.; Shuid, A.N.; Das, S.; Soelaiman, I.N. The use of traditional treatment modalities with special mention of Piper sarmentosum in treatment of bone fracture. J. Med. Plants Res. 2011, 5, 7132–7139. [Google Scholar] [CrossRef]
- Estai, M.A.; Suhaimi, F.; Shuid, A.N.; Das, S.; Abdullah, S.; Soelaiman, I.N. Biomechanical evaluation of fracture healing following administration of Piper sarmentosum in ovariectomised rats. Afr. J. Pharm. Pharmacol. 2012, 6, 144–147. [Google Scholar] [CrossRef]
- Maizura, M.Z.; Zakaria, Z.; Nor Anita Megat, M.N.; Othman, F. Does oral ingestion of Piper sarmentosum cause toxicity in experimental animals? Evid. Based Complement. Altern. Med. 2013, 2013, 705950. [Google Scholar] [CrossRef] [Green Version]
- Chanwitheesuk, A.; Teerawutgulrag, A.; Rakariyatham, N. Screening of antioxidant activity and antioxidant compounds of some edible plants of Thailand. Food Chem. 2005, 92, 491–497. [Google Scholar] [CrossRef]



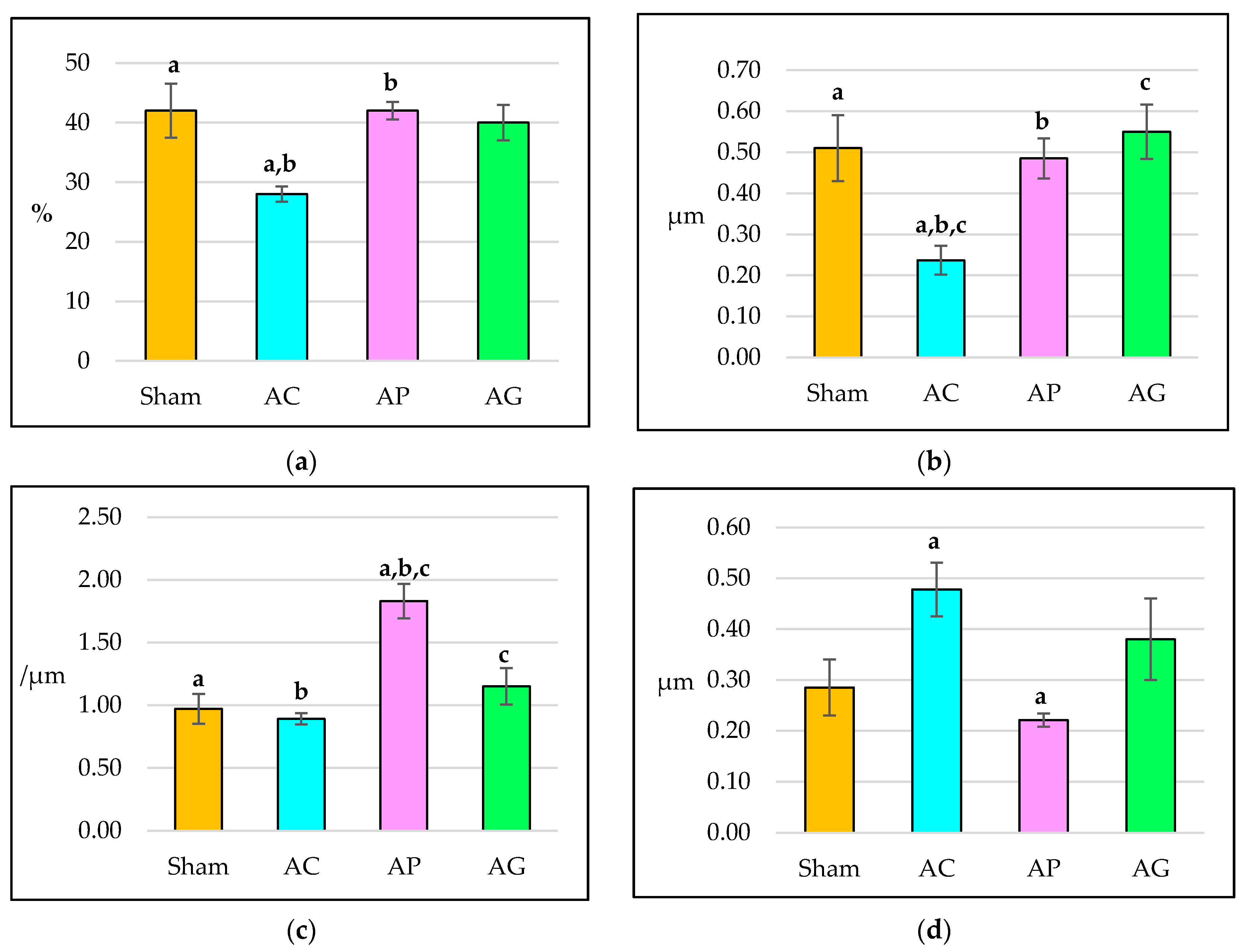


| Parameter | Sham | AC | AP | AG | ||||
|---|---|---|---|---|---|---|---|---|
| Mean ± SEM | SD | Mean ± SEM | SD | Mean ± SEM | SD | Mean ± SEM | SD | |
| BV/TV (%) | 42 ± 4.53 | 11.09 | 28 ± 1.29 | 3.16 | 42 ± 1.49 | 3.66 | 40 ± 2.97 | 7.27 |
| Tb.Th (μm) | 0.51 ± 0.08 | 0.20 | 0.24 ± 0.04 | 0.09 | 0.49 ± 0.05 | 0.12 | 0.55 ± 0.07 | 0.16 |
| Tb.N (/μm) | 0.97 ± 0.12 | 0.18 | 0.89 ± 0.05 | 0.12 | 1.83 ± 0.14 | 0.21 | 1.15 ± 0.15 | 0.22 |
| Tb.Sp (μm) | 0.29 ± 0.06 | 0.13 | 0.48 ± 0.05 | 0.13 | 0.22 ± 0.01 | 0.03 | 0.38 ± 0.08 | 0.20 |
| Parameter | Sham | AC | AP | AG | ||||
|---|---|---|---|---|---|---|---|---|
| Mean ± SEM | SD | Mean ± SEM | SD | Mean ± SEM | SD | Mean ± SEM | SD | |
| OV/BV (%) | 15.01 ± 2.21 | 5.42 | 10.40 ± 1.08 | 2.65 | 19.85 ± 3.10 | 7.59 | 16.60 ± 3.35 | 8.21 |
| OS/BS (%) | 11.04 ± 1.08 | 2.64 | 8.63 ± 0.82 | 2.00 | 14.27 ± 1.39 | 3.41 | 14.58 ± 1.55 | 3.80 |
| Ob.S (%) | 2.51 ± 0.35 | 0.86 | 1.28 ± 0.10 | 0.25 | 2.47 ± 0.31 | 0.76 | 2.30 ± 0.29 | 0.71 |
| Oc.S (%) | 0.97 ± 0.16 | 0.39 | 1.25 ± 0.09 | 0.23 | 0.63 ± 0.08 | 0.19 | 0.72 ± 0.04 | 0.09 |
| Parameter | Sham | AC | AP | AG | ||||
|---|---|---|---|---|---|---|---|---|
| Mean ± SEM | SD | Mean ± SEM | SD | Mean ± SEM | SD | Mean ± SEM | SD | |
| sLS/BS (%) | 4.06 ± 0.24 | 0.59 | 2.57 ± 0.26 | 0.64 | 3.80 ± 0.29 | 0.70 | 3.66 ± 0.28 | 0.69 |
| dLS/BS (%) | 4.37 ± 0.42 | 1.04 | 2.68 ± 0.29 | 0.70 | 4.55 ± 0.36 | 0.87 | 4.37 ± 0.36 | 0.87 |
| MS/BS (%) | 16.19 ± 1.34 | 3.29 | 9.47 ± 1.32 | 3.22 | 18.67 ± 2.46 | 6.01 | 13.01 ± 1.43 | 3.49 |
| MAR (μm/day) | 0.63 ± 0.03 | 0.01 | 0.56 ± 0.04 | 0.01 | 0.76 ± 0.06 | 0.01 | 0.87 ± 0.05 | 0.01 |
| BFR/BS (µm3/µm2/d) | 0.03 ± 0.004 | 0.01 | 0.02 ± 0.003 | 0.01 | 0.05 ± 0.010 | 0.02 | 0.04 ± 0.006 | 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohamad Asri, S.F.; Soelaiman, I.N.; Mohd Moklas, M.A.; Mohd Nor, N.H.; Mohamad Zainal, N.H.; Mohd Ramli, E.S. The Role of Piper sarmentosum Aqueous Extract as a Bone Protective Agent, a Histomorphometric Study. Int. J. Mol. Sci. 2020, 21, 7715. https://doi.org/10.3390/ijms21207715
Mohamad Asri SF, Soelaiman IN, Mohd Moklas MA, Mohd Nor NH, Mohamad Zainal NH, Mohd Ramli ES. The Role of Piper sarmentosum Aqueous Extract as a Bone Protective Agent, a Histomorphometric Study. International Journal of Molecular Sciences. 2020; 21(20):7715. https://doi.org/10.3390/ijms21207715
Chicago/Turabian StyleMohamad Asri, Siti Fadziyah, Ima Nirwana Soelaiman, Mohamad Aris Mohd Moklas, Nurul Huda Mohd Nor, Nurul Hayati Mohamad Zainal, and Elvy Suhana Mohd Ramli. 2020. "The Role of Piper sarmentosum Aqueous Extract as a Bone Protective Agent, a Histomorphometric Study" International Journal of Molecular Sciences 21, no. 20: 7715. https://doi.org/10.3390/ijms21207715
APA StyleMohamad Asri, S. F., Soelaiman, I. N., Mohd Moklas, M. A., Mohd Nor, N. H., Mohamad Zainal, N. H., & Mohd Ramli, E. S. (2020). The Role of Piper sarmentosum Aqueous Extract as a Bone Protective Agent, a Histomorphometric Study. International Journal of Molecular Sciences, 21(20), 7715. https://doi.org/10.3390/ijms21207715





